Lipidic Cubic-Phase Nanoparticles (Cubosomes) Loaded with Doxorubicin and Labeled with 177Lu as a Potential Tool for Combined Chemo and Internal Radiotherapy for Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Radionuclides
2.2. Reagents
2.3. Instrumentation
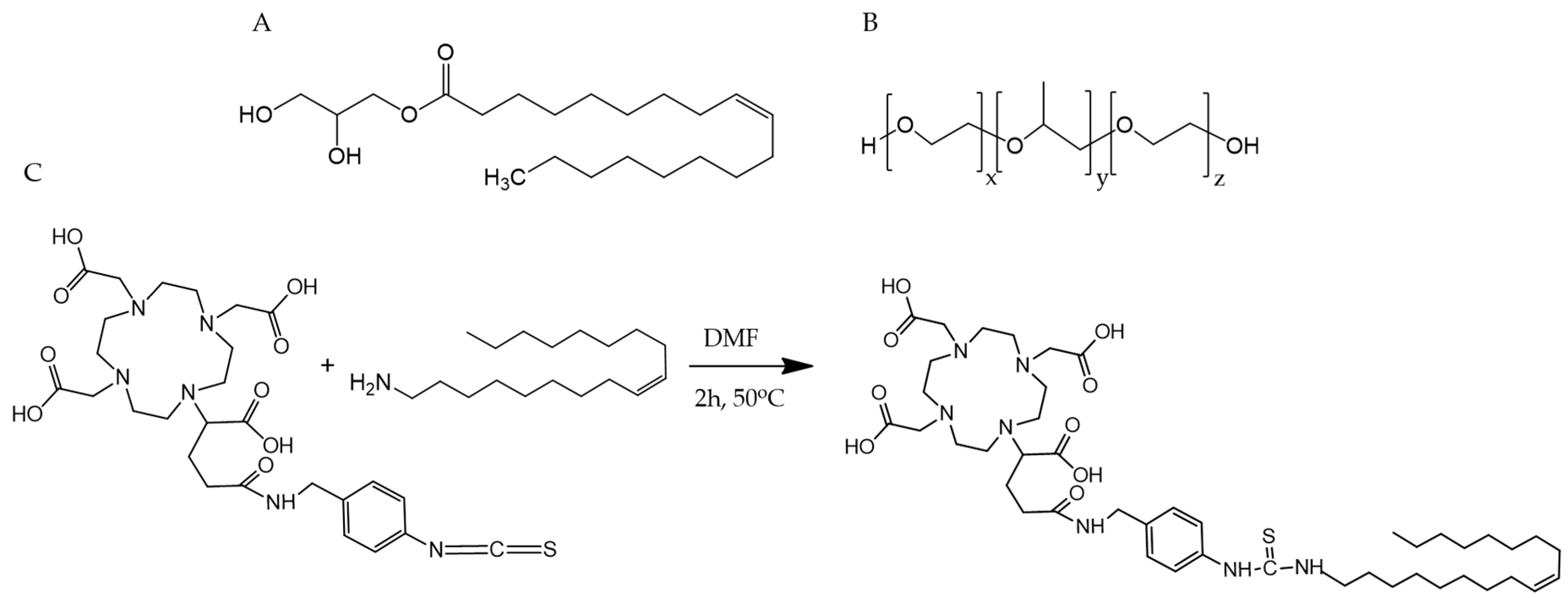
2.4. Synthesis of p-NCS-benzyl-DOTA-GA-Oleylamine Conjugate (DOTAGA-OA)
2.5. Lipophilicity of DOTAGA-OA-177Lu Complex
2.6. Preparation of Cubosomes Loaded with DOTAGA-OA and DOX
2.7. Radiolabeling of Cubosomes Loaded with DOX and DOTAGA-OA
2.8. Stability Studies of Cubosomes with 177Lu
2.9. Cytotoxicity Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of DOTAGA-OA Conjugate
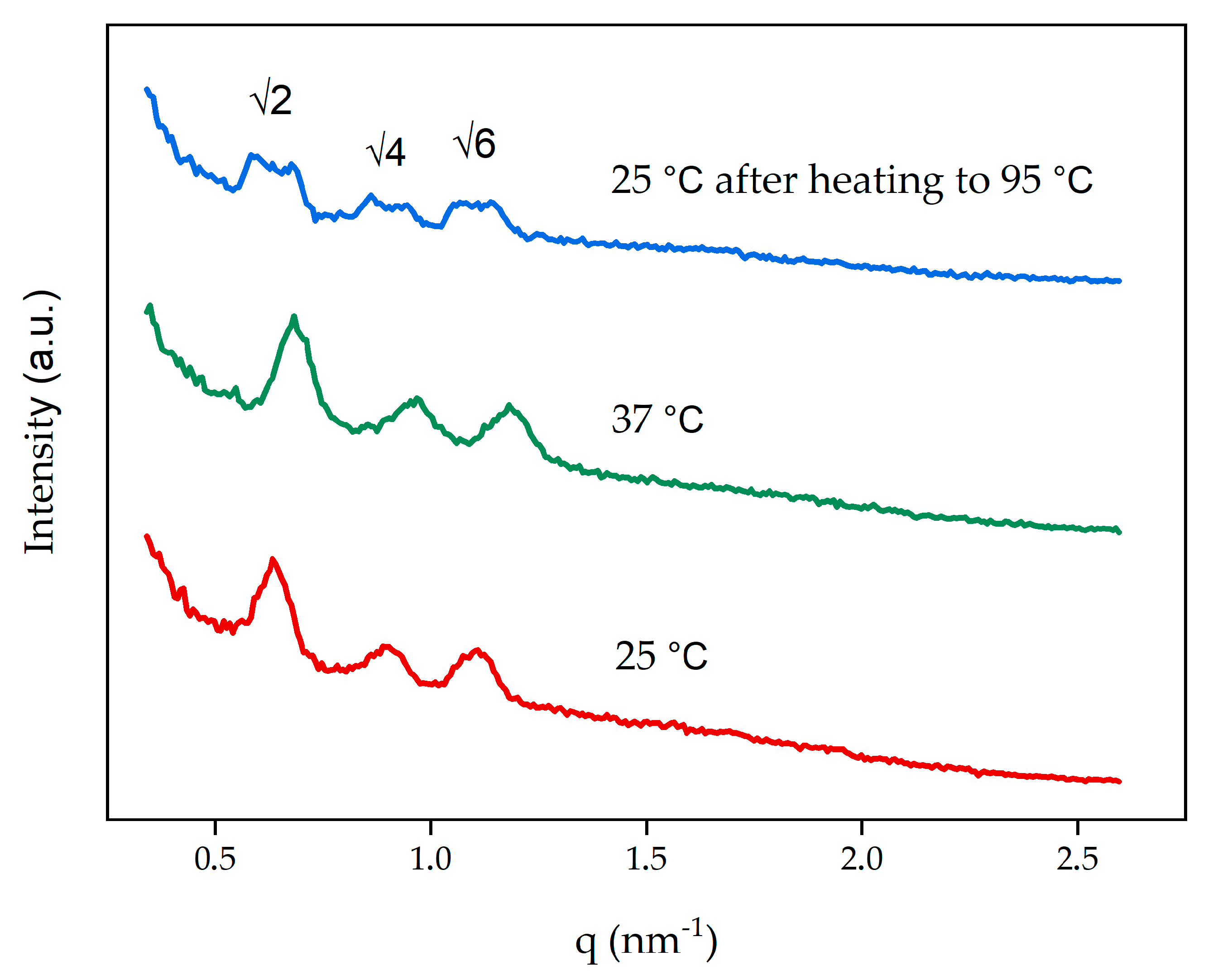
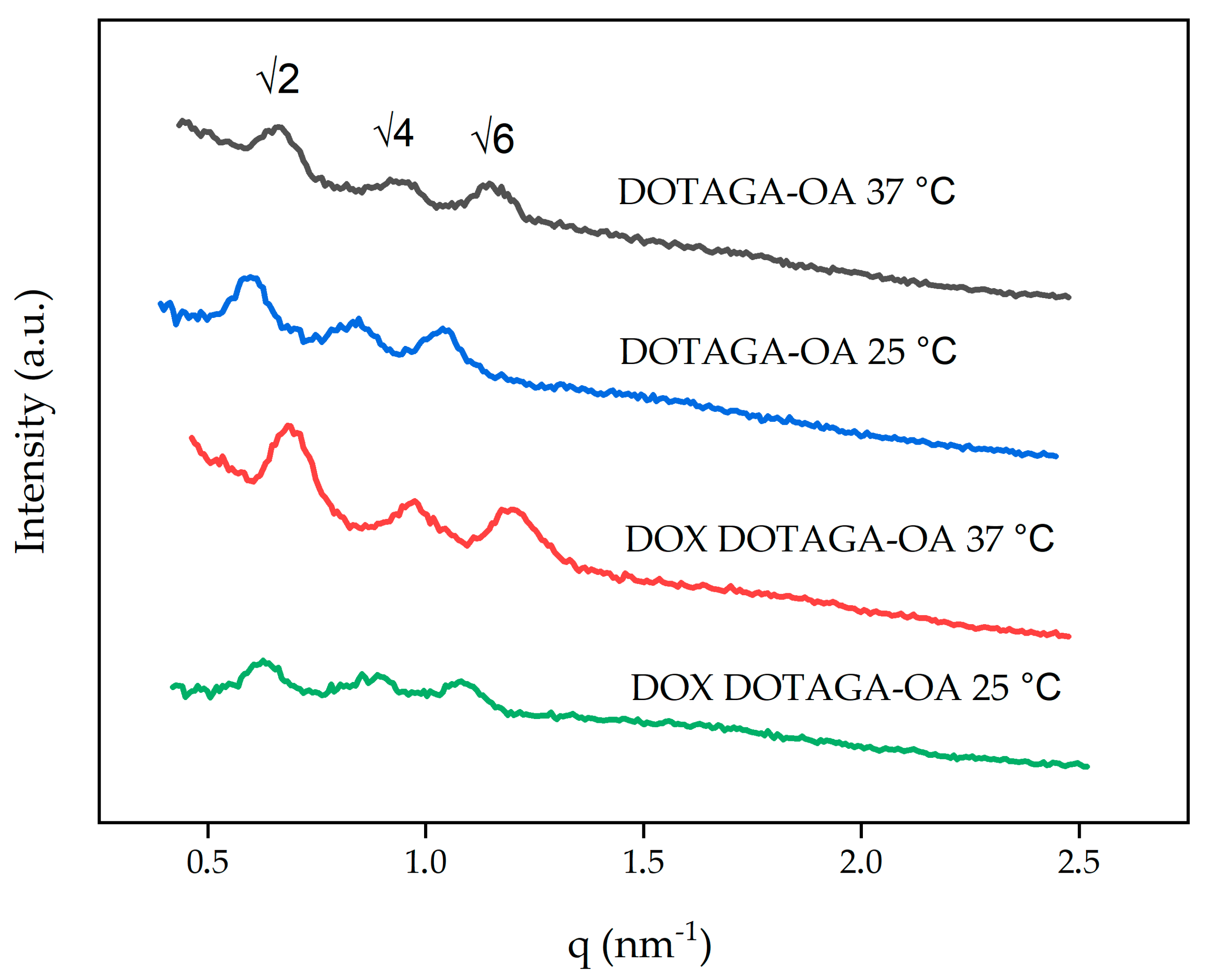
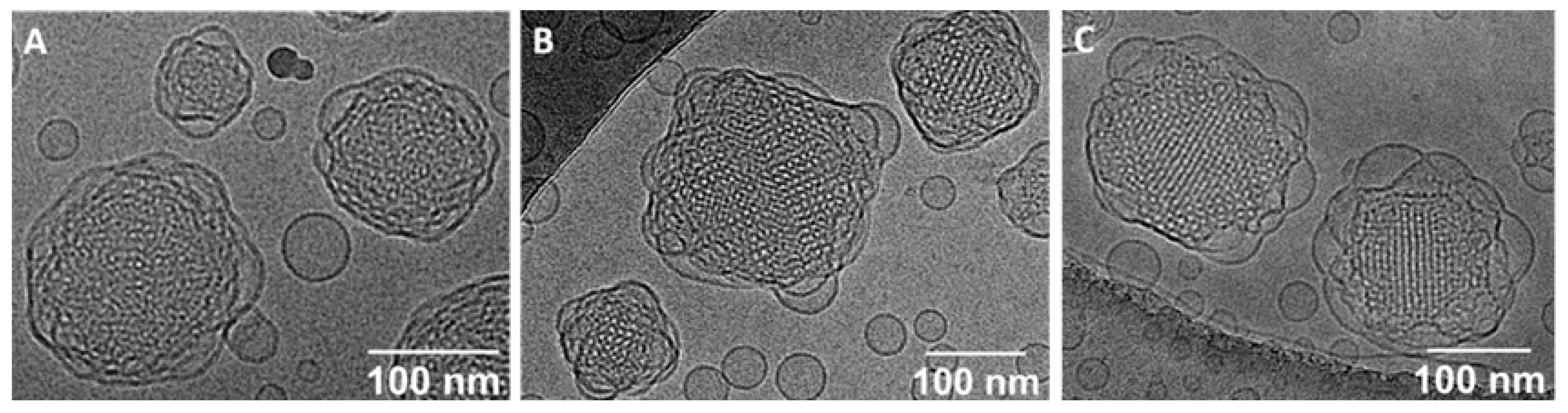
3.2. Characterization of Cubosomes
3.3. Radiolabeling of Cubosomes Loaded with DOX and DOTAGA-OA and Carrier Stability
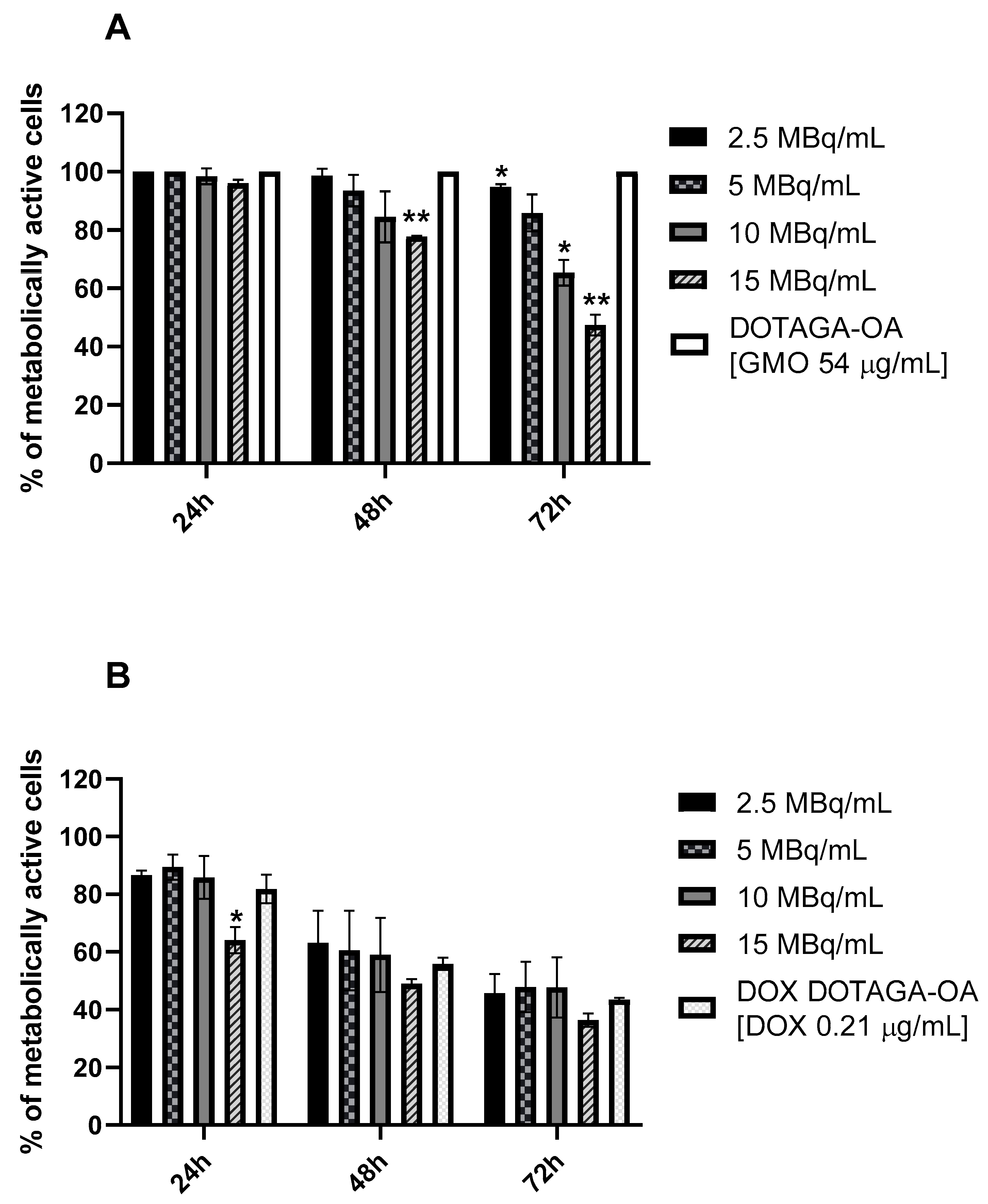
3.4. Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Prosnitz, R.G.; Schwartz, A.L.; Carver, J.R. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer 2012, 118, 2270–2276. [Google Scholar] [CrossRef]
- Lo, S.S.; Teh, B.S.; Jiang, G.-L.; Mayr, N.A. Controversies in Radiation Oncology, 1st ed.; Lo, S.S., Teh, B.S., Jiang, G.-L., Mayr, N.A., Eds.; Medical Radiology Series; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-319-51194-8. [Google Scholar]
