A Novel Nanocomposite of Activated Serpentine Mineral Decorated with Magnetic Nanoparticles for Rapid and Effective Adsorption of Hazardous Cationic Dyes: Kinetics and Equilibrium Studies
Abstract
1. Introduction
2. Materials and Methods
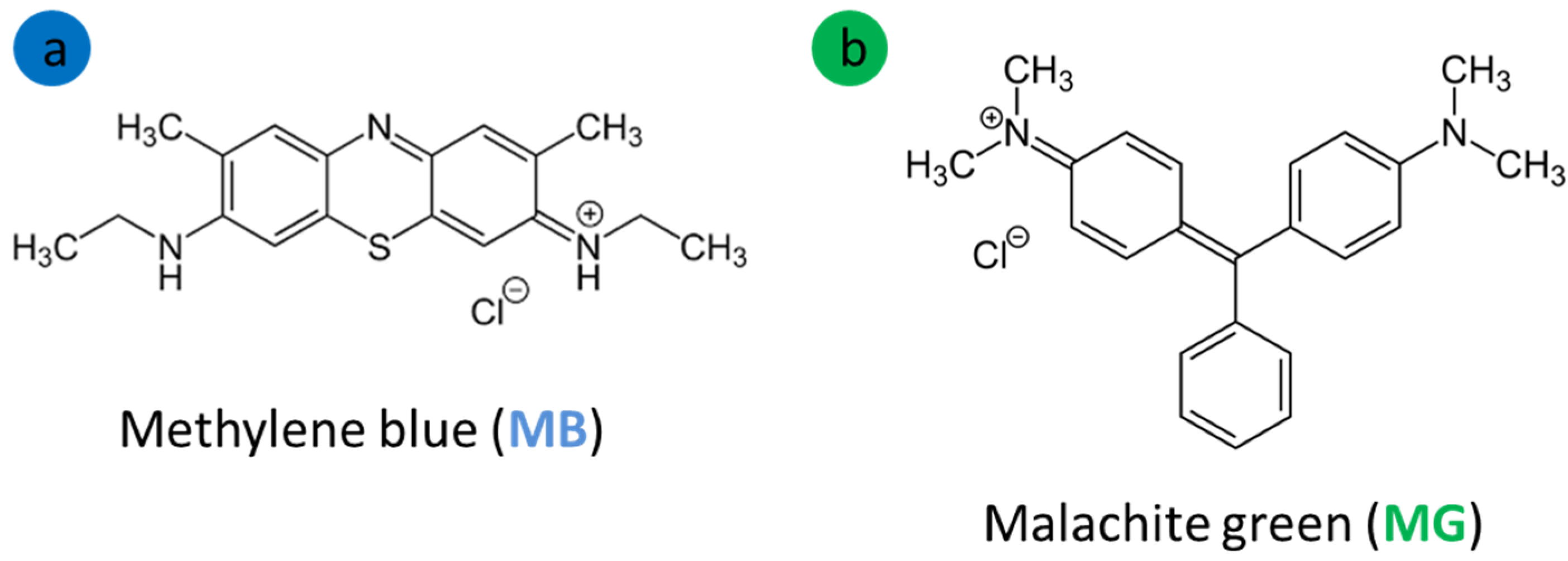
2.1. Materials
2.2. Preparation of the Composite Adsorbent
2.3. Characterizations
2.4. Adsorption Experiments
2.5. Fitting of the Adsorption Data
2.6. Salinity Effect on Adsorption
2.7. Reusability – Adsorption/Desorption Cycles
3. Results and Discussions
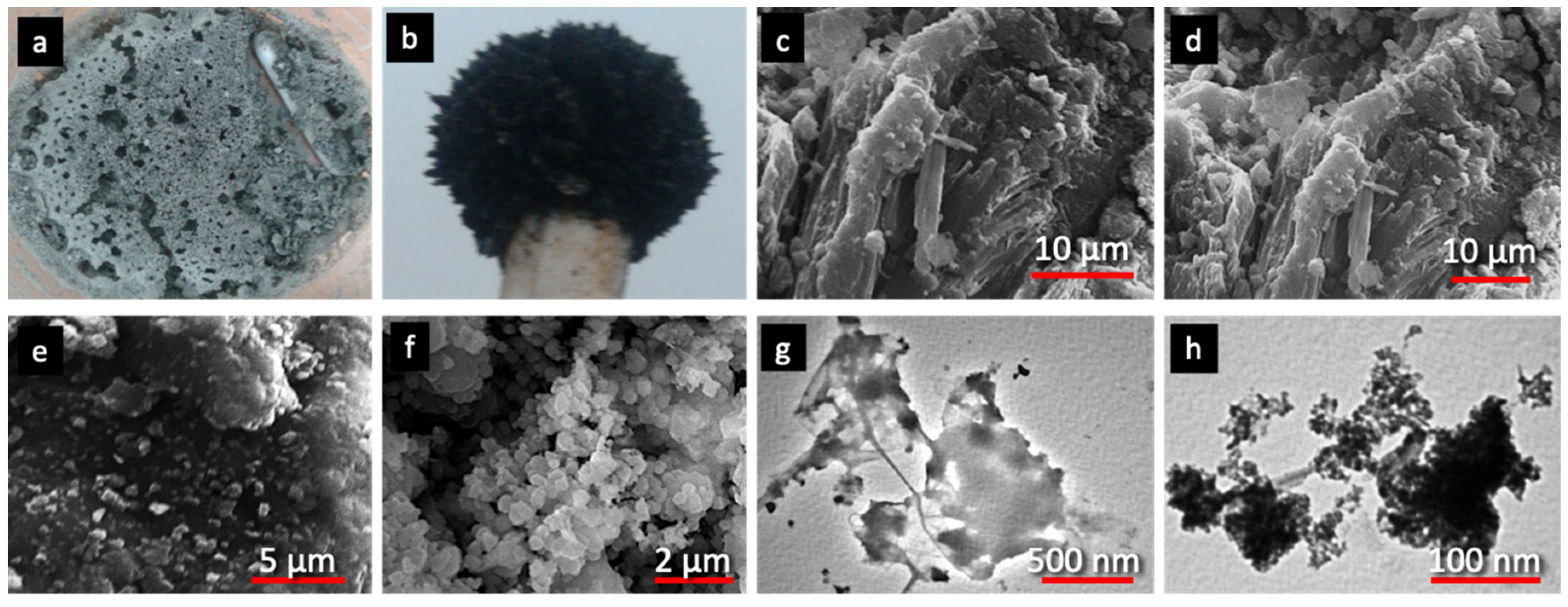
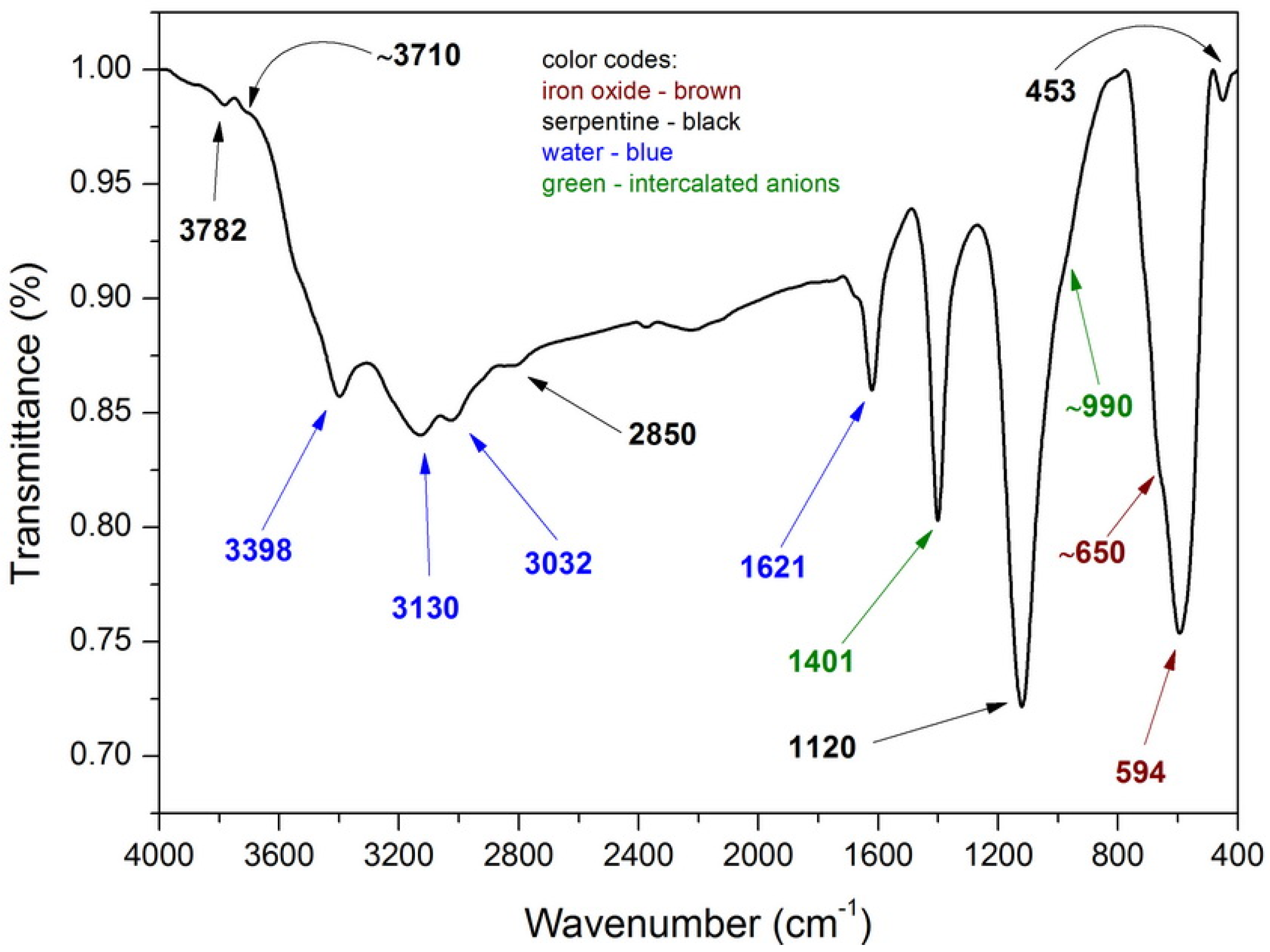
3.1. Characterization of the Fe3O4/activated serpentine (MNP/SP) Adsorbent
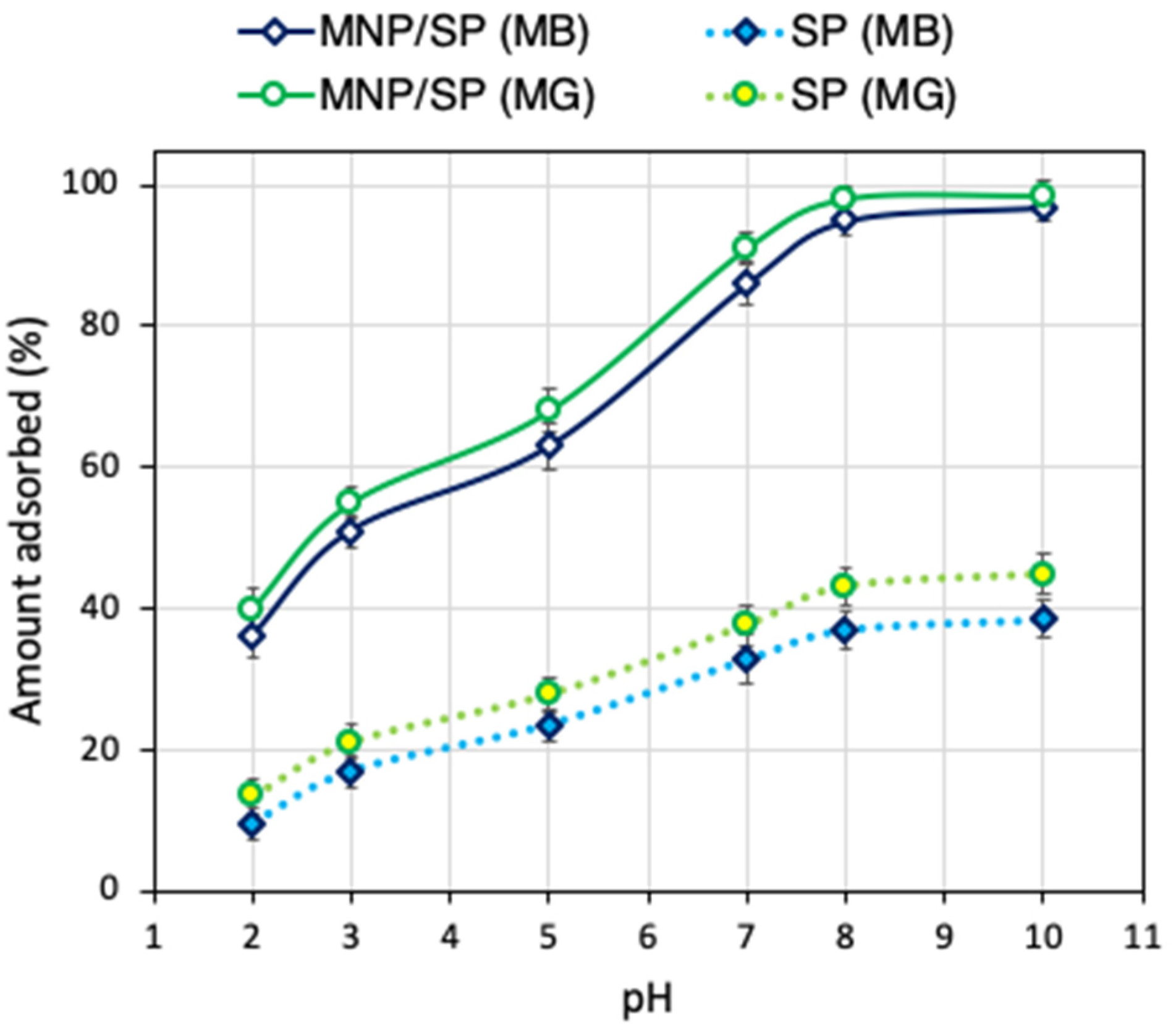
3.2. Effect of pH on Methylene Blue and Malachite Green Adsorption
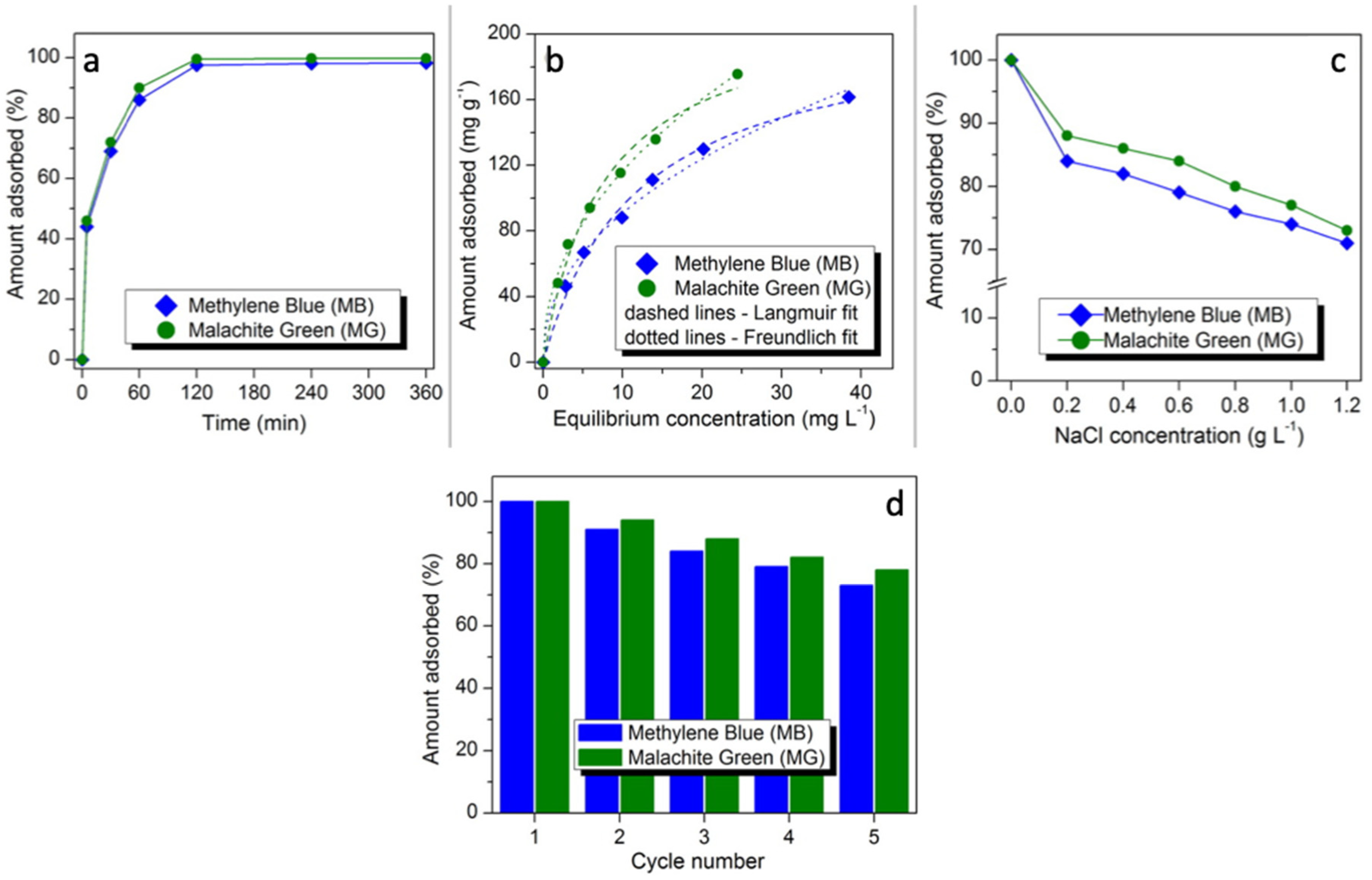
3.3. Effect of Contact Time on MB and MG Adsorption
3.4. Adsorption Kinetics and Diffusion Mechanism
3.5. Adsorption Isotherms
3.6. Comparison of the MNP/SP Adsorbent with Other Studies
3.7. Temperature Effect on MB and MG Adsorption
3.8. Effect of Salinity on MB and MG Adsorption
3.9. The Reusability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Wang, G.; Zhai, K.; He, C.; Li, Q.; Guo, P. Methylene blue adsorption from aqueous solution by loofah sponge-based porous carbons. Colloids Surf. A 2018, 538, 28–35. [Google Scholar] [CrossRef]
- Mobarak, M.; Selim, A.Q.; Mohamed, E.A.; Seliem, M.K. Experimental results and theoretical statistical modeling of malachite green adsorption onto MCM–41silica/rice husk composite modified by beta radiation. J. Mol. Liq. 2019, 273, 68–82. [Google Scholar] [CrossRef]
