Controlling the Oxidation of Magnetic and Electrically Conductive Solid-Solution Iron-Rhodium Nanoparticles Synthesized by Laser Ablation in Liquids
Abstract
1. Introduction
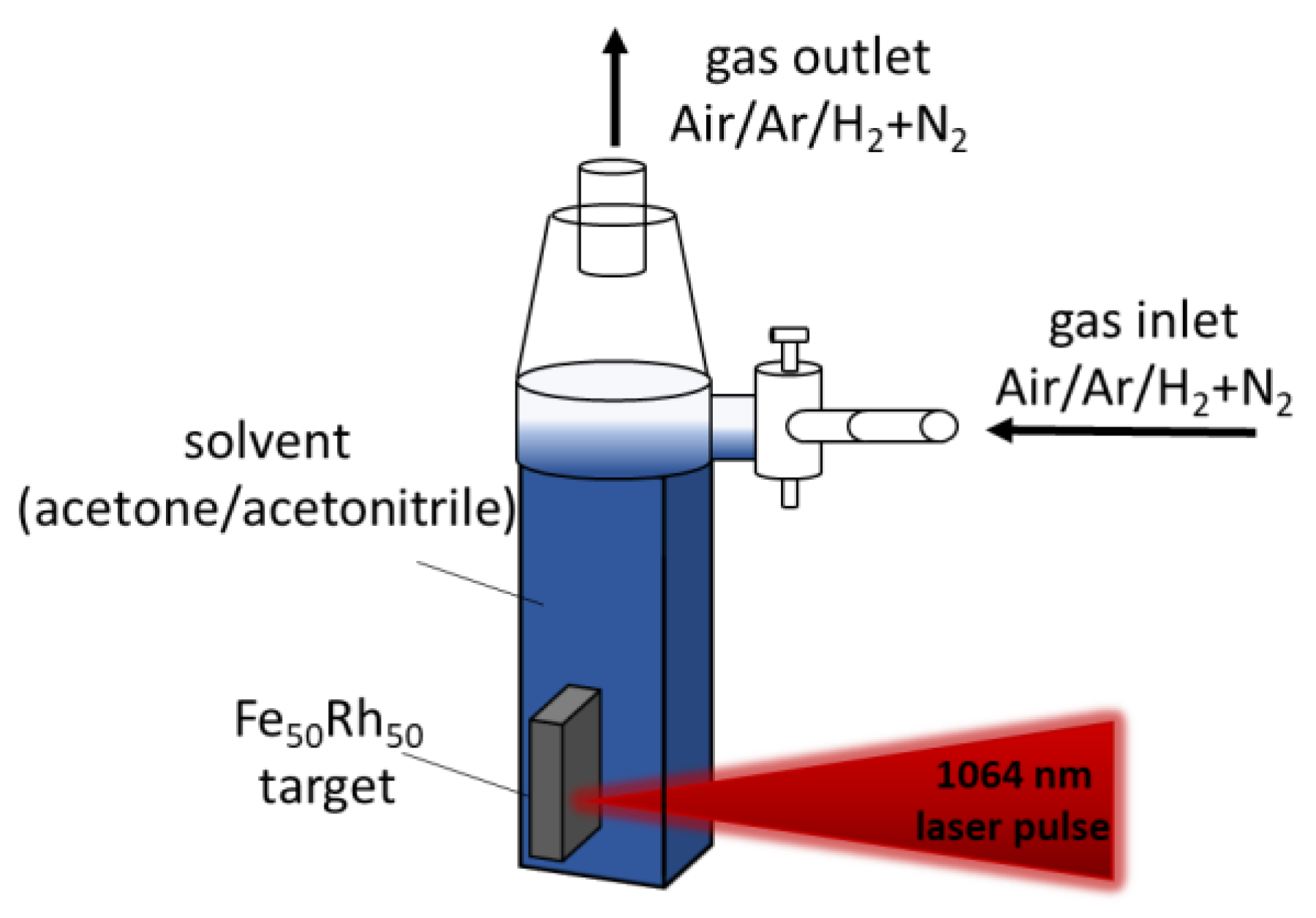
2. Materials and Methods
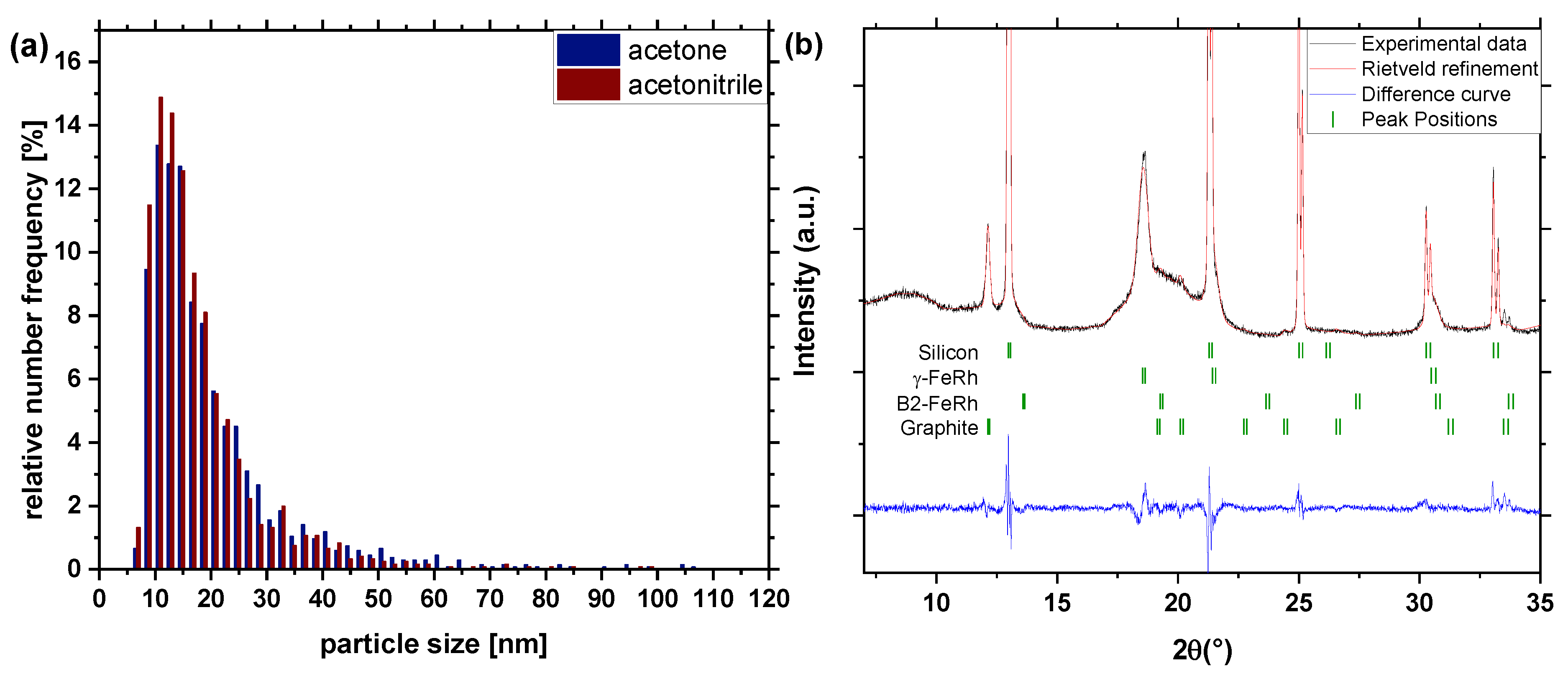
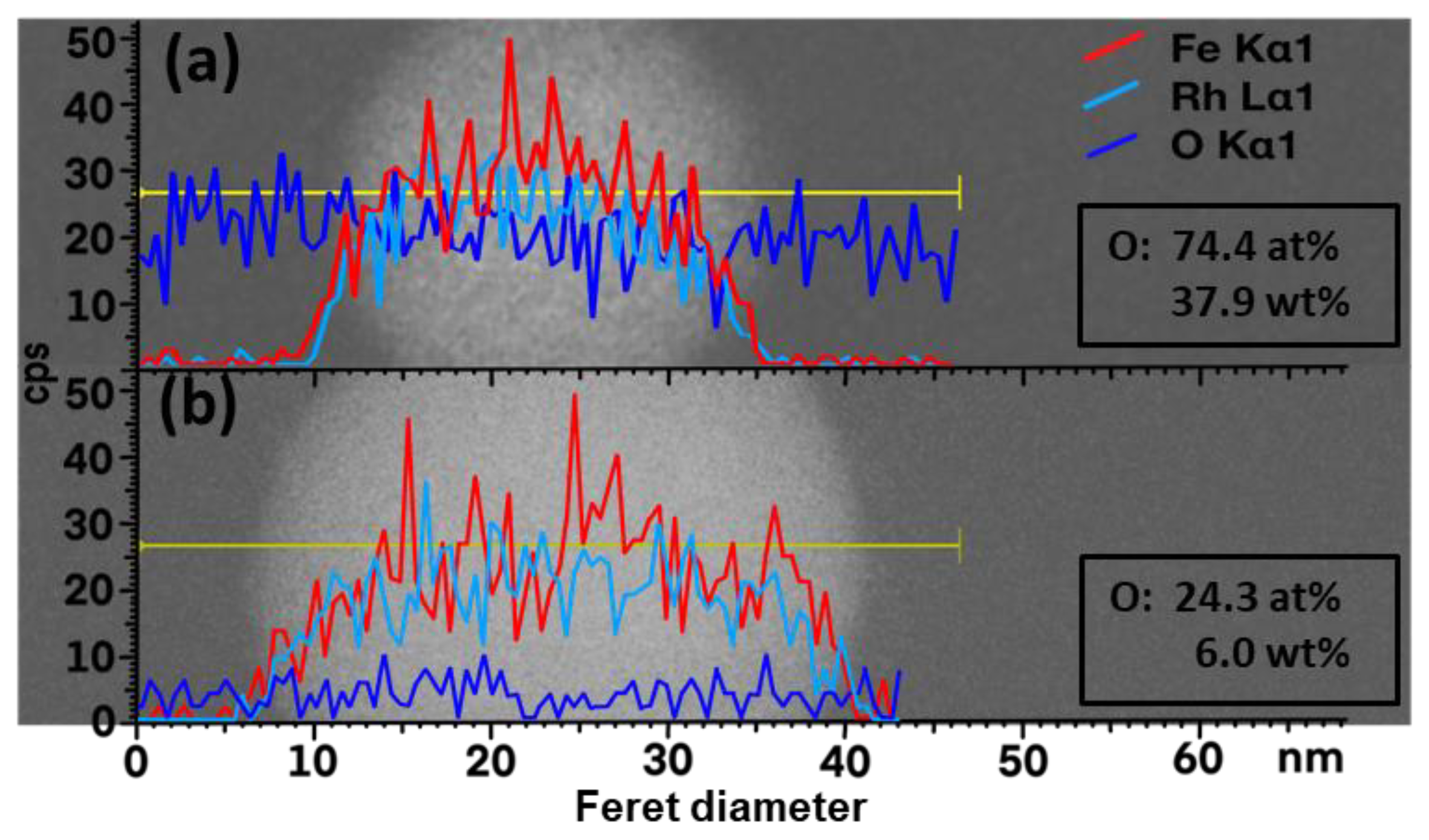
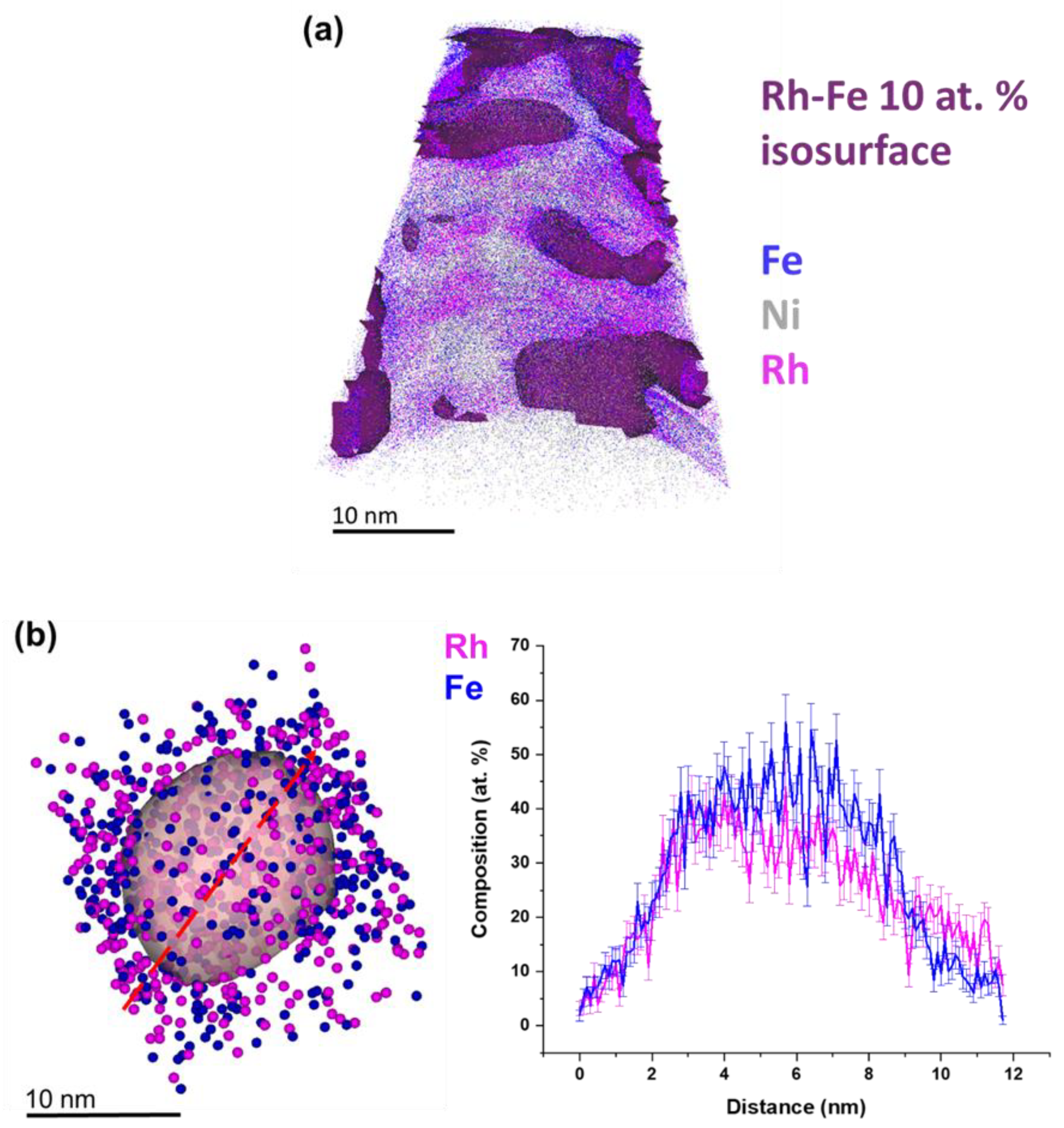
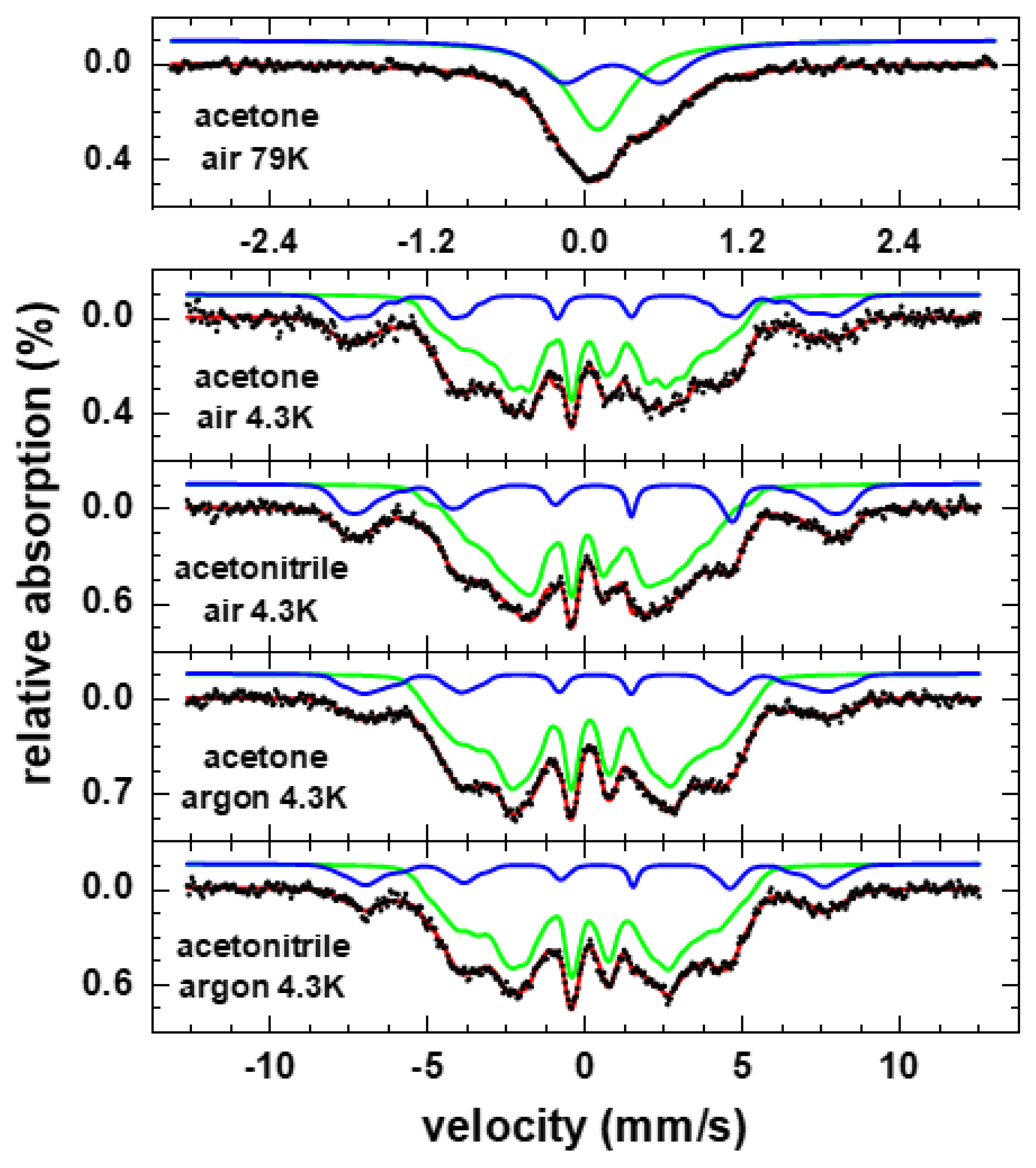
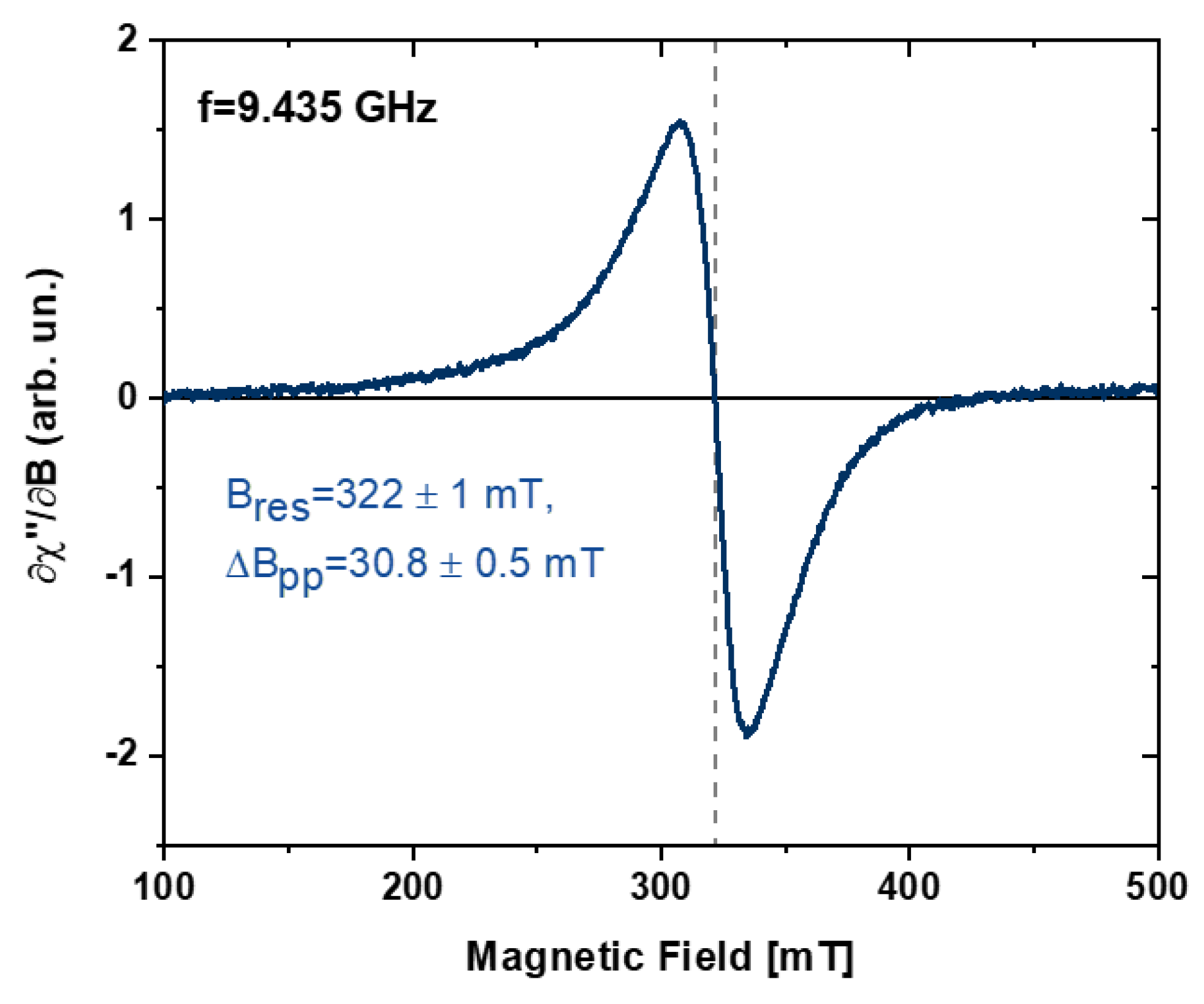
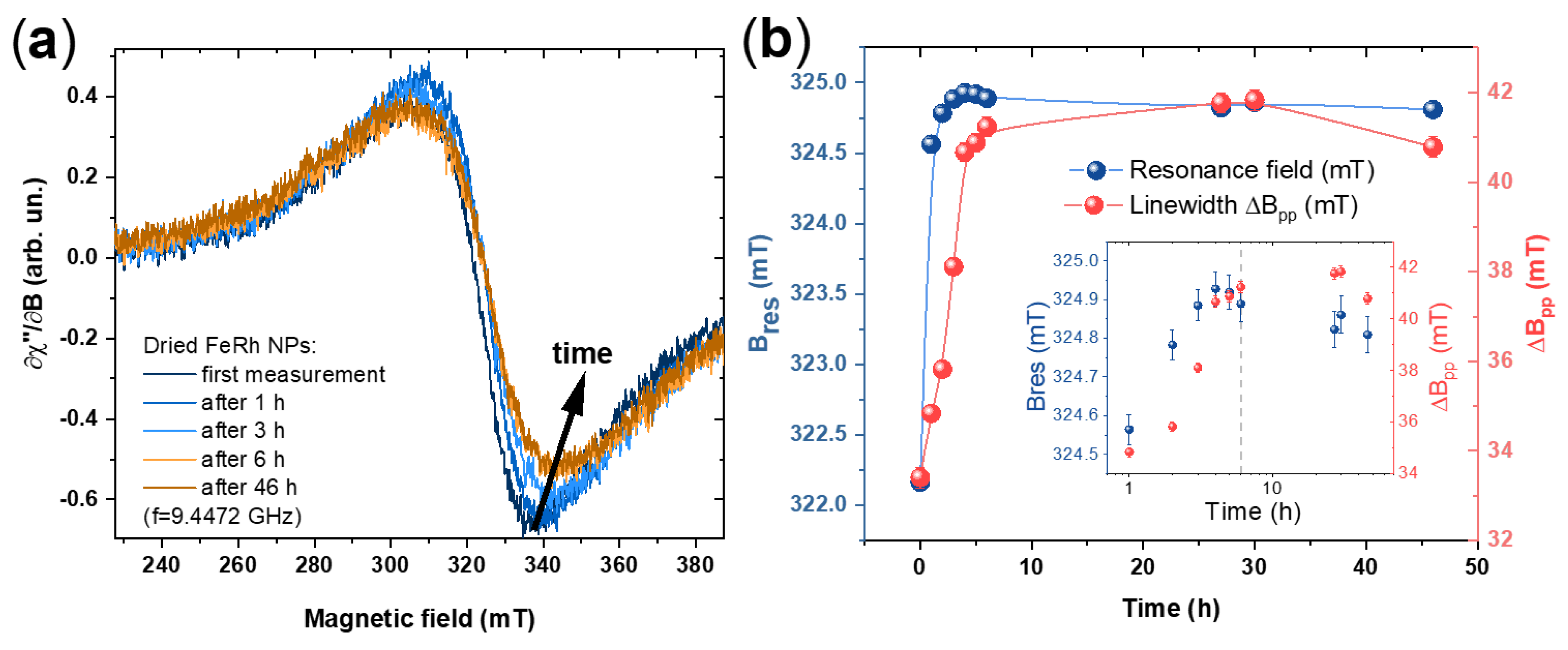
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakamula, I.; Yamanoi, Y.; Imaoka, T.; Yamamoto, K.; Nishihara, H. A Uniform Bimetallic Rhodium/Iron Nanoparticle Catalyst for the Hydrogenation of Olefins and Nitroarenes. Angew. Chem. Int. Ed. 2011, 50, 5830–5833. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, I.; Mashaly, M.; Eltaweel, F.; Jackson, M.A.; Hassan, E.B. Dehydration of glucose to 5-hydroxymethylfurfural by a core-shell Fe3O4@SiO2-SO3H magnetic nanoparticle catalyst. Fuel 2018, 221, 407–416. [Google Scholar] [CrossRef]
- Yiu, H.H.P.; Keane, M.A. Enzyme-magnetic nanoparticle hybrids: New effective catalysts for the production of high value chemicals. J. Chem. Technol. Biotechnol. 2012, 87, 583–594. [Google Scholar] [CrossRef]
- Wilhelm, C.; Gazeau, F. Universal cell labelling with anionic magnetic nanoparticles. Biomaterials 2008, 29, 3161–3174. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Nanotechnology for bone materials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikov, A.V.; Grachev, A.A.; Gubanov, V.A.; Odintsov, S.A.; Martyshkin, A.A.; Sheshukova, S.E.; Sharaevskii, Y.P.; Nikitov, S.A. Spin-wave intermodal coupling in the interconnection of magnonic units. Appl. Phys. Lett. 2018, 112, 142402. [Google Scholar] [CrossRef]
- Swisher, J.; Robbins, M.; Sherwood, R.; Fuchs, E.; Lockwood, W.; Keilp, J. Polymer-coated metal and alloy particles for magnetic recording. IEEE Trans. Magn. 1971, 7, 155–158. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Liu, Y.; Sun, S. Synthesis and assembly of magnetic nanoparticles for information and energy storage applications. Front. Phys. China 2010, 5, 347–356. [Google Scholar] [CrossRef]