- Dimanche-Boitrel, M.-T.; Pelletier, H.; Genne, P.; Petit, J.-M.; Le Grimellec, C.; Canal, P.; Ardiet, C.; Bastian, G.; Chauffert, B. Confluence-dependent resistance in human colon cancer cells: Role of reduced drug accumulation and low intrinsic chemosensitivity of resting cells. Int. J. Cancer 1992, 50, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Steel, G.G.; Peckham, M.J. Exploitable mechanisms in combined radiotherapy-chemotherapy: The concept of additivity. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 85–91. [Google Scholar] [CrossRef]
- Nelson, D.F. Radiotherapy in the treatment of primary central nervous system lymphoma (PCNSL). J. Neurooncol. 1999, 43, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fang, L.; Sun, D.; Shen, Y.; Hu, Y.; Li, N.; Chang, J.; Li, W.; Tan, J. 131I-labeled and DOX-loaded multifunctional nanoliposomes for radiotherapy and chemotherapy in brain gliomas. Brain Res. 2020, 1739, 145218. [Google Scholar] [CrossRef]
- Chen, M.H.; Chang, C.H.; Chang, Y.J.; Chen, L.C.; Yu, C.Y.; Wu, Y.H.; Lee, W.C.; Yeh, C.H.; Lin, F.H.; Lee, T.W.; et al. MicroSPECT/CT imaging and pharmacokinetics of 188Re-(DXR)-liposome in human colorectal adenocarcinoma-bearing mice. Anticancer Res. 2010, 30, 65–72. [Google Scholar]
- Ludwig, J.M.; Xing, M.; Gai, Y.; Sun, L.; Zeng, D.; Kim, H.S. Targeted yttrium 89-doxorubicin drug-eluting bead—A safety and feasibility pilot study in a rabbit liver cancer model. Mol. Pharm. 2017, 14, 2824–2830. [Google Scholar] [CrossRef]
- Huang, F.Y.J.; Lee, T.W.; Chang, C.H.; Chen, L.C.; Hsu, W.H.; Chang, C.W.; Lo, J.M. Evaluation of 188Re-labeled PEGylated nanoliposome as a radionuclide therapeutic agent in an orthotopic glioma-bearing rat model. Int. J. Nanomed. 2015, 10, 463–473. [Google Scholar] [CrossRef]
