Zinc Gallium Oxide—A Review from Synthesis to Applications
Abstract
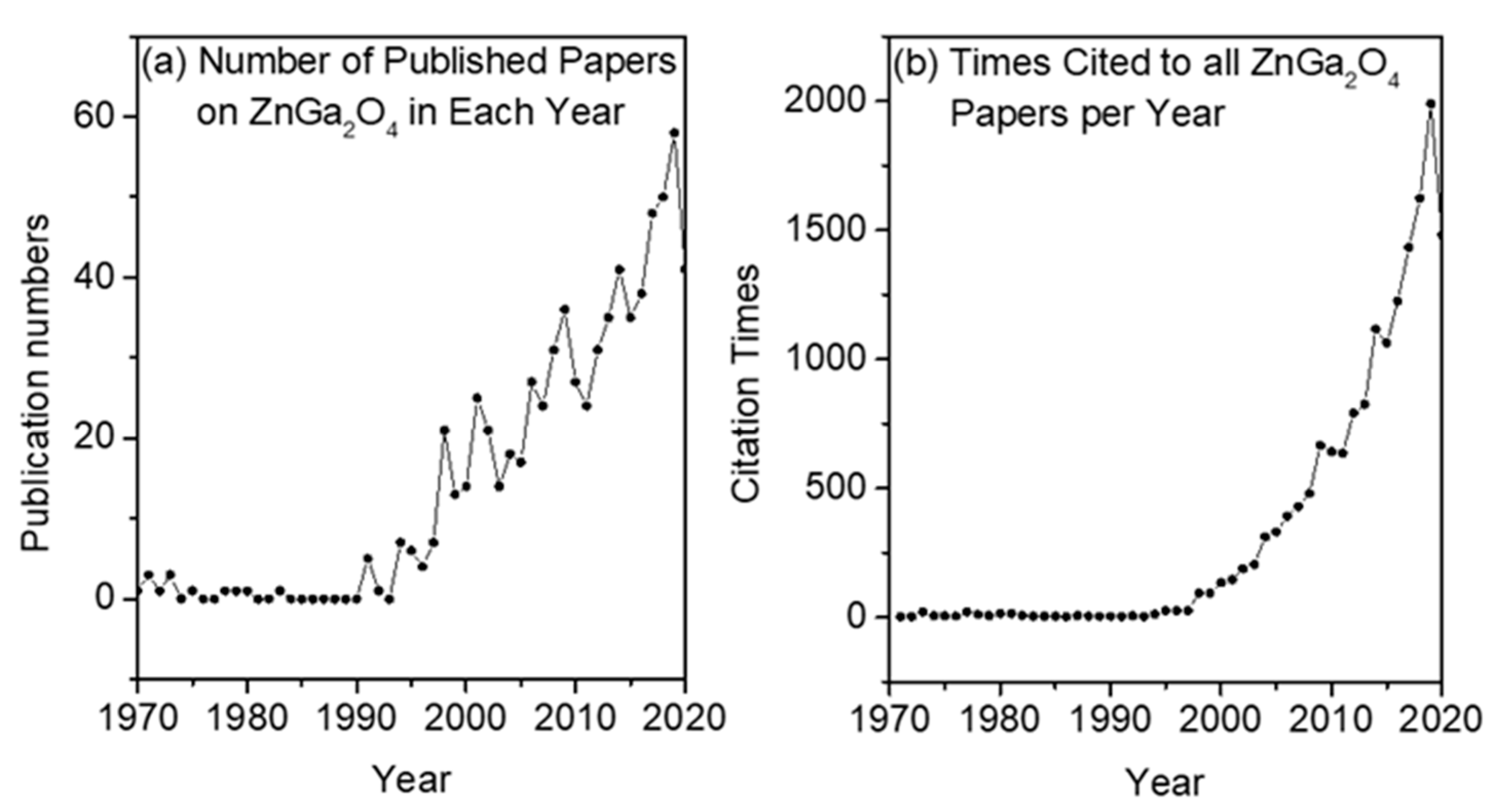
1. Introduction
2. Basic Properties of ZnGa2O4
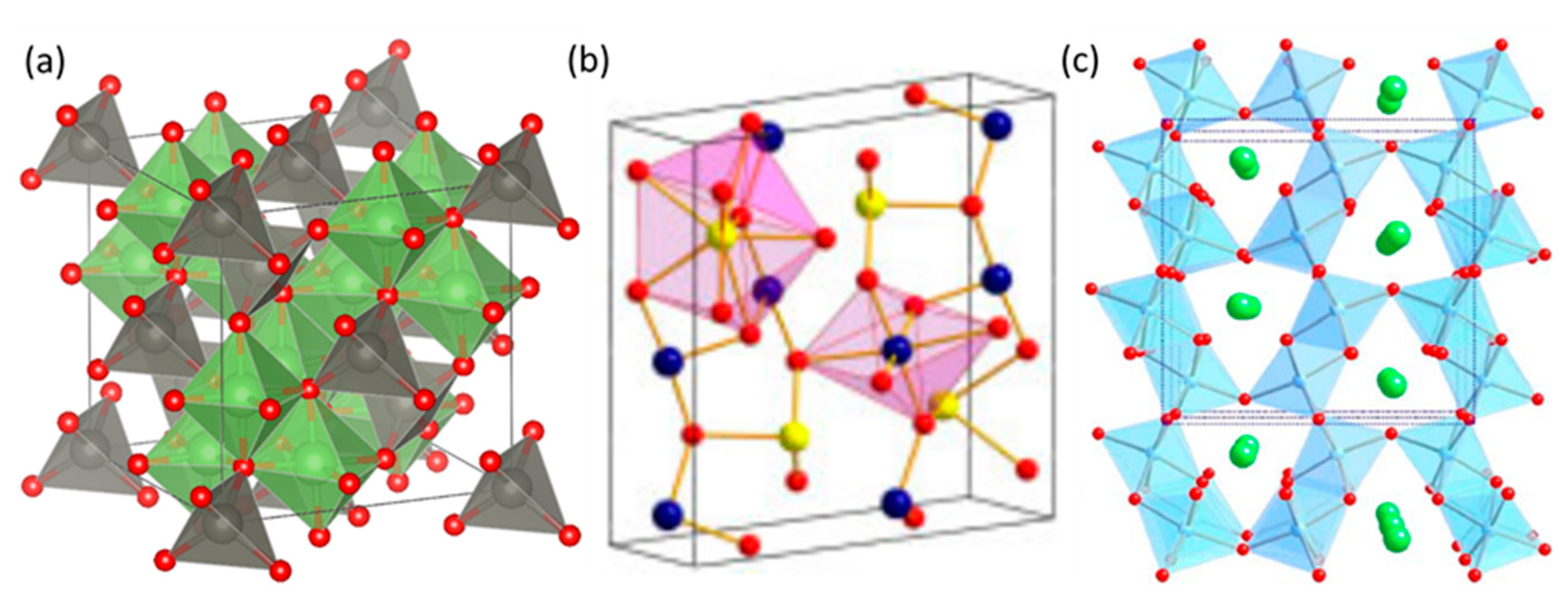
2.1. Crystalline Structure of ZnGa2O4
2.2. Mechanical Properties of ZnGa2O4 under External Pressure
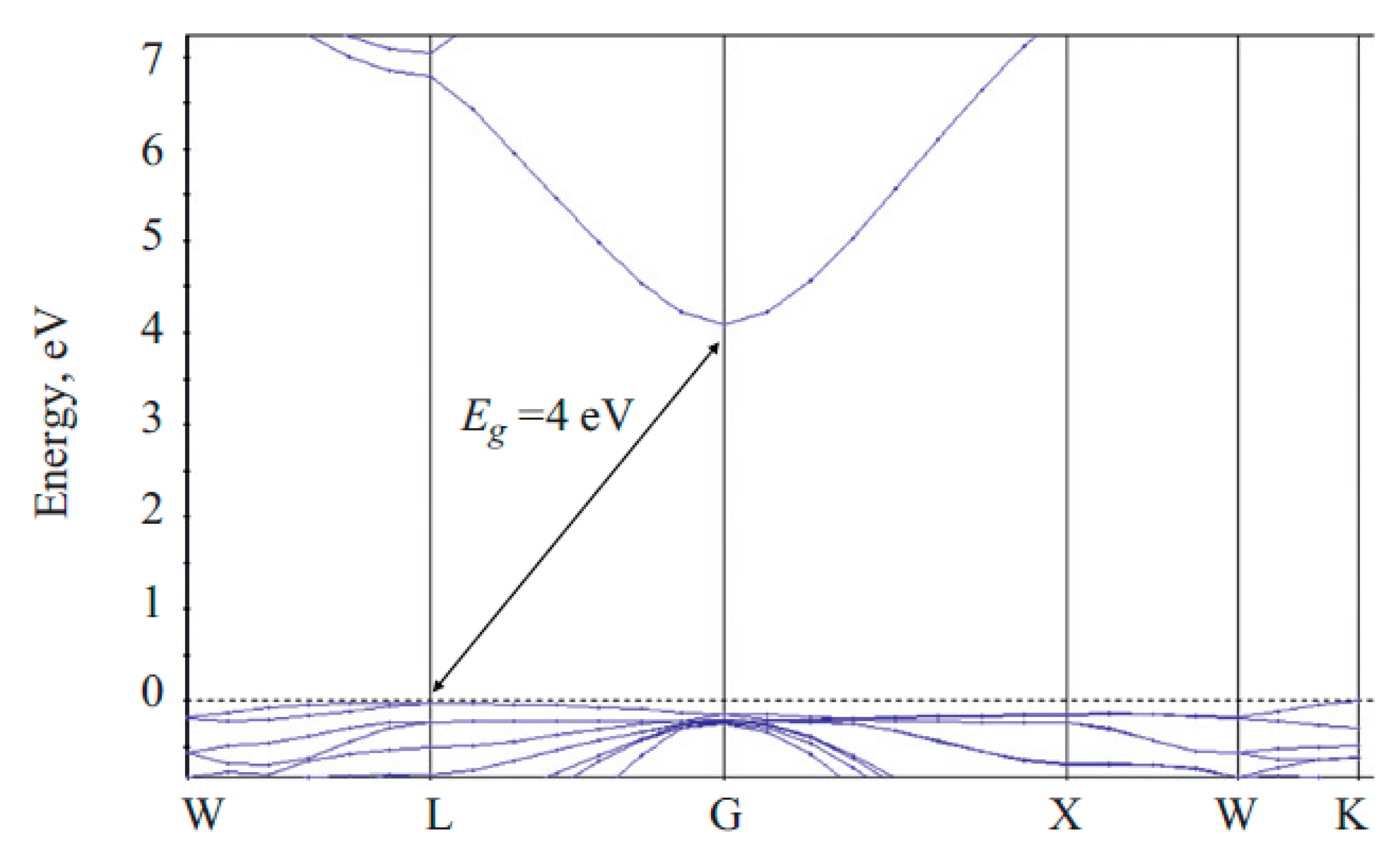
2.3. Band Structure of ZnGa2O4
