Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation, Characterization and Labeling of Mesoporous SiO2–CaO Nanospheres
2.2. Antiosteoporotic Drug Loading
2.3. Cell Culture of MC3T3-E1 Pre-Osteoblasts for FITC–NanoMBG Incorporation. Evaluation of the Endocytic Mechanisms for FITC-NanoMBG Cell Entry
2.4. Cell Size and Complexity Analysis
2.5. Cell Viability Studies
2.6. Cell-Cycle Analysis and Apoptosis Detection by Flow Cytometry
2.7. Intracellular Reactive Oxygen Species (ROS) Content
2.8. Confocal Microscopy Studies
2.9. Intracellular Calcium Content
2.10. Alkaline Phosphatase Activity
2.11. Mineralization Assay
2.12. Interleukin 6 (IL-6) Detection
2.13. Statistics
3. Results and Discussion
3.1. Characterization of Mesoporous Nanospheres
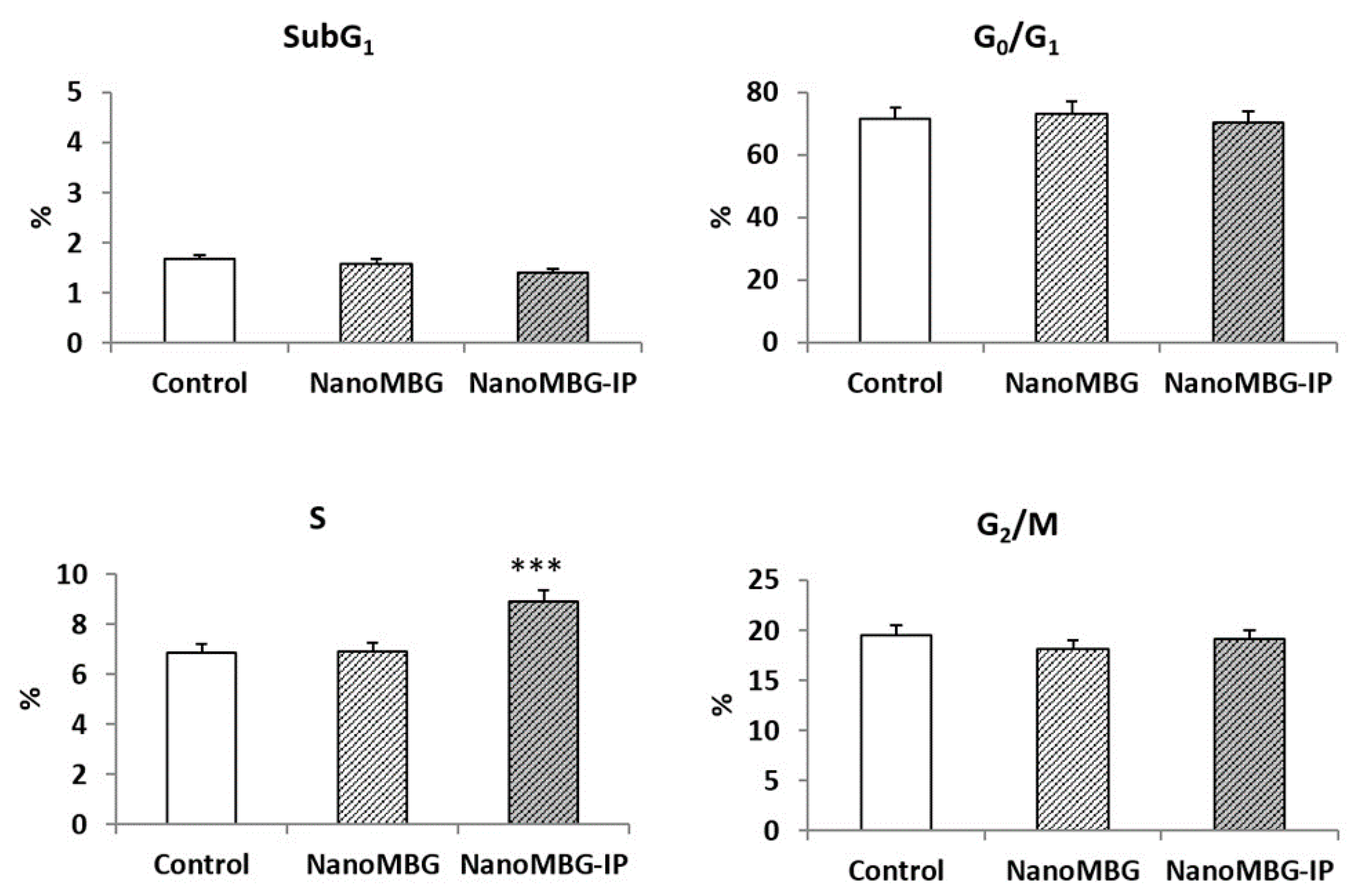
3.2. Effects of NanoMBGs and NanoMBG-IPs on Size, Complexity, Apoptosis and Cell Cycle of MC3T3-E1 Pre-Osteoblasts
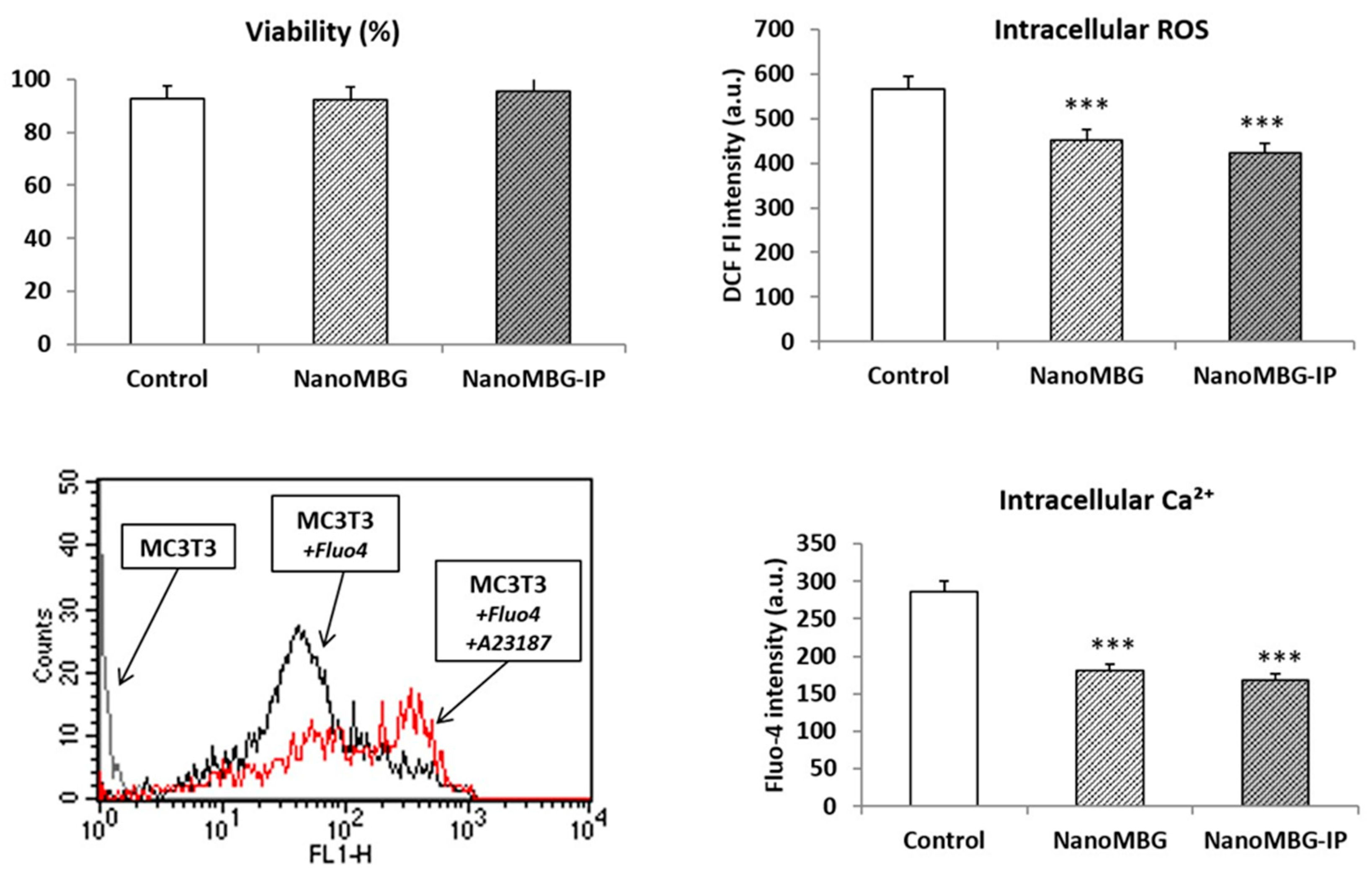
3.3. Effects of NanoMBGs and NanoMBG-IPs on Viability, Intracellular Reactive Oxygen Species (ROS) and Calcium Content of MC3T3-E1 Pre-Osteoblasts
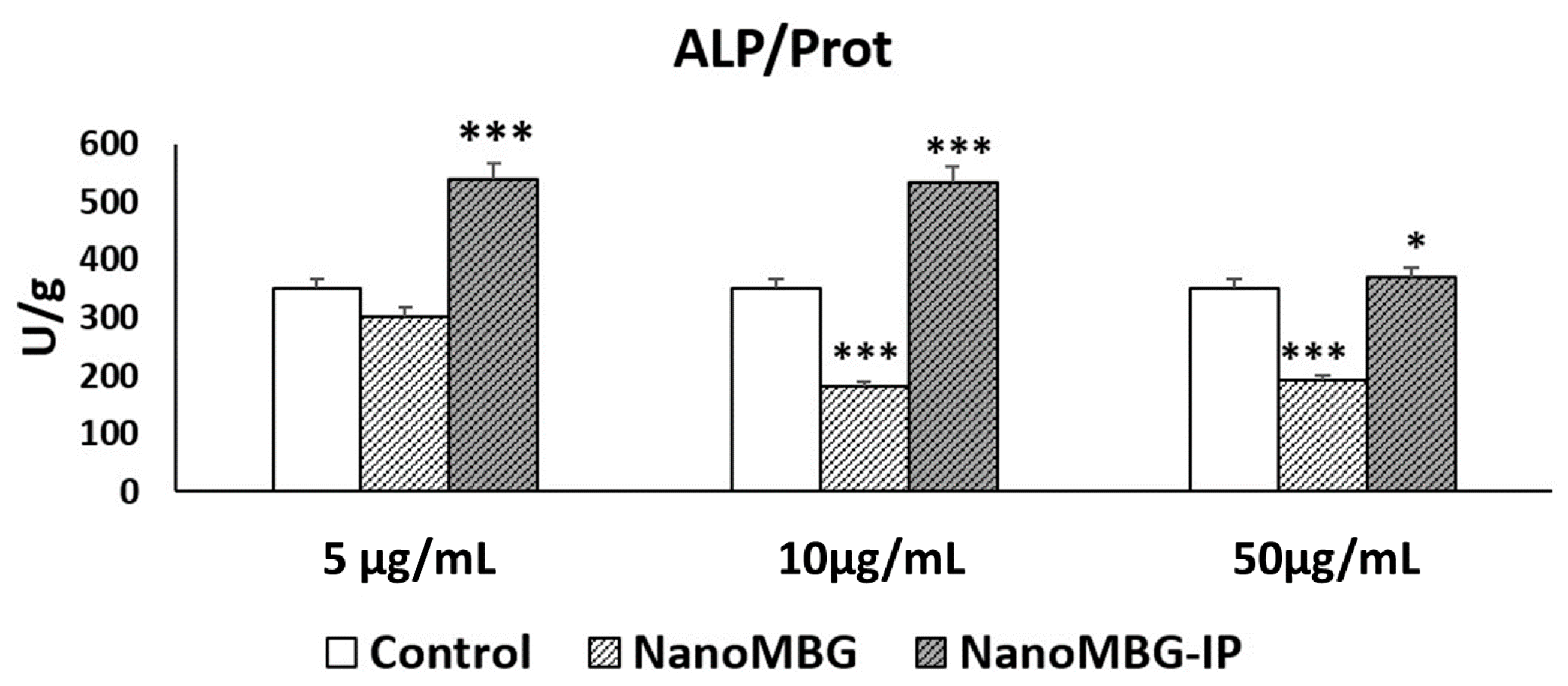
3.4. Effects of NanoMBGs and NanoMBG-IPs on Differentiation of MC3T3-E1 Pre-Osteoblasts
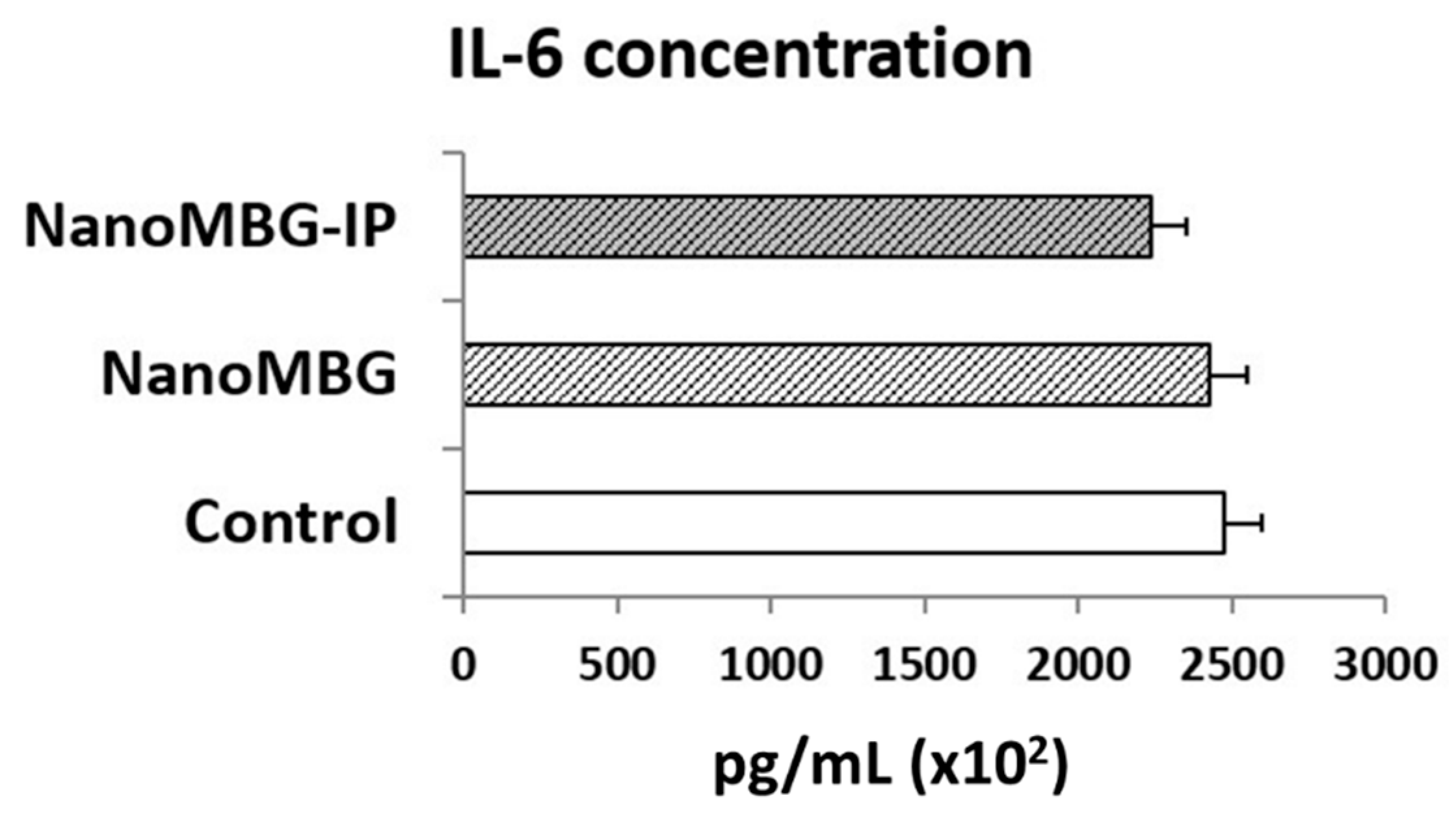
3.5. Effects of NanoMBGs and NanoMBG-IPs on Interleukin 6 (IL-6) Production by MC3T3-E1 Pre-Osteoblasts