- Hsu, W.H.; Liu, S.Y.; Chang, Y.J.; Chang, C.H.; Ting, G.; Lee, T.W. The PEGylated liposomal doxorubicin improves the delivery and therapeutic efficiency of 188Re-Liposome by modulating phagocytosis in C26 murine colon carcinoma tumor model. Nucl. Med. Biol. 2014, 41, 765–771. [Google Scholar] [CrossRef]
- Soundararajan, A.; Bao, A.; Phillips, W.T.; McManus, L.M.; Goins, B.A. Chemoradionuclide therapy with 186Re-labeled liposomal doxorubicin: Toxicity, dosimetry, and therapeutic response. Cancer Biother. Radiopharm. 2011, 26, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.M.; Sun, R.J.; Pan, X.L. Folate-functionalized lipid nanoemulsion to deliver chemo-radiotherapeutics together for the effective treatment of nasopharyngeal carcinoma. AAPS PharmSciTech 2017, 18, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering–ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.; Caffrey, M. Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. J. Control. Release 2005, 107, 97–111. [Google Scholar] [CrossRef]
- Hinton, T.M.; Grusche, F.; Acharya, D.; Shukla, R.; Bansal, V.; Waddington, L.J.; Monaghan, P.; Muir, B.W. Bicontinuous cubic phase nanoparticle lipid chemistry affects toxicity in cultured cells. Toxicol. Res. 2014, 3, 11–22. [Google Scholar] [CrossRef]
- Engström, S.; Nordén, T.P.; Nyquist, H. Cubic phases for studies of drug partition into lipid bilayers. Eur. J. Pharm. Sci. 1999, 8, 243–254. [Google Scholar] [CrossRef]
