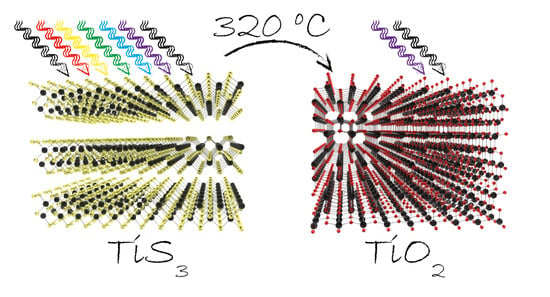
Tunable Photodetectors via In Situ Thermal Conversion of TiS3 to TiO2
Abstract
1. Introduction
2. Materials Synthesis
3. Experimental Results and Discussion
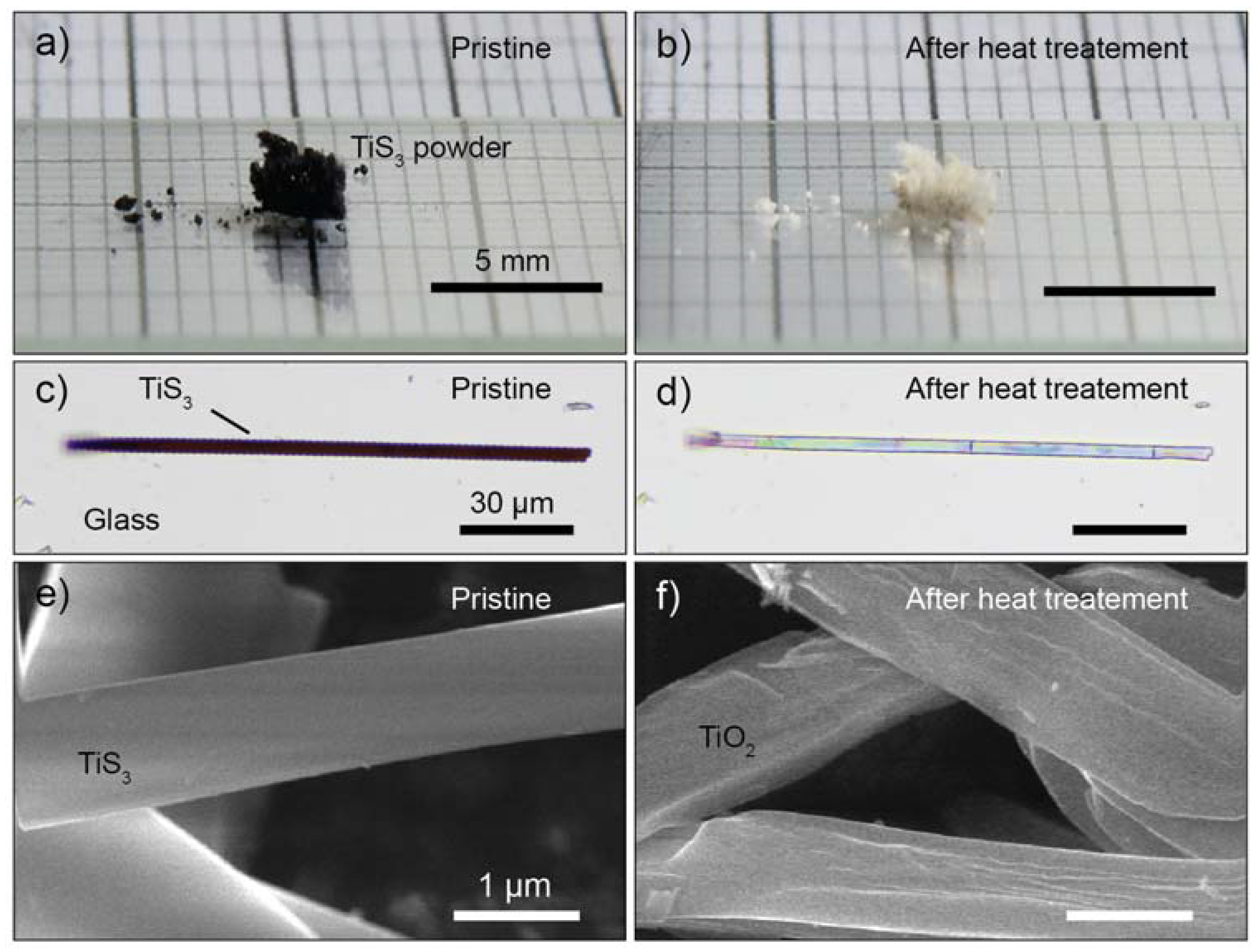
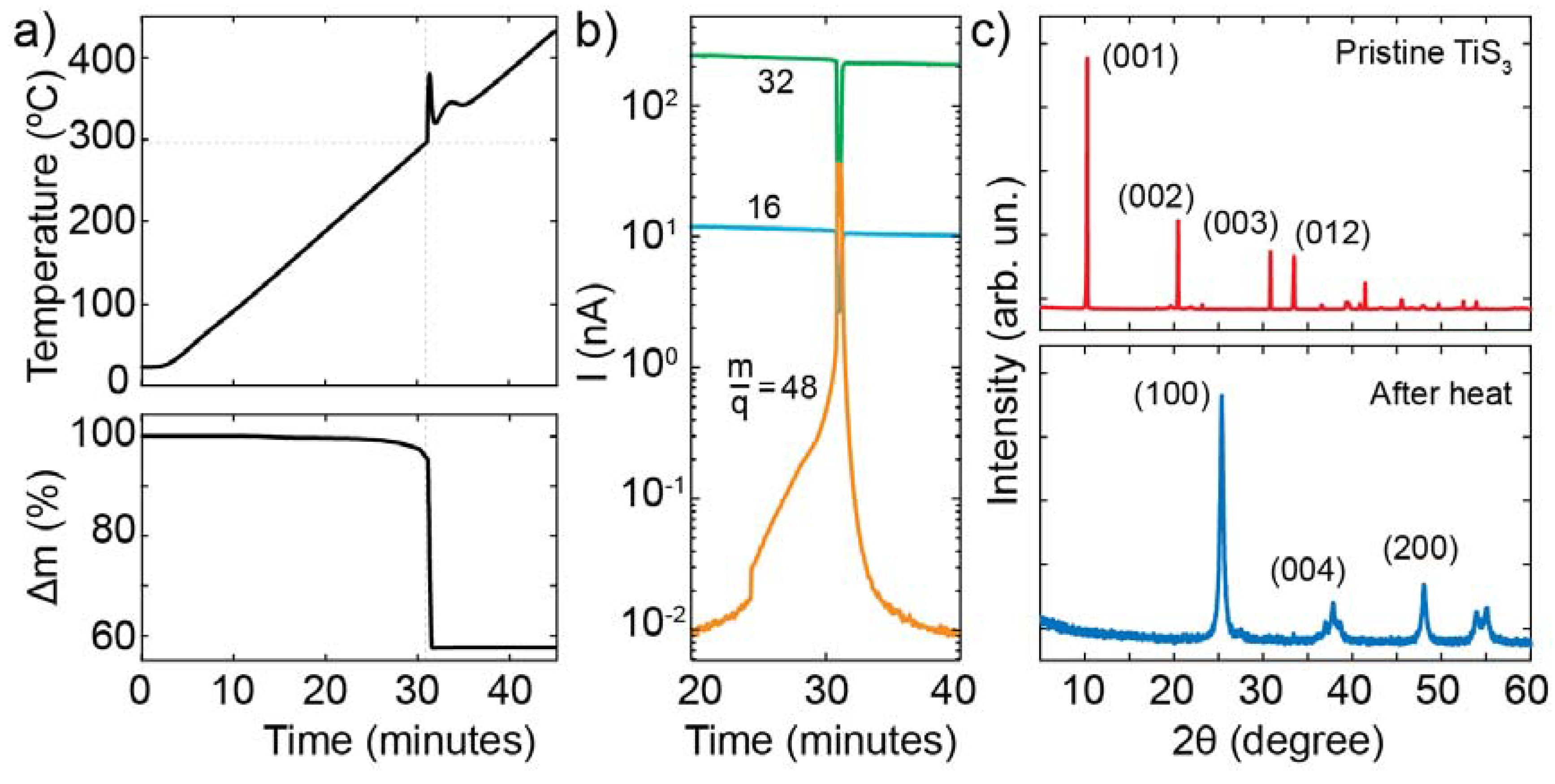
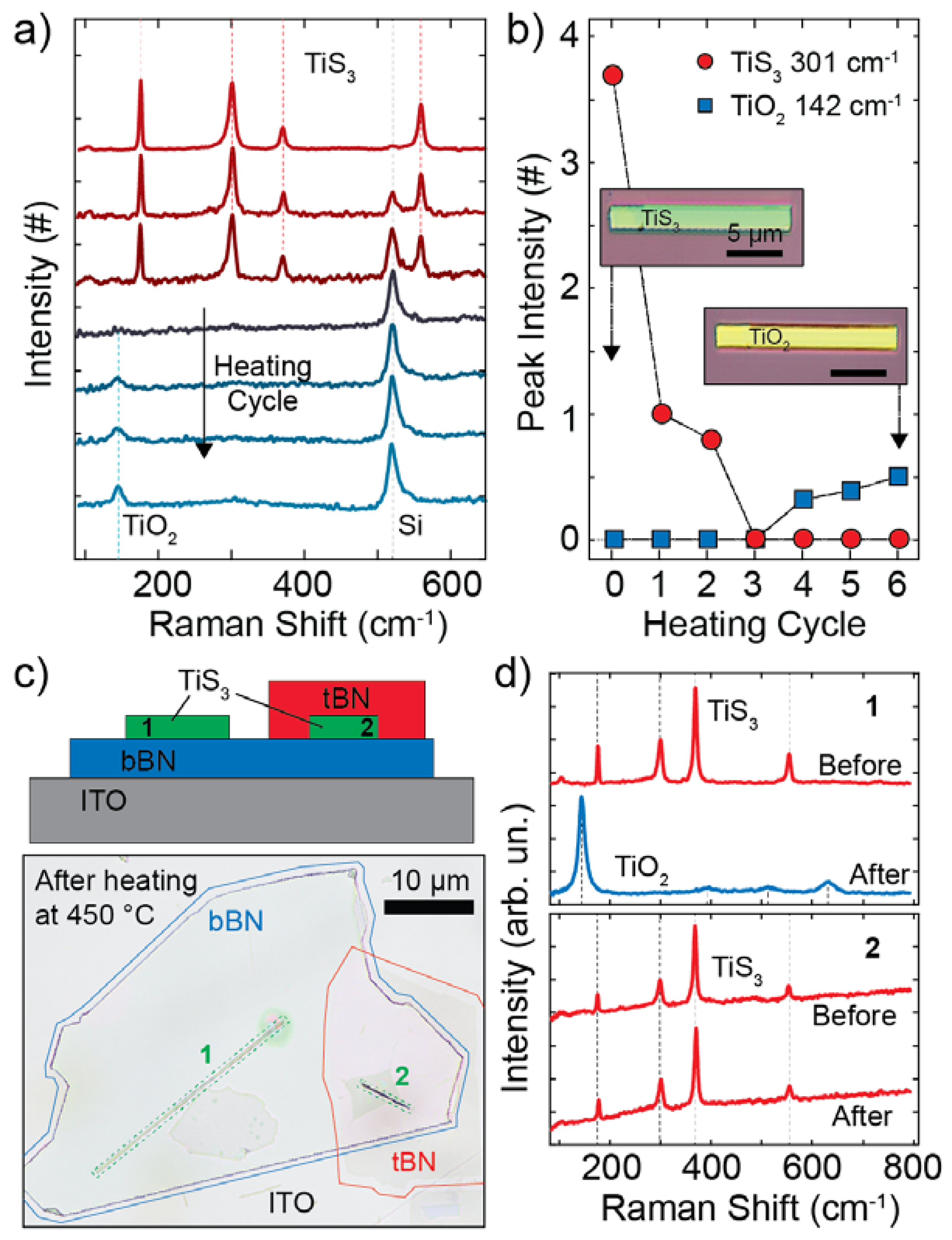
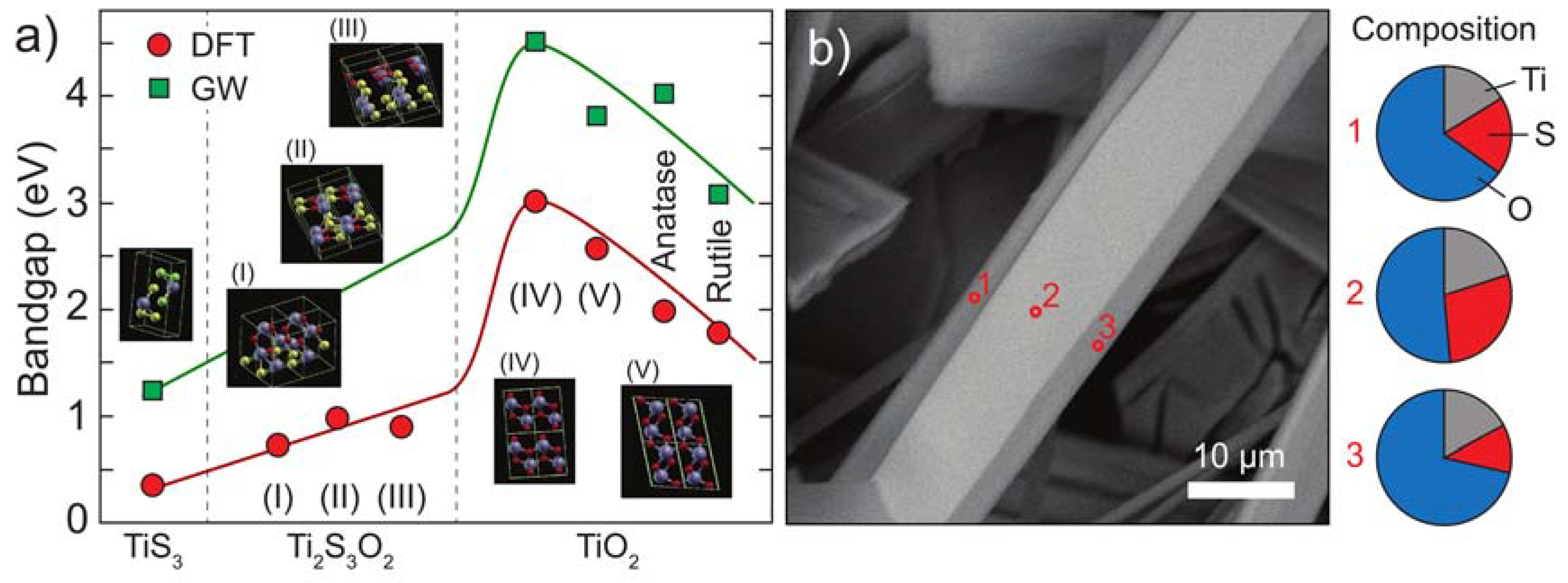
3.1. Thermal Oxidation Analysis
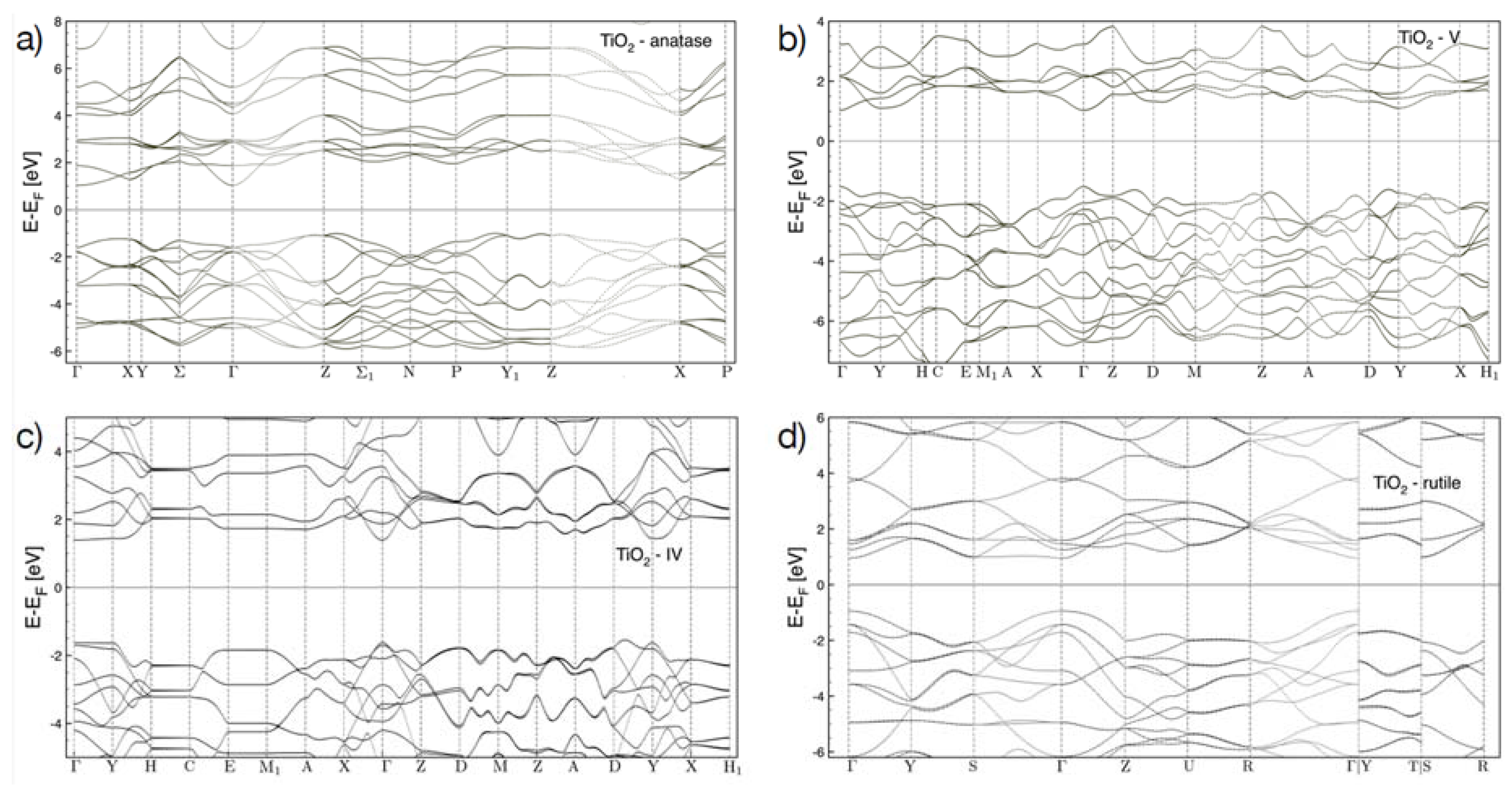
3.2. Bandgap Energy Calculation
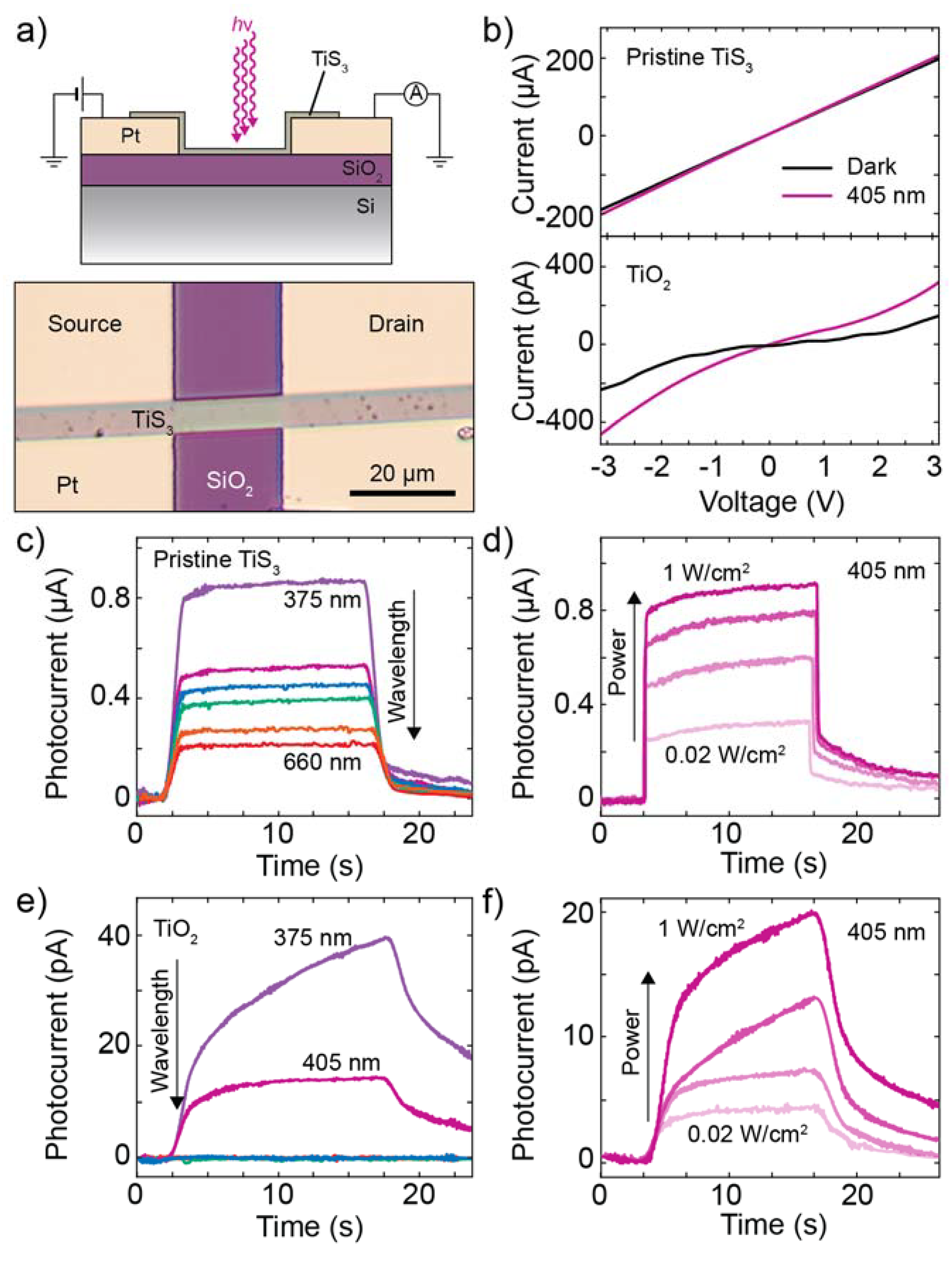
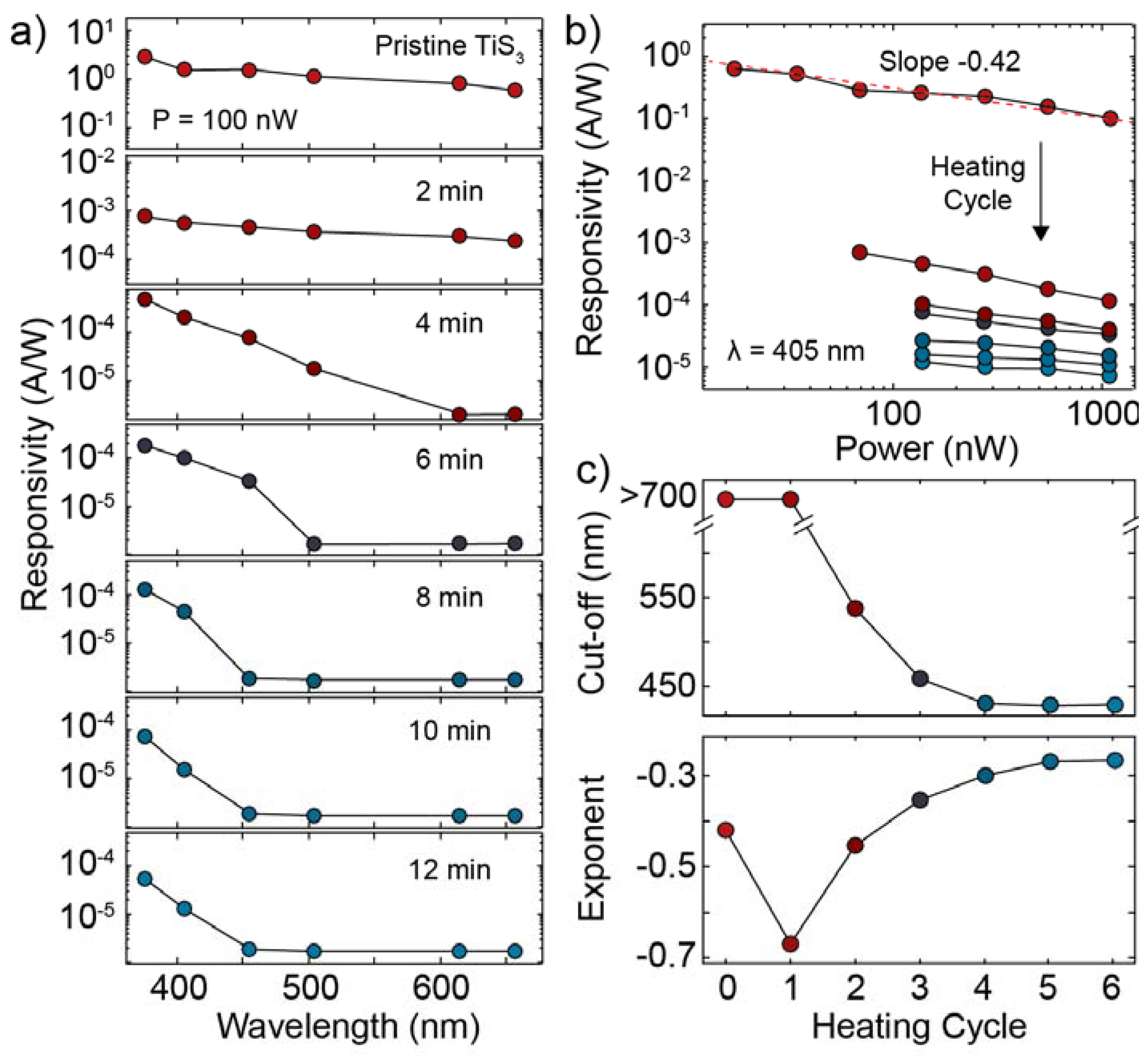
3.3. Electronic and Optoelectronic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, H.; Giustiniano, F.; Eda, G. ChemInform Abstract: Electronic Transport Properties of Transition Metal Dichalcogenide Field-Effect Devices: Surface and Interface Effects. Chem. Soc. Rev. 2015, 46, 7715–7736. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Mishchenko, A.; Carvalho, A.; Neto, A.C. 2D materials and van der Waals heterostruc-tures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed]
- Frinsenda, R.; Molina-Mendoza, A.J.; Mueller, T.; Castellanos-Gómez, A.; Van Der Zant, H.S.J. Atomically thin p–n junctions based on two-dimensional materials. Chem. Soc. Rev. 2018, 47, 3339–3358. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Favron, A.; Gaufrès, E.; Fossard, F.; Phaneuf-L’Heureux, A.-L.; Tang, N.Y.-W.; Levesque, P.L.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat. Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef]