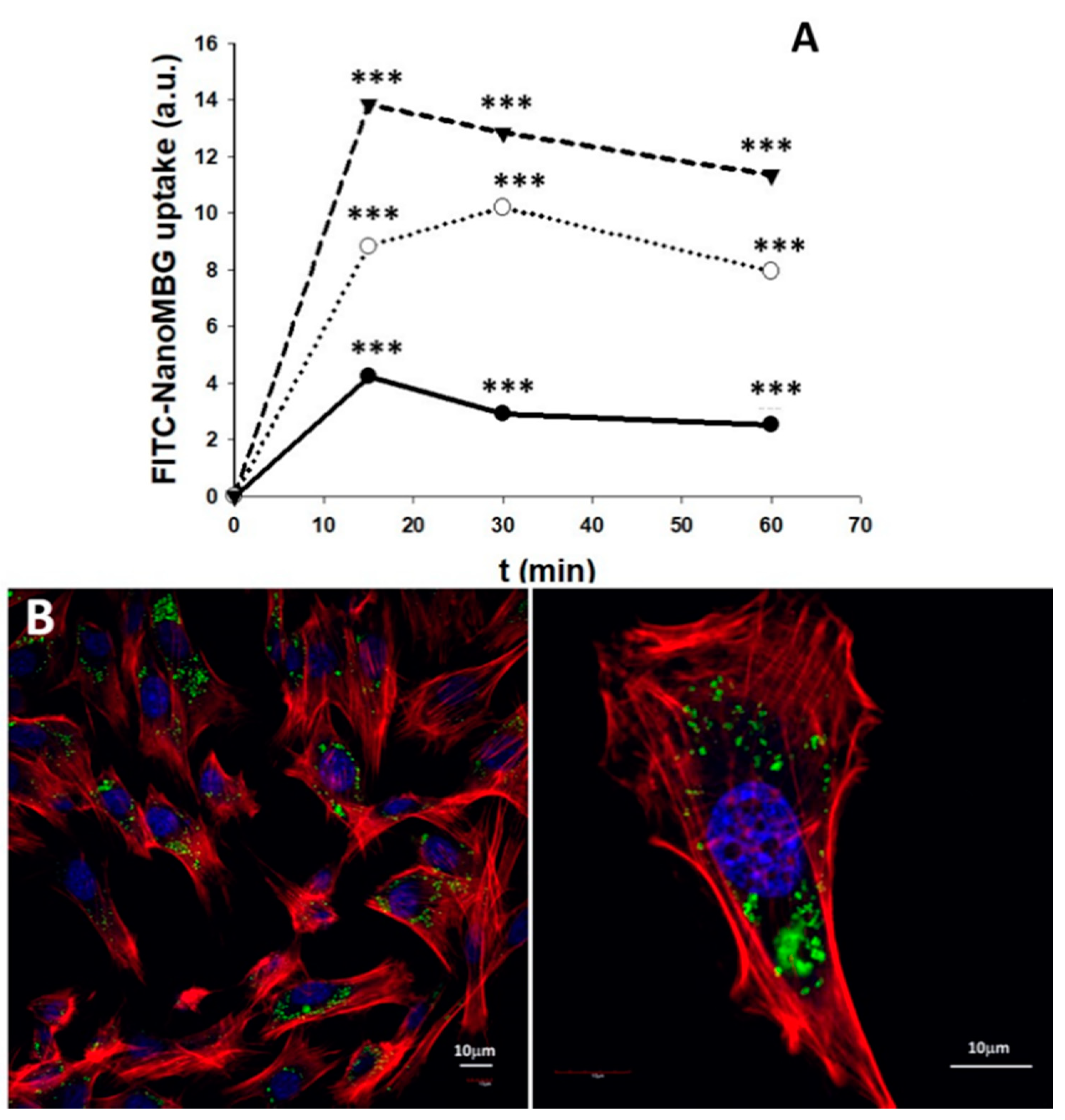
3.6. Uptake of NanoMBGs by MC3T3-E1 Pre-Osteoblasts
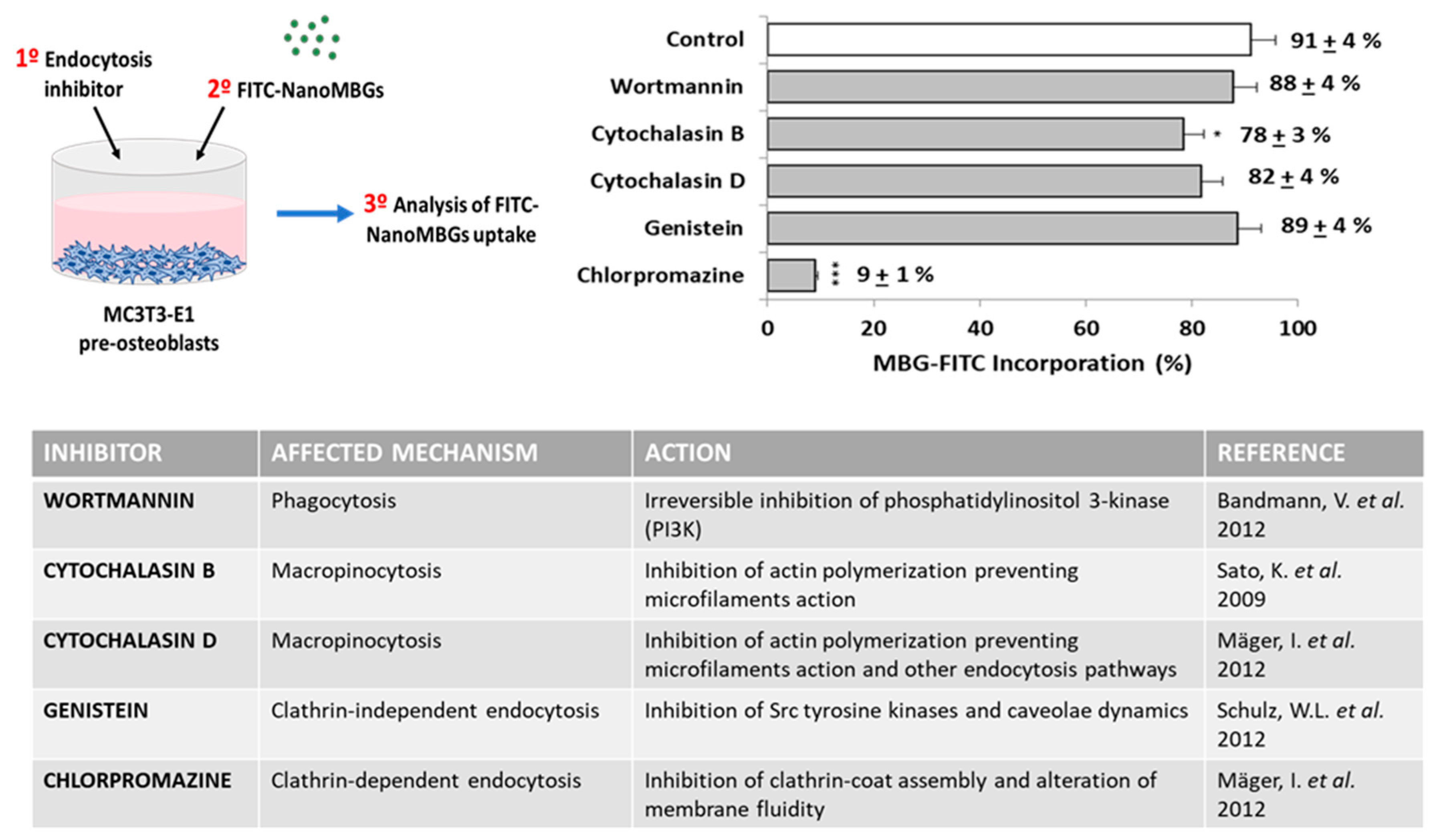
3.7. Endocytic Mechanisms for FITC–NanoMBG Entry into MC3T3-E1 Pre-Osteoblasts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, D.C. Bioglass and Bioactivity: A Brief Look Back in Bioactive Glasses: Properties, Composition and Recent Applications; Arcos, D., Vallet-Regí, M., Eds.; Novascience Publishers: New York, NY, USA, 2020. [Google Scholar]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Arcos, D.; Delgado, M.R.; Ruiz, E.; Gil, F.J.; Vallet-Regí, M. Bioactive Star Gels. Chem. Mater. 2006, 18, 5696–5703. [Google Scholar] [CrossRef]
- Gómez-Cerezo, M.N.; Lozano, D.; Arcos, D.; Vallet-Regí, M.; Vaquette, C. The effect of biomimetic mineralization of 3D-printed mesoporous bioglass scaffolds on physical properties and in vitro osteogenicity. Mater. Sci. Eng. C 2020, 109, 110572. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; De Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.; Portolés, M.T.; et al. Mesoporous bioactive glass/ε-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater. 2019, 90, 393–402. [Google Scholar] [CrossRef]
