Metal Exsolution to Enhance the Catalytic Activity of Electrodes in Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Towards an Optimal Anode for SOFC
3. Conductive Oxides and Exsolution Hosts
4. Manner of Lattice Substitution
5. Mechanisms and Energetics of the Exsolution Process
Thermodynamic Considerations
6. Properties of the Exsolved Particles
Catalytic Properties
7. Summary
8. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Pihlatie, M.; Ramos, T.; Kaiser, A. Testing and improving the redox stability of Ni-based solid oxide fuel cells. J. Power Sources 2009, 193, 322–330. [Google Scholar] [CrossRef]
- Hanna, J.; Lee, W.Y.; Shi, Y.; Ghoniem, A. Fundamentals of electro-and thermochemistry in the anode of solid-oxide fuel cells with hydrocarbon and syngas fuels. Prog. Energy Combust. Sci. 2014, 40, 74–111. [Google Scholar] [CrossRef]
- He, H.; Gorte, R.J.; Vohs, J.M. Highly sulfur tolerant Cu-ceria anodes for SOFCs. Electrochem. Solid-State Lett. 2005, 8, A279. [Google Scholar] [CrossRef]
- Ahn, K.; Jung, S.; Vohs, J.M.; Gorte, R.J. A support layer for solid oxide fuel cells. Ceram. Int. 2007, 33, 1065–1070. [Google Scholar] [CrossRef][Green Version]
- Tucker, M.C.; Lau, G.Y.; Jacobson, C.P.; DeJonghe, L.C.; Visco, S.J. Performance of metal-supported SOFCs with infiltrated electrodes. J. Power Sources 2007, 171, 477–482. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.; Shin, J.; Corre, G.; Irvine, J.; Vohs, J.; Gorte, R.J. Investigation of the structural and catalytic requirements for high-performance SOFC anodes formed by infiltration of LSCM. Electrochem. Solid State Lett. 2009, 12, B48–B52. [Google Scholar] [CrossRef]
- Simwonis, D.; Tietz, F.; Stöver, D. Nickel coarsening in annealed Ni/8YSZ anode substrates for solid oxide fuel cells. Solid State Ion. 2000, 132, 241–251. [Google Scholar] [CrossRef]
- Tanaka, H.; Taniguchi, M.; Uenishi, M.; Kajita, N.; Tan, I.; Nishihata, Y.; Mizuki, J.I.; Narita, K.; Kimura, M.; Kaneko, K. Self-regenerating Rh-and Pt-based perovskite catalysts for automotive-emissions control. Angewante Chem. Int. Ed. 2006, 45, 5998–6002. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, L.; Balaji Padmanabhan, V.; Küngas, R.; Gorte, R.J.; Vohs, J.M. Transition metal-doped rare earth vanadates: A regenerable catalytic material for SOFC anodes. J. Mater. Chem. 2012, 22. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.; Saccoccio, M.; Lu, Z.; Ciucci, F. From material design to mechanism study: Nanoscale Ni exsolution on a highly active A-site deficient anode material for solid oxide fuel cells. Nano Energy 2016, 27, 499–508. [Google Scholar] [CrossRef]
- Madsen, B.D.; Kobsiriphat, W.; Wang, Y.; Marks, L.D.; Barnett, S.A. Nucleation of nanometer-scale electrocatalyst particles in solid oxide fuel cell anodes. J. Power Sources 2007, 166, 64–67. [Google Scholar] [CrossRef]
- Tanner, C.W.; Fung, K.Z.; Virkar, A.V. The effect of porous composite electrode structure on solid oxide fuel cell performance: I. theoretical analysis. J. Electrochem. Soc. 1997, 144, 21–30. [Google Scholar] [CrossRef]
- Vohs, J.M.; Gorte, R.J. High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 2009, 21, 943–956. [Google Scholar] [CrossRef]
- Gorte, R.J.; Park, S.; Vohs, J.M.; Wang, C. Anodes for direct oxidation of dry hydrocarbons in a solid-oxide fuel cell. Adv. Mater. 2000, 12, 1465–1469. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of liquid fuels in a solid oxide fuel cell. J. Electrochem. Soc. 2001, 148, A693. [Google Scholar] [CrossRef]
- Zhou, N.; Yin, Y.-M.; Chen, Z.; Song, Y.; Yin, J.; Zhou, D.; Ma, Z.-F. A regenerative coking and sulfur resistant composite anode with Cu exsolution for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2018, 165, F629. [Google Scholar] [CrossRef]
- Jung, S.; Lu, C.; He, H.; Ahn, K.; Gorte, R.J.; Vohs, J.M. Influence of composition and Cu impregnation method on the performance of Cu/CeO2/YSZ SOFC anodes. J. Power Sources 2006, 154, 42–50. [Google Scholar] [CrossRef]
- Gasper, P.; Lu, Y.; Basu, S.N.; Gopalan, S.; Pal, U.B. Effect of anodic current density on the spreading of infiltrated nickel nanoparticles in nickel-yttria stabilized zirconia cermet anodes. J. Power Sources 2019, 410, 196–203. [Google Scholar] [CrossRef]
- Corre, G.; Kim, G.; Cassidy, M.; Vohs, J.; Gorte, R.; Irvine, J. Activation and ripening of impregnated manganese containing perovskite SOFC electrodes under redox cycling. Chem. Mater. 2009, 21, 1077–1084. [Google Scholar] [CrossRef]
- Adijanto, L.; Sampath, A.; Yu, A.S.; Cargnello, M.; Fornasiero, P.; Gorte, R.J.; Vohs, J.M. Synthesis and Stability of Pd@CeO2 Core–Shell Catalyst Films in Solid Oxide Fuel Cell Anodes. ACS Catal. 2013, 3, 1801–1809. [Google Scholar] [CrossRef]
- Graham, G.; Jen, H.-W.; Chun, W.; Sun, H.; Pan, X.; McCabe, R. Coarsening of Pt particles in a model NO x trap. Catal. Lett. 2004, 93, 129–134. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- He, J.-J.; Wang, C.-X.; Zheng, T.-T.; Zhao, Y.-K. Thermally induced deactivation and the corresponding strategies for improving durability in automotive three-way catalysts. Johns. Matthey Technol. Rev. 2016, 60, 196–203. [Google Scholar] [CrossRef]