- Saffari, R.; Shariatinia, Z.; Jourshabani, M. Synthesis and photocatalytic degradation activities of phosphorus containing ZnO microparticles under visible light irradiation for water treatment applications. Environ. Pollut. 2020, 259, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, F.; Feng, Z.; Deng, S.; Wang, Y. Adsorptive removal and kinetics of methylene blue from aqueous solution using NiO/MCM-41composite. Phys. E 2015, 65, 4–12. [Google Scholar] [CrossRef]
- VGupta, K.; Ali, S.I.; Saini, V.K. Removal of Rhodamine B, Fast Green, and Methylene Blue from Waste water Using Red Mud, an Aluminum Industry Waste. Ind. Eng. Chem. Res. 2004, 43, 1740–1747. [Google Scholar]
- Ahmad, M.A.; Alrozi, R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 171, 510–516. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Mashhadi, S.; Ghasemi, M. Kinetics and thermodynamics of malachite green dye removal from aqueous phase using iron nanoparticles loaded on ash. J. Mol. Liq. 2016, 223, 1340–1347. [Google Scholar] [CrossRef]
- Seliem, M.K.; Mohamed, E.A.; Selim, A.Q.; Shahien, M.G.; Abukhadra, M.R. Synthesis of Na-A zeolites from natural and thermally activated Egyptian kaolinite: Characterization and competitive adsorption of copper ions from aqueous solutions. Int. J. Bioassays 2015, 4, 4423–4430. [Google Scholar]
- Tehrani, A.R.; Nikkar, H.; Mahmoodi, N.M.; Makazi, M.; Menger, F.M. The sorption of cationic dyes onto kaolin: Kinetic, isotherm and thermodynamic studies. Desalination 2011, 266, 274–280. [Google Scholar] [CrossRef]
- Hajjaji, M.; Alami, A. Influence of operating conditions on methylene blue uptake by a smectite rich clay fraction. Appl. Clay Sci. 2009, 44, 127–129. [Google Scholar] [CrossRef]