- Wang, G.; Pandey, R.; Karna, S.P. Physics and chemistry of oxidation of two-dimensional nanomaterials by molecular oxygen. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 7, e1280. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S.-H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Shi, L.; Chen, Q.; Wang, J. Recent advances in oxidation and degradation mechanisms of ultrathin 2D materials under ambient conditions and their passivation strategies. J. Mater. Chem. A 2019, 7, 4291–4312. [Google Scholar] [CrossRef]
- Island, J.; A Steele, G.; Van Der Zant, H.S.J.; Castellanos-Gómez, A. Environmental instability of few-layer black phosphorus. 2D Mater. 2015, 2, 11002. [Google Scholar] [CrossRef]
- Kuriakose, S.; Ahmed, T.; Balendhran, S.; Bansal, V.; Sriram, S.; Bhaskaran, M.; Walia, S. Black phos-phorus: Ambient degradation and strategies for protection. 2D Mater. 2018, 5, 032001. [Google Scholar] [CrossRef]
- Cartamil-Bueno, S.J.; Steeneken, P.G.; Tichelaar, F.D.; Moratalla, E.N.; Venstra, W.J.; Van Leeuwen, R.; Coronado, E.; Van Der Zant, H.S.J.; Steele, G.A.; Castellanos-Gómez, A. High-quality-factor tantalum oxide nanomechanical resonators by laser oxidation of TaSe2. Nano Res. 2015, 8, 2842–2849. [Google Scholar] [CrossRef]
