Antibacterial Effect of Chitosan–Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan–Gold Nanoparticles Preparation
2.3. Characterization of the Chitosan-Gold Nanoparticles
2.3.1. Dynamic Light Scattering (DLS)
2.3.2. UV-Vis Spectroscopy
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. Antibacterial Assay
2.4. Computer Simulation Parameters
2.4.1. Lipid Bilayer Model
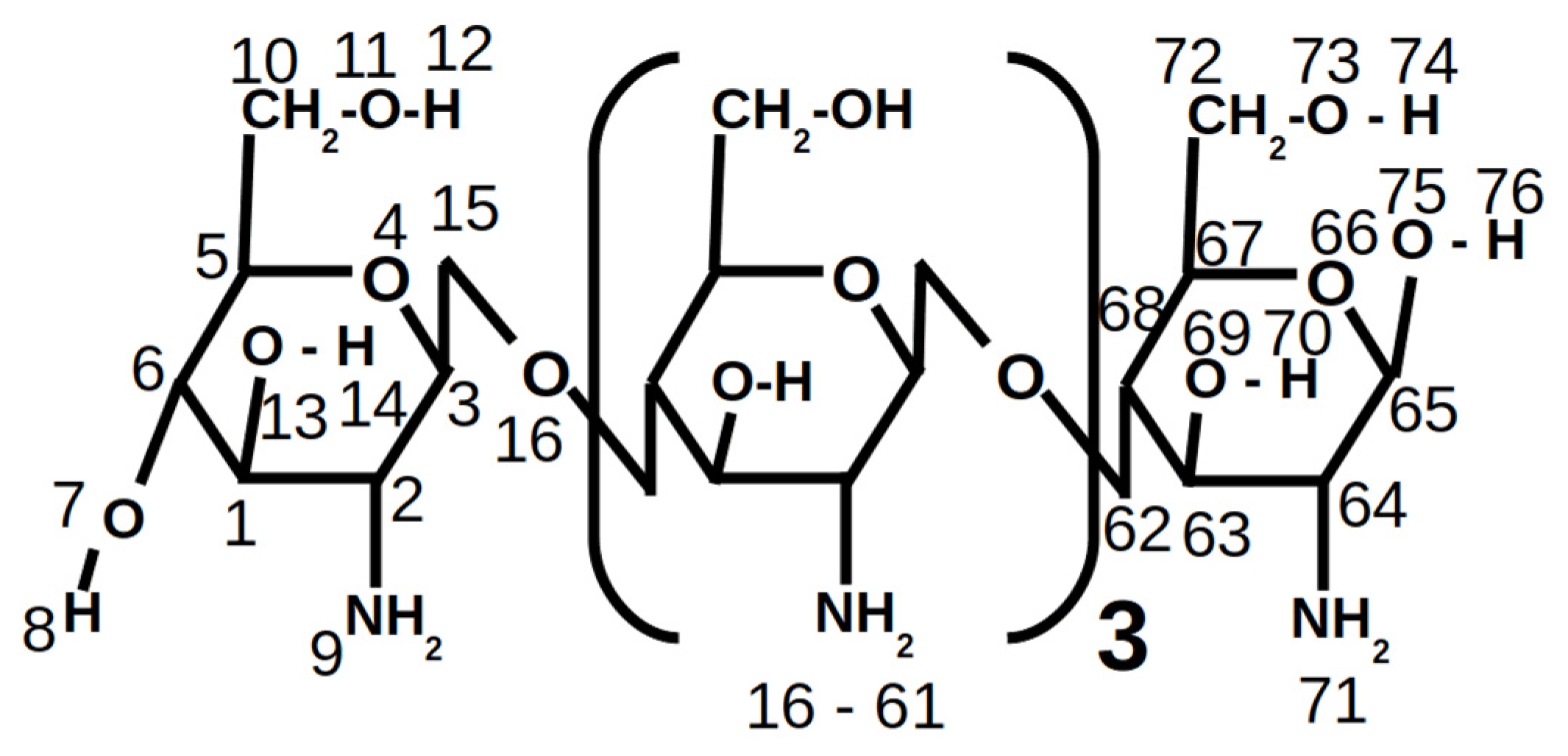
2.4.2. Chitosan Model
2.4.3. Simulation Box
- System in the presence of CT+1
- System in the presence of CT+5
2.5. Statistical Analysis
3. Results and Discussion
3.1. Influence of Chitosan Molecular Weight on the Physical Features of Cs-AuNPs
3.2. Influence of the Chitosan Concentration on the Biosynthesis of Cs-AuNPs
3.3. Influence of the Gold Concentration on the Biosynthesis of Cs-AuNPs
3.4. Influence of the Reaction Solvent on the Biosynthesis of Cs-AuNPs
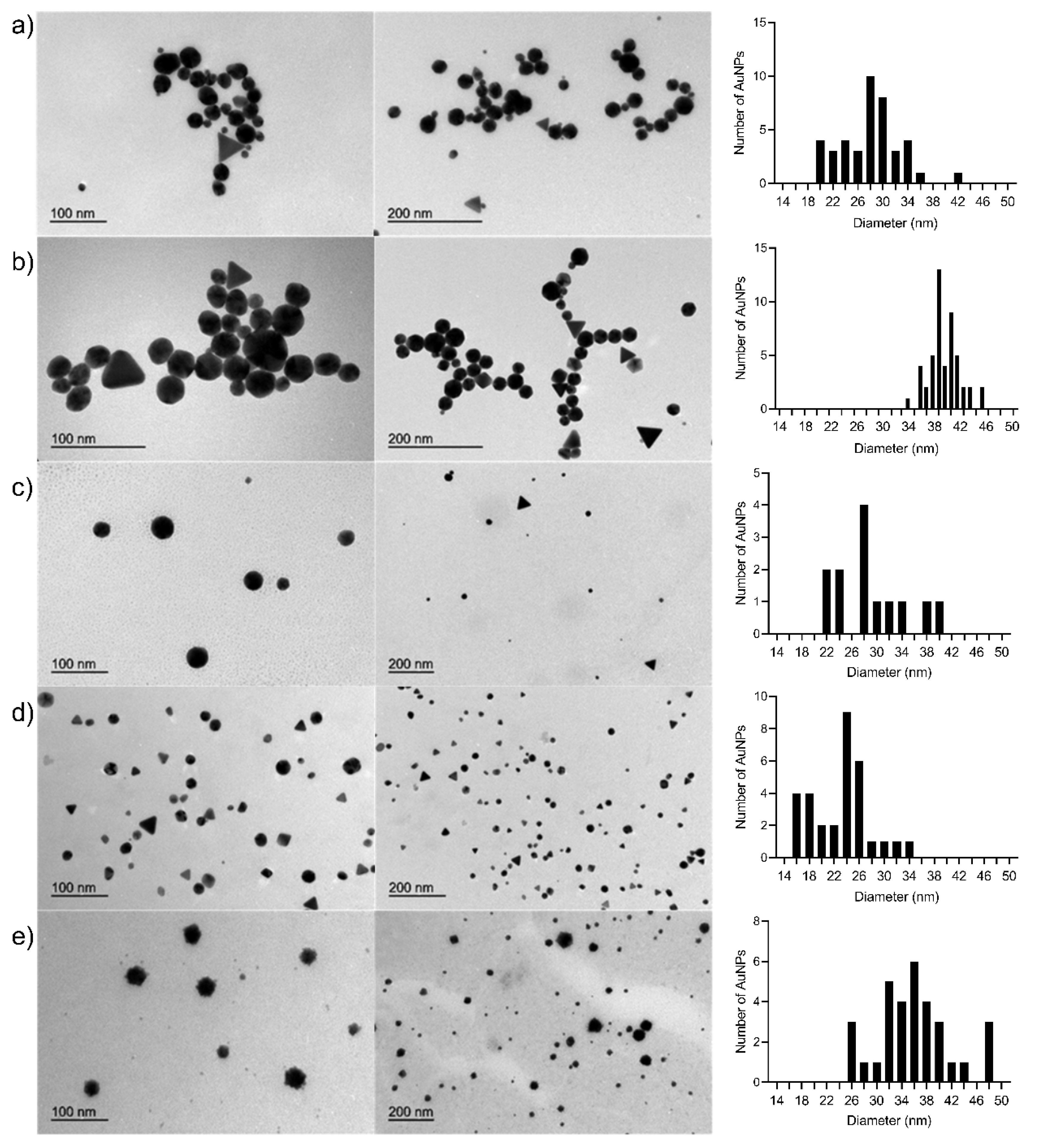
3.5. Transmission Electron Microscopy
3.6. Antibacterial Activity
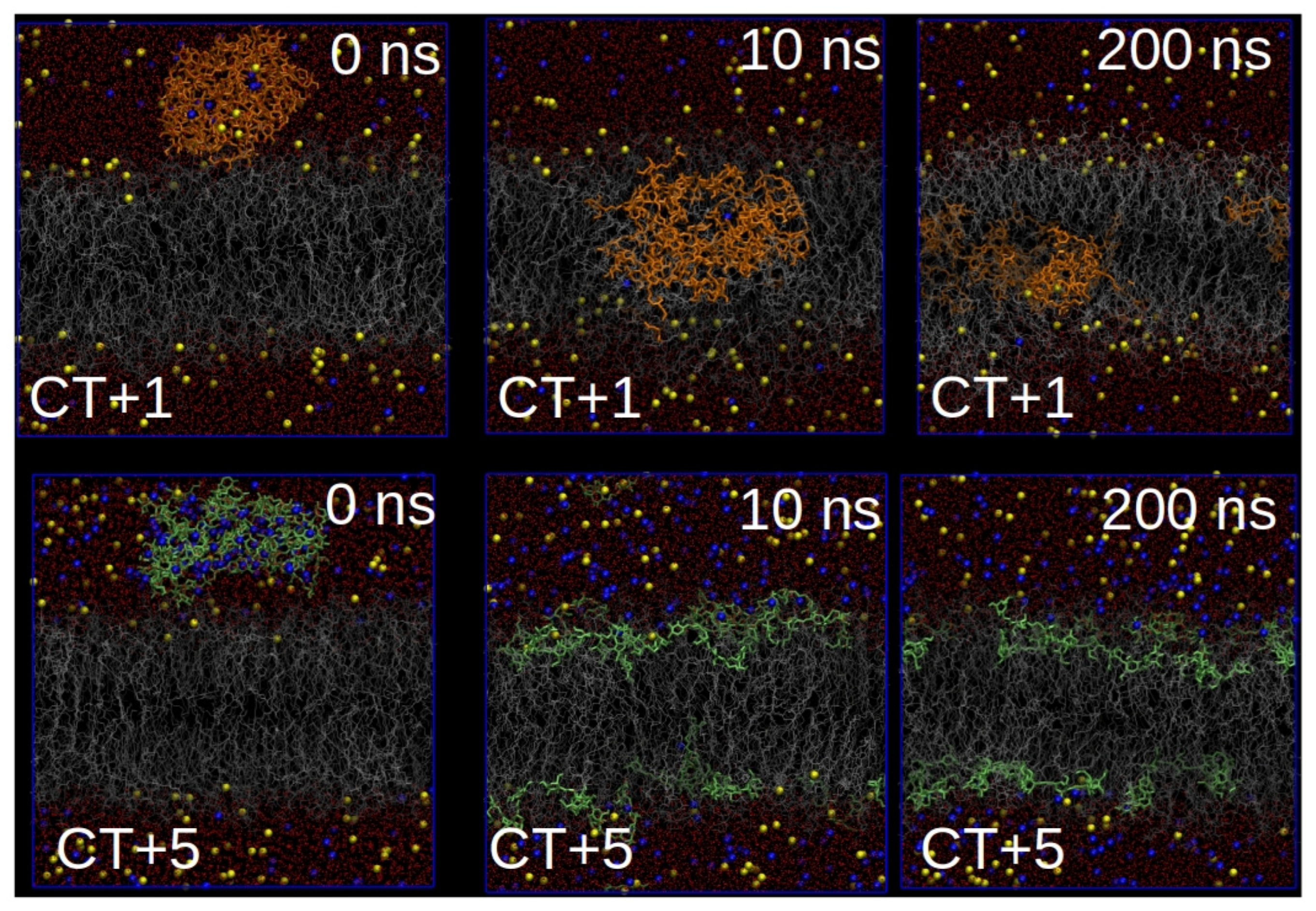
3.7. Simulation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Majoumouo, M.S.; Sibuyi, N.R.S.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced Anti-Bacterial Activity of Biogenic Silver Nanoparticles Synthesized from Terminalia mantaly Extracts. Int. J. Nanomed. 2019, 14, 9031–9046. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Paitan, Y. Current Trends in Antimicrobial Resistence of Escherichia coli. In Escherichia coli, a Versatile Pathogen, Current Topics in Microbiology and Immunology; Frankel, G., Ron, E., Eds.; Springer: New York, NY, USA, 2018; pp. 181–211. [Google Scholar]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Lim, C.S.; Nor Azlan, A.Y.H.; Buang, F.; Mh Busra, M.F. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Maji, J.; Pandey, S.; Basu, S. Synthesis and evaluation of antibacterial properties of magnesium oxide nanoparticles. Bull. Mater. Sci. 2019, 43, 25. [Google Scholar] [CrossRef]
- Esther, J.; Sridevi, V. Synthesis and characterization of chitosan-stabilized gold nanoparticles through a facile and green approach. Gold Bull. 2017, 50, 1–5. [Google Scholar] [CrossRef]