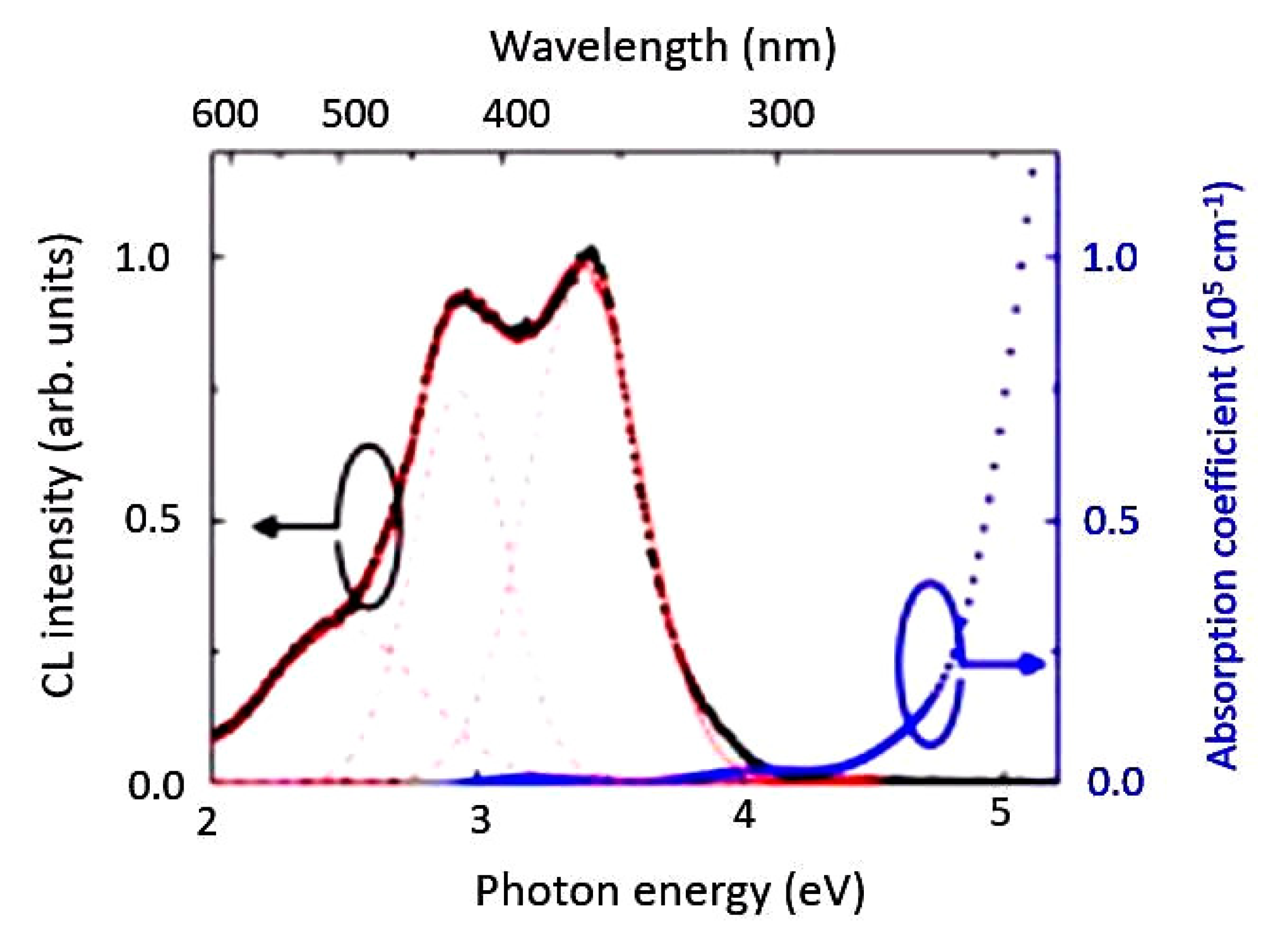
2.4. Bond Distance and Its Electronic Properties
3. Bulk Growth Mechanisms
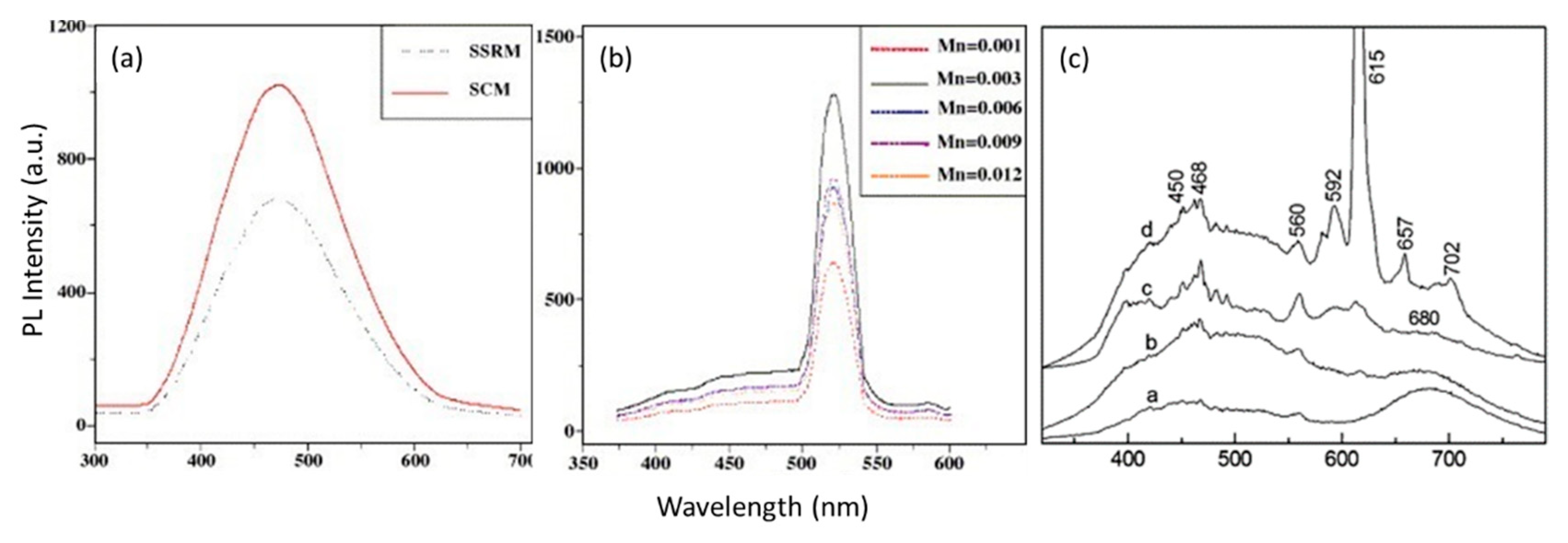
3.1. Solid-State Method
3.2. Flux Growth Method
3.3. Czochralski Method
3.4. Laser Heat Pedestal Growth Method
3.5. Hydrothermal Method
- It can synthesize oxide materials in crystalline phases that are not found to be stable at the melting point.
