Transformation and Cytotoxicity of Surface-Modified Silver Nanoparticles Undergoing Long-Term Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of AgNPs
2.2. Media Impact on Physicochemical Properties of AgNPs
2.3. Aging of AgNPs in 4 °C Storage
2.4. Cell Lines and Cell Culture
2.5. Cell Viability
2.6. Statistical Analysis
3. Results
3.1. Characterization of AgNPs
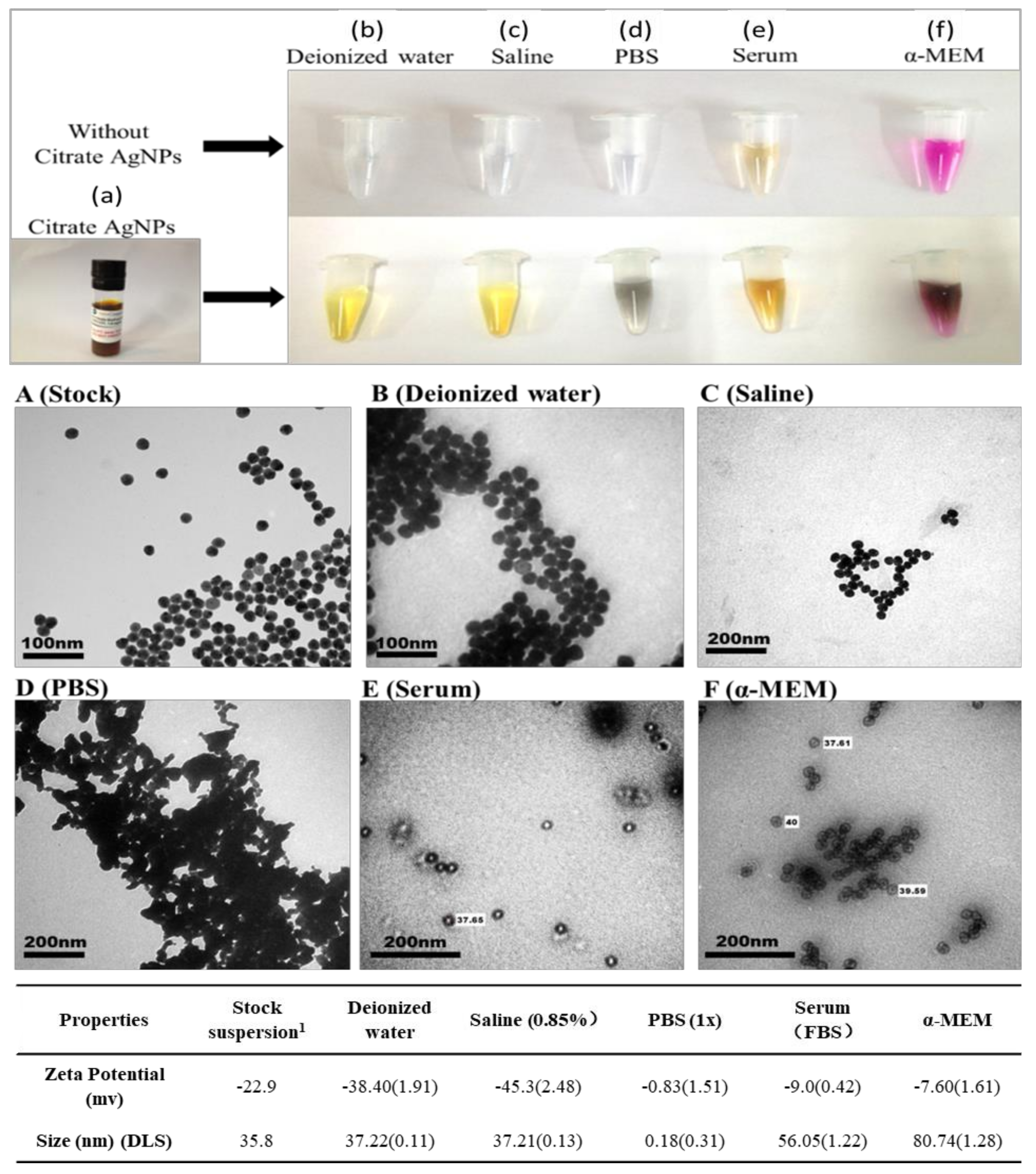