- De Sanctis, A.; Amit, I.; Hepplestone, S.; Craciun, M.F.; Russo, S. Strain-engineered inverse charge-funnelling in layered semiconductors. Nat. Commun. 2018, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Peimyoo, N.; Barnes, M.D.; Mehew, J.D.; De Sanctis, A.; Amit, I.; Escolar, J.; Anastasiou, K.; Rooney, A.P.; Haigh, S.J.; Russo, S.; et al. Laser-writable high-k dielectric for van der Waals nanoelectronics. Sci. Adv. 2019, 5, eaau0906. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, R.; Matsumoto, M.; Mitoma, N. Gate-controlled photo-oxidation of graphene for electronic structure modification. J. Mater. Chem. C 2019, 7, 1904–1912. [Google Scholar] [CrossRef]
- Island, J.; Barawi, M.; Biele, R.; Almazán, A.; Clamagirand, J.M.; Ares, J.R.; Sanchez, C.; Van Der Zant, H.S.J.; Alvarez, J.; D’Agosta, R.; et al. TiS3Transistors with Tailored Morphology and Electrical Properties. Adv. Mater. 2015, 27, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Island, J.; Biele, R.; Barawi, M.; Clamagirand, J.M.; Ares, J.R.; Sánchez, C.; Van Der Zant, H.S.J.; Ferrer, I.J.; D’Agosta, R.; Castellanos-Gómez, A. Titanium trisulfide (TiS3): A 2D semiconductor with quasi-1D optical and electronic properties. Sci. Rep. 2016, 6, 22214. [Google Scholar] [CrossRef]
- Island, J.; Buscema, M.; Barawi, M.; Clamagirand, J.M.; Ares, J.R.; Sanchez, C.; Ferrer, I.J.; Steele, G.A.; Van Der Zant, H.S.J.; Castellanos-Gómez, A. Ultrahigh Photoresponse of Few-Layer TiS3Nanoribbon Transistors. Adv. Opt. Mater. 2014, 2, 641–645. [Google Scholar] [CrossRef]