- Mierzwa, M.; Cytryniak, A.; Krysiński, P.; Bilewicz, R. Lipidic liquid crystalline cubic phases and magnetocubosomes as methotrexate carriers. Nanomaterials 2019, 9, 636. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Meli, V.; Caltagirone, C.; Sinico, C.; Lai, F.; Falchi, A.M.; Monduzzi, M.; Obiols-Rabasa, M.; Picci, G.; Rosa, A.; Schmidt, J.; et al. Theranostic hexosomes for cancer treatments: An in vitro study. New J. Chem. 2017, 41, 1558–1565. [Google Scholar] [CrossRef]
- Nazaruk, E.; Szlezak, M.; Górecka, E.; Bilewicz, R.; Osornio, Y.M.; Uebelhart, P.; Landau, E.M. Design and assembly of pH-sensitive lipidic cubic phase matrices for drug release. Langmuir 2014, 30, 1383–1390. [Google Scholar] [CrossRef]
- Azhari, H.; Strauss, M.; Hook, S.; Boyd, B.J.; Rizwan, S.B. Stabilising cubosomes with Tween 80 as a step towards targeting lipid nanocarriers to the blood–brain barrier. Eur. J. Pharm. Biopharm. 2016, 104, 148–155. [Google Scholar] [CrossRef]
- Meli, V.; Caltagirone, C.; Falchi, A.M.; Hyde, S.T.; Lippolis, V.; Monduzzi, M.; Obiols-Rabasa, M.; Rosa, A.; Schmidt, J.; Talmon, Y.; et al. Docetaxel-loaded fluorescent liquid-crystalline nanoparticles for cancer theranostics. Langmuir 2015, 31, 9566–9575. [Google Scholar] [CrossRef]
- Godlewska, M.; Majkowska-Pilip, A.; Stachurska, A.; Biernat, J.F.; Gaweł, D.; Nazaruk, E. Voltammetric and biological studies of folate-targeted non-lamellar lipid mesophases. Electrochim. Acta 2019, 299, 1–11. [Google Scholar] [CrossRef]
- Aleandri, S.; Bandera, D.; Mezzenga, R.; Landau, E.M. Biotinylated cubosomes: A versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye. Langmuir 2015, 31, 12770–12776. [Google Scholar] [CrossRef]