- Tanaka, H.; Tan, I.; Uenishi, M.; Kimura, M.; Dohmae, K. Regeneration of palladium subsequent to solid solution and segregation in a perovskite catalyst: An intelligent catalyst. Top. Catal. 2001, 16, 63–70. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164. [Google Scholar] [CrossRef]
- Tanaka, H.; Taniguchi, M.; Kajita, N.; Uenishi, M.; Tan, I.; Sato, N.; Narita, K.; Kimura, M. Design of the intelligent catalyst for Japan ULEV standard. Top. Catal. 2004, 30, 389–396. [Google Scholar] [CrossRef]
- Uenishi, M.; Taniguchi, M.; Tanaka, H.; Kimura, M.; Nishihata, Y.; Mizuki, J.; Kobayashi, T. Redox behavior of palladium at start-up in the Perovskite-type LaFePdOx automotive catalysts showing a self-regenerative function. Appl. Catal. B Environ. 2005, 57, 267–273. [Google Scholar] [CrossRef]
- Adijanto, L.; Padmanabhan, V.B.; Gorte, R.J.; Vohs, J.M. Polarization-Induced Hysteresis in CuCo-Doped Rare Earth Vanadates SOFC Anodes. J. Electrochem. Soc. 2012, 159, F751–F756. [Google Scholar] [CrossRef]
- Myung, J.H.; Neagu, D.; Miller, D.N.; Irvine, J.T. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.K.; Nenning, A.; Rameshan, C.; Rameshan, R.; Blume, R.; Havecker, M.; Knop-Gericke, A.; Rupprechter, G.; Fleig, J.; Klotzer, B. Enhancing electrochemical water-splitting kinetics by polarization-driven formation of near-surface iron(0): An in situ XPS study on perovskite-type electrodes. Angewante Chem. Int. Ed. 2015, 54, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.K.; Nenning, A.; Vonk, V.; Volkov, S.; Bertram, F.; Summerer, H.; Schwarz, S.; Steiger-Thirsfeld, A.; Bernardi, J.; Stierle, A. Understanding electrochemical switchability of perovskite-type exsolution catalysts. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Tsekouras, G.; Neagu, D.; Irvine, J.T.S. Step-change in high temperature steam electrolysis performance of perovskite oxide cathodes with exsolution of B-site dopants. Energy Environ. Sci. 2013, 6, 256–266. [Google Scholar] [CrossRef]
- Kwon, O.; Sengodan, S.; Kim, K.; Kim, G.; Jeong, H.Y.; Shin, J.; Ju, Y.-W.; Han, J.W.; Kim, G. Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Shen, X.; Chen, T.; Bishop, S.; Perry, N.H.; Tuller, H.; Sasaki, K. Redox cycling induced Ni exsolution in Gd0.1Ce0.8Ni0.1O2-(Sr0.9La0.1)0.9Ti0.9Ni0.1O3 composite solid oxide fuel cell anodes. J. Power Sources 2017, 370, 122–130. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Yin, Y.-M.; Zhou, N.; Ma, Z.-F. A novel high performance composite anode with in situ growth of Fe-Ni alloy nanoparticles for intermediate solid oxide fuel cells. Electrochim. Acta 2017, 235, 317–322. [Google Scholar] [CrossRef]
- Oemar, U.; Ang, M.; Hee, W.; Hidajat, K.; Kawi, S. Perovskite LaxM1−xNi0.8Fe0.2O3 catalyst for steam reforming of toluene: Crucial role of alkaline earth metal at low steam condition. Appl. Catal. B Environ. 2014, 148, 231–242. [Google Scholar] [CrossRef]
- Thalinger, R.; Gocyla, M.; Heggen, M.; Dunin-Borkowski, R.; Grünbacher, M.; Stöger-Pollach, M.; Schmidmair, D.; Klötzer, B.; Penner, S. Ni–perovskite interaction and its structural and catalytic consequences in methane steam reforming and methanation reactions. J. Catal. 2016, 337, 26–35. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.-S.; Rahani, E.K.; Neagu, D.; Irvine, J.T.; Shenoy, V.B.; Gorte, R.J.; Vohs, J.M. Evidence and model for strain-driven release of metal nanocatalysts from perovskites during exsolution. J. Phys. Chem. Lett. 2015, 6, 5106–5110. [Google Scholar] [CrossRef] [PubMed]
- Neagu, D.; Papaioannou, E.I.; Ramli, W.K.; Miller, D.N.; Murdoch, B.J.; Ménard, H.; Umar, A.; Barlow, A.J.; Cumpson, P.J.; Irvine, J.T. Demonstration of chemistry at a point through restructuring and catalytic activation at anchored nanoparticles. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-M.; Chan, S.-H.; Liu, Q.-L.; Sun, Q. Solid Oxide Fuel Cell Anode Materials for Direct Hydrocarbon Utilization. Adv. Energy Mater. 2012, 2, 1156–1181. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.; Ghoniem, A.F.; Yildiz, B. In situ catalyst exsolution on perovskite oxides for the production of CO and synthesis gas in ceramic membrane reactors. Sustain. Energy Fuels 2019, 3, 2347–2355. [Google Scholar] [CrossRef]
- Steele, B.; Middleton, P.; Rudkin, R. Material science aspects of SOFC technology with special reference to anode development. Solid State Ion. 1990, 40, 388–393. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T. A redox-stable efficient anode for solid-oxide fuel cells. Nat. Mater. 2003, 2, 320–323. [Google Scholar] [CrossRef]
- Sun, Y.F.; Zhang, Y.Q.; Chen, J.; Li, J.H.; Zhu, Y.T.; Zeng, Y.M.; Amirkhiz, B.S.; Li, J.; Hua, B.; Luo, J.L. New Opportunity for in Situ Exsolution of Metallic Nanoparticles on Perovskite Parent. Nano Lett. 2016, 16, 5303–5309. [Google Scholar] [CrossRef]
- Kwon, O.; Joo, S.; Choi, S.; Sengodan, S.; Kim, G. Review on exsolution and its driving forces in perovskites. J. Phys. Energy 2020, 2, 032001. [Google Scholar] [CrossRef]