- Polo, L.; Gómez-Cerezo, N.; García-Fernández, A.; Aznar, E.; Vivancos, J.-L.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Mesoporous bioactive glasses equipped with stimuli-responsive molecular gates for the controlled delivery of levofloxacin against bacteria. Chem. Eur. J. 2018, 24, 18944. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly Ordered Mesoporous Bioactive Glasses with Superior In Vitro Bone-Forming Bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Dharmadhikari, S.; Abdulla, A.M.; Bapat, P.; Alexander, A.; Dubey, S.K.; Kesharwani, P. Recent advances of gold nanoparticles as biomaterial in dentistry. Int. J. Pharm. 2020, 586, 119596. [Google Scholar] [CrossRef] [PubMed]
- Zakrewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected nanomaterials’ application enhaced with the use of stem cells in acceleration of alveolar bone regeneration during augmentation process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. Natural enamel wear—A physiological source of hydroxylapatite nanoparticles for biofilm management and tooth repair? Med. Hypotheses 2010, 74, 670–672. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kang, M.-S.; Mahapatra, C.; Kim, H.-W. Effect of Aminated Mesoporous Bioactive Glass Nanoparticles on the Differentiation of Dental Pulp Stem Cells. PLoS ONE 2016, 11, e0150727. [Google Scholar] [CrossRef]
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef]