- Barcikowski, S.; Baranowski, T.; Durmus, Y.; Wiedwald, U.; Gökce, B. Solid solution magnetic FeNi nanostrand-polymer composites by connecting-coarsening assembly. J. Mater. Chem. C 2015, 3, 10699–10704. [Google Scholar] [CrossRef]
- Beck, G.; Barcikowski, S.; Chakravadhanula, V.S.K.; Comesaña-Hermo, M.; Deng, M.; Farle, M.; Hilgendorff, M.; Jakobi, J.; Janek, J.; Kienle, L.; et al. An approach for transparent and electrically conducting coatings: A transparent plastic varnish with nanoparticulate magnetic additives. Thin Solid Film. 2015, 595, 96–107. [Google Scholar] [CrossRef]
- Rashid, H.; Mansoor, M.A.; Haider, B.; Nasir, R.; Hamid, S.B.A.; Abdulrahman, A. Synthesis and characterization of magnetite nano particles with high selectivity using in-situ precipitation method. Sep. Sci. Technol. 2020, 55, 1207–1215. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Jamshidiyan, M.; Shirani, A.; Alahyarizadeh, G. Solvothermal synthesis and characterization of magnetic Fe3O4 nanoparticle by different sodium salt sources. Mater. Sci. 2017, 35, 50–57. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, F.; Hong, R. Solvothermal synthesis of magnetic Fe3O4 microparticles via self-assembly of Fe3O4 nanoparticles. Particuology 2011, 9, 179–186. [Google Scholar] [CrossRef]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef]
- Shen, B.; Sun, S. Chemical Synthesis of Magnetic Nanoparticles for Permanent Magnet Applications. Chemistry 2020, 26, 6757–6766. [Google Scholar] [CrossRef]
- De Carvalho, J.; Medeiros, S.N.; Morales, M.; Dantas, A.L.; Carriço, A. Synthesis of magnetite nanoparticles by high energy ball milling. Appl. Surf. Sci. 2013, 275, 84–87. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Streubel, R.; Barcikowski, S.; Gokce, B. Continuous multigram nanoparticle synthesis by high-power, high-repetition-rate ultrafast laser ablation in liquids. Opt. Lett. 2016, 41, 1486–1489. [Google Scholar] [CrossRef]