- Zhou, J.; Shin, T.-H.; Ni, C.; Chen, G.; Wu, K.; Cheng, Y.; Irvine, J.T.S. In Situ Growth of Nanoparticles in Layered Perovskite La0.8Sr1.2Fe0.9Co0.1O4−δ as an Active and Stable Electrode for Symmetrical Solid Oxide Fuel Cells. Chem. Mater. 2016, 28, 2981–2993. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Luo, J.-L. CO2-to-CO conversion on layered perovskite with in situ exsolved Co–Fe alloy nanoparticles: An active and stable cathode for solid oxide electrolysis cells. J. Mater. Chem. A 2016, 4, 17521–17528. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Yi, S.; Xia, Q.; Gong, Y.; Zhang, Y.; Cheng, X.; Li, Y.; Gu, L.; Swierczek, K. High-Performance Anode Material Sr2FeMo0.65Ni0.35O6-delta with In Situ Exsolved Nanoparticle Catalyst. ACS Nano 2016, 10, 8660–8669. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Sengodan, S.; Park, S.; Ju, Y.W.; Kim, J.; Hyodo, J.; Jeong, H.Y.; Ishihara, T.; Shin, J.; Kim, G. A robust symmetrical electrode with layered perovskite structure for direct hydrocarbon solid oxide fuel cells: PrBa0.8Ca0.2Mn2O5+δ. J. Mater. Chem. A 2016, 4, 1747–1753. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, S.; Kim, J.; Park, S.; Jun, A.; Sengodan, S.; Kim, J.; Shin, J.; Jeong, H.Y.; Choi, Y.; et al. Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa0.5Sr0.5Co2−xFexO5+δ. Sci. Rep. 2013, 3, 2426. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef]
- Lv, H.; Lin, L.; Zhang, X.; Song, Y.; Matsumoto, H.; Zeng, C.; Ta, N.; Liu, W.; Gao, D.; Wang, G.; et al. In Situ Investigation of Reversible Exsolution/Dissolution of CoFe Alloy Nanoparticles in a Co-Doped Sr2Fe1.5Mo0.5O6-δ Cathode for CO2 Electrolysis. Adv. Mater. 2020, 32, e1906193. [Google Scholar] [CrossRef]
- Adijanto, L.; Balaji Padmanabhan, V.; Holmes, K.J.; Gorte, R.J.; Vohs, J.M. Physical and electrochemical properties of alkaline earth doped, rare earth vanadates. J. Solid State Chem. 2012, 190, 12–17. [Google Scholar] [CrossRef]
- Petit, C.T.G.; Lan, R.; Cowin, P.I.; Irvine, J.T.S.; Tao, S. Novel redox reversible oxide, Sr-doped cerium orthovanadate to metavanadate. J. Mater. Chem. 2011, 21, 525–531. [Google Scholar] [CrossRef]
- Boulfrad, S.; Cassidy, M.; Irvine, J.T.S. NbTi0.5Ni0.5O4 as anode compound material for SOFCs. Solid State Ion. 2011, 197, 37–41. [Google Scholar] [CrossRef]
- Zenou, V.Y.; Fowler, D.E.; Gautier, R.; Barnett, S.A.; Poeppelmeier, K.R.; Marks, L.D. Redox and phase behavior of Pd-substituted (La,Sr)CrO3 perovskite solid oxide fuel cell anodes. Solid State Ion. 2016, 296, 90–105. [Google Scholar] [CrossRef]
- Lindenthal, L.; Rameshan, R.; Summerer, H.; Ruh, T.; Popovic, J.; Nenning, A.; Löffler, S.; Opitz, A.K.; Blaha, P.; Rameshan, C. Modifying the Surface Structure of Perovskite-Based Catalysts by Nanoparticle Exsolution. Catalysts 2020, 10, 268. [Google Scholar] [CrossRef]
- Zhang, S.; Katz, M.B.; Dai, S.; Zhang, K.; Du, X.; Graham, G.W.; Pan, X. New Atomic-Scale Insight into Self-Regeneration of Pt-CaTiO3 Catalysts: Incipient Redox-Induced Structures Revealed by a Small-Angle Tilting STEM Technique. J. Phys. Chem. C 2017, 121, 17348–17353. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, S.; Katz, M.B.; Graham, G.W.; Pan, X. In Situ Observation of Rh-CaTiO3 Catalysts during Reduction and Oxidation Treatments by Transmission Electron Microscopy. ACS Catal. 2017, 7, 1579–1582. [Google Scholar] [CrossRef]
- Han, H.; Park, J.; Nam, S.Y.; Kim, K.J.; Choi, G.M.; Parkin, S.S.P.; Jang, H.M.; Irvine, J.T.S. Lattice strain-enhanced exsolution of nanoparticles in thin films. Nat. Commun. 2019, 10, 1471. [Google Scholar] [CrossRef]
- Kim, K.J.; Han, H.; Defferriere, T.; Yoon, D.; Na, S.; Kim, S.J.; Dayaghi, A.M.; Son, J.; Oh, T.S.; Jang, H.M.; et al. Facet-Dependent in Situ Growth of Nanoparticles in Epitaxial Thin Films: The Role of Interfacial Energy. J. Am. Chem. Soc. 2019, 141, 7509–7517. [Google Scholar] [CrossRef] [PubMed]
- Neagu, D.; Kyriakou, V.; Roiban, I.-L.; Aouine, M.; Tang, C.; Caravaca, A.; Kousi, K.; Schreur-Piet, I.; Metcalfe, I.S.; Vernoux, P. In Situ Observation of Nanoparticle Exsolution from Perovskite Oxides; from Atomic Scale Mechanistic Insight to Nanostructure Tailoring. ACS Nano 2019, 13, 12995–13005. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.; Mogni, L.V.; Santaya, M.; Han, M.; Barnett, S.A. Exsolution and electrochemistry in perovskite solid oxide fuel cell anodes: Role of stoichiometry in Sr(Ti, Fe, Ni)O3. J. Power Sources 2019, 439, 227077. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.E.; Mogni, L.V.; Han, M.; Barnett, S.A. Ni-Substituted Sr(Ti,Fe)O3 SOFC Anodes: Achieving High Performance via Metal Alloy Nanoparticle Exsolution. Joule 2018, 2, 478–496. [Google Scholar] [CrossRef]
- Götsch, T.; Köpfle, N.; Grünbacher, M.; Bernardi, J.; Carbonio, E.A.; Hävecker, M.; Knop-Gericke, A.; Bekheet, M.F.; Schlicker, L.; Doran, A. Crystallographic and electronic evolution of lanthanum strontium ferrite (La0.6 Sr0.4 FeO3−δ) thin film and bulk model systems during iron exsolution. Phys. Chem. Chem. Phys. 2019, 21, 3781–3794. [Google Scholar] [CrossRef]
- Joo, S.; Kwon, O.; Kim, K.; Kim, S.; Kim, H.; Shin, J.; Jeong, H.Y.; Sengodan, S.; Han, J.W.; Kim, G. Cation-swapped homogeneous nanoparticles in perovskite oxides for high power density. Nat. Commun. 2019, 10, 697. [Google Scholar] [CrossRef]