- Jeyarani, S.; Vinita, N.M.; Puja, P.; Senthamilselvi, S.; Devan, U.; Velangani, A.J.; Biruntha, M.; Pugazhendhi, A.; Kumar, P. Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J. Photochem. Photobiol. B 2020, 202, 111715. [Google Scholar] [CrossRef]
- Sun, I.C.; Na, J.H.; Jeong, S.Y.; Kim, D.E.; Kwon, I.C.; Choi, K.; Ahn, C.H.; Kim, K. Biocompatible glycol chitosan-coated gold nanoparticles for tumor-targeting CT imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef]
- Collado-González, M.; Fernández Espín, V.; Montalbán, M.G.; Pamies, R.; Hernández Cifre, J.G.; Díaz Baños, F.G.; Víllora, G.; García de la Torre, J. Aggregation behaviour of gold nanoparticles in presence of chitosan. J. Nanopart. Res. 2015, 17, 268. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, S.; Georgiev, P.; Exner, K.S.; Mihaylov, L.; Nihtianova, D.; Koynov, K.; Balashev, K. Kinetic study of gold nanoparticles synthesized in the presence of chitosan and citric acid. Colloids Surf. A 2018, 557, 106–115. [Google Scholar] [CrossRef]
- Sun, C.; Qu, R.; Chen, H.; Ji, C.; Wang, C.; Sun, Y.; Wang, B. Degradation behavior of chitosan chains in the “green” synthesis of gold nanoparticles. Carbohyd. Res. 2018, 343, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- León, Y.; Cárdenas, G.; Arias, M. Synthesis and characterizations of metallic nanoparticles in chitosan by chemical reduction. J. Chil. Chem. Soc. 2017, 62, 3760–3764. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohyd. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef]
- Jaouen, V.; Brayner, R.; Lantiat, D.; Steunou, N.; Coradin, T. In situ growth of gold colloids within alginate films. Nanotechnology 2010, 21, 185605. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of noncytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Inter. 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Cai, J.; Zhong, L.; Ren, G.; Ma, Q. One pot synthesis of gold nanoparticles using chitosan with varying degree of deacetylation and molecular weight. Carbohyd. Polym. 2017, 178, 105–114. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Shih, P.-Y.; Liao, Y.-T.; Tseng, Y.-K.; Deng, F.-S.; Lin, C.