- Kohsakowski, S.; Santagata, A.; Dell’Aglio, M.; De Giacomo, A.; Barcikowski, S.; Wagener, P.; Gökce, B. High productive and continuous nanoparticle fabrication by laser ablation of a wire-target in a liquid jet. Appl. Surf. Sci. 2017, 403, 487–499. [Google Scholar] [CrossRef]
- Jakobi, J.; Petersen, S.; Menéndez-Manjón, A.; Wagener, P.; Barcikowski, S. Magnetic Alloy Nanoparticles from Laser Ablation in Cyclopentanone and Their Embedding into a Photoresist. Langmuir 2010, 26, 6892–6897. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Fallot, M.; Hocart, R. On the Appearance of Ferromagnetism upon Elevation of the Temperature of Iron and Rhodium. Rev. Sci. 1939, 8, 498–500. [Google Scholar]
- Vogler, C.; Abert, C.; Bruckner, F.; Suess, D. Noise Reduction Based on an Fe−Rh Interlayer in Exchange-Coupled Heat-Assisted Recording Media. Phys. Rev. Appl. 2017, 8, 054021. [Google Scholar] [CrossRef]
- Huang, P.-W.; Victora, R.H. Approaching the Grain-Size Limit for Jitter Using FeRh/FePt in Heat-Assisted Magnetic Recording. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Wolloch, M.; Gruner, M.E.; Keune, W.; Mohn, P.; Redinger, J.; Hofer, F.; Suess, D.; Podloucky, R.; Landers, J.; Salamon, S.; et al. Impact of lattice dynamics on the phase stability of metamagnetic FeRh: Bulk and thin films. Phys. Rev. B 2016, 94, 174435. [Google Scholar] [CrossRef]
- Eggert, B.; Schmeink, A.; Lill, J.; Liedke, M.; Kentsch, U.; Butterling, M.; Wagner, A.; Pascarelli, S.; Potzger, K.; Lindner, J.; et al. Magnetic response of FeRh to static and dynamic disorder. RSC Adv. 2020, 10, 14386–14395. [Google Scholar] [CrossRef]
- Astefanoaei, I.; Gimaev, R.; Zverev, V.; Stancu, A. Modelling of working parameters of Gd and FeRh nanoparticles for magnetic hyperthermia. Mater. Res. Express 2019, 6, 125089. [Google Scholar] [CrossRef]
- Astefanoaei, I.; Dumitru, I.; Chiriac, H.; Stancu, A. Controlling temperature in magnetic hyperthermia with low Curie temperature particles. J. Appl. Phys. 2014, 115, 17B531. [Google Scholar] [CrossRef]