- Crystal growth occurs with lower thermal strain; hence it has lower dislocation density than the melt grown methods where the large value of temperature gradient is required.
- A large volume of high-quality crystal can be obtained by keeping control of composition.
4. Thin Films of ZnGa2O4
4.1. Physical Vapor Deposition (Sputter and PLD)
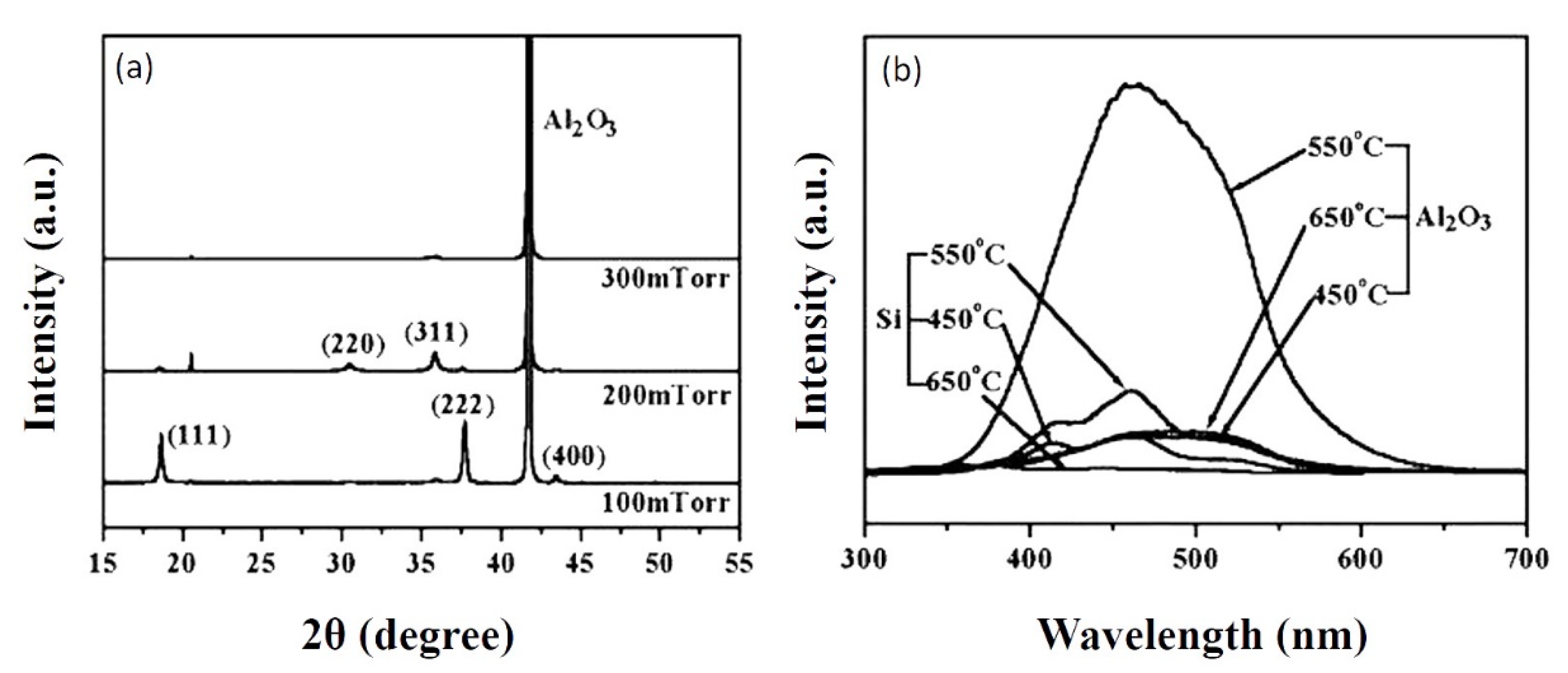
4.1.1. Effect of Substrate Materials
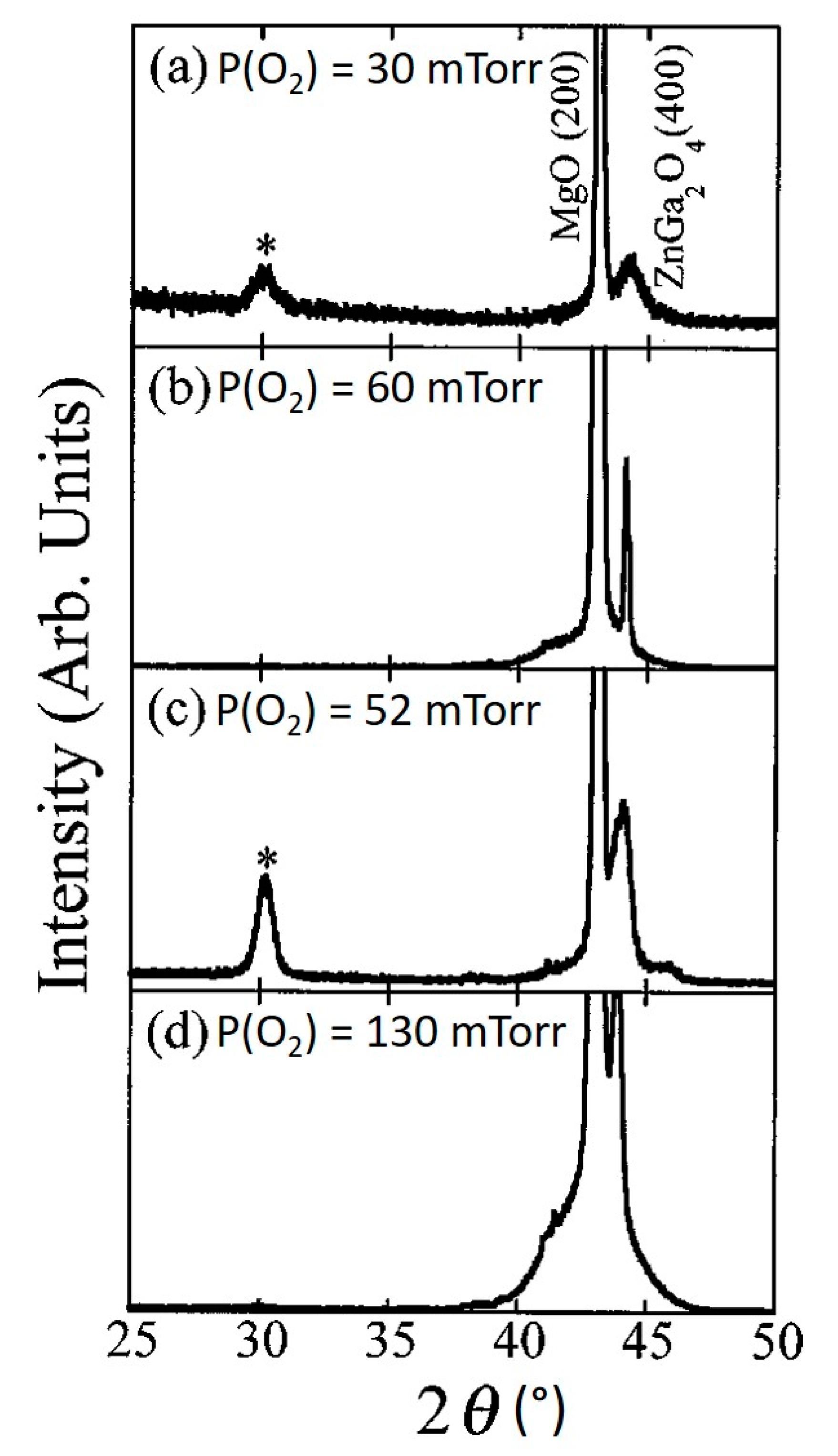
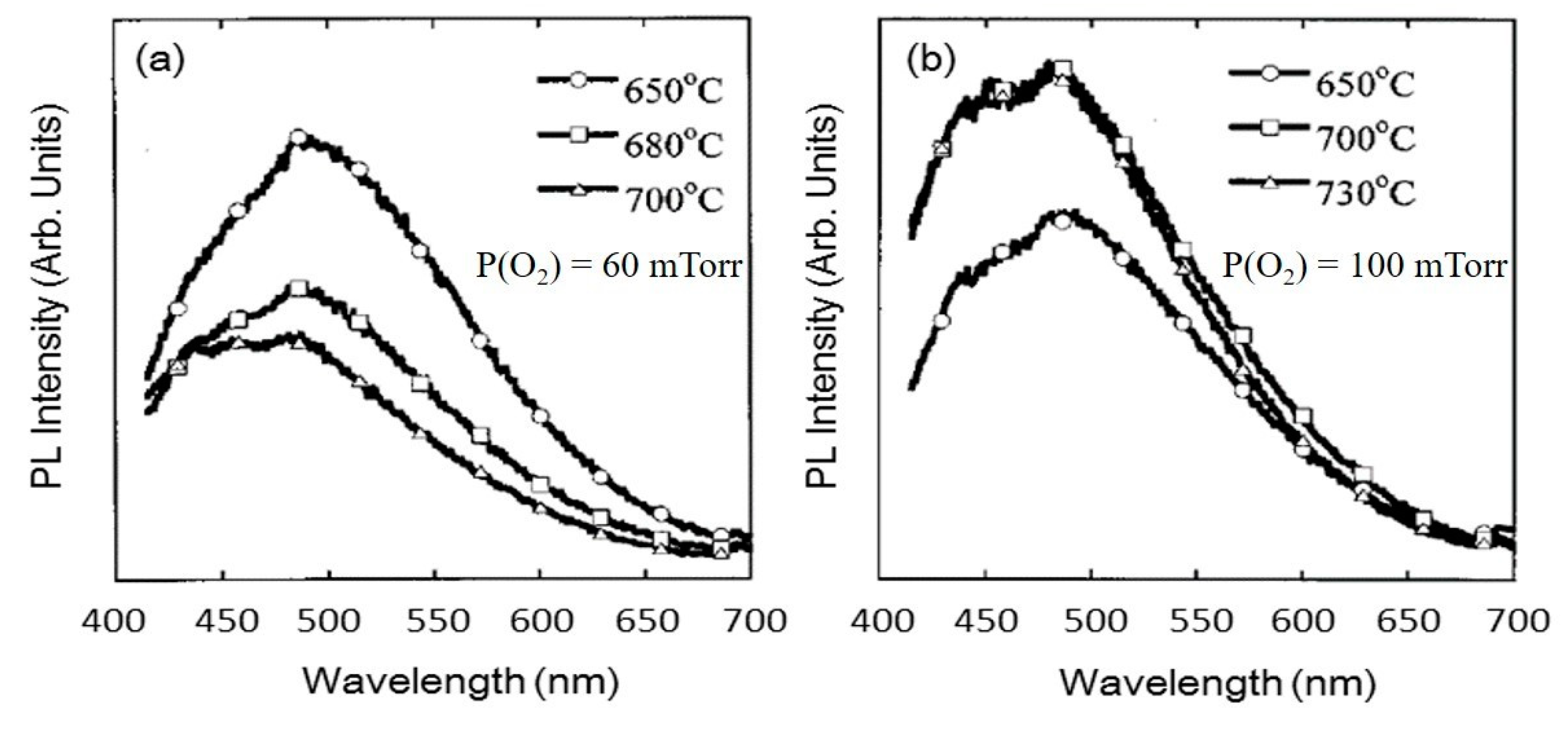