- Lipatov, A.; Wilson, P.M.; Shekhirev, M.; Teeter, J.; Netusil, R.; Sinitskii, A. Few-layered titanium trisulfide (TiS3) field-effect transistors. Nanoscale 2015, 7, 12291–12296. [Google Scholar] [CrossRef]
- Molina-Mendoza, A.J.; Barawi, M.; Biele, R.; Flores, E.; Ares, J.R.; Sánchez, C.; Rubio-Bollinger, G.; Agraït, N.; D’Agosta, R.; Ferrer, I.J.; et al. Electronic Bandgap and Exciton Binding Energy of Layered Semiconductor TiS3. Adv. Electron. Mater. 2015, 1, 1500126. [Google Scholar] [CrossRef]
- Biele, R.; Flores, E.; Ares, J.-R.; Sánchez, C.; Ferrer, I.J.; Rubio-Bollinger, G.; Castellanos-Gomez, A.; D’Agosta, R. Strain induced bang-gap engineering in layered TiS3TiS3. Nano Res. 2015, 11, 225–232. [Google Scholar] [CrossRef]
- Dai, J.; Zeng, X.C. Titanium Trisulfide Monolayer: Theoretical Prediction of a New Direct-Gap Semi-conductor with High and Anisotropic Carrier Mobility. Angew. Chem. 2015, 127, 7682–7686. [Google Scholar] [CrossRef]
- Ferrer, I.J.; Ares, J.; Clamagirand, J.; Barawi, M.; Sanchez, C. Optical properties of titanium trisulphide (TiS3) thin films. Thin Solid Films 2013, 535, 398–401. [Google Scholar] [CrossRef]