- Carrillo, P.; Shi, R.; Teeluck, K.; Senanayake, S.D.; White, M.G. In Situ Formation of FeRh Nanoalloys for Oxygenate Synthesis. ACS Catal. 2018, 8, 7279–7286. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, Y.; Li, K.; Tang, H.; Wang, Y.; Wu, Z. FeRh and Nitrogen Codoped Graphene, a Highly Efficient Bifunctional Catalyst toward Oxygen Reduction and Oxygen Evolution Reactions. J. Phys. Chem. C 2020, 124, 9142–9150. [Google Scholar] [CrossRef]
- Swartzendruber, L.J. The Fe−Rh (Iron-Rhodium) system. Bull. Alloy Phase Diagr. 1984, 5, 456–462. [Google Scholar] [CrossRef]
- Jia, Z.; Harrell, J.W.; Misra, R.D.K. Synthesis and magnetic properties of self-assembled FeRh nanoparticles. Appl. Phys. Lett. 2008, 93, 022504. [Google Scholar] [CrossRef]
- Ciuculescu, D.; Amiens, C.; Respaud, M.; Falqui, A.; Lecante, P.; Benfield, R.E.; Jiang, L.; Fauth, K.; Chaudret, B. One-pot synthesis of core-shell FeRh nanoparticles. Chem. Mater. 2007, 19, 4624–4626. [Google Scholar] [CrossRef]
- Ciuculescu, D.; Amiens, C.; Respaud, M.; Lecante, P.; Falqui, A.; Chaudret, B. Synthesis and Characterization of FeRh Nanoparticles. Mod. Phys. Lett. B 2007, 21, 1153–1159. [Google Scholar] [CrossRef]
- Ko, H.Y.Y.; Suzuki, T. Self-assembly and magnetic properties of FePt, FeRh nanoparticles, and FePt/FeRh nanocomposite particles. IEEE Trans. Magn. 2007, 43, 885–887. [Google Scholar] [CrossRef]
- Palmer, M.; Martinez, K.A.; Gadgil, M.G.; Campbell, D.J. Demonstrations of Magnetism and Oxidation by Combustion of Iron Supplement Tablets. J. Chem. Educ. 2018, 95, 423–427. [Google Scholar] [CrossRef]
- Biacchi, A.J.; Schaak, R.E. The Solvent Matters: Kinetic versus Thermodynamic Shape Control in the Polyol Synthesis of Rhodium Nanoparticles. ACS Nano 2011, 5, 8089–8099. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kawaguchi, K.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. Preparation of Fe-Pt alloy particles by pulsed laser ablation in liquid medium. Chem. Phys. Lett. 2006, 428, 426–429. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Carraro, F.; Cattaruzza, E. Formation of alloy nanoparticles by laser ablation of Au/Fe multilayer films in liquid environment. J. Colloid Interface Sci. 2017, 489, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Scaramuzza, S.; Agnoli, S.; Granozzi, G.; Meneghetti, M.; Campo, G.; Bonanni, V.; Pineider, F.; Sangregorio, C.; Ghigna, P.; et al. Laser generation of iron-doped silver nanotruffles with magnetic and plasmonic properties. Nano Res. 2015, 8, 4007–4023. [Google Scholar] [CrossRef]
- Amendola, V.; Riello, P.; Meneghetti, M. Magnetic Nanoparticles of Iron Carbide, Iron Oxide, Iron@Iron Oxide, and Metal Iron Synthesized by Laser Ablation in Organic Solvents. J. Phys. Chem. C 2011, 115, 5140–5146. [Google Scholar] [CrossRef]
- Marzun, G.; Bönnemann, H.; Lehmann, C.; Spliethoff, B.; Weidenthaler, C.; Barcikowski, S. Role of Dissolved and Molecular Oxygen on Cu and PtCu Alloy Particle Structure during Laser Ablation Synthesis in Liquids. ChemPhysChem 2017, 18, 1175–1184. [Google Scholar] [CrossRef]
- Wagener, P.; Schwenke, A.; Chichkov, B.N.; Barcikowski, S. Pulsed Laser Ablation of Zinc in Tetrahydrofuran: Bypassing the Cavitation Bubble. J. Phys. Chem. C 2010, 114, 7618–7625. [Google Scholar] [CrossRef]
- Lim, J.; Kim, S.; Armengol, R.A.; Kasian, O.; Choi, P.; Stephenson, L.T.; Gault, B.; Scheu, C. Atomic-Scale Mapping of Impurities in Partially Reduced Hollow TiO2 Nanowires. Angew. Chem. Int. Ed. 2020, 59, 5651–5655. [Google Scholar] [CrossRef]
- Thompson, K.; Lawrence, D.; Larson, D.; Olson, J.; Kelly, T.; Gorman, B. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 2007, 107, 131–139. [Google Scholar] [CrossRef]
- Kalus, M.R.; Barsch, N.; Streubel, R.; Gokce, E.; Barcikowski, S.; Gokce, B. How persistent microbubbles shield nanoparticle productivity in laser synthesis of colloids—Quantification of their volume, dwell dynamics, and gas composition. Phys. Chem. Chem. Phys. 2017, 19, 7112–7123. [Google Scholar] [CrossRef]