- Hajjaji, M.; el Arfaoui, H. Adsorption of methylene blue and zinc ions on raw and acid-activated bentonite from Morocco. Appl. Clay Sci. 2009, 46, 418–421. [Google Scholar] [CrossRef]
- Hajjaji, M.; Alami, A.; el Bouadili, A. Removal of methylene blue from aqueous solution by fibrous clay minerals. J. Hazard. Mater. 2006, 135, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bulut, E.; Ozacar, M.; Sengil, I.A. Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microporous Mesoporous Mater. 2008, 115, 234–246. [Google Scholar] [CrossRef]
- Kiani, G.; Dostali, M.; Rostami, A.; Khataee, A.R. Adsorption studies on the removal of malachite green from aqueous solutions onto halloysite nanotubes. Appl. Clay Sci. 2011, 54, 34–39. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Sun, Q.; Wang, L.; Song, J.; He, X.; Dou, C. Malachite green adsorption onto natural zeolite and reuse by microwave irradiation. J. Hazard. Mater. 2010, 175, 1056–1061. [Google Scholar] [CrossRef]
- Khattria, S.D.; Singh, M.K. Removal of malachite green from dye wastewater using neem sawdust by adsorption. J. Hazard. Mater. 2009, 167, 1089–1094. [Google Scholar] [CrossRef]
- Keyhanian, F.; Shariati, S.; Faraji, M.; Hesabi, M. Magnetite nanoparticles with surface modification for removal of methyl violet from aqueous solutions. Arab. J. Chem. 2016, 9, S348–S354. [Google Scholar] [CrossRef]