-H. A Potential Antifungal Effect of Chitosan Against Candida albicans Is Mediated via the Inhibition of SAGA Complex Component Expression and the Subsequent Alteration of Cell Surface Integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Young, D.H.; Kauss, H. Release of Calcium from Suspension-Cultured Glycine max Cells by Chitosan, Other Polycations, and Polyamines in Relation to Effects on Membrane Permeability. Plant Physiol. 1983, 73, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial Activity, Interactions with Food Components and Applicability as a Coating on Fruit and Vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Sebti, I.; Martial-Gros, A.; Carnet-Pantiez, A.; Grelier, S.; Coma, V. Chitosan Polymer as Bioactive Coating and Film against Aspergillus niger Contamination. J. Food Sci. 2005, 70, M100–M104. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan- metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Lu, B.; Lu, F.; Ran, L.; Yu, K.; Xiao, Y.; Li, Z.; Dai, F.; Wu, D.; Lan, G. Imidazole-molecule-capped chitosan–gold nanocomposites with enhanced antimicrobial activity for treating biofilm-related infections. J. Colloid Interface Sci. 2018, 531, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Nakamura, H.; Watamo, S. MD simulation study of direct permeation of a nanoparticle across the cell membrane under an external electric field. Nanoscale 2016, 8, 11897–11906. [Google Scholar] [CrossRef] [PubMed]
- Pak, I.; Bhattacharyya, D.; Kar, R.K.; Zarena, D.; Bhunia, A.; Atreya, H.S. A Peptide-Nanoparticle System with Improved Efficacy against Multidrug Resistant Bacteria. Sci. Rep. 2019, 9, 4485. [Google Scholar]
- Petretto, E.; Campomanes, P.; Stellacci, F.; Rothen-Rustishauser, B.; Petri-Fink, A.; Vanni, S. An Atomistic Look into Bio-inspired Nanoparticles and their Molecular Interactions with Cells. Chimia 2019, 73, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Melby, E.S.; Allen, C.; Foreman-Ortiz, I.U.; Caudill, E.R.; Kuech, T.R.; Vartanian, A.M.; Zhang, X.; Murphy, C.J.; Hernandez, R.; Pedersen, J.A. Peripheral Membrane Proteins Facilitate Nanoparticle Binding at Lipid Bilayer Interfaces. Langmuir 2018, 34, 10793–10805. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Irudayanathan, F.J.; Jiang, W.; Nangia, S. Simulating Gram-Negative Bacterial Outer Membrane: A Coarse Grain Model. J. Phys. Chem. B 2015, 119, 14668–14682. [Google Scholar] [CrossRef] [PubMed]