- Joo, S.; Kwon, O.; Kim, S.; Jeong, H.Y.; Kim, G. Ni-Fe Bimetallic Nanocatalysts Produced by Topotactic Exsolution in Fe deposited PrBaMn1.7Ni0.3O5+δ for Dry Reforming of Methane. J. Electrochem. Soc. 2020, 167. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, eabb1573. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Foucher, A.C.; Stach, E.A.; Gorte, R.J. Changes in Ni-NiO equilibrium due to LaFeO3 and the effect on dry reforming of CH4. J. Catal. 2020, 381, 561–569. [Google Scholar] [CrossRef]
- Hamada, I.; Uozumi, A.; Morikawa, Y.; Yanase, A.; Katayama-Yoshida, H. A density functional theory study of self-regenerating catalysts LaFe(1−x)M(x)O(3−y) (M = Pd, Rh, Pt). J. Am. Chem. Soc. 2011, 133, 18506–18509. [Google Scholar] [CrossRef]
- Raman, A.S.; Vojvodic, A. Modeling Exsolution of Pt from ATiO3 Perovskites (A = Ca/Sr/Ba) Using First-Principles Methods. Chem. Mater. 2020, 32, 9642–9649. [Google Scholar] [CrossRef]
- Mao, X.; Lin, C.; Graham, G.W.; Gorte, R.J. A Perspective on Thin-Film Perovskites as Supports for Metal Catalysts. ACS Catal. 2020, 10, 8840–8849. [Google Scholar] [CrossRef]
- Mao, X.; Foucher, A.C.; Montini, T.; Stach, E.A.; Fornasiero, P.; Gorte, R.J. Epitaxial and Strong Support Interactions between Pt and LaFeO3 Films Stabilize Pt Dispersion. J. Am. Chem. Soc. 2020, 142, 10373–10382. [Google Scholar] [CrossRef]
- Lin, C.; Foucher, A.C.; Ji, Y.; Curran, C.D.; Stach, E.A.; McIntosh, S.; Gorte, R.J. “Intelligent” Pt Catalysts Studied on High-Surface-Area CaTiO3 Films. ACS Catal. 2019, 9, 7318–7327. [Google Scholar] [CrossRef]
- Lin, C.; Foucher, A.C.; Ji, Y.; Stach, E.A.; Gorte, R.J. Investigation of Rh–titanate (ATiO3) interactions on high-surface-area perovskite thin films prepared by atomic layer deposition. J. Mater. Chem. A 2020, 8, 16973–16984. [Google Scholar] [CrossRef]
- Helveg, S.; Sehested, J.; Rostrup-Nielsen, J. Whisker carbon in perspective. Catal. Today 2011, 178, 42–46. [Google Scholar] [CrossRef]
- Papargyriou, D.; Miller, D.N.; Irvine, J.T.S. Exsolution of Fe–Ni alloy nanoparticles from (La, Sr)(Cr, Fe, Ni) O 3 perovskites as potential oxygen transport membrane catalysts for methane reforming. J. Mater. Chem. A 2019, 7, 15812–15822. [Google Scholar] [CrossRef]
- McIntosh, S.; Gorte, R.J. Direct hydrocarbon solid oxide fuel cells. Chem. Rev. 2004, 104, 4845–4866. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jang, J.B.; Zhang, L.; Stach, E.A.; Gorte, R.J. Improved Coking Resistance of “Intelligent” Ni Catalysts Prepared by Atomic Layer Deposition. ACS Catal. 2018, 8, 7679–7687. [Google Scholar] [CrossRef]
- Mao, X.; Foucher, A.C.; Stach, E.A.; Gorte, R.J. “Intelligent” Pt Catalysts Based on Thin LaCoO3 Films Prepared by Atomic Layer Deposition. Inorganics 2019, 7, 113. [Google Scholar] [CrossRef]
- Kishimoto, H.; Horita, T.; Yamaji, K.; Brito, M.E.; Xiong, Y.-P.; Yokokawa, H. Sulfur poisoning on SOFC Ni anodes: Thermodynamic analyses within local equilibrium anode reaction model. J. Electrochem. Soc. 2010, 157, B802. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Hansen, J.; Helveg, S.; Christiansen, N.; Jannasch, A.-K. Sites for catalysis and electrochemistry in solid oxide fuel cell (SOFC) anode. Appl. Phys. A 2006, 85, 427–430. [Google Scholar] [CrossRef]
- Brightman, E.; Ivey, D.; Brett, D.; Brandon, N. The effect of current density on H2S-poisoning of nickel-based solid oxide fuel cell anodes. J. Power Sources 2011, 196, 7182–7187. [Google Scholar] [CrossRef]
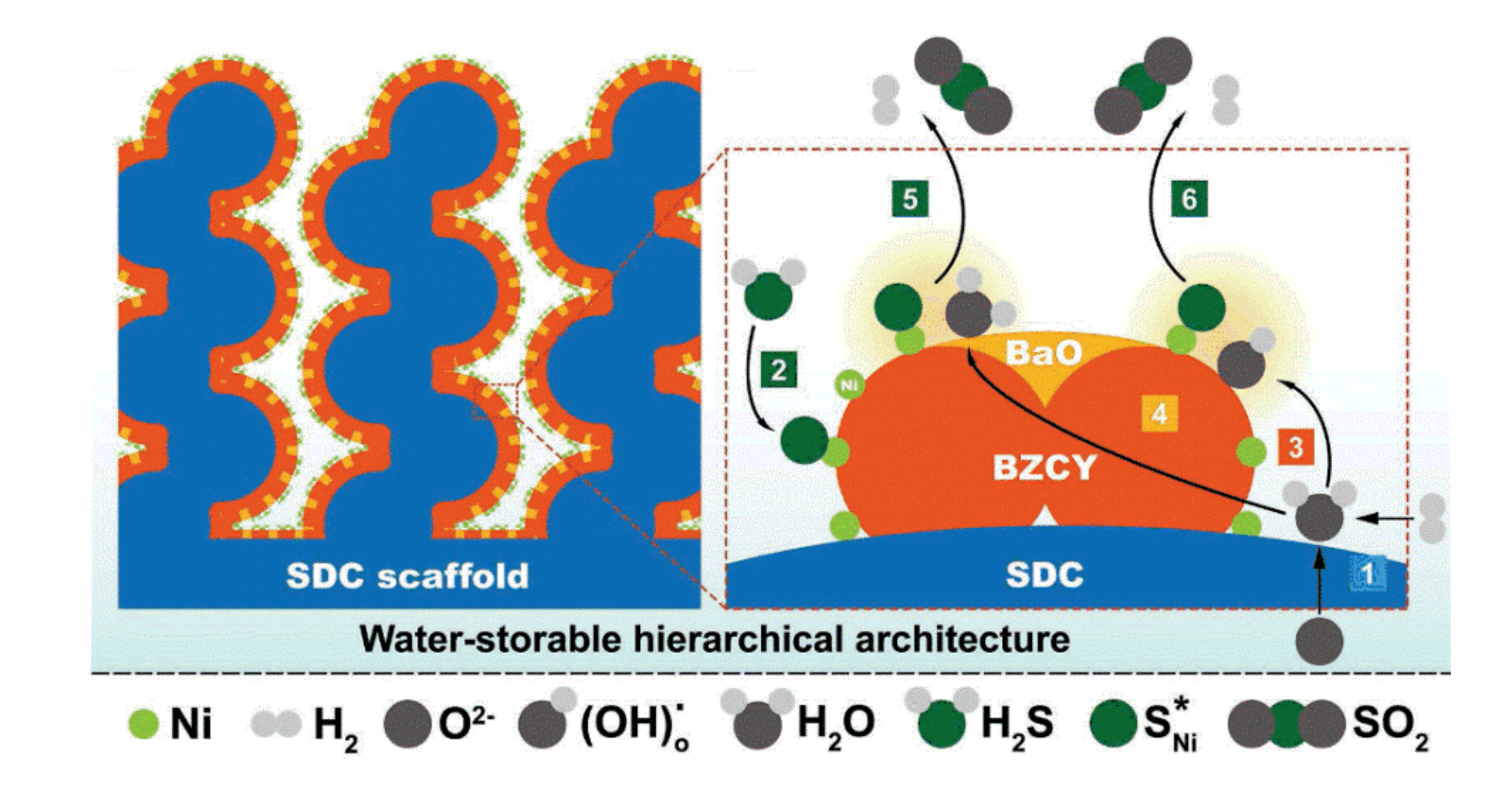
- Song, Y.; Wang, W.; Ge, L.; Xu, X.; Zhang, Z.; Juliao, P.S.B.; Zhou, W.; Shao, Z. Rational Design of a Water-Storable Hierarchical Architecture Decorated with Amorphous Barium Oxide and Nickel Nanoparticles as a Solid Oxide Fuel Cell Anode with Excellent Sulfur Tolerance. Adv. Sci. 2017, 4, 1700337. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.; Behnamian, Y.; Luo, J. A-site deficient perovskite: The parent for in situ exsolution of highly active, regenerable nano-particles as SOFC anodes. J. Mater. Chem. A 2015, 3, 11048–11056. [Google Scholar] [CrossRef]
- Cui, S.-H.; Li, J.-H.; Zhou, X.-W.; Wang, G.-Y.; Luo, J.-L.; Chuang, K.T.; Bai, Y.; Qiao, L.-J. Cobalt doped LaSrTiO3−δ as an anode catalyst: Effect of Co nanoparticle precipitation on SOFCs operating on H2S-containing hydrogen. J. Mater. Chem. A 2013, 1. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Zhang, W.; Zheng, Y.; Lou, X.; Yu, B.; Chen, J.; Chen, Y.; Liu, M.; Wang, J. Heterointerface engineering for enhancing the electrochemical performance of solid oxide cells. Energy Environ. Sci. 2020, 13, 53–85. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, L.; Li, J.; Chi, B.; Pu, J.; Li, J. High-performance Ni in-situ exsolved Ba(Ce0.9Y0.1)0.8Ni0.2O3−δ/Gd0.1Ce0.9O1.95 composite anode for SOFC with long-term stability in methane fuel. Compos. Part B Eng. 2020, 193. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Chen, Y.; Tadé, M.O.; Shao, Z. Nano La0.6Ca0.4Fe0.8Ni0.2O3−δ decorated porous doped ceria as a novel cobalt-free electrode for “symmetrical” solid oxide fuel cells. J. Mater. Chem. A 2014, 2, 19526–19535. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhou, X.-W.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.-N.; Zhang, L.-Z.; Hua, B.; Li, J.; Li, J.-H.; Luo, J.-L. An ingenious Ni/Ce co-doped titanate based perovskite as a coking-tolerant anode material for direct hydrocarbon solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 22830–22838. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, C.; Pan, C.; Lin, W.; Lemmon, J.P.; Chen, F.; Li, C.; Xie, K. Highly efficient electrochemical reforming of CH4/CO2 in a solid oxide electrolyser. Sci. Adv. 2018, 4, eaar5100. [Google Scholar] [CrossRef]