3.2. Media Impact on Citrate AgNPs’ Physicochemical Properties
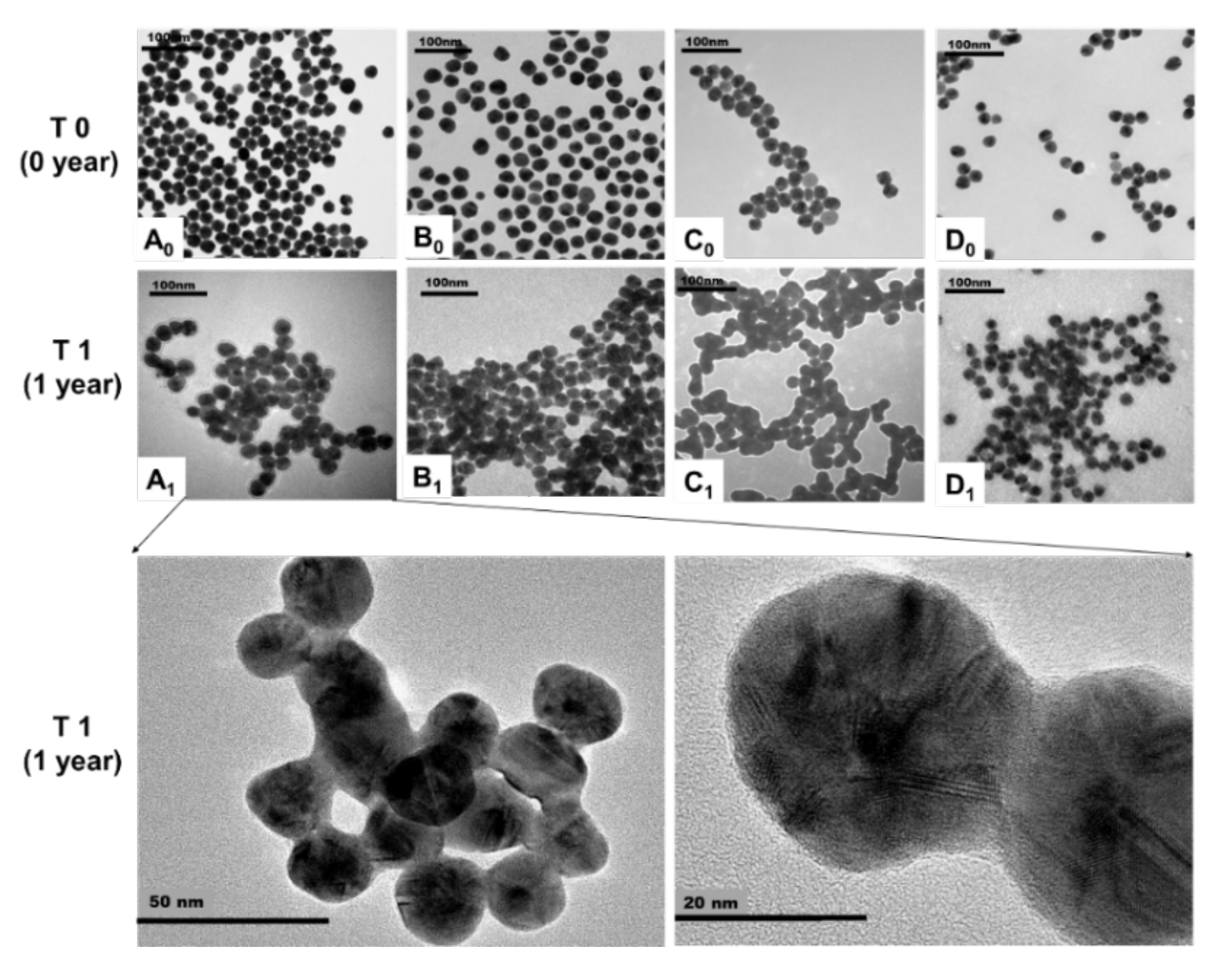
3.3. Transformation of AgNPs with Different Surface Coatings during Storage
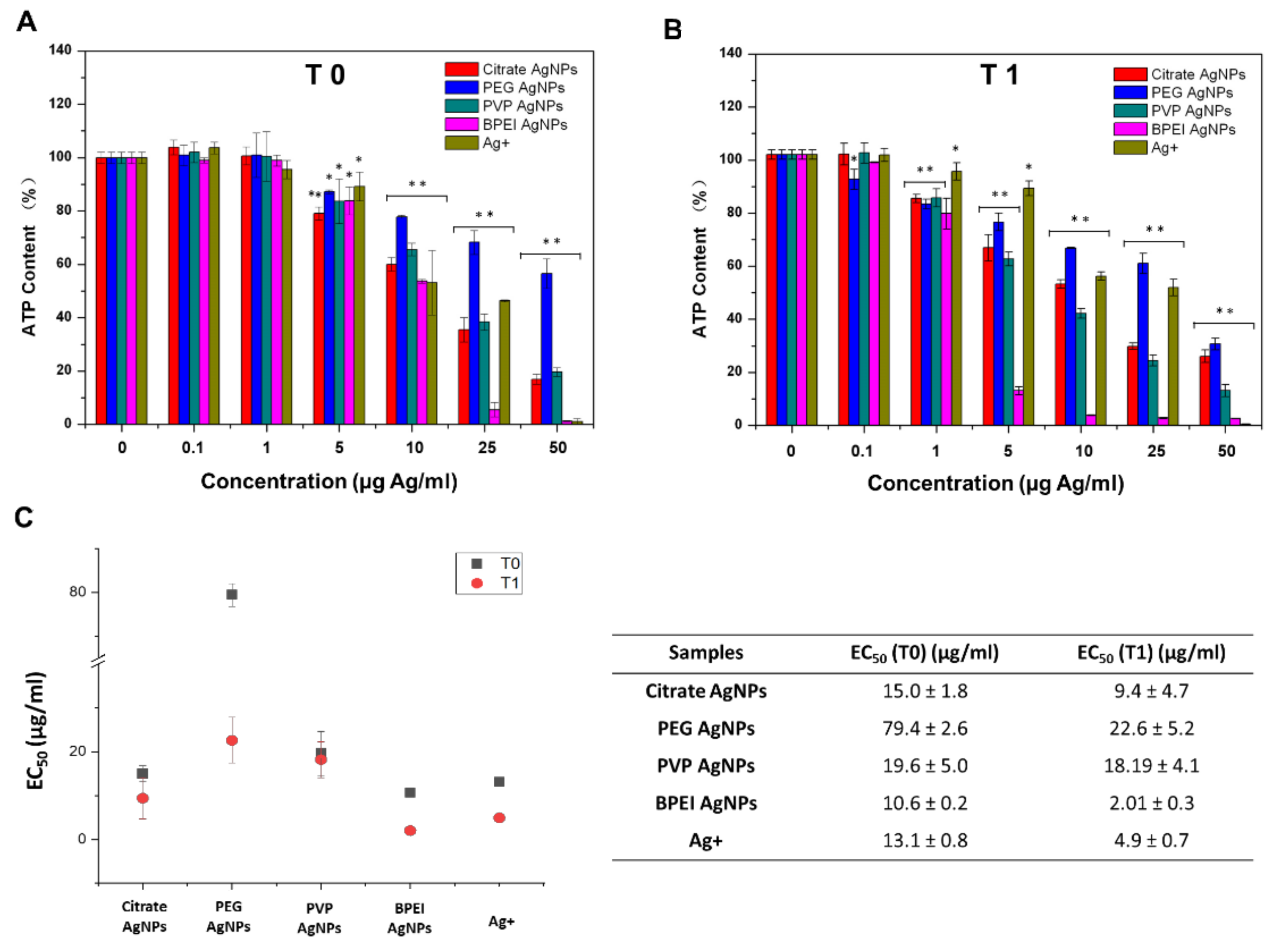
3.4. Dynamics of AgNPs’ Toxicity to Hepa1c1c7 Cells
4. Discussion
4.1. Media Impact on Citrate AgNPs’ Transformation
4.2. Impact of Aging on AgNPs’ Aggregation, Shape, Surface Charge, Size, and Dissolution
4.2.1. TEM Image Analysis for Aggregation and Shape of AgNPs
4.2.2. Hydrodynamic Size and Zeta Potential of AgNPs by DLS Analysis
4.2.3. Dissolution of AgNPs
4.3. Aging Impact on the Toxicity of AgNPs’ to Hepa1c1c7 Cells
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DTU Environment. The Nanodatabase. 2013. Available online: http://nanodb.dk/products (accessed on 17 December 2013).