- Pestov, A.; Nazirov, A.; Modin, E.; Mironenko, A.; Bratskaya, S. Mechanism of Au(III) reduction by chitosan: Comprehensive study with 13C and 1H NMR analysis of chitosan degradation products. Carbohyd. Polym. 2015, 117, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; M100-S22; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Bellemans, A. Molecular dynamics of liquid alkanes. Faraday Discuss. 1978, 66, 95–106. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces: Proceedings of the Fourteenth Jerusalem Symposium on Quantum Chemistry and Biochemistry; Pullman, B., Ed.; Reidel Publ. Company: Dordrecht, The Netherlands, 1981; pp. 331–342. [Google Scholar]
- van Gunsteren, W.F.; Berendsen, H.J.C. Groningen Molecular Simulation (GROMOS) Library Manual; Biomos: Groningen, The Netherlands, 1987. [Google Scholar]
- Ahumada-Gutierrez, H.; Peñalva, D.A.; Enriz, R.D.; Antollini, S.S.; Cascales, J.J.L. Mechanical properties of bilayers containing sperm sphingomyelins and ceramides with very long-chain polyunsaturated fatty acids. Chem. Phys. Lipids 2019, 218, 178–186. [Google Scholar] [CrossRef]
- Cascales, J.J.L.; Costa, S.D.O.; Porasso, R.D. Thermodynamic study of benzocaine insertion into different lipid bilayers. J. Chem. Phys. 2011, 135, 135103. [Google Scholar] [CrossRef]
- Egberts, E.; Marrink, S.-J.; Berendsen, H.J.C. Molecular dynamics simulation of a phospholipid membrane. Eur. Biophys. J. 1994, 22, 423–436. [Google Scholar] [CrossRef]
- Bahamonde-Padilla, V.E.; Espinoza, J.; Weiss-López, B.E.; Cascales, J.J.L.; Montecinos, R.; Araya-Maturana, R. Effect of lithium on the properties of a liquid crystal formed by sodium dodecylsulphate and decanol in aqueous solution. J. Chem. Phys. 2013, 139, 14703. [Google Scholar] [CrossRef] [PubMed]
- Cascales, J.J.L.; Costa, S.D.O.; Garro, A.; Enriz, R.D. Mechanical properties of binary DPPC/DPPS bilayers. RSC Adv. 2012, 2, 11743–11750. [Google Scholar] [CrossRef]