- Madsen, B.D.; Kobsiriphat, W.; Wang, Y.; Marks, L.D.; Barnett, S. SOFC anode performance enhancement through precipitation of nanoscale catalysts. ECS Trans. 2007, 7, 1339. [Google Scholar] [CrossRef]
- Thommy, L.; Joubert, O.; Hamon, J.; Caldes, M.-T. Impregnation versus exsolution: Using metal catalysts to improve electrocatalytic properties of LSCM-based anodes operating at 600 °C. Int. J. Hydrog. Energy 2016, 41, 14207–14216. [Google Scholar] [CrossRef]
- Kobsiriphat, W.; Madsen, B.; Wang, Y.; Shah, M.; Marks, L.; Barnett, S.A. Nickel-and ruthenium-doped lanthanum chromite anodes: Effects of nanoscale metal precipitation on solid oxide fuel cell performance. J. Electrochem. Soc. 2010, 157, B279–B284. [Google Scholar] [CrossRef]
- Vert, V.B.; Melo, F.V.; Navarrete, L.; Serra, J.M. Redox stability and electrochemical study of nickel doped chromites as anodes for H2/CH4-fueled solid oxide fuel cells. Appl. Catal. B Environ. 2012, 115–116, 346–356. [Google Scholar] [CrossRef]
- Sun, Y.F.; Li, J.H.; Cui, L.; Hua, B.; Cui, S.H.; Li, J.; Luo, J.L. A-site-deficiency facilitated in situ growth of bimetallic Ni-Fe nano-alloys: A novel coking-tolerant fuel cell anode catalyst. Nanoscale 2015, 7, 11173–11181. [Google Scholar] [CrossRef]
- Wu, N.; Wang, W.; Zhong, Y.; Yang, G.; Qu, J.; Shao, Z. Nickel-Iron Alloy Nanoparticle-Decorated K2NiF4 -Type Oxide as an Efficient and Sulfur-Tolerant Anode for Solid Oxide Fuel Cells. ChemElectroChem 2017, 4, 2378–2384. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Y.-M.; Yu, Y.; Song, Y.; Ma, Z.-F.; Yin, J. Roles of Fe Ni nanoparticles and SrLaFeO4 substrate in the performance and reliability of a composite anode prepared through in-situ exsolution for intermediate temperature solid oxide fuel cells (I). Int. J. Hydrog. Energy 2018, 43, 10440–10447. [Google Scholar] [CrossRef]
- Park, B.H.; Choi, G.M. Ex-solution of Ni nanoparticles in a La0.2Sr0.8Ti1−xNixO3−δ alternative anode for solid oxide fuel cell. Solid State Ion. 2014, 262, 345–348. [Google Scholar] [CrossRef]
- Park, B.H.; Choi, G.M. Effect of anode firing on the performance of lanthanum and nickel co-doped SrTiO3 (La0.2Sr0.8Ti0.9Ni0.1O3−δ) anode of solid oxide fuel cell. J. Power Sources 2015, 293, 684–691. [Google Scholar] [CrossRef]
- Arrivé, C.; Delahaye, T.; Joubert, O.; Gauthier, G. Exsolution of nickel nanoparticles at the surface of a conducting titanate as potential hydrogen electrode material for solid oxide electrochemical cells. J. Power Sources 2013, 223, 341–348. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, M.; Huang, P.; Guo, G.; Hong, M.; Li, C.; Irvine, J.T.; Xie, K. Enhancing CO2 electrolysis through synergistic control of non-stoichiometry and doping to tune cathode surface structures. Nat. Commun. 2017, 8, 14785. [Google Scholar] [CrossRef]
- Bahout, M.; Managutti, P.B.; Dorcet, V.; Le Gal La Salle, A.; Paofai, S.; Hansen, T.C. In situ exsolution of Ni particles on the PrBaMn2O5 SOFC electrode material monitored by high temperature neutron powder diffraction under hydrogen. J. Mater. Chem. A 2020, 8, 3590–3597. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, K.; Joo, S.; Jeong, H.Y.; Shin, J.; Han, J.W.; Sengodan, S.; Kim, G. Self-assembled alloy nanoparticles in a layered double perovskite as a fuel oxidation catalyst for solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 15947–15953. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Li, M.; Xia, C.; Zhou, B.; Chen, F. Exsolved Fe–Ni nano-particles from Sr2Fe1.3Ni0.2Mo0.5O6 perovskite oxide as a cathode for solid oxide steam electrolysis cells. J. Mater. Chem. A 2016, 4, 14163–14169. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, S.; Lin, Y.; Zhang, Y.; An, K.; Chen, F. Releasing metal catalysts via phase transition: (NiO)0.05-(SrTi0.8Nb0.2O3)0.95 as a redox stable anode material for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2014, 6, 19990–19996. [Google Scholar] [CrossRef]
- Kyriakou, V.; Neagu, D.; Zafeiropoulos, G.; Sharma, R.K.; Tang, C.; Kousi, K.; Metcalfe, I.S.; van de Sanden, M.C.M.; Tsampas, M.N. Symmetrical Exsolution of Rh Nanoparticles in Solid Oxide Cells for Efficient Syngas Production from Greenhouse Gases. ACS Catal. 2019, 10, 1278–1288. [Google Scholar] [CrossRef]
- Monteiro, N.K.; Noronha, F.B.; da Costa, L.O.O.; Linardi, M.; Fonseca, F.C. A direct ethanol anode for solid oxide fuel cell based on a chromite-manganite with catalytic ruthenium nanoparticles. Int. J. Hydrogen Energy 2012, 37, 9816–9829. [Google Scholar] [CrossRef]