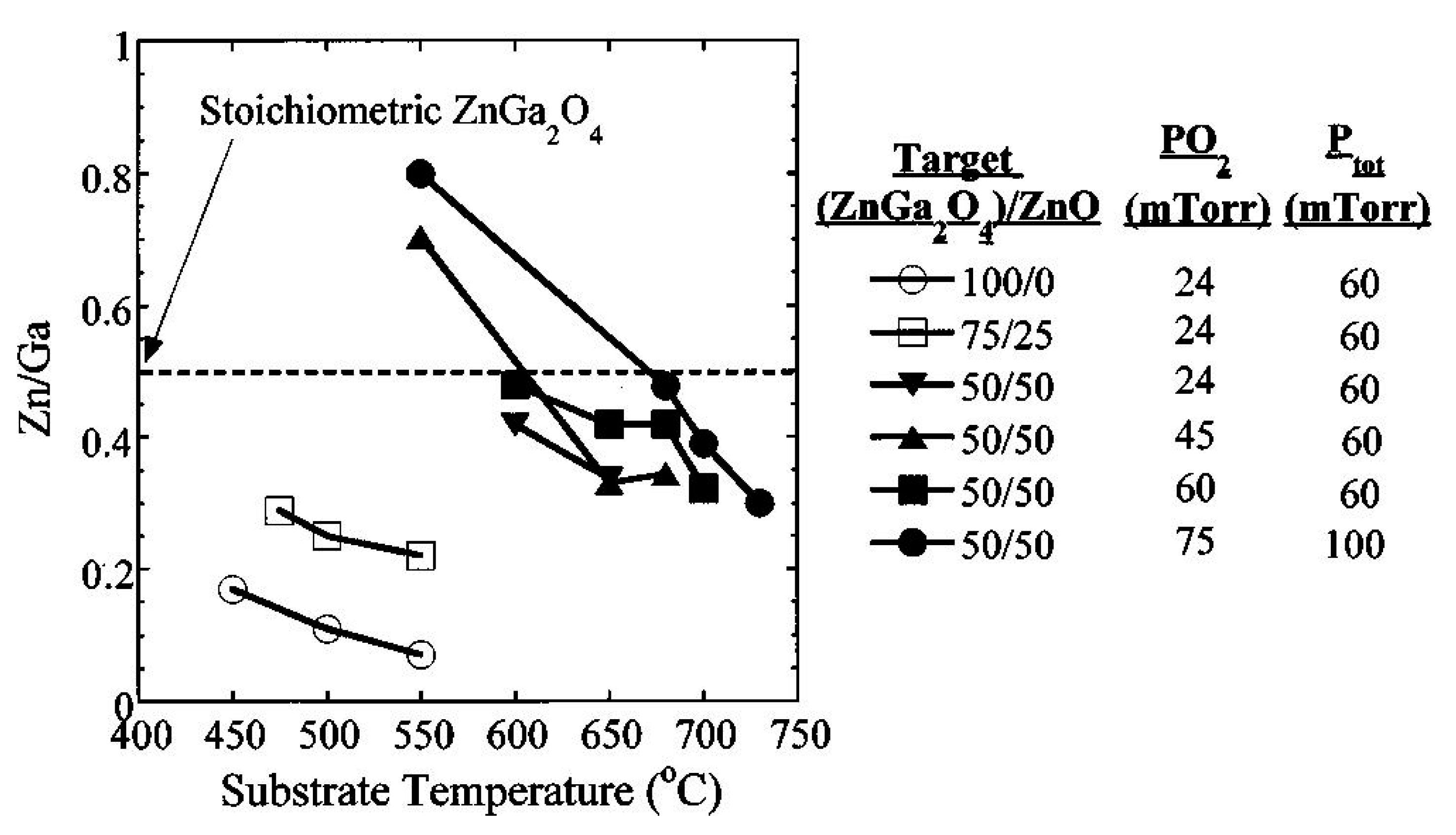
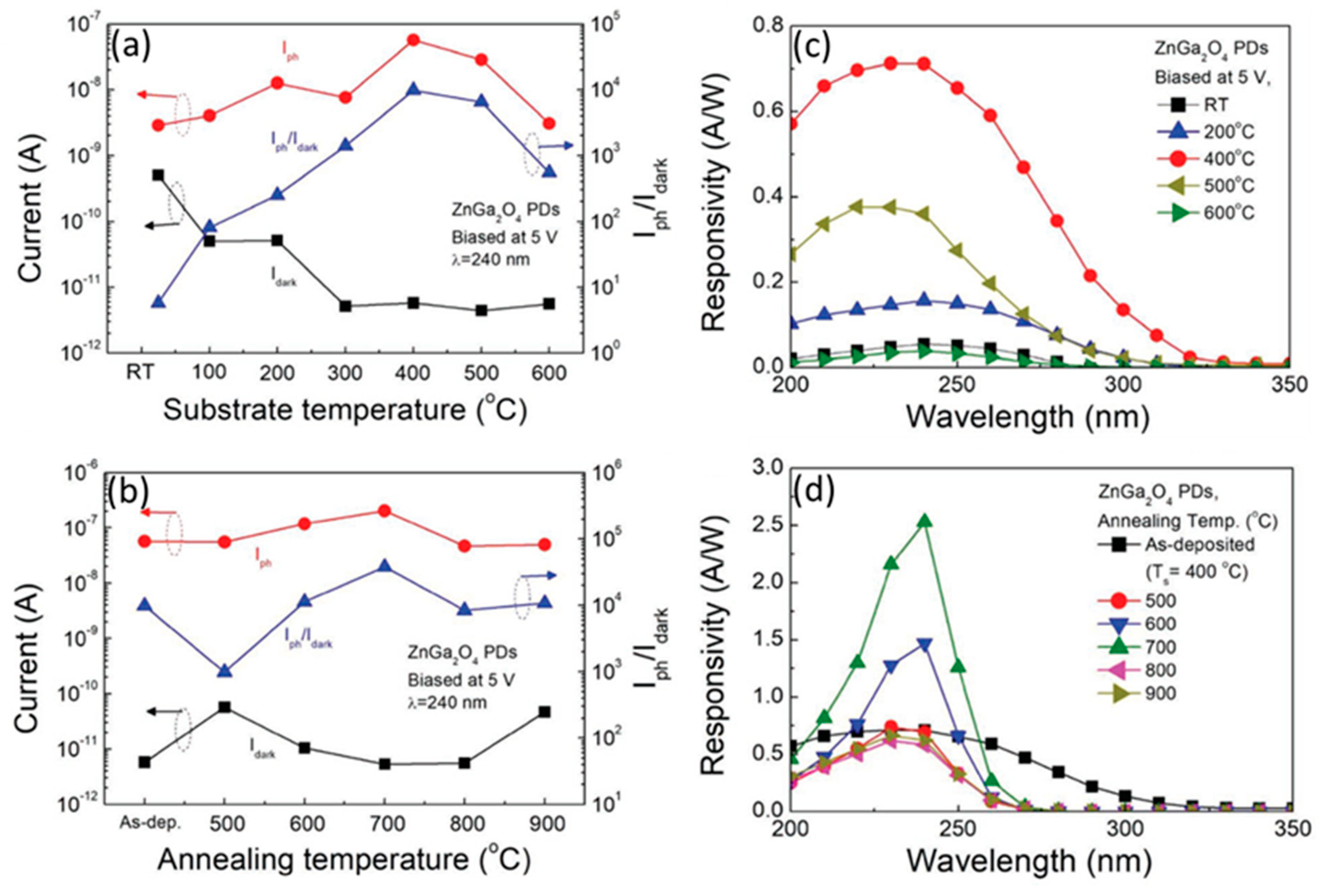
4.1.2. Effects of Substrate Temperature and Oxygen Pressure
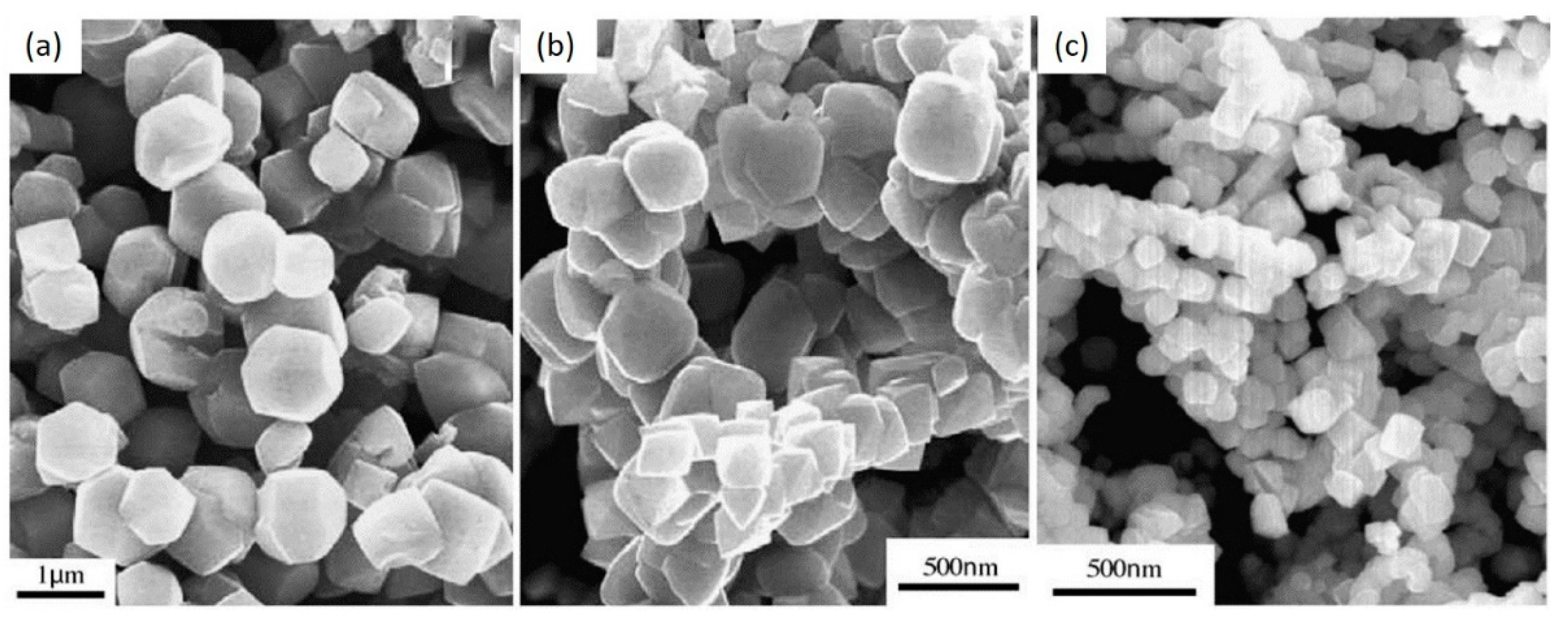
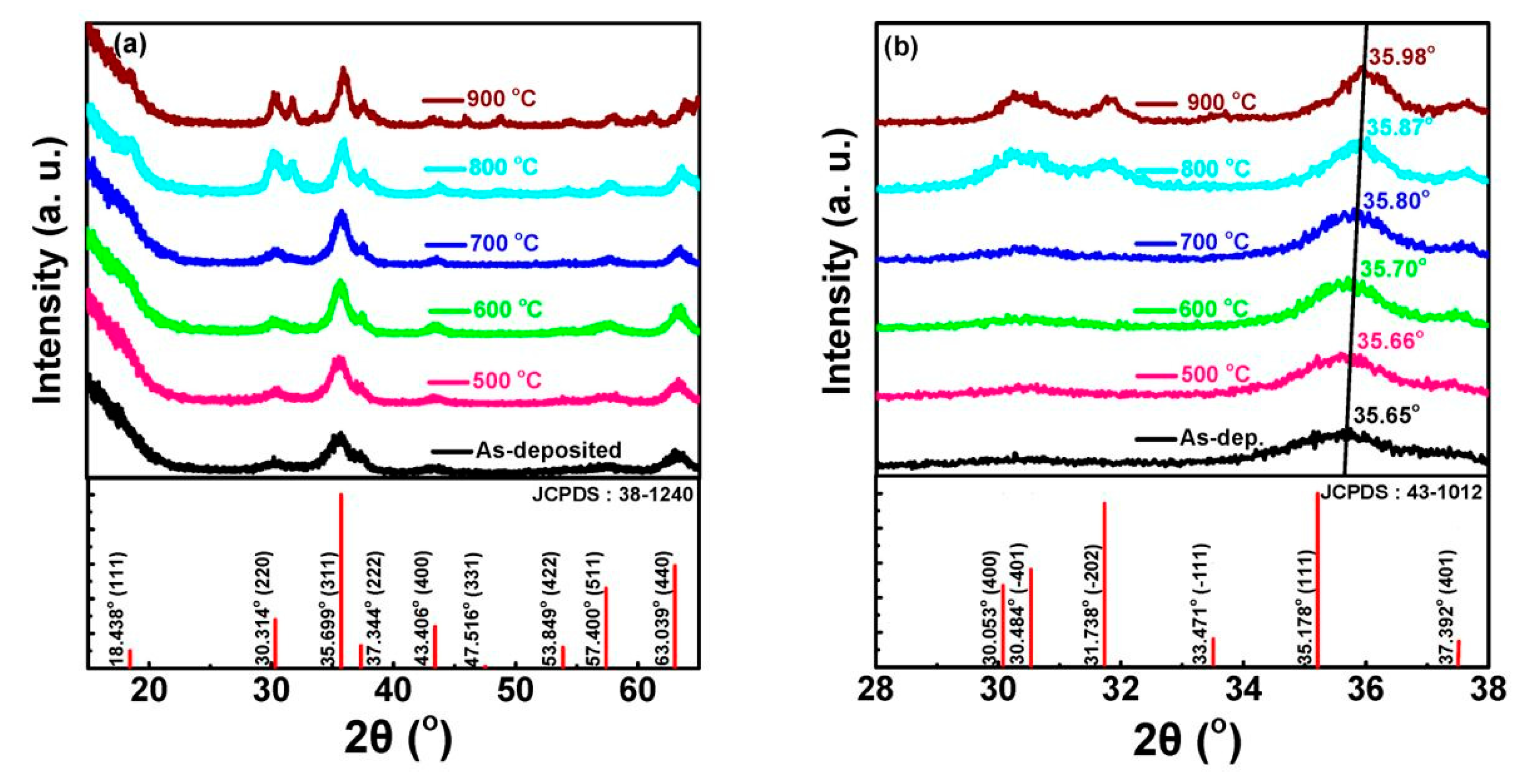
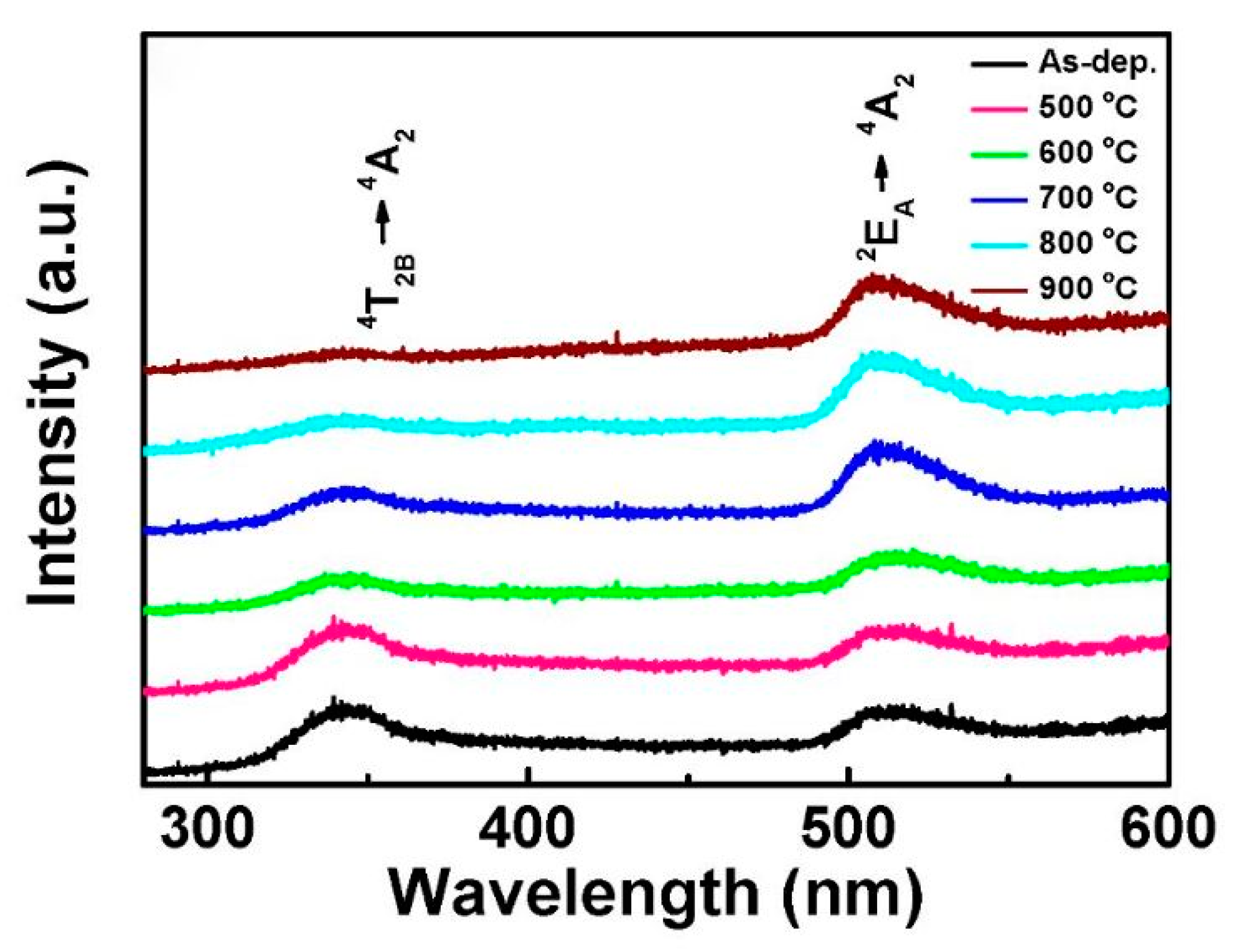