- López Cascales, J.J.; Hernández Cifre, J.G.; García de la Torre, J. Anaesthetic Mechanism on a Model Biological Membrane: A Molecular Dynamics Simulation Study. J. Phys Chem. B 1998, 102, 625–631. [Google Scholar] [CrossRef]
- López Cascales, J.J.; Garro, A.; Porasso, R.D.; Enriz, R.D. The dynamic action mechanism of small cationic antimicrobial peptides. Phys. Chem. Chem. Phys. 2014, 16, 21694–21705. [Google Scholar] [CrossRef]
- Seelig, A.; Seelig, J. Dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry 1974, 13, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Watts, A.; Marsh, D. Titration of the phase transition of phosphatidylserine bilayer membranes. Effects of pH, surface electrostatics, ion binding, and head-group hydration. Biochemistry 1981, 20, 4955–4965. [Google Scholar] [CrossRef]
- Luna, E.J.; McConnell, H.M. Lateral phase separations in binary mixtures of phospholipids having different charges and different crystalline structures. BBA Biomembranes 1977, 470, 303–316. [Google Scholar] [CrossRef]
- Pople, J.A.; Segal, G.A. Approximate Self-Consistent Molecular Orbital Theory. III. CNDO Results for AB2 and AB3 Systems. J. Chem. Phys. 1966, 44, 3289–3296. [Google Scholar] [CrossRef]
- HyperChem; Package developed by and licensed from HyperCube, Inc.; HyperCube, Inc.: Gainesville, FL, USA., 1992.
- Potara, M.; Maniu, D.; Astilean, S. The synthesis of biocompatible and SERS-active gold nanoparticles using chitosan. Nanotechnology 2009, 20, 315602. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of chitosan-stabilized gold nanoparticles in the absence/presence of tripolyphosphate. Biomacromolecules 2004, 5, 2340–2346. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal–chitosan nanocomposites. Colloids Surf. B 2004, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Curulli, A.; Toro, R.G.; Bianchini, C.; De Caro, T.; Padeletti, G.; Zane, D.; Ingo, G.M. Green Synthesis of Gold–Chitosan Nanocomposites for Caffeic Acid Sensing. Langmuir 2012, 28, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Prema, P.; Thangapandiyan, S. In-vitro antibacterial activity of gold nanoparticles capped with polysaccharide stabilizing agents. Int. J. Pharm. Pharm. Sci. 2013, 5, 310–314. [Google Scholar]