- Liao, Y.; Bierschenk, D.M.; Barnett, S.A.; Marks, L.D. Operational Inhomogeneities in La0.9Sr0.1Ga0.8Mg0.2O3-δ Electrolytes and La0.8Sr0.2Cr0.82Ru0.18O3−δ-Ce0.9Gd0.1O2−δ Composite Anodes for Solid Oxide Fuel Cells. Fuel Cells 2011, 11, 635–641. [Google Scholar] [CrossRef]
- Kobsiriphat, W.; Madsen, B.D.; Wang, Y.; Marks, L.D.; Barnett, S.A. La0.8Sr0.2Cr1−xRuxO3−δ–Gd0.1Ce0.9O1.95 solid oxide fuel cell anodes: Ru precipitation and electrochemical performance. Solid State Ion. 2009, 180, 257–264. [Google Scholar] [CrossRef]
- Bierschenk, D.M.; Potter-Nelson, E.; Hoel, C.; Liao, Y.; Marks, L.; Poeppelmeier, K.R.; Barnett, S.A. Pd-substituted (La,Sr)CrO3−δ–Ce0.9Gd0.1O2−δ solid oxide fuel cell anodes exhibiting regenerative behavior. J. Power Sources 2011, 196, 3089–3094. [Google Scholar] [CrossRef]
- Marcucci, A.; Zurlo, F.; Sora, I.N.; Placidi, E.; Casciardi, S.; Licoccia, S.; Di Bartolomeo, E. A redox stable Pd-doped perovskite for SOFC applications. J. Mater. Chem. A 2019, 7, 5344–5352. [Google Scholar] [CrossRef]
- Shin, T.H.; Okamoto, Y.; Ida, S.; Ishihara, T. Self-recovery of Pd nanoparticles that were dispersed over La(Sr)Fe(Mn)O3 for intelligent oxide anodes of solid-oxide fuel cells. Chem. Eur. J. 2012, 18, 11695–11702. [Google Scholar] [CrossRef]
- Glaser, R.; Zhu, T.; Troiani, H.; Caneiro, A.; Mogni, L.; Barnett, S. The enhanced electrochemical response of Sr(Ti0.3Fe0.7Ru0.07)O3−δ anodes due to exsolved Ru–Fe nanoparticles. J. Mater. Chem. A 2018, 6, 5193–5201. [Google Scholar] [CrossRef]
- Chanthanumataporn, M.; Hui, J.; Yue, X.; Kakinuma, K.; Irvine, J.T.S.; Hanamura, K. Electrical reduction of perovskite electrodes for accelerating exsolution of nanoparticles. Electrochim. Acta 2019, 306, 159–166. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-H.; Wang, M.-N.; Hua, B.; Li, J.; Luo, J.-L. A-site deficient chromite perovskite with in situ exsolution of nano-Fe: A promising bi-functional catalyst bridging the growth of CNTs and SOFCs. J. Mater. Chem. A 2015, 3, 14625–14630. [Google Scholar] [CrossRef]
- Qi, H.; Yang, T.; Li, W.; Ma, L.; Hu, S.; Shi, W.; Sabolsky, E.M.; Zondlo, J.W.; Hart, R.; Hackett, G.A. Reversible In-Situ Exsolution of Fe Catalyst in La0.5Sr1.5Fe1.5Mo0.5O6−δ Anode for SOFCs. ECS Trans. 2019, 91, 1701. [Google Scholar] [CrossRef]
- Qi, H.; Xia, F.; Yang, T.; Li, W.; Li, W.; Ma, L.; Collins, G.; Shi, W.; Tian, H.; Hu, S.; et al. In Situ Exsolved Nanoparticles on La0.5Sr1.5Fe1.5Mo0.5O6−δ Anode Enhance the Hydrogen Oxidation Reaction in SOFCs. J. Electrochem. Soc. 2020, 167. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, C.; Xie, K.; Gan, L. High performance, coking-resistant and sulfur-tolerant anode for solid oxide fuel cell. J. Power Sources 2018, 406, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Wei, B.; Cao, Z.; Yue, X.; Zhang, Y.; Lu, Z. Niobium Doped Lanthanum Strontium Ferrite as A Redox-Stable and Sulfur-Tolerant Anode for Solid Oxide Fuel Cells. ChemSusChem 2018, 11, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ni, W.; Wang, J.; Zhong, Q.; Han, M.; Zhu, T. Exploration of Co-Fe alloy precipitation and electrochemical behavior hysteresis using Lanthanum and Cobalt co-substituted SrFeO3−δ SOFC anode. Electrochim. Acta 2018, 277, 226–234. [Google Scholar] [CrossRef]
- Lai, K.-Y.; Manthiram, A. Self-Regenerating Co–Fe Nanoparticles on Perovskite Oxides as a Hydrocarbon Fuel Oxidation Catalyst in Solid Oxide Fuel Cells. Chem. Mater. 2018, 30, 2515–2525. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Lin, Y.; Liu, J.; Chen, F.; Liu, M. In situ fabrication of CoFe alloy nanoparticles structured (Pr0.4Sr0.6)3(Fe0.85Nb0.15)2O7 ceramic anode for direct hydrocarbon solid oxide fuel cells. Nano Energy 2015, 11, 704–710. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Z.; Jin, C.; Xiao, G.; Chen, F.; Han, M. Sulfur-tolerant redox-reversible anode material for direct hydrocarbon solid oxide fuel cells. Adv. Mater. 2012, 24, 1439–1443. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, Z.; Lei, Z.; Jin, C.; Liu, Y.; Wang, Y.; Peng, S. Co-substituted Sr2Fe1.5Mo0.5O6-δ as anode materials for solid oxide fuel cells: Achieving high performance via nanoparticle exsolution. J. Power Sources 2019, 438. [Google Scholar] [CrossRef]
- Xi, X.; Cao, Z.-S.; Shen, X.-Q.; Lu, Y.; Li, J.; Luo, J.-L.; Fu, X.-Z. In situ embedding of CoFe nanocatalysts into Sr3FeMoO7 matrix as high-performance anode materials for solid oxide fuel cells. J. Power Sources 2020, 459, 228071. [Google Scholar] [CrossRef]
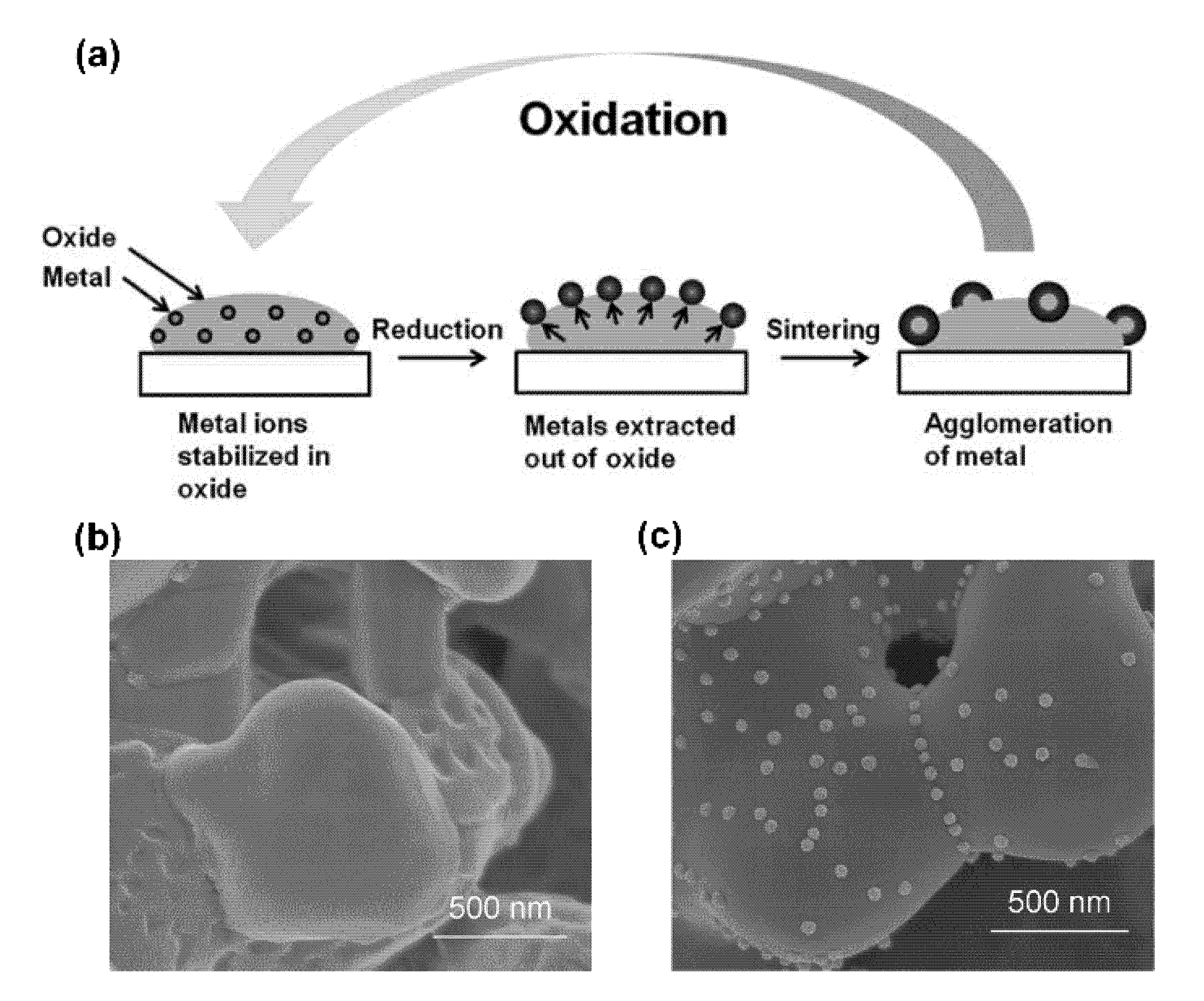
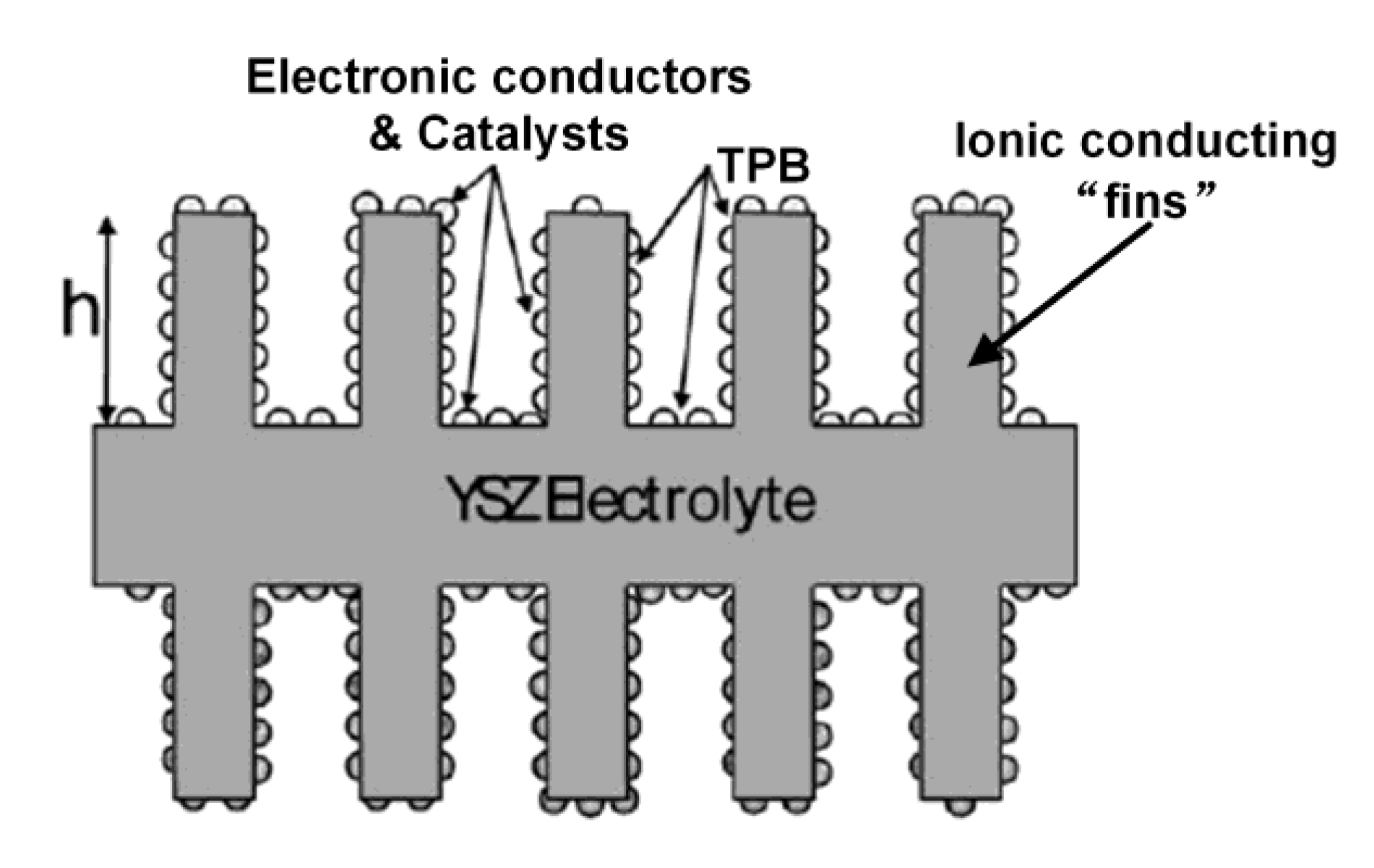
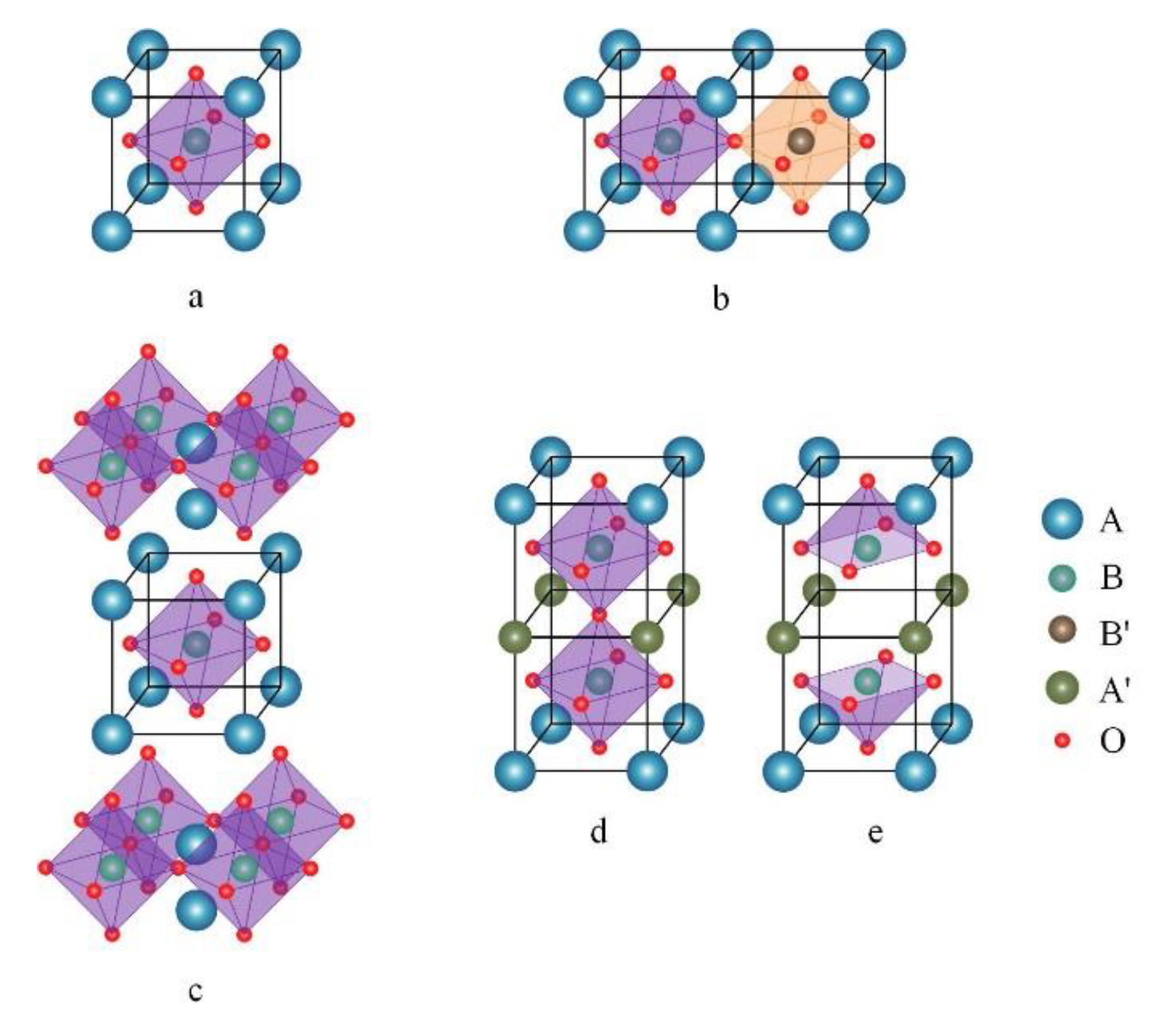
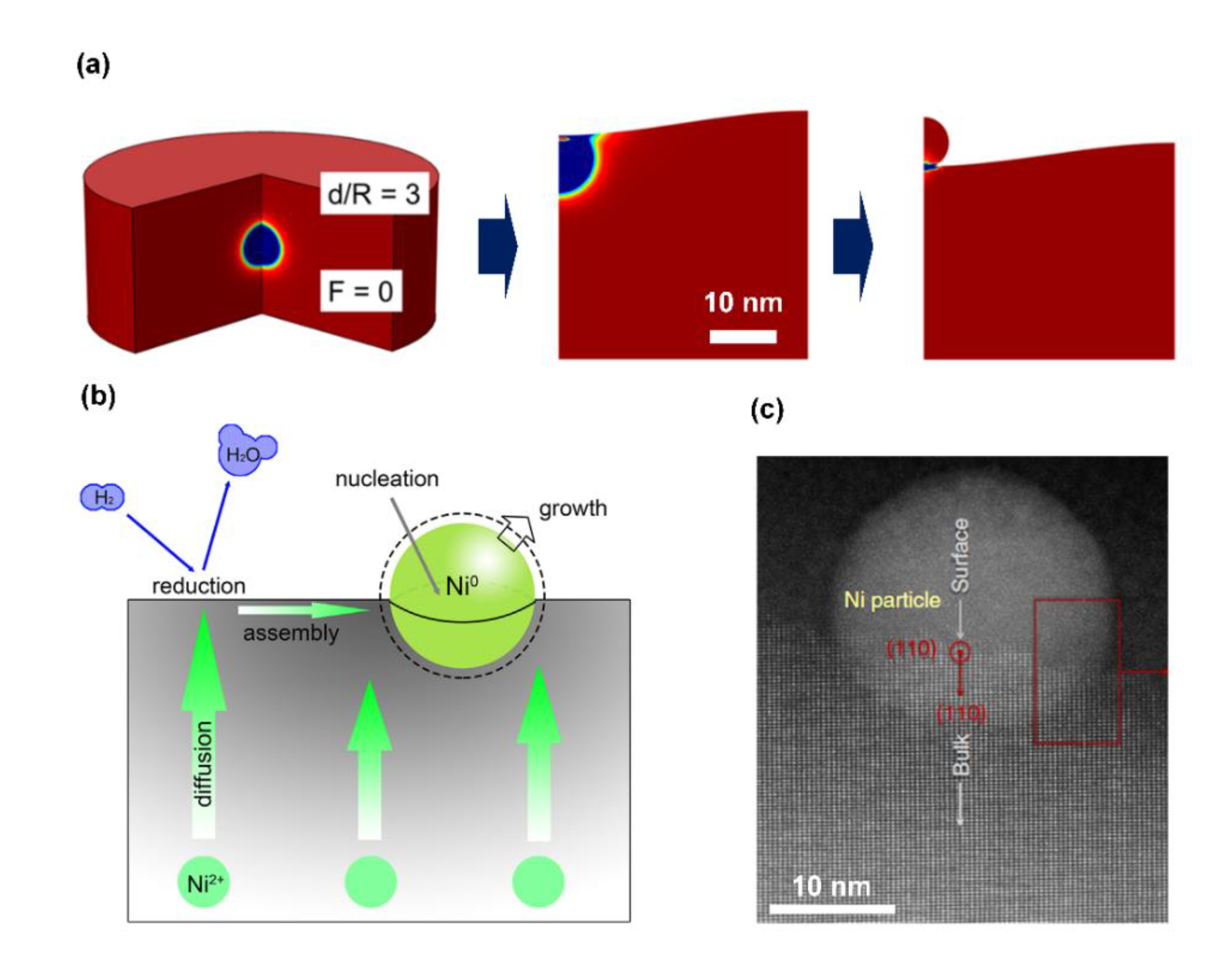
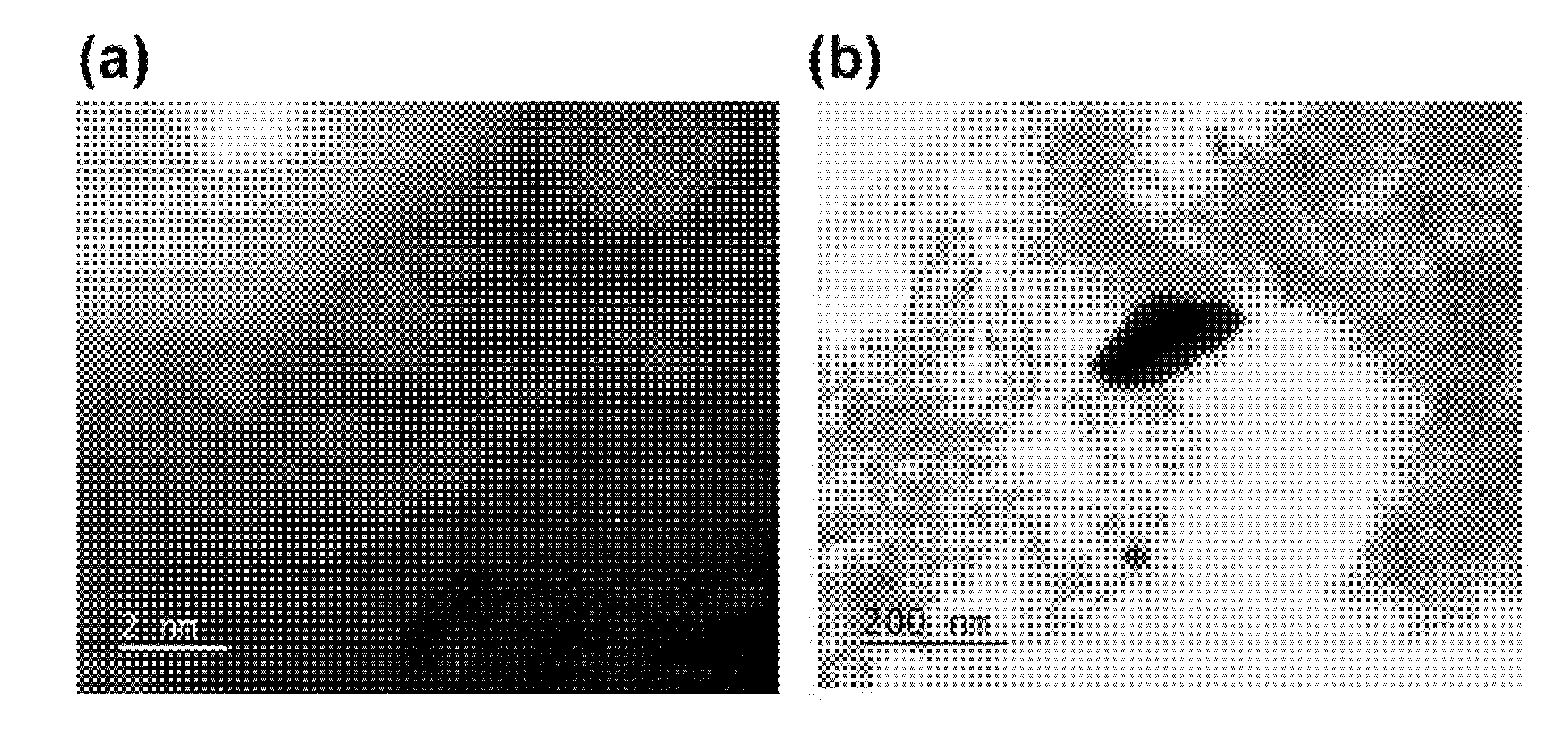
| Pristine Phase | Operation Mode | Metal | Hosting Phase | Particle Parameters | Cell Configuration |
|---|---|---|---|---|---|
| BaCe0.72Y0.08Ni0.2O3−δ [92] | SOFC | Ni | simple | -- | BCYNi infiltrated GDC (Gd0.1Ce0.9O1.95)|GDC|LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ)-GDC |