- Jung, H.J.; Choi, M.Y. Specific Solvent Produces Specific Phase Ni Nanoparticles: A Pulsed Laser Ablation in Solvents. J. Phys. Chem. C 2014, 118, 14647–14654. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Aschauer, U.J.; Braddell, R.; Brechbühl, S.A.; Derlet, P.M.; Spaldin, N.A. Strain-induced structural instability in FeRh. Phys. Rev. B 2016, 94, 014109. [Google Scholar] [CrossRef]
- Semisalova, A. FMR Study of the Magnetic Anisotropy in Fe50Rh50 Core/Shell Nanoparticles. Master’s (Diploma) Thesis, Lomonosov Moscow State University, Moscow, Russia, 2009. [Google Scholar]
- Kuncser, V.; Rosenberg, M.; Principi, G.; Russo, U.; Hernando, A.; Navarro, E.; Filoti, G. Magnetic interactions in nanocrystalline FeRh alloys studied by in field Mössbauer spectroscopy. J. Alloys Compd. 2000, 308, 21–29. [Google Scholar] [CrossRef]
- Filoti, G.; Kuncsea, V.; Navarro, E.; Hernando, A.; Rosenberg, M. Hyperfine fields and Fe magnetic moments in Fe-Rh alloys; a Mössbauer spectroscopy study. J. Alloy Compd. 1998, 278, 60–68. [Google Scholar] [CrossRef]
- Trunova, A.; Lindner, J.; Meckenstock, R.; Spasova, M.; Farle, M.; Ciuculescu, D.; Amiens, C.; Chaudret, B.; Respaud, M. Temperature dependent magnetic characterisation of core/shell nanoparticles. J. Magn. Magn. Mater. 2009, 321, 3502–3506. [Google Scholar] [CrossRef]
- Trunova, A.; Meckenstock, R.; Barsukov, I.; Hassel, C.; Margeat, O.; Spasova, M.; Lindner, J.; Farle, M. Magnetic characterization of iron nanocubes. J. Appl. Phys. 2008, 104, 093904. [Google Scholar] [CrossRef]
- Platow, W.; Anisimov, A.N.; Farle, M.; Baberscheke, K. Magnetic Anisotropy and the Temperature Dependent Magnetic Order-Disorder Transition in Fe/Cu(001). Phys. Status Solidi A 1999, 173, 145–151. [Google Scholar] [CrossRef]
- Sidorov, S.N.; Bronstein, L.M.; Davankov, V.A.; Tsyurupa, M.P.; Solodovnikov, S.P.; Valetsky, P.M.; Wilder, E.A.; Spontak, R.J. Cobalt Nanoparticle Formation in the Pores of Hyper-Cross-Linked Polystyrene: Control of Nanoparticle Growth and Morphology. Chem. Mater. 1999, 11, 3210–3215. [Google Scholar] [CrossRef]