- Jin, Y.; Li, X.; Yang, J. Single layer of MX 3 (M = Ti, Zr; X = S, Se, Te): A new platform for nano-electronics and optics. Phys. Chem. Chem. Phys. 2015, 17, 18665–18669. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Sahin, H.; Ozaydin, H.D.; Senger, T.; Peeters, F.M. TiS3nanoribbons: Width-independent band gap and strain-tunable electronic properties. Phys. Rev. B 2015, 92, 075413. [Google Scholar] [CrossRef]
- Li, M.; Dai, J.; Zeng, X.C. Tuning the electronic properties of transition-metal trichalcogenides via tensile strain. Nanoscale 2015, 7, 15385–15391. [Google Scholar] [CrossRef]
- Iyikanat, F.; Sahin, H.; Senger, R.T.; Peeters, F.M.; Senger, T. Vacancy Formation and Oxidation Characteristics of Single Layer TiS3. J. Phys. Chem. C 2015, 119, 10709–10715. [Google Scholar] [CrossRef]
- Miro, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photo-catalyst than rutile?-Model studies on epitaxial TiO 2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Mattsson, A.; Österlund, L. Adsorption and Photoinduced Decomposition of Acetone and Acetic Acid on Anatase, Brookite, and Rutile TiO2 Nanoparticles. J. Phys. Chem. C 2010, 114, 14121–14132. [Google Scholar] [CrossRef]
- Flores, E.; Ares, J.; Ferrer, I.; Sánchez, C. Synthesis and characterization of a family of layered trichalco-genides for assisted hydrogen photogeneration. Rapid Res. Lett. 2016, 10, 802–806. [Google Scholar]