- Czechowska-Biskup, R.; Rokita, B.; Ulański, P.; Rosiak, J.M. Preparation of gold nanoparticles stabilized by chitosan using irradiation and sonication methods. Prog. Chem. Appl. Chitin Deriv. 2015, 20, 18–33. [Google Scholar] [CrossRef]
- Abrica-González, P.; Zamora-Justo, J.A.; Sotelo-López, A.; Vázquez-Martínez, G.R.; Balderas-López, J.A.; Muñoz-Diosdado, A.; Ibáñez-Hernández, M. Gold nanoparticles with chitosan, N-acylated chitosan, and chitosan oligosaccharide as DNA carriers. Nanoscale Res. Lett. 2019, 14, 258. [Google Scholar] [CrossRef]
- Adlim, A.; Bakar, M.A. Preparation of chitosan-gold nanoparticles: Part 1 (of 2). Effect of reducing technique. Indones. J. Chem. 2008, 8, 184–188. [Google Scholar] [CrossRef]
- López Cascales, J.J.; Zenak, S.; García de la Torre, J.; Lezama, O.G.; Garro, A.; Enriz, R.D. Small Cationic Peptides: Influence of Charge on Their Antimicrobial Activity. ACS Omega 2018, 3, 5390–5398. [Google Scholar] [CrossRef]
| Experiment | Cs Molecular Weight | Cs Concentration (w/v %) | Au Concentration (mM) | Solvent | Cs:Au Molar Ratio | pH |
|---|---|---|---|---|---|---|
| 1 | VLMw | 0.1 | 0.13 | Acetic acid | 40:1 | 3.22 |
| 2 | LMw | 0.1 | 0.13 | Acetic acid | 40:1 | 3.01 |
| 3 | MMw | 0.1 | 0.13 | Acetic acid | 40:1 | 2.95 |
| 4 | HMw | 0.1 | 0.13 | Acetic acid | 40:1 | 2.97 |
| 5 | VLMw | 0.4 | 0.13 | Acetic acid | 160:1 | 3.41 |
| 6 | LMw | 0.4 | 0.13 | Acetic acid | 160:1 | 3.45 |
| 7 | MMw | 0.4 | 0.13 | Acetic acid | 160:1 | 3.48 |
| 8 | HMw | 0.4 | 0.13 | Acetic acid | 160:1 | 3.44 |
| 9 | MMw | 0.1 | 0.26 | Acetic acid | 20:1 | 2.98 |
| 10 | MMw | 0.1 | 0.13 | Acetic acid/methanol | 40:1 | 3.41 |
| 11 | MMw | 0.1 | 0.26 | acetic acid/isopropanol | 20:1 | 3.36 |
| 12 | MMw | 0.4 | 0.26 | Acetic acid | 80:1 | 3.41 |
| 13 | MMw | 0.4 | 0.26 | Acetic acid/methanol | 80:1 | 4.13 |
| Exp. | Cs Concentration (w/v %) | Cs:Au Molar Ratio | Cs Molec Weight | Z-Average (nm) | Zeta Potential (mV) | PdI | Electrophoretic Mobility (µm·cm/Vs) |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 40:1 | VLMW | 64.02 ± 0.14 a | 46.3 ± 2.25 ac | 0.317 ± 0.006 a | 3.632 ± 0.176 ab |