- Pang, C.P.; Hsieh, C.T.; Lue, J.T. A study of magneto-optical effect in dilute Fe3O4 ferrofluid by attenuated total reflection, ferromagnetic resonance and Faraday rotation. J. Phys. D Appl. Phys. 2003, 36, 1764–1768. [Google Scholar] [CrossRef]
- Noginova, N.; Chen, F.; Weaver, T.; Giannelis, E.P.; Bourlinos, A.B.; Atsarkin, V.A. Magnetic resonance in nanoparticles: Between ferro- and paramagnetism. J. Phys. Condens. Matter 2007, 19, 246208. [Google Scholar] [CrossRef]
- Antoniak, C.; Lindner, J.; Farle, M. Magnetic anisotropy and its temperature dependence in iron-rich FexPt1-x nanoparticles. EPL Europhys. Lett. 2005, 70, 250–256. [Google Scholar] [CrossRef]
- Mancini, E.; Pressacco, F.; Haertinger, M.; Fullerton, E.E.; Suzuki, T.; Woltersdorf, G.; Back, C. Magnetic phase transition in iron-rhodium thin films probed by ferromagnetic resonance. J. Phys. D Appl. Phys. 2013, 46, 245302. [Google Scholar] [CrossRef]
- Merkel, D.G.; Lengyel, A.; Nagy, D.L.; Németh, A.; Horváth, Z.E.; Bogdán, C.; Gracheva, M.A.; Hegedűs, G.; Sajti, S.; Radnóczi, G.Z.; et al. Reversible control of magnetism in FeRh thin films. Sci. Rep. 2020, 10, 13923. [Google Scholar] [CrossRef] [PubMed]
- Zingsem, B.W.; Feggeler, T.; Terwey, A.; Ghaisari, S.; Spoddig, D.; Faivre, D.; Meckenstock, R.; Farle, M.; Winklhofer, M. Biologically encoded magnonics. Nat. Commun. 2019, 10, 4345. [Google Scholar] [CrossRef]
- Stashkevich, A.A.; Roussigneé, Y.; Djemia, P.; Billet, D.; Stognij, A.I.; Novitskii, N.N.; Wurtz, G.; Zayats, A.V.; Viau, G.; Chaboussant, G.; et al. Brillouin light scattering observation of the transition from the superparamagnetic to the superferromagnetic state in nanogranular (SiO2)Co films. J. Appl. Phys. 2008, 104, 93912. [Google Scholar] [CrossRef]
- Voronin, D.V.; Sadovnikov, A.V.; Shchukin, D.G.; Gorin, D.A.; Beginin, E.N.; Sharaevskii, Y.P.; Nikitov, S.A. Studying the spectra of thermal magnons in composite materials with embedded magnetite nanoparticles using Brillouin light-scattering spectroscopy. Tech. Phys. Lett. 2013, 39, 715–718. [Google Scholar] [CrossRef]