- Pirbazari, A.E.; Saberikhaha, E.; Kozani, S.S.H. Fe3O4–wheat straw: Preparation, characterization and its application for methylene blue adsorption. Water Resour. Ind. 2014, 7–8, 23–37. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiaoa, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Mobarak, M.; Mohamed, E.A.; Selim, A.Q.; Sellaoui, L.; Laminec, A.B.; Erto, A.; Bonilla-Petriciolete, A.; Seliem, M.K. Surfactant–modified serpentine for fluoride and Cr(VI) adsorption in single and binary systems: Experimental studies and theoretical modeling. Chem. Eng. J. 2019, 360, 333–343. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel (II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.Q.; Mohamed, E.A.; Mobarak, M.; Zayed, A.M.; Seliem, M.K.; Komarneni, S. Cr(VI) uptake by a composite of processed diatomite with MCM-41: Isotherm, kinetic and thermodynamic studies. Microporous Mesoporous Mater. 2018, 260, 84–92. [Google Scholar] [CrossRef]
- Barakat, M.A.; Kumar, R.; Balkhyour, M.; Taleb, M.A. Novel AlO3/GO/halloysite nanotube composite for sequestration of anionic and cationic dyes. Rsc Adv. 2019, 9, 13916–13926. [Google Scholar] [CrossRef]
- Langergren, S.; Svenska, B.K. Veternskapsakad, Zur theorie der sogenannten adsorption geloester stoffe. Handlingar 1898, 24, 1–39. [Google Scholar]
- YHo, S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar]
- Weber, J.C.; Morris, W.J. Proceedings of International Conference on Water Pollution Symposium; Pergamon Press: Oxford, UK, 1962. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Mobarak, M.; Selim, A.Q.; Mohamed, E.; Seliem, M.K. Modification of organic matter-rich clay by a solution of cationic surfactant/H2O2: A new product for fluoride adsorption from solutions. J. Clean. Prod. 2018, 192, 712–721. [Google Scholar] [CrossRef]