| 2 | LMW | 49.48 ± 0.14 b | 44.83 ± 1.47 a | 0.334 ± 0.002 a | 3.516 ± 0.112 b | ||
| 3 | MMW | 52.57 ± 1.11 b | 46.1 ± 1.06 ac | 0.315 ± 0.005 a | 3.613 ± 0.082 ab | ||
| 4 | HMW | 54.86 ± 0.08 b | 48.66 ± 2.11 ac | 0.319 ± 0.004 a | 3.812 ± 0.166 ab | ||
| 5 | 0.4 | 160:1 | VLMW | 98.1 ± 3.76 c | 49.53 ± 3.48 abc | 0.491 ± 0.039 b | 3.881 ± 0.273 abc |
| 6 | LMW | 185.13 ± 7.12 d | 55.23 ± 2.14 bd | 0.486 ± 0.023 b | 4.328 ± 0.166 c | ||
| 7 | MMW | 175.76 ± 2.97 e | 49.76 ± 2.4 ad | 0.494 ± 0.006 b | 3.902 ± 0.185 abc | ||
| 8 | HMW | 194 ± 2.08 d | 51.3 ± 1.99 cd | 0.456 ± 0.002 b | 4.020 ± 0.158 ac |
| Exp. | Cs Concentration (w/v %) | Au Concentration (mM) | Cs:Au Molar Ratio | Z-Average (nm) | Zeta Potential (mV) | PdI | Electrophoretic Mobility (µm·cm/Vs) |
|---|---|---|---|---|---|---|---|
| 3 | 0.1 | 0.13 | 40:1 | 52.57 ± 1.11 a | 46.1 ± 1.06 a | 0.315 ± 0.005 a | 3.613 ± 0.082 a |
| 9 | 0.26 | 20:1 | 44.21 ± 0.51 b | 46.5 ± 2 a | 0.309 ± 0.008 a | 3.646 ± 0.158 a | |
| 7 | 0.4 | 0.13 | 160:1 | 175.76 ± 2.97 c | 49.76 ± 2.4 a | 0.494 ± 0.006 b | 3.902 ± 0.185 a |
| 12 | 0.26 | 80:1 | 112 ± 1.65 d | 48.4 ± 3.27 a | 0.339 ± 0.037 a | 3.792 ± 0.257 a |
| Experiments | Cs Concentration (w/v %) | Au Concentration (mM) | Cs:Au Molar Ratio | Reaction Solvent | Z-Average (nm) | Zeta Potential (mV) | PdI | Electrophoretic Mobility (µm·cm/Vs) |
|---|---|---|---|---|---|---|---|---|
| 3 | 0.1 | 0.13 | 40:1 | Acetic acid | 52.57 ± 1.11 a | 46.1 ± 1.06 a | 0.315 ± 0.005 a | 3.613 ± 0.082 a |
| 10 | Acetic acid/methanol | 81.76 ± 0.87 b | 24.9 ± 1.70 b | 0.550 ± 0.006 b | 1.950 ± 0.132 b | |||
| 12 | 0.4 | 0.26 | 80:1 | Acetic acid | 112 ± 1.65 c | 48.4 ± 3.27 a | 0.339 ± 0.037 a | 3.792 ± 0.257 a |
| 13 | Acetic acid/methanol | 345 ± 1.1 d | 27.6 ± 1.59 b | 0.347 ± 0.043 a | 2.179 ± 0.124 b | |||
| 9 | 0.1 | 0.26 | 20:1 | Acetic acid | 44.21 ± 0.50 e | 46.5 ± 2 a | 0.309 ± 0.008 a | 3.646 ± 0.158 a |
| 11 | Acetic acid/isopropanol | 301 ± 4.85 f | 11.4 ± 0.2 c | 0.206 ± 0.017 c | 0.893 ± 0.017 c |
| Bacteria | Cs-AuNPs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Cef | ||