- Caltagirone, C.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Sgarzi, M.; Talmon, Y.; et al. Cancer-cell-targeted theranostic cubosomes. Langmuir 2014, 30, 6228–6236. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. PH responsiveness of hexosomes and cubosomes for combined delivery of Brucea javanica oil and doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Liu, G.; Conn, C.E.; Waddington, L.J.; Mudie, S.T.; Drummond, C.J. Colloidal amphiphile self-assembly particles composed of gadolinium oleate and myverol: Evaluation as contrast agents for magnetic resonance imaging. Langmuir 2010, 26, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Bye, N.; Moffat, B.A.; Wright, D.K.; Cuddihy, A.; Hinton, T.M.; Hawley, A.M.; Reynolds, N.P.; Waddington, L.J.; Mulet, X.; et al. Dual-modality NIRF-MRI cubosomes and hexosomes: High throughput formulation and in vivo biodistribution. Mater. Sci. Eng. C 2017, 71, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Majkowska-Pilip, A.; Bilewicz, R. Lipidic cubic-phase nanoparticles-cubosomes for efficient drug delivery to cancer cells. ChemPlusChem 2017, 82, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [PubMed]
- Murgia, S.; Biffi, S.; Mezzenga, R. Recent advances of non-lamellar lyotropic liquid crystalline nanoparticles in nanomedicine. Curr. Opin. Colloid Interface Sci. 2020, 48, 28–39. [Google Scholar] [CrossRef]
- Van’t Hag, L.; Gras, S.L.; Conn, C.E.; Drummond, C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space-ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017, 46, 2705–2731. [Google Scholar] [CrossRef]