- Tan, T.C.N.; Sen, T.K. Aqueous-phase methylene blue (MB) dye removal by mixture of eucalyptus bark (EB) biomass and kaolin clay (KC) adsorbents: Kinetics, thermodynamics, and isotherm modeling. Sep. Sci. Technol. 2019, 55, 1036–1050. [Google Scholar] [CrossRef]
- Selim, A.Q.; Sellaoui, L.; Mobarak, M. Statistical physics modeling of phosphate adsorption onto chemically modified carbonaceous clay. J. Mol. Liq. 2019, 279, 94–107. [Google Scholar] [CrossRef]
- Wang, G.; Hua, Y.; Su, X.; Komarneni, S.; Ma, S.; Wang, Y. Cr(VI) adsorption by montmorillonite nanocomposites. Appl. Clay Sci. 2016, 124–125, 111–118. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Selim, A.Q.; Zayed, A.M.; Komarneni, S.; Mobarak, M.; Seliem, M.K. Enhancing adsorption capacity of Egyptian diatomaceous earth by thermo-chemical purification: Methylene blue uptake. J. Colloid Interface Sci. 2019, 534, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; El-Khaiary, M.I. Malachite green adsorption by rattan sawdust: Isotherm, kinetic and mechanism modeling. J. Hazard. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef]
- Seliem, M.K.; Komarneni, S. Equilibrium and kinetic studies for adsorption of iron from aqueous solution by synthetic Na-A zeolites: Statistical modeling and optimization. Micropor. Mesopor. Mater. 2016, 228, 266–274. [Google Scholar] [CrossRef]
- Seliem, M.K.; Komarneni, V.; Byrne, T.; Cannon, F.S.; Shahien, M.G.; Khalil, A.A.; El-Gaid, I.M.A. Removal of Perchlorate by synthetic organosilica and organoclays: Kinetic and Isotherm Studies. Appl. Clay Sci. 2013, 71, 21–26. [Google Scholar] [CrossRef]