- Molina-Mendoza, A.J.; Island, J.O.; Paz, W.S.; Clamagirand, J.M.; Ares, J.R.; Flores, E.; Leardini, F.; Sánchez, C.; Agraït, N.; Rubio-Bollinger, G. High Current Density Electrical Breakdown of TiS3 Nano-ribbon-Based Field-Effect Transistors. Adv. Funct. Mater. 2017, 27, 1605647. [Google Scholar] [CrossRef]
- Frinsenda, R.; Moratalla, E.N.; Gant, P.; De Lara, D.P.; Jarillo-Herrero, P.; Gorbachev, R.V.; Castellanos-Gómez, A. Recent progress in the assembly of nanodevices and van der Waals heterostructures by deterministic placement of 2D materials. Chem. Soc. Rev. 2018, 47, 53–68. [Google Scholar] [CrossRef]
- Castellanos-Gómez, A.; Buscema, M.; Molenaar, R.; Singh, V.; Janssen, L.; Van Der Zant, H.S.J.; A Steele, G. Deterministic transfer of two-dimensional materials by all-dry viscoelastic stamping. 2D Mater. 2014, 1, 11002. [Google Scholar] [CrossRef]
- Pawbake, A.S.; Island, J.O.; Flores, E.; Ares, J.R.; Sanchez, C.; Ferrer, I.J.; Jadkar, S.R.; van der Zant, H.S.; Castellanos-Gomez, A.; Late, D.J. Temperature-Dependent Raman Spectroscopy of Titanium Tri-sulfide (TiS3) Nanoribbons and Nanosheets. ACS Appl. Mater. Interfaces 2015, 7, 24185–24190. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Liu, C.; Yu, A.; Peng, M.; Zhai, J.; Zhang, K. Raman study of 2D anatase TiO 2 nanosheets. Phys. Chem. Chem. Phys. 2016, 18, 32178–32184. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and open-source software project for quan-tum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Hartwigsen, C.; Goedecker, S.; Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 1998, 58, 3641–3662. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, K.; Kalish, I.; Beams, R.; Tavazza, F. High-throughput Identification and Characterization of Two-dimensional Materials using Density functional theory. Sci. Rep. 2017, 7, 5179. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, I.G.; Pokrovskii, V.Y.; Zybtsev, S.G.; Titov, A.; Timofeev, V.N. Features of the conductivity of the quasi-one-dimensional compound TiS3. J. Exp. Theor. Phys. 2010, 111, 298–303. [Google Scholar] [CrossRef]
- Molina-Mendoza, A.; Moya, A.; Frisenda, R.; Svatek, S.A.; Gant, P.; Gonzalez-Abad, S.; Antolín, E.; Agrait, N.; Bollinger, G.R.; de Lara, D.P. Highly responsive UV-photodetectors based on single electro-spun TiO2 nanofibres. J. Mater. Chem. C 2016, 4, 10707–10714. [Google Scholar] [CrossRef]
- Albert, R. Concepts in Photoconductivity and Allied Problems; No. 19; Interscience Publishers: New York, NY, USA, 1963. [Google Scholar]
- Bube, R.H. Photoelectronic Properties of Semiconductors; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Arsentev, M.; Petrov, A.; Missyul, A.; Hammouri, M. Exfoliation, point defects and hydrogen storage properties of monolayer TiS3: An ab initio study. RSC Adv. 2018, 8, 26169–26179. [Google Scholar] [CrossRef]