- Mertins, O.; Mathews, P.D.; Angelova, A. Advances in the design of ph-sensitive cubosome liquid crystalline nanocarriers for drug delivery applications. Nanomaterials 2020, 10, 963. [Google Scholar] [CrossRef]
- Nazaruk, E.; Majkowska-Pilip, A.; Godlewska, M.; Salamończyk, M.; Gawel, D. Electrochemical and biological characterization of lyotropic liquid crystalline phases—Retardation of drug release from hexagonal mesophases. J. Electroanal. Chem. 2018, 813, 208–215. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Kozminski, P.; Wawrzynowska, A.; Budlewski, T.; Kostkiewicz, B.; Gniazdowska, E. Application of neurokinin-1 receptor in targeted strategies for glioma treatment. Part I: Synthesis and evaluation of substance P fragments labeled with 99mTc and 177Lu as potential receptor radiopharmaceuticals. Molecules 2018, 23, 2542. [Google Scholar] [CrossRef]
- Nazaruk, E.; Miszta, P.; Filipek, S.; Górecka, E.; Landau, E.M.; Bilewicz, R. Lyotropic cubic phases for drug delivery: Diffusion and sustained release from the mesophase evaluated by electrochemical methods. Langmuir 2015, 31, 12753–12761. [Google Scholar] [CrossRef]
- Dong, Y.D.; Tilley, A.J.; Larson, I.; Lawrence, M.J.; Amenitsch, H.; Rappolt, M.; Hanley, T.; Boyd, B.J. Nonequilibrium effects in self-assembled mesophase materials: Unexpected supercooling effects for cubosomes and hexosomes. Langmuir 2010, 26, 9000–9010. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, T.E.; Ladewig, K.; O’Connor, A.J.; Hartley, P.G.; McLean, K.M. Physicochemical and cytotoxicity analysis of glycerol monoolein-based nanoparticles. RSC Adv. 2015, 5, 26543–26549. [Google Scholar] [CrossRef]