| BaZr0.32Ce0.32Y0.16Ni0.2O3−δ [88] | SOFC | Ni | simple | ~30 nm dia. | BZCYNi infiltrated SDC (Sm0.2Ce0.8O1.9)|SDC|BSCF (Ba0.5Sr0.5Co0.8Fe0.2O3−δ) infiltrated SDC |
| Ce1−xNixVO4−δ family and Ce0.7(Sr/Ca)0.1Ni0.2VO4−δ [12] | SOFC | Ni | simple | 10–20 nm dia. | doped vanadate-YSZ|YSZ|LSF (La0.8Sr0.2FeO3)-YSZ |
| La0.6Ca0.4Fe0.8Ni0.2O3−δ [93] | SOFC | Ni | simple | ~10 nm | LCFNi infiltrated SDC (Sm0.2Ce0.8O1.9)|SDC|LCFNi infiltrated SDC symmetric cell |
| La0.3Sr0.6Ce0.1Ni0.1Ti0.9O3−δ and La0.4Sr0.6Ni0.1Ti0.9O3−δ [94] | SOFC | Ni | simple | ~20 nm dia. | LSCNT/LSNT|YSZ|LSM-YSZ |
| La0.675Sr0.225Cr0.45Mn0.45Ni0.1−xCuxO3−δ family [95] | SOEC | Ni-Cu | simple | ~40 nm dia. | LSCMNiCu-SDC (Ce0.8Sm0.2O2−δ)|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3)|LSM (La0.76Sr0.19MnO3−δ)-SDC |
| La0.8Sr0.2Cr1-xNixO3−δ [96] | SOFC | Ni | simple | 15–20 nm dia. | LSCrNi-GDC (Ce0.9Gd0.1O1.95)|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)|LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ)-GDC |
| La0.4Sr0.6Fe0.75Ni0.1Nb0.15O3−δ [38] | SOFC | Fe-Ni | RP-simple | 20–40 nm dia. | LSFeNiN|SDC (Sm0.2Ce0.8O1.9)|ScCeSZ ((Sc0.2O0.3)0.1(CeO2)0.01(ZrO2)0.89)|SDC|LSFCN (La0.5Sr0.5Fe0.8Cu0.15Nb0.05O3−δ) |
| La0.4Sr0.4Ni0.06Ti0.94O3−δ [35] | SOEC | Ni | simple | 60–90 nm dia. | LSNiT|YSZ|LSM (La0.76Sr0.19MnO3)-YSZ |
| La0.75Sr0.25Cr0.5Mn0.3Ni0.2O3−δ [97] | SOFC | Ni | simple | ~15 nm dia. | LSCMNi|Ce0.9Gd0.1O1.95 (GDC)|LSCMNi symmetric cell for impedance testing |
| La0.8Sr0.2Cr0.69Ni0.31O3−δ family [98] | SOFC | Ni | simple | 10–50 nm dia. | LSCrNi-GDC|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)|LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ)-GDC |
| La0.85Sr0.15Cr1−yNiyO3−δ family [99] | SOFC | Ni | simple | 15–20 nm dia. | LSCrN|YSZ|LSCrN symmetric cell for impedance testing |
| La0.7Sr0.3Cr0.85Ni0.15O3−δ and La0.7Sr0.3Cr0.85Ni0.1125Fe0.0375O3−δ [100] | SOFC | Ni/Ni-Fe | simple | 25–30 nm dia. | LSCN/LSCNF-YSZ|YSZ|LSM-YSZ |
| LaSrFeNiO6−δ [101] | SOFC | Ni-Fe | RP | 30–50 nm dia. | LSFeNi infiltrated SDC (Sm0.2Ce0.8O1.9)|SDC|SSC (Sm0.5Sr0.5CoO3−δ) infiltrated SDC |
| La0.5Sr0.5Fe0.8Ni0.2O3−δ [102] | SOFC | Ni-Fe | RP | ~25 nm dia. | LSFN|SDC (Sm0.2Ce0.8O1.9)|ScCeSZ ((Sc0.2O0.3)0.1(CeO2)0.01(ZrO2)0.89)|SDC|LSFCN (La0.5Sr0.5Fe0.8Cu0.15Nb0.05O3−δ) |
| La0.4Sr0.4Sc0.9Ni0.1O3−δ [13] | SOFC | Ni | simple | ~50 nm dia. | LSSN-SDC (samarium-doped ceria)|SDC|LSSN-SDC symmetric cell for impedance testing |
| La0.2Sr0.8Ti1−xNixO3−δ [103] | SOFC | Ni | simple | ~7 nm dia. | LSTNi|(ScCeSZ) ((Sc2O3)0.1(CeO2)0.01(ZrO2)0.89)|Gd0.2Ce0.8O2 (GDC)|La0.6Sr0.4Co0.2Fe0.8O3 (LSCF)-Gd0.1Ce0.9O2 (GDC) electrolyte-supported |
| La0.2Sr0.8Ti0.9Ni0.1O3−δ [104] | SOFC | Ni | simple | ~7 nm dia. | LSTNi|(Sc2O3)0.1(CeO2)0.01(ZrO2)0.89 (ScCeSZ)|Gd0.2Ce0.8O2 (GDC)|La0.6Sr0.4Co0.2Fe0.8O3 (LSCF)-Gd0.1Ce0.9O2 (GDC) electrolyte-supported |
| La0.5Sr0.5Ti0.75Ni0.25O3−δ [105] | SOFC | Ni | simple | 5–50 nm dia. | LSTNi|yttria-doped ceria (YDC)|YSZ|YDC|LSTNi symmetric cell for impedance testing |
| La0.19Sr0.76Ti0.85Cr0.1Ni0.05O3+δ/La0.19Sr0.76Ti0.85Mn0.1Ni0.05O3+δ [106] | SOEC | Ni | simple | ~60 nm dia. | LSTCNi/LSTMNi-SDC (Ce0.8Sm0.2O2-δ)|YSZ|LSM (La0.76Sr0.19MnO3−δ)-YSZ |
| NbTi0.5Ni0.5O4 [59] | SOFC | Ni | rutile | ~80 nm dia. | NTNO-YSZ|YSZ|NTNO-YSZ symmetric cell for impedance tests |
| Pr0.65Ba0.35Mn0.975Ni0.025O3 [107] | SOFC | Ni | layered double | ~80 nm dia. | PBMNi|Ce0.9Gd0.1O1.95 (GDC)|YSZ|GDC|PBMNi symmetric cell for impedance tests |
| PrBaMn1.7Co0.1Ni0.2O5+δ [108] | SOFC | Co-Ni | layered double | ~40 nm dia. | PBMCNO|La0.4Ce0.6O2−δ (LDC)|La0.9Sr0.1Ga0.8Mg0.2O3−δ (LSGM)|NdBa0.5Sr0.5Co1.5Fe0.5O5+δ–Ce0.9Gd0.1O2-δ (NBSCF50-GDC) |
| Pr0.5Ba0.5Mn0.85(Ni/Co)0.15O3−δ [36] | SOFC | Ni, Co, respectively | layered double | 20–50 nm dia. | PBMNi/Co|LDC (La0.4Ce0.6O2-δ)|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)|NdBSCF (NdBa0.5Sr0.5Co1.5Fe0.5O5-δ)-GDC (Ce0.9Gd0.1O2−δ) |
| Sr2FeMo1−xNixO6−δ family [52] | SOFC | Fe-Ni | RP and simple | 50–60 nm dia. | SFMNi|La0.4Ce0.6O2−δ(LDC)|La0.8Sr0.2Ga0.87Mg0.13O3 (LSGM)|La0.58Sr0.4Co0.2Fe0.8O3−δ (LSCF) |
| Sr2Fe1.3Mo0.5Ni0.2O6−δ [109] | SOEC | Fe-Ni | simple | ~30 nm dia., ~500 μm−2 | SFMNi–Sm0.2Ce0.8O1.9 (SDC)|La0.5Ce0.5O1.5 (LDC)|La0.8Sr0.2Ga0.87Mg0.13O3 (LSGM)|SDC–La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) |
| Sr0.95Ti0.3Fe0.63Ni0.07O3−δ [68], SrTi0.3Fe0.63Ni0.07O3−δ [67] | SOFC | Fe-Ni | simple | STFN: 40–70 nm dia. ~240 μm−2 S95TFN: 20–70 nm dia. ~210 μm−2 | STFeNi|LDC (La0.4Ce0.6O2−δ)|LSGM (La0.8Sr0.2Ga0.83Mg0.17O3−δ)|LSCF (La0.6Sr0.4Co0.2Fe0.8O33−δ)-GDC (Gd0.1Ce0.9O1.95) |
| Sr0.95Ti0.76Nb0.19Ni0.05O3−δ [110] | SOFC | Ni | simple | ~50 nm dia. | STNNi- Ce0.8Sm0.2O2 (SDC)|La0.4Ce0.6O2 (LDC)|La0.8Sr0.2Ga0.87Mg0.13O3 (LSGM)|La0.6Sr0.4Co0.2Fe0.8O3 (LSCF) |
| La0.43Ca0.37Rh0.06Ti0.94O3 [111] | SOEC | Rh | simple | 2.1–3.2 nm dia.; 11,000–5100 μm−2 | LCRhT|GDC|ScCeSZ|GDC|LCRhT |
| La0.75Sr0.25Cr0.50−xMn0.50-yRux,yO3 family [112] | SOFC | Ru | simple | 5–10 nm dia. | LSCMRu|Ni-YSZ|YSZ|La0.65Sr0.3MnO3 (LSM) |
| La0.8Sr0.2Cr0.82Ru0.18O3−δ [98,113] | SOFC | Ru | simple | ~2.9 nm | LSCrRu-GDC (Ce0.9Gd0.1O1.95)|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)|LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ)-GDC |