- Huang, W.; Yang, J.; Feng, Q.; Shu, Y.; Liu, C.; Zeng, S.; Guan, H.; Ge, L.; Pathak, J.L.; Zeng, S. Mesoporous Bioactive Glass Nanoparticles Promote Odontogenesis and Neutralize Pathophysiological Acidic pH. Front. Mater. 2020, 7, 241. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol–gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312. [Google Scholar] [CrossRef]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S.-H. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Mediero, A.; Esbrit, P.; Sánchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. Unexpected Bone Formation Produced by RANKL Blockade. Trends Endocrinol. Metab. 2017, 28, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, T.; Germershaus, O.; Groll, J.; Meinel, L. Bone targeting for the treatment of osteoporosis. J. Control. Release 2012, 161, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.-Y.L. Ipriflavone: Pharmacological properties and usefulness in postmenopausal osteoporosis. Bone Miner. 1993, 23, 223–232. [Google Scholar] [CrossRef]
- Mazzuoli, G.; Romagnoli, E.; Carnevale, V.; Scarda, A.; Scarnecchia, L.; Pacitti, M.T.; Rosso, R.; Minisola, S. Effects of ipriflavone on bone remodeling in primary hyperparathyroidism. Bone Miner. 1992, 19 (Suppl. 1), S27–S33. [Google Scholar] [CrossRef]
- Duan, H.; Diao, J.; Zhao, N.; Ma, Y. Synthesis of hollow mesoporous bioactive glass microspheres with tunable shell thickness by hydrothermal-assisted self-transformation method. Mater. Lett. 2016, 167, 201–204. [Google Scholar] [CrossRef]