- Rejeski, D. Nanotechnology and Consumer Products. 2009. Available online: http://www.nanotechproject.org/process/assets/files/8278/pen_submission_cpsc.pdf (accessed on 18 September 2013).
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Avellan, A.; Lowry, G.V. Effect of initial speciation of copper-and silver-based nanoparticles on their long-term fate and phytoavailability in freshwater wetland mesocosms. Environ. Sci. Technol. 2017, 51, 12114–12122. [Google Scholar] [CrossRef] [PubMed]
- Siemer, S.; Westmeier, D.; Barz, M.; Eckrich, J.; Wünsch, D.; Seckert, C.; Thyssen, C.; Schilling, O.; Hasenberg, M.; Pang, E.; et al. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: Implications for the design of improved nanoantibiotics. Biomaterials 2019, 192, 551–559. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Pan, J.; Jiang, X.; Ji, Y.; Li, Y.; Qu, Y.; Zhao, Y.; Wu, X.; Chen, C. Revealing the binding structure of the protein corona on gold nanorods using synchrotron radiation-based techniques: Understanding the reduced damage in cell membranes. J. Am. Chem. Soc. 2013, 135, 17359–17368. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Richards, L.; Rippstein, P.; Massarsky, A.; Moon, T.W.; Tayabali, A.F. Toxicological evaluation of representative silver nanoparticles in macrophages and epithelial cells. Toxicol. In Vitro 2016, 33, 163–173. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Pang, C.; Selck, H.; Misra, S.K.; Berhanu, D.; Dybowska, A.; Valsami-Jones, E.; Forbes, V.E. Effects of sediment-associated copper to the deposit-feeding snail, Potamopyrgus antipodarum: A comparison of Cu added in aqueous form or as nano- and micro-CuO particles. Aquat. Toxicol. 2012, 106-107, 114–122. [Google Scholar] [CrossRef]
- Pang, C.; Brunelli, A.; Zhu, C.; Hristozov, D.; Liu, Y.; Semenzin, E.; Wang, W.; Tao, W.; Liang, J.; Marcomini, A.; et al. Demonstrating approaches to chemically modify the surface of Ag nanoparticles in order to influence their cytotoxicity and biodistribution after single dose acute intravenous administration. Nanotoxicology 2016, 10, 129–139. [Google Scholar] [CrossRef]