- Seliem, M.K.; Komarneni, S.; Khadra, M.R.A. Phosphate removal from solution by composite of MCM-41 silica with rice husk: Kinetic and equilibrium studies. Microporous Mesoporous. Mater. 2016, 224, 51–57. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, X.; Kang, Y.; Shu, Y.; Sun, Q.; Li, L. Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution. J. Colloid Interface Sci. 2014, 429, 25–33. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Highly efficient methylene blue dyes removal from aqueous systems by chitosan coated magnetic mesoporous silica nanoparticles. J. Porous Mater. 2015, 22, 1383–1392. [Google Scholar] [CrossRef]
- Rahman, I.A.; Saad, B.; Shaidan, S.; Rizal, E.S.S. Adsorption characteristics of malachite green on activated carbon derived from rice husks produced by chemical–thermal process. Bioresour. Technol. 2005, 96, 1578–1583. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, C.; Jing, Y. Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root. J. Hazard. Mater. 2008, 150, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
- Baek, M.; Ijagbemi, C.O.; Jin, S.; Kim, D. Removal of malachite green from aqueous solution using degreased coffee bean. J. Hazard. Mater. 2010, 176, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Mangwandi, C.; Muhtaseb, A.A.; Walker, G.; Allen, S.; Ahmad, M.N.M. Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye adsorption by resins: Effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Barczak, M.; Dobrowolski, R.; Borowski, P.; Giannakoudakis, D.A. Pyridine-, thiol- and amine-functionalized mesoporous silicas for adsorptive removal of pharmaceuticals. Microporous Mesoporous Mater. 2020, 299, 1–8. [Google Scholar] [CrossRef]