- Li, W.; Sun, D.; Li, N.; Shen, Y.; Hu, Y.; Tan, J. Therapy of cervical cancer using 131 I-labeled nanoparticles. J. Int. Med. Res. 2018, 46, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.J.; Lim, S.H.; Ho, A.L.; Ghossein, R.A.; Fury, M.G.; Shaha, A.R.; Rivera, M.; Lin, O.; Wolden, S.; Lee, N.Y.; et al. Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: A critical re-evaluation including uniform pathologic review. Radiother. Oncol. 2011, 101, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yook, S.; Lu, Y.; Bergstrom, D.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Local radiation treatment of HER2-positive breast cancer using trastuzumab-modified gold nanoparticles labeled with 177Lu. Pharm. Res. 2017, 34, 579–590. [Google Scholar] [CrossRef] [PubMed]
| Cubosome Formulations | Final Compositions | Components Ratio (wt.%) |
|---|---|---|
| Blank | buffer/GMO/F-127 | 94.62/4.84/0.54 |
| DOTAGA-OA | buffer/GMO/DOTAGA-OA/F-127 | 94.18/4.81/0.47/0.54 |
| DOX DOTAGA-OA | buffer/GMO/DOX/DOTAGA-OA/F-127 | 94.15/4.82/0.02/0.47/0.54 |
| Cubosomes Formulations | Symmetry | Unit Cell (nm) |
|---|---|---|
| Blank 25 °C | Im | 14.0 |
| Blank 37 °C | Im | 13.0 |
| Blank 25 °C (after heating to 95 °C) | Im | 14.0 |
| Cubosome Formulations | Symmetry | Unit Cell (nm) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| Blank | Im | 14.0 | 181 ± 10 | 0.23 ± 0.01 | −27 ± 0.9 |
| DOTAGA-OA | Im | 14.9 A (13.5 B) | 153 ± 12 | 0.19 ± 0.03 | −11 ± 0.7 |