4.1.3. Effect of Annealing Temperature
4.2. Chemical Vapor Deposition (Mist CVD and MOCVD)
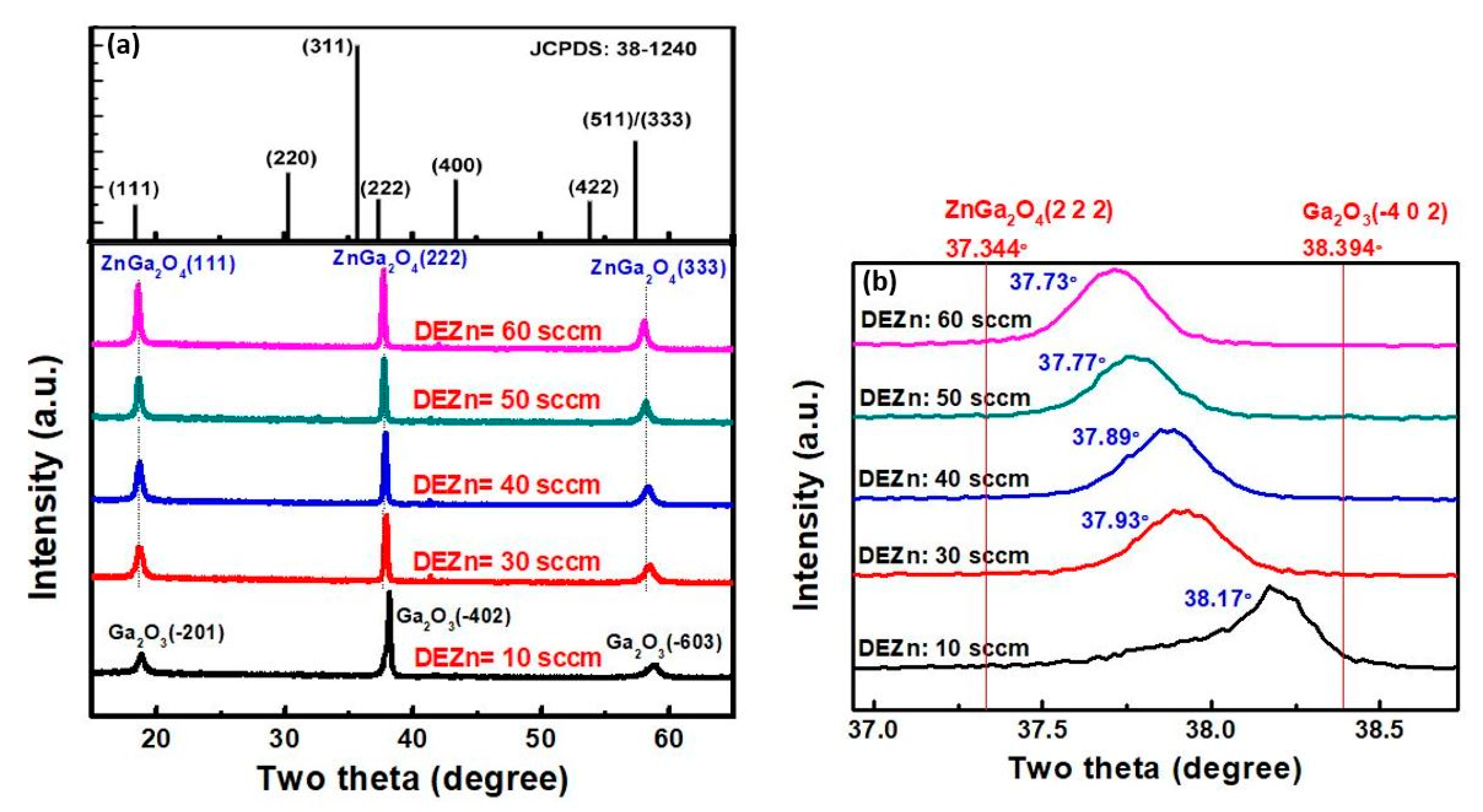
4.2.1. Effect of Zn/Ga Precursor Ratio
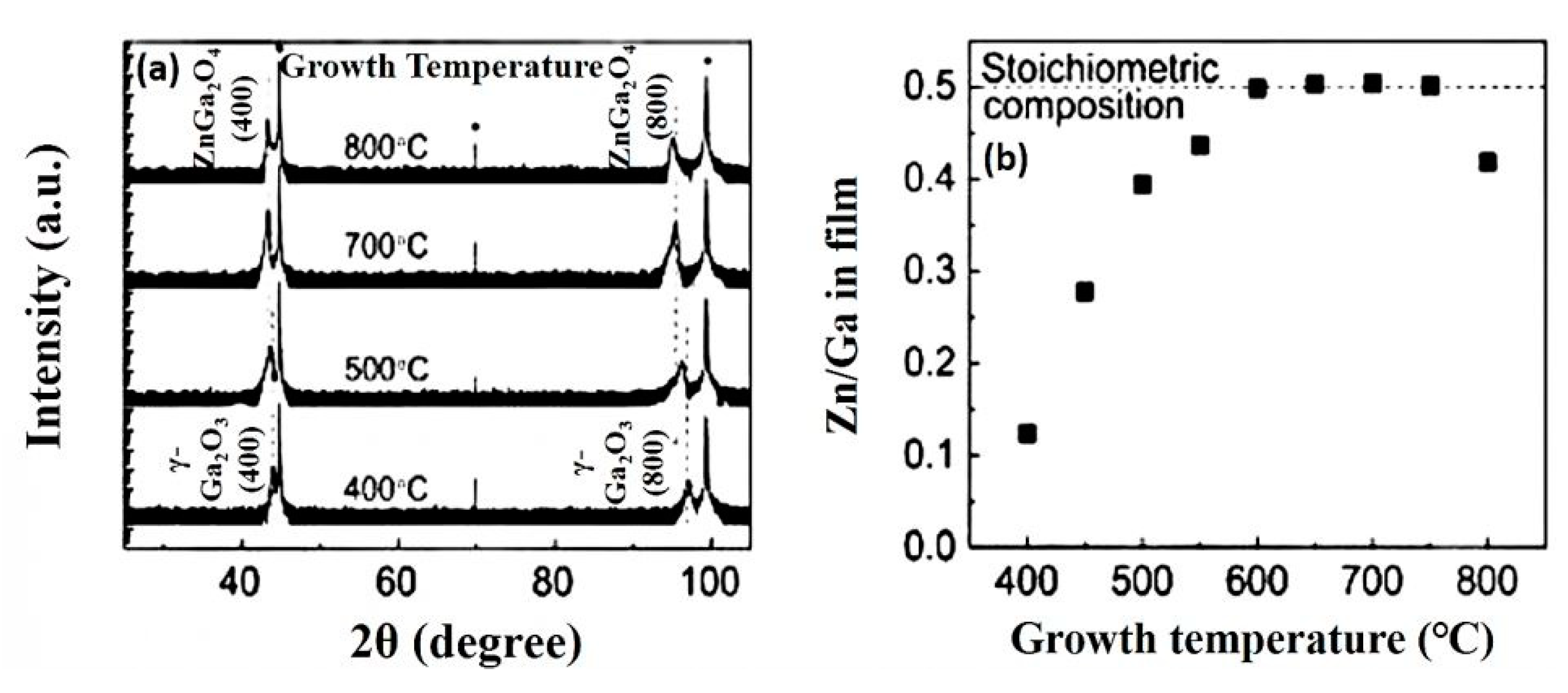
4.2.2. Effect of Substrate Temperature
5. Applications of ZnGa2O4