- Barczak, M. Amine-modified mesoporous silicas: Morphology-controlled synthesis toward efficient removal of pharmaceuticals. Microporous Mesoporous Mater. 2019, 278, 354–365. [Google Scholar] [CrossRef]
- Geczo, A.; Giannakoudakis, D.A.; Triantafyllidis, K.; Elshaer, M.R.; Rodríguez-Aguado, E.; Bashkova, S. Mechanistic insights into acetaminophen removal on cashew nut shell biomass-derived activated carbons. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Saroyan, H.S.; Bele, S.; Giannakoudakis, D.A.; Samanidou, V.F.; Bandosz, T.J.; Deliyanni, E.A. Degradation of endocrine disruptor, bisphenol-A, on mixed oxidation state manganese oxide/modified graphite oxide composite: A role of carbonaceous phase. J. Colloid Interface Sci. 2019, 539, 516–524. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hosseini-Bandegharaei, A.; Tsafrakidou, P.; Triantafyllidis, K.S.; Kornaros, M.; Anastopoulos, I. Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: A review. J. Environ. Manag. 2018, 227, 354–364. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Escudero, L.B.; Zhou, Y.; Colmenares, J.C.; Núñez-Delgado, A.; et al. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
| Adsorbate | Adsorbent | Sorption Capacity (mg g−1) | Reference |
|---|---|---|---|
| MB | Montmorillonite | 64 | [20] |
| MB | Fe3O4/montmorillonite | 106 | [20] |
| MB | Fibrous clay minerals | 39–85 | [12] |
| MB | Mn-doped mesoporous MCM-41 silica | 132 | [39] |
| MB | Purified diatomite | 105 | [34] |
| MB | Chitosan/magnetic silica | 201 | [40] |
| MB | Fe3O4/serpentine composite | 201 | Current study |
| MG | Bentonite | 179 | [14] |
| MG | Activated carbon | 57 | [41] |
| MG | Natural zeolite | 24 | [16] |