- Stauber, R.H.; Siemer, S.; Becker, S.; Ding, G.B.; Strieth, S.; Knauer, S.K. Small meets smaller: Effects of nanomaterials on microbial biology, pathology, and ecology. ACS Nano 2018, 12, 6351–6359. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of protein modification on the protein corona on nanoparticles and nanoparticle-cell interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Köller, M.; Epple, M. The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.M.; Scheckel, K.G.; Suidan, M.; Tolaymat, T. The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles. Sci. Total Environ. 2012, 429, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Blunk, T.; Hochstrasser, D.F.; Sanchez, J.C.; Muller, B.W.; Muller, R.H. Colloidal carriers for intravenous drug targeting: Plasma protein adsorption patterns on surface-modified latex particles evaluated by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 1993, 14, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, M.S.; Friedman, A.E.; Finkelstein, J.N.; Oberdorster, G.; McGrath, J.L. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials 2009, 30, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Lai, X.; Ke, P.C.; Podila, R.; Brown, J.M.; Witzmann, F.A. Silver nanoparticle protein corona composition in cell culture media. PLoS ONE 2013, 8, e74001. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Thomas, M.B.; Thomas, R.; Raichur, A.M.; Chakravortty, D. Interaction of silver nanoparticles with serum proteins affects their antimicrobial activity in vivo. Antimicrob. Agents Chemother. 2013, 57, 4945–4955. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Schekel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Mackevica, A.; Tian, J.; Feng, H.; Li, Z.; Baun, A. Release of Ag/ZnO Nanomaterials and associated risks of a novel water sterilization technology. Water 2019, 11, 2276. [Google Scholar] [CrossRef]
- Gorham, J.M.; Rohlfing, A.B.; Lippa, K.A.; MacCuspie, R.I.; Hemmati, A.; Holbrook, R.D. Storage Wars: How citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions. J. Nanopart. Res. 2014, 16, 2339. [Google Scholar] [CrossRef]
- Izak-Nau, E.; Huk, A.; Reidy, B.; Uggerud, H.; Vadset, M.; Eiden, S.; Voetz, M.; Himly, M.; Duschl, A.; Dusinska, M.; et al. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. Rsc. Adv. 2015, 5, 84172–84185. [Google Scholar] [CrossRef]