5.1. Deep-Ultraviolet Photodetectors
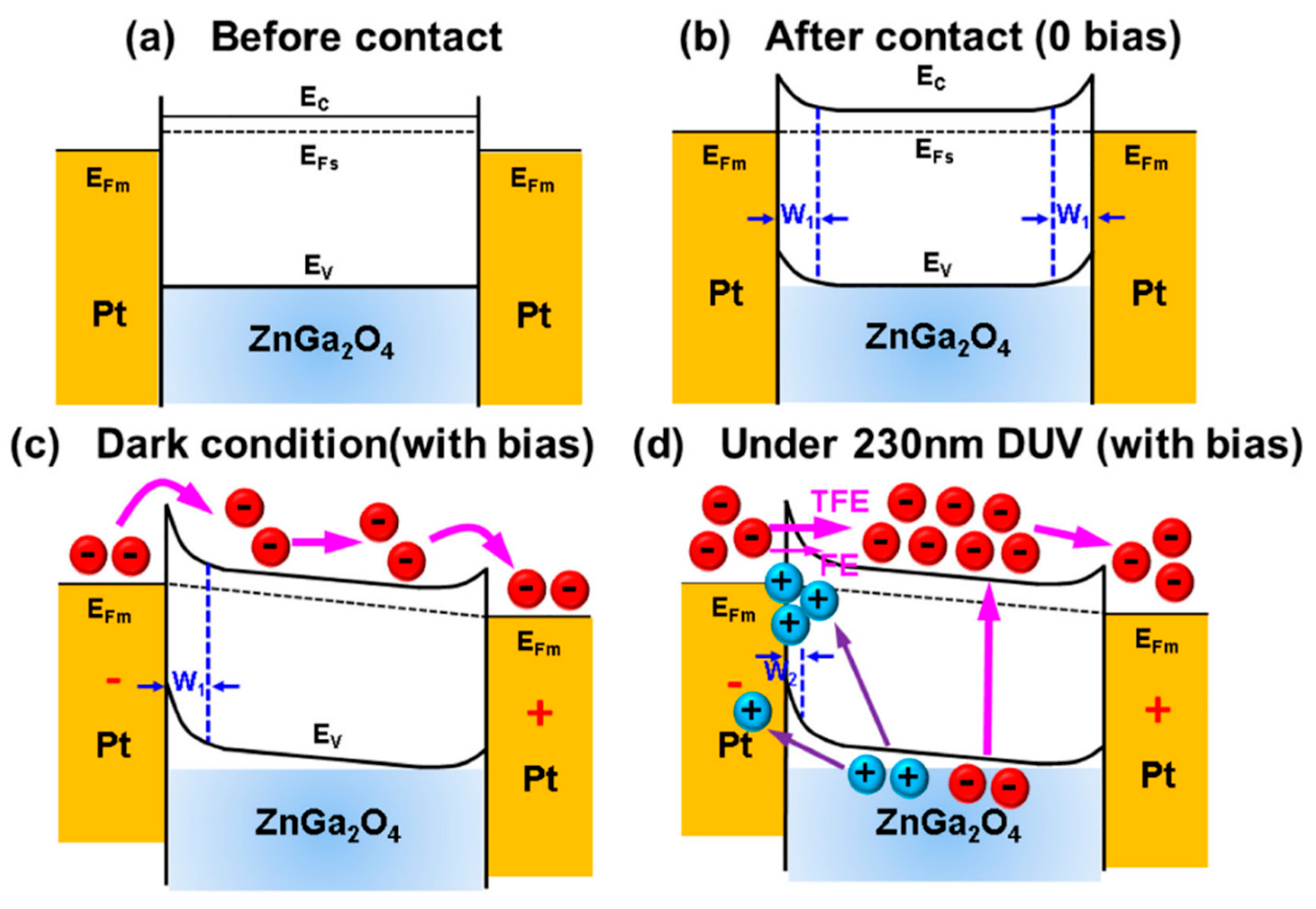
5.1.1. MSM DUV Photo-Detecting Mechanism
5.1.2. Dark Current and Photocurrent
5.1.3. Spectral Response
5.1.4. Response Time of Photodetectors
5.2. Gas Sensors
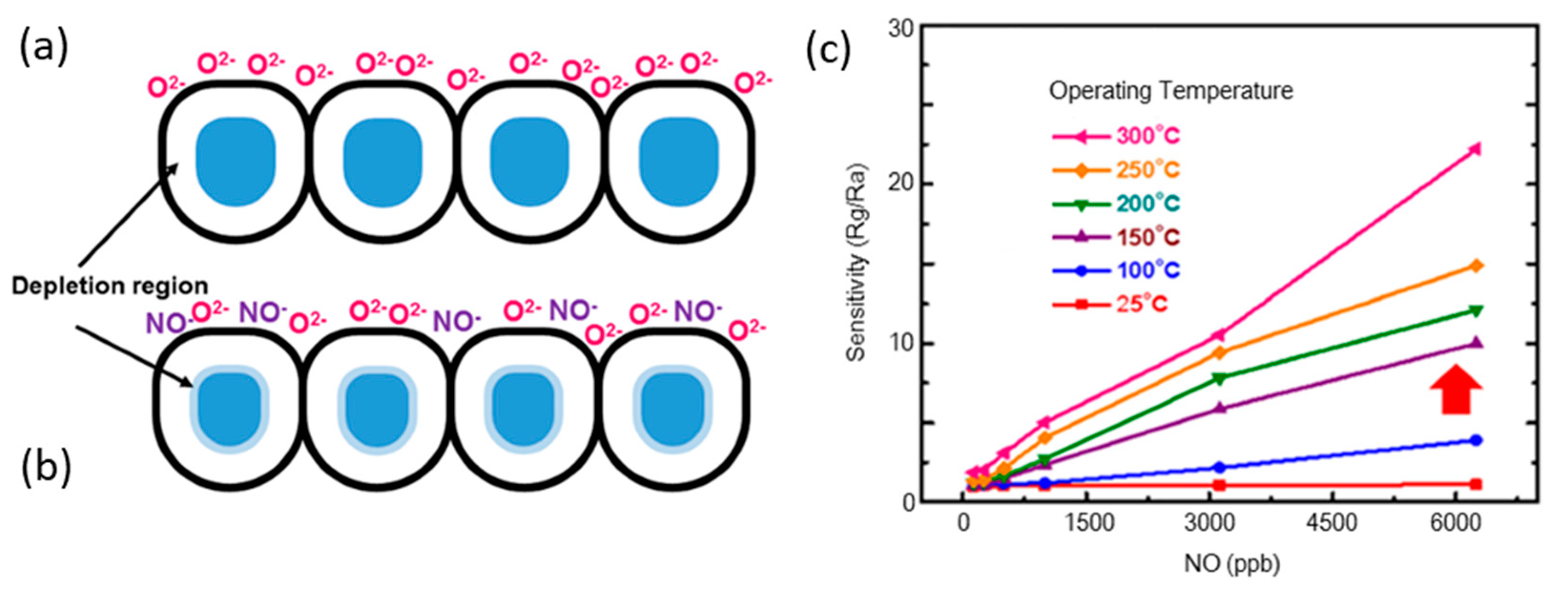
5.2.1. Gas Sensing Mechanism
5.2.2. Effects on Gas Sensitivity
- Particles originally adsorbed on the surface desorb due to high temperature, which creates more states on the ZnGa2O4 surface to react with the target gas.
- Increasing temperature