| Gram (+) | |||||||||||||||
| MSSA | MIC | 65 | 65 | 65 | >65 | 32.5 | 16.2 | 32.5 | 32.5 | >65 | >65 | 65 | 65 | 65 | 0.50 |
| MBC | >65 | >65 | >65 | - | 65 | 32.5 | 32.5 | 32.5 | - | - | 65 | 65 | 65 | 0.80 | |
| MRSA | MIC | 65 | 32.5 | >65 | >65 | 65 | 32.5 | 32.5 | 32.5 | >65 | >65 | 65 | 65 | 65 | 0.50 |
| MBC | >65 | 65 | - | - | - | 32.5 | 32.5 | 32.5 | - | - | 65 | 65 | 65 | 0.50 | |
| Gram (−) | |||||||||||||||
| EC | MIC | 65 | 65 | >65 | >65 | 65 | 65 | 65 | 65 | >65 | >65 | >65 | 65 | 65 | 1.9 |
| MBC | 65 | >65 | - | - | 65 | 65 | 65 | - | - | - | 65 | 65 | 2.5 | ||
| CI-EC | MIC | 65 | 65 | 65 | 65 | 32.5 | 32.5 | 16.2 | 32.5 | >65 | >65 | 65 | 32.5 | 65 | 1 |
| MBC | >65 | >65 | >65 | >65 | 32.5 | 32.5 | 32.5 | 32.5 | - | - | >65 | 65 | >65 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuster, M.G.; Montalbán, M.G.; Carissimi, G.; Lima, B.; Feresin, G.E.; Cano, M.; Giner-Casares, J.J.; López-Cascales, J.J.; Enriz, R.D.; Víllora, G. Antibacterial Effect of Chitosan–Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model. Nanomaterials 2020, 10, 2340. https://doi.org/10.3390/nano10122340
Fuster MG, Montalbán MG, Carissimi G, Lima B, Feresin GE, Cano M, Giner-Casares JJ, López-Cascales JJ, Enriz RD, Víllora G. Antibacterial Effect of Chitosan–Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model. Nanomaterials. 2020; 10(12):2340. https://doi.org/10.3390/nano10122340
Chicago/Turabian StyleFuster, M. G., M. G. Montalbán, G. Carissimi, B. Lima, G. E. Feresin, M. Cano, J. J. Giner-Casares, J. J. López-Cascales, R. D. Enriz, and G. Víllora. 2020. "Antibacterial Effect of Chitosan–Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model" Nanomaterials 10, no. 12: 2340. https://doi.org/10.3390/nano10122340
APA StyleFuster, M. G., Montalbán, M. G., Carissimi, G., Lima, B., Feresin, G. E., Cano, M., Giner-Casares, J. J., López-Cascales, J. J., Enriz, R. D., & Víllora, G. (2020). Antibacterial Effect of Chitosan–Gold Nanoparticles and Computational Modeling of the Interaction between Chitosan and a Lipid Bilayer Model. Nanomaterials, 10(12), 2340. https://doi.org/10.3390/nano10122340