| DOX DOTAGA-OA | Im | 14.2 A (13.0 B) | 160 ± 5 | 0.19 ± 0.02 | −19 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cytryniak, A.; Nazaruk, E.; Bilewicz, R.; Górzyńska, E.; Żelechowska-Matysiak, K.; Walczak, R.; Mames, A.; Bilewicz, A.; Majkowska-Pilip, A. Lipidic Cubic-Phase Nanoparticles (Cubosomes) Loaded with Doxorubicin and Labeled with 177Lu as a Potential Tool for Combined Chemo and Internal Radiotherapy for Cancers. Nanomaterials 2020, 10, 2272. https://doi.org/10.3390/nano10112272
Cytryniak A, Nazaruk E, Bilewicz R, Górzyńska E, Żelechowska-Matysiak K, Walczak R, Mames A, Bilewicz A, Majkowska-Pilip A. Lipidic Cubic-Phase Nanoparticles (Cubosomes) Loaded with Doxorubicin and Labeled with 177Lu as a Potential Tool for Combined Chemo and Internal Radiotherapy for Cancers. Nanomaterials. 2020; 10(11):2272. https://doi.org/10.3390/nano10112272
Chicago/Turabian StyleCytryniak, Adrianna, Ewa Nazaruk, Renata Bilewicz, Emilia Górzyńska, Kinga Żelechowska-Matysiak, Rafał Walczak, Adam Mames, Aleksander Bilewicz, and Agnieszka Majkowska-Pilip. 2020. "Lipidic Cubic-Phase Nanoparticles (Cubosomes) Loaded with Doxorubicin and Labeled with 177Lu as a Potential Tool for Combined Chemo and Internal Radiotherapy for Cancers" Nanomaterials 10, no. 11: 2272. https://doi.org/10.3390/nano10112272
APA StyleCytryniak, A., Nazaruk, E., Bilewicz, R., Górzyńska, E., Żelechowska-Matysiak, K., Walczak, R., Mames, A., Bilewicz, A., & Majkowska-Pilip, A. (2020). Lipidic Cubic-Phase Nanoparticles (Cubosomes) Loaded with Doxorubicin and Labeled with 177Lu as a Potential Tool for Combined Chemo and Internal Radiotherapy for Cancers. Nanomaterials, 10(11), 2272. https://doi.org/10.3390/nano10112272