| MG | Biocarbon prepared from plant root | 8 | [42] |
| MG | Halloysite nanotubes | 100 | [15] |
| MG | Modified rice husk | 12 | [43] |
| MG | Degreased coffee bean | 55 | [44] |
| MG | Fe3O4/serpentine composite | 218 | Current study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seliem, M.K.; Barczak, M.; Anastopoulos, I.; Giannakoudakis, D.A. A Novel Nanocomposite of Activated Serpentine Mineral Decorated with Magnetic Nanoparticles for Rapid and Effective Adsorption of Hazardous Cationic Dyes: Kinetics and Equilibrium Studies. Nanomaterials 2020, 10, 684. https://doi.org/10.3390/nano10040684
Seliem MK, Barczak M, Anastopoulos I, Giannakoudakis DA. A Novel Nanocomposite of Activated Serpentine Mineral Decorated with Magnetic Nanoparticles for Rapid and Effective Adsorption of Hazardous Cationic Dyes: Kinetics and Equilibrium Studies. Nanomaterials. 2020; 10(4):684. https://doi.org/10.3390/nano10040684
Chicago/Turabian StyleSeliem, Moaaz K., Mariusz Barczak, Ioannis Anastopoulos, and Dimitrios A. Giannakoudakis. 2020. "A Novel Nanocomposite of Activated Serpentine Mineral Decorated with Magnetic Nanoparticles for Rapid and Effective Adsorption of Hazardous Cationic Dyes: Kinetics and Equilibrium Studies" Nanomaterials 10, no. 4: 684. https://doi.org/10.3390/nano10040684
APA StyleSeliem, M. K., Barczak, M., Anastopoulos, I., & Giannakoudakis, D. A. (2020). A Novel Nanocomposite of Activated Serpentine Mineral Decorated with Magnetic Nanoparticles for Rapid and Effective Adsorption of Hazardous Cationic Dyes: Kinetics and Equilibrium Studies. Nanomaterials, 10(4), 684. https://doi.org/10.3390/nano10040684