- Lu, H.; Liang, F.; Gou, J. Nanopaper enabled shape-memory nanocomposite with vertically aligned nickel nanostrand: Controlled synthesis and electrical actuation. Soft Matter 2011, 7, 7416–7423. [Google Scholar] [CrossRef]
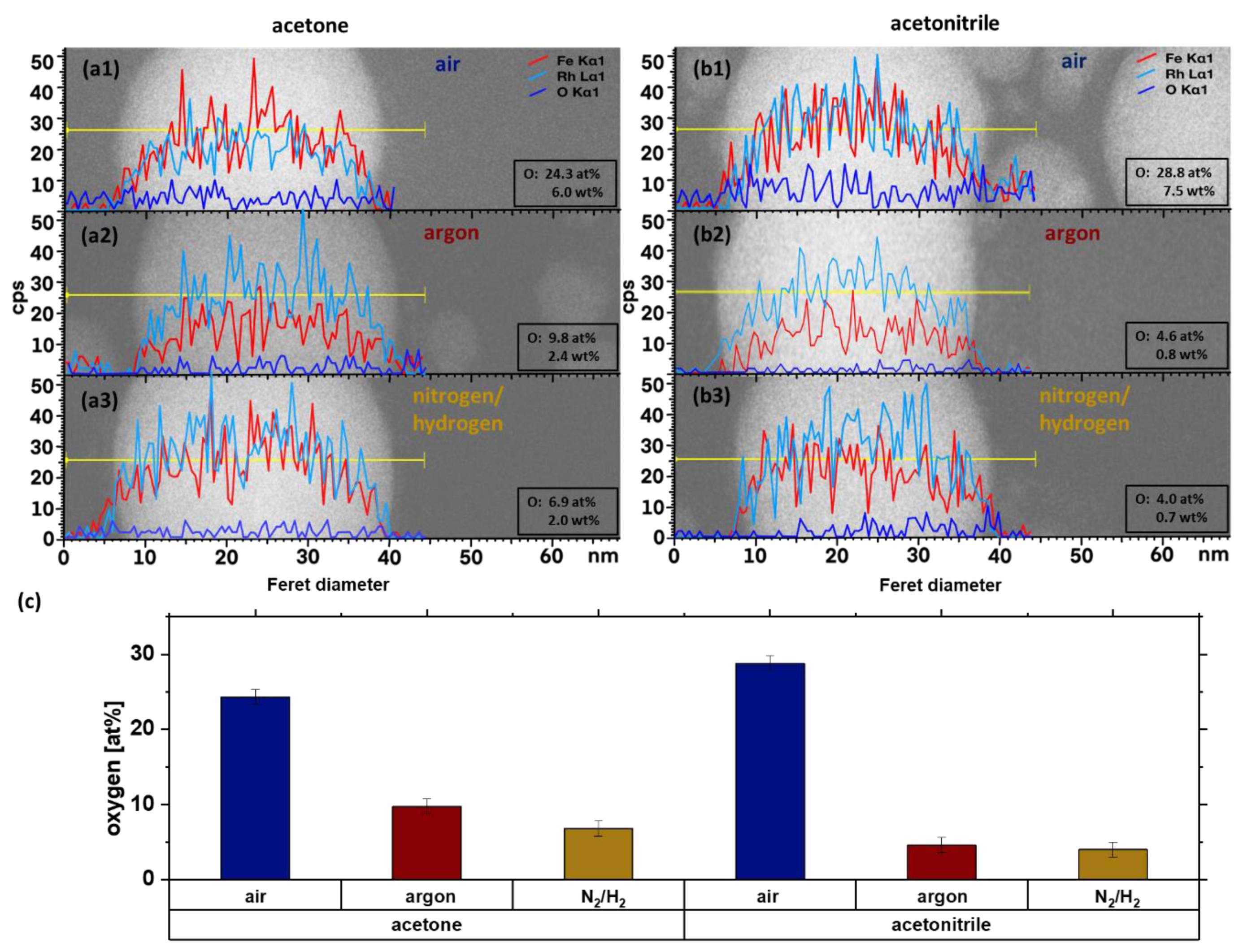

| Configuration | Solvent | Atmosphere |
|---|---|---|
| a1 | acetone | air |
| a2 | acetone | argon |
| a3 | acetone | N2/H2 |
| b1 | acetonitrile | air |
| b2 | acetonitrile | argon |
| b3 | acetonitrile | N2/H2 |
| Acetone | Acetonitrile | |
|---|---|---|
| air | 20.3 ± 1.7 at% | 21.5 ± 1.9 at% |
| argon | 15.7 ± 1.6 at% | 15.9 ± 1.4 at% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadarajah, R.; Tahir, S.; Landers, J.; Koch, D.; Semisalova, A.S.; Wiemeler, J.; El-Zoka, A.; Kim, S.-H.; Utzat, D.; Möller, R.; et al. Controlling the Oxidation of Magnetic and Electrically Conductive Solid-Solution Iron-Rhodium Nanoparticles Synthesized by Laser Ablation in Liquids. Nanomaterials 2020, 10, 2362. https://doi.org/10.3390/nano10122362
Nadarajah R, Tahir S, Landers J, Koch D, Semisalova AS, Wiemeler J, El-Zoka A, Kim S-H, Utzat D, Möller R, et al. Controlling the Oxidation of Magnetic and Electrically Conductive Solid-Solution Iron-Rhodium Nanoparticles Synthesized by Laser Ablation in Liquids. Nanomaterials. 2020; 10(12):2362. https://doi.org/10.3390/nano10122362
Chicago/Turabian StyleNadarajah, Ruksan, Shabbir Tahir, Joachim Landers, David Koch, Anna S. Semisalova, Jonas Wiemeler, Ayman El-Zoka, Se-Ho Kim, Detlef Utzat, Rolf Möller, and et al. 2020. "Controlling the Oxidation of Magnetic and Electrically Conductive Solid-Solution Iron-Rhodium Nanoparticles Synthesized by Laser Ablation in Liquids" Nanomaterials 10, no. 12: 2362. https://doi.org/10.3390/nano10122362
APA StyleNadarajah, R., Tahir, S., Landers, J., Koch, D., Semisalova, A. S., Wiemeler, J., El-Zoka, A., Kim, S.-H., Utzat, D., Möller, R., Gault, B., Wende, H., Farle, M., & Gökce, B. (2020). Controlling the Oxidation of Magnetic and Electrically Conductive Solid-Solution Iron-Rhodium Nanoparticles Synthesized by Laser Ablation in Liquids. Nanomaterials, 10(12), 2362. https://doi.org/10.3390/nano10122362