- Gera, S.; Sampathi, S.; Dodoala, S. Role of Nanoparticles in Drug Delivery and Regenerative Therapy for Bone Diseases. Curr. Drug Deliv. 2017, 14, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.; Nelea, V.; Chicatun, F.; Chien, Y.-C.; Tran-Khanh, N.; Buschmann, M.D.; Nazhat, S.; Kaartinen, M.T.; Vali, H.; Tecklenburg, M.; et al. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: An ultrastructural, compositional and comparative analysis with mouse bone. Bone 2015, 71, 244–256. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Incorporation and effects of mesoporous SiO2–CaO nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef]
- Li, Y.; Bastakoti, B.P.; Yamauchi, Y. Smart Soft-Templating Synthesis of Hollow Mesoporous Bioactive Glass Spheres. Chem. A Eur. J. 2015, 21, 8038–8042. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Vallet-Regí, M. Functionalizing Mesoporous Bioglasses for Long-Term Anti-Osteoporotic Drug Delivery. Chem. Eur. J. 2010, 16, 10879–10886. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Silva, V.S.; Hortigüela, M.J.; Matesanz, M.C.; Vila, M.; Portolés, M.T. MC3T3-E1 pre-osteoblast response and differentiation after graphene oxide nanosheet uptake. Colloids Surf. B Biointerfaces 2017, 158, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Udall, J.N.; Moscicki, R.A.; Preffer, F.I.; Ariniello, P.D.; Carter, E.A.; Bhan, A.K.; Bloch, K.J. Flow Cytometry: A New Approach to the Isolation and Characterization of Kupffer Cells. Adv. Exp. Med. Biol. 1987, 216, 821–827. [Google Scholar] [CrossRef]
- Matesanz, M.-C.; Vila, M.; Feito, M.-J.; Linares, J.; Gonçalves, G.; Vallet-Regí, M.; Marques, P.A.A.P.; Portolés, M.-T. The effects of graphene oxide nanosheets localized on F-actin filaments on cell-cycle alterations. Biomaterials 2013, 34, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef]
- Beck, G.R.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, D.; Li, N.; Wen, J.; Jiang, X.; Liu, C.; Li, Y. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 2016, 6, srep19361. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Casarrubios, L.; Morales, I.; Feito, M.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of a mesoporous bioactive glass on osteoblasts, osteoclasts and macrophages. J. Colloid Interface Sci. 2018, 528, 309–320. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Proliferation/Differentiation Interrelationships During Progressive Development of the Osteoblast Phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Ara, T.; Declerck, Y.A. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer 2010, 46, 1223–1231. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, N.; Katagiri, T.; Tamura, T.; Wada, S.; Findlay, D.M.; Martin, T.J.; Hirota, H.; Taga, T.; Kishimoto, T.; et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J. Exp. Med. 1995, 182, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M. Osteotropic Agents Regulate the Expression of Osteoclast Differentiation Factor and Osteoprotegerin in Osteoblastic Stromal Cells. Endocrinol. 1998, 139, 4743. [Google Scholar] [CrossRef]
- Kuroyanagi, G.; Adapala, N.S.; Yamaguchi, R.; Kamiya, N.; Deng, Z.; Aruwajoye, O.; Kutschke, M.; Chen, E.; Jo, C.; Ren, Y.; et al. Interleukin-6 deletion stimulates revascularization and new bone formation following ischemic osteonecrosis in a murine model. Bone 2018, 116, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef]
- Bandmann, V.; Müller, J.D.; Köhler, T.; Homann, U. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett. 2012, 586, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nagai, J.; Mitsui, N.; Yumoto, R.; Takano, M. Effects of endocytosis inhibitors on internalization of human IgG by Caco-2 human intestinal epithelial cells. Life Sci. 2009, 85, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, Ü. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef]
- Schulz, W.L.; Haj, A.K.; Schiff, L.A. Reovirus Uses Multiple Endocytic Pathways for Cell Entry. J. Virol. 2012, 86, 12665–12675. [Google Scholar] [CrossRef]