- Furchi, M.M.; Polyushkin, D.K.; Pospischil, A.; Mueller, T. Mechanisms of photoconductivity in atomi-cally thin MoS2. Nano Lett. 2014, 14, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, F.; Frisenda, R.; Flores, E.; Papadopoulos, N.; Biele, R.; Perez de Lara, D.; van der Zant, H.S.J.; Watanabe, K.; Taniguchi, T.; D’Agosta, R.; et al. Tunable Photodetectors via In Situ Thermal Conversion of TiS3 to TiO2. Nanomaterials 2020, 10, 711. https://doi.org/10.3390/nano10040711
Ghasemi F, Frisenda R, Flores E, Papadopoulos N, Biele R, Perez de Lara D, van der Zant HSJ, Watanabe K, Taniguchi T, D’Agosta R, et al. Tunable Photodetectors via In Situ Thermal Conversion of TiS3 to TiO2. Nanomaterials. 2020; 10(4):711. https://doi.org/10.3390/nano10040711
Chicago/Turabian StyleGhasemi, Foad, Riccardo Frisenda, Eduardo Flores, Nikos Papadopoulos, Robert Biele, David Perez de Lara, Herre S. J. van der Zant, Kenji Watanabe, Takashi Taniguchi, Roberto D’Agosta, and et al. 2020. "Tunable Photodetectors via In Situ Thermal Conversion of TiS3 to TiO2" Nanomaterials 10, no. 4: 711. https://doi.org/10.3390/nano10040711
APA StyleGhasemi, F., Frisenda, R., Flores, E., Papadopoulos, N., Biele, R., Perez de Lara, D., van der Zant, H. S. J., Watanabe, K., Taniguchi, T., D’Agosta, R., Ares, J. R., Sánchez, C., Ferrer, I. J., & Castellanos-Gomez, A. (2020). Tunable Photodetectors via In Situ Thermal Conversion of TiS3 to TiO2. Nanomaterials, 10(4), 711. https://doi.org/10.3390/nano10040711