- Wiley, B. Polyol Synthesis of Silver Nanoparticles: Use of Chloride and Oxygen to Promote the Formation of Single-Crystal, Truncated Cubes and Tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- Jin, R. Photoinduced Conversion of Silver Nanospheres to Nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef]
- Yang, J. Dissolution-recrystallization mechanism for the conversion of silver nanospheres to triangular nanoplates. J. Colloid Interface Sci. 2007, 308, 157–161. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
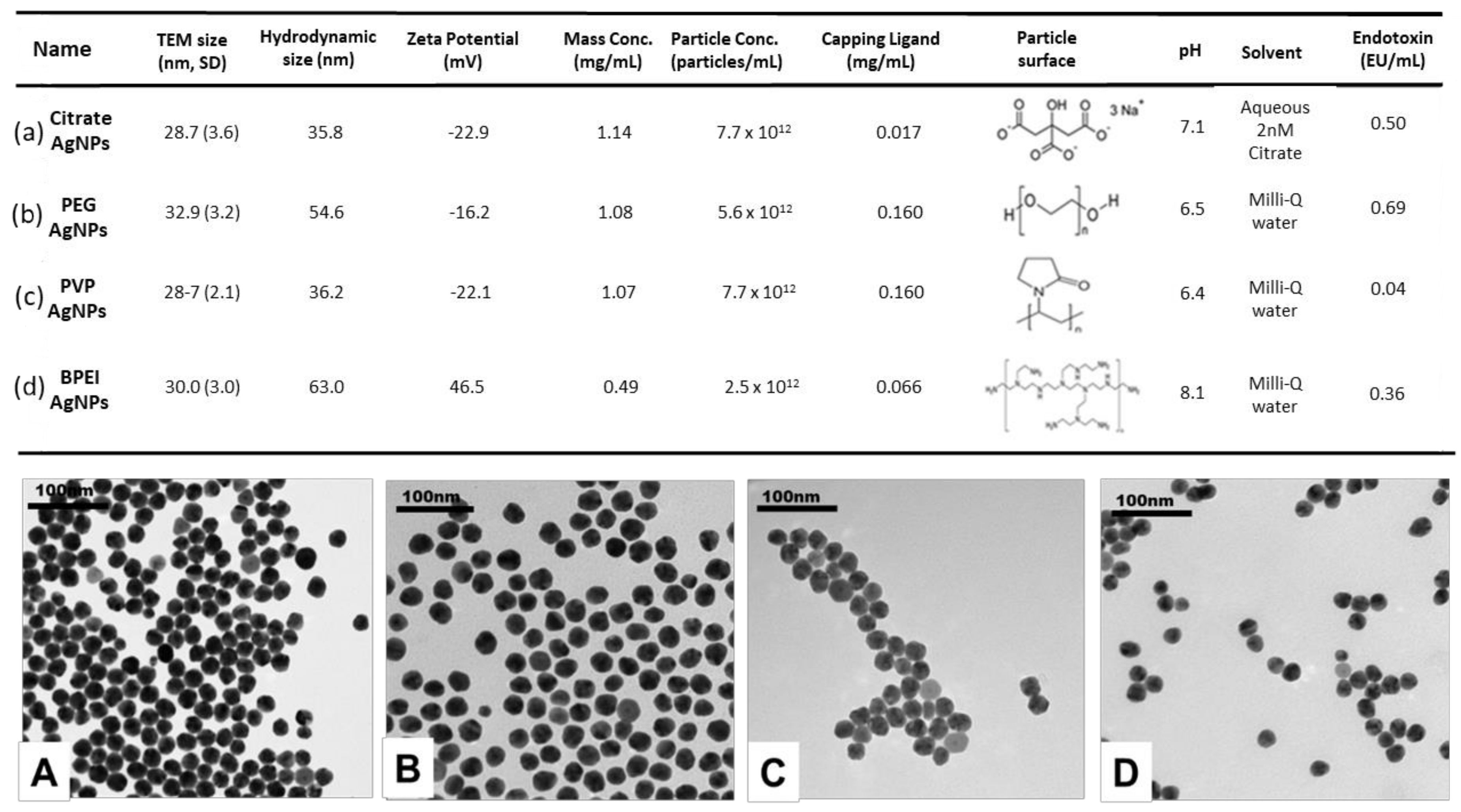
| Name | TEM Size (nm) | Hydrodynamic Size (nm) | Zeta Potential (mV) | Mass Conc. (mg/mL) | Released Ag+ (µg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (Year) | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 |
| Citrate AgNPs | 28.7(3.6) | 76.2(34.0) | 35.8 | 34.4 | −22.9 | −48.5 | 1.14 (0.06) | 1.09 (0.02) | 0.43 (0.10) | 3.12 (0.14) |
| PEG AgNPs | 32.9(3.2) | 60.5(30.5) | 54.6 | 55.8 | −16.2 | −2.71 | 1.08 (0.01) | 1.01 (0.03) | 0.45 (0.03) | 67.36 (4.26) |
| PVP AgNPs | 28.7(2.1) | 31.3(34.9) | 36.2 | 40.5 | −22.1 | −31.5 | 1.07 (0.03) | 0.91 (0.03) | 0.58 (0.10) | 130.60 (5.40) |
| BPEI AgNPs | 30.0(3.0) | 26.6(4.3) | 63.0 | 70.4 | 46.5 | 37 | 0.49 (0.03) | 0.39 (0.02) | 0.80 (0.07) | 371.10 (14.82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, C.; Zhang, P.; Mu, Y.; Ren, J.; Zhao, B. Transformation and Cytotoxicity of Surface-Modified Silver Nanoparticles Undergoing Long-Term Aging. Nanomaterials 2020, 10, 2255. https://doi.org/10.3390/nano10112255
Pang C, Zhang P, Mu Y, Ren J, Zhao B. Transformation and Cytotoxicity of Surface-Modified Silver Nanoparticles Undergoing Long-Term Aging. Nanomaterials. 2020; 10(11):2255. https://doi.org/10.3390/nano10112255
Chicago/Turabian StylePang, Chengfang, Panhong Zhang, Yunsong Mu, Jingzheng Ren, and Bin Zhao. 2020. "Transformation and Cytotoxicity of Surface-Modified Silver Nanoparticles Undergoing Long-Term Aging" Nanomaterials 10, no. 11: 2255. https://doi.org/10.3390/nano10112255
APA StylePang, C., Zhang, P., Mu, Y., Ren, J., & Zhao, B. (2020). Transformation and Cytotoxicity of Surface-Modified Silver Nanoparticles Undergoing Long-Term Aging. Nanomaterials, 10(11), 2255. https://doi.org/10.3390/nano10112255