- Linares, J.; Matesanz, M.C.; Vila, M.; Feito, M.J.; Gonçalves, G.; Vallet-Regí, M.; Marques, P.A.A.P.; Portolés, M.T. Endocytic Mechanisms of Graphene Oxide Nanosheets in Osteoblasts, Hepatocytes and Macrophages. ACS Appl. Mater. Interfaces 2014, 6, 13697–13706. [Google Scholar] [CrossRef]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
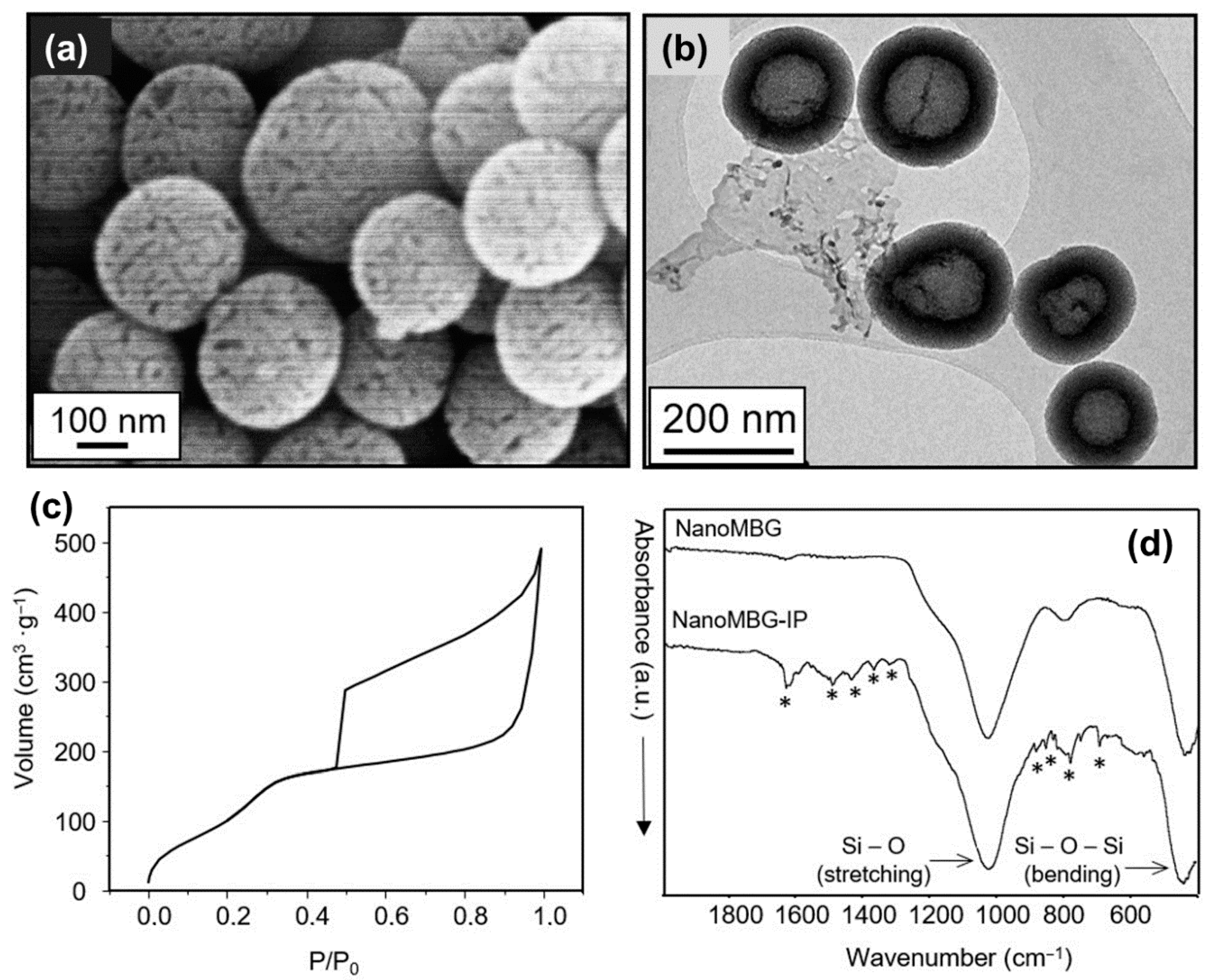
| Sample | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|
| nanoMBG | 543.6 | 0.435 | −2.5 nm |
| nanoMBG–IP | 14.4 | 0.057 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials 2020, 10, 2573. https://doi.org/10.3390/nano10122573
Casarrubios L, Gómez-Cerezo N, Feito MJ, Vallet-Regí M, Arcos D, Portolés MT. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials. 2020; 10(12):2573. https://doi.org/10.3390/nano10122573
Chicago/Turabian StyleCasarrubios, Laura, Natividad Gómez-Cerezo, María José Feito, María Vallet-Regí, Daniel Arcos, and María Teresa Portolés. 2020. "Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment" Nanomaterials 10, no. 12: 2573. https://doi.org/10.3390/nano10122573
APA StyleCasarrubios, L., Gómez-Cerezo, N., Feito, M. J., Vallet-Regí, M., Arcos, D., & Portolés, M. T. (2020). Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials, 10(12), 2573. https://doi.org/10.3390/nano10122573