| La0.8Sr0.2Cr1−xRuxO3−δ family [14,96,114] | SOFC | Ru | simple | ≤5 nm dia. | LSCrRu-GDC (Ce0.9Gd0.1O1.95)|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)|LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ)-GDC |
| La0.8Sr0.2Cr0.8Pd0.2O3−δ, La0.8Sr0.2Cr0.95Pd0.05O3−δ [115] | SOFC | Pd | simple | ~8 nm dia. | LSCPd-GDC (Ce0.9Gd0.1O2−δ)|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)|LSCF (La0.6Sr0.4Fe0.8Co0.2O3−δ)-GDC |
| La0.6Sr0.4Fe0.95Pd0.05O3−δ [116] | SOFC | Pd and Fe separately | RP and simple | 10–15 nm dia. | LSFPd-Ce0.9Gd0.1O2 (GDC)|La0.8Sr0.2Ga0.8Mg0.2O3−δ (LSGM)|LSFPd |
| La0.6Sr0.4Fe0.85Pd0.05Mn0.9O3 [117] | SOFC | Pd | simple | ~10 nm dia. | LSFMP|La0.8Sr0.2Ga0.8Mg0.15Co0.05O3 (LSGMC)|Sm0.5Sr0.5CoO3 (SSC) electrolyte-supported |
| SrTi0.3Fe0.7Ru0.07O3−δ [118] | SOFC | Ru-Fe | simple | 5–20 nm dia. | STFRu|LDC (La0.4Ce0.6O2)|LSGM (La0.8Sr0.2Ga0.83Mg0.17O3)|LSCF (La0.6Sr0.4Fe0.8Co0.2O3)-GDC (Ce0.9Gd0.1O2) |
| Ce1−x(Co/Cu)xVO4−δ families [12] | SOFC | Co, Cu | simple | 10–20 nm dia. | CeTMV-YSZ|YSZ|LSF (La0.8Sr0.2FeO3)-YSZ |
| Ce0.8Sr0.1Cu0.05Co0.05VO4-δ [31] | SOFC | Cu, Co mixture with separate phases | simple | 10–20 nm dia. | CeSrCuCV-YSZ|YSZ|LSF (La0.8Sr0.2FeO3)-YSZ |
| La0.43Ca0.37Ni0.06Ti0.94O3−δ/La0.43Ca0.37Ni0.03Fe0.03Ti0.94O3−δ [119] | SOFC and SOEC | Ni/Ni-Fe alloy | simple | 30–100 nm dia. | LCNiT/LCNiFeT|ScCeSZ|LSM (La0.76Sr0.19MnO3)-ScCeSZ |
| La0.3Sr0.7Co0.07Ti0.93O4−δ [90] | SOFC | Co | simple | ~5 nm dia. | LSCT-YSZ|YSZ|LSM-YSZ |
| La0.7Sr0.3Cr0.85Fe0.15O3−δ, La0.6Sr0.3Cr0.85Fe0.15O3−δ [120] | SOFC | Fe | simple | ~25 nm dia. | LSCrF-YSZ|YSZ|LSM-YSZ |
| La0.5Sr1.5Fe1.5Mo0.5O6-δ [121,122] | SOFC | Fe | RP and simple | ~100 nm dia. | LSFM|LSGM|LSFM symmetric cell for impedance testing |
| La0.675Sr0.225Cr0.45Mn0.45Cu0.1-xFexO3−δ family [123] | SOFC | Cu-Fe | simple | ~30 nm dia. | LSCMCuFe-SDC (Ce0.8Sm0.2O2-δ)|LDC (Ce0.6La0.4O2)|LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)|LSM (La0.8Sr0.2MnO3−δ)-SDC |
| La0.5Sr0.5Fe0.8Cu0.15Nb0.05O3−δ [19] | SOFC | Cu | RP-simple | 5–10 nm dia. | LSFCuN|SDC (Sm0.2Ce0.8O1.9)|ScCeSZ ((Sc2O3)0.1(CeO2)0.01(ZrO2)0.89)|SDC|LSFCuN |
| La0.8Sr0.2Fe0.9Nb0.1O3−δ [124] | SOFC | Fe | simple | less than 50 nm dia. | LSFNb|Sm0.2Ce0.8O2−δ (SDC)|(Sc2O3)0.1(CeO2)0.01(ZrO2)0.89 (ScCeSZ)|(La0.75Sr0.25)0.95MnO3−δ-ScCeSZ |
| La0.5Sr0.5Co0.45Fe0.45Nb0.1O3−δ [125] | SOFC | Co-Fe | simple | 30–50 nm dia. | LSCoFeN|LSGM (La0.8Sr0.2Ga0.83Mg0.17O3−δ)|LSCoFeN symmetric cell for impedance testing |
| La0.3Sr0.7Cr0.3Fe0.6Co0.1O3−δ [126] | SOFC | Co-Fe | simple | ~30 nm dia. | LSCrFeCo-GDC|La0.4Ce0.6O2−δ (LDC)|La0.8Sr0.2Ga0.8Mg0.2O3−δ (LSGM)|LSCF-GDC |
| La0.8Sr1.2Fe0.9Co0.1O4-δ [50] | SOFC | Co | RP | ~10 nm dia. | LSFC-LSGM (La0.9Sr0.1Ga0.8Mg0.2O3−δ)-GDC multilayered anode |LSGM|LSFC-LSGM-GDC symmetric cell for impedance testing |
| La0.4Sr0.4Fe0.06Ti0.94O3−δ [35] | SOEC | Fe | simple | 30–60 nm dia. | LSFeTi|YSZ|LSM (La0.76Sr0.19MnO3)-YSZ |
| Pr0.5Ba0.5Mn0.9Co0.1O3−δ [48] | SOEC/SOFC | Co | layered double | 20–30 nm dia. | PBMCo-GDC|YSZ|LSM-DGC |
| Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ [127] | SOFC | Co-Fe | RP | ~30 nm dia. | PSCFN|LDC (La0.4Ce0.6O2-δ)|LSGM (La0.8Sr0.2Ga0.83Mg0.17O3−δ)|BCFN (Ba0.9Co0.7Fe0.2Nb0.1O3−δ) |
| Pr0.4Sr0.6Co0.2Fe0.7Nb0.1O3−δ [128] | SOFC | Co-Fe | RP | ~50 nm dia. | PSCoFeN|LSGM (La0.8Sr0.2Ga0.83Mg0.17O3−δ)|PSCoFeN, PSCFN|LSGM|BCFN (Ba0.9Co0.7Fe0.2Nb0.1O3−δ) and PSCoFeN|YSZ|BCFN |
| Pr0.4Sr0.6Co0.2Fe0.7Mo0.1O3−δ [51] | SOEC | Co-Fe | RP | ~50 nm dia. | PSCFM-GDC (Gd0.2Ce0.8O2-δ)|GDC|YSZ|GDC|LSCF (La0.58Sr0.38Co0.20Fe0.80O3−δ)-GDC |
| Sr2Fe1.3Co0.2Mo0.5O6−δ [129] | SOFC | Co | RP | ~50 nm dia. | SFCM|LSGM (La0.8Sr0.2Ga0.83Mg0.17O3−δ)|LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ) |
| Sr2Fe1.35Mo0.45Co0.2O6−δ [56] | SOEC | Co-Fe | RP and double | exsolved under H2 at 850 °C for different length of time. 1 h: ~12 nm, ~750 μm−2; 2 h: ~18 nm, ~780 μm−2; 4 h: ~25 nm; 680 μm−2 | LCRhT|GDC|ScCeSZ|GDC|LCRhT |
| Sr2FeMo0.67Co0.33O6−δ [130] | SOFC | Co-Fe | RP | 10–50 nm | SFMCo-SDC (Sm0.2Ce0.8O1.9)|LDC (La0.4Ce0.6O2−δ)|LSGM (La0.8Sr0.2Ga0.8Mg0.2O3−δ)|LSCF (La0.58Sr0.4Co0.2Fe0.8O3−δ)-SDC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.; Kwon, O.; Gorte, R.J.; Vohs, J.M. Metal Exsolution to Enhance the Catalytic Activity of Electrodes in Solid Oxide Fuel Cells. Nanomaterials 2020, 10, 2445. https://doi.org/10.3390/nano10122445
Cao T, Kwon O, Gorte RJ, Vohs JM. Metal Exsolution to Enhance the Catalytic Activity of Electrodes in Solid Oxide Fuel Cells. Nanomaterials. 2020; 10(12):2445. https://doi.org/10.3390/nano10122445
Chicago/Turabian StyleCao, Tianyu, Ohhun Kwon, Raymond J. Gorte, and John M. Vohs. 2020. "Metal Exsolution to Enhance the Catalytic Activity of Electrodes in Solid Oxide Fuel Cells" Nanomaterials 10, no. 12: 2445. https://doi.org/10.3390/nano10122445
APA StyleCao, T., Kwon, O., Gorte, R. J., & Vohs, J. M. (2020). Metal Exsolution to Enhance the Catalytic Activity of Electrodes in Solid Oxide Fuel Cells. Nanomaterials, 10(12), 2445. https://doi.org/10.3390/nano10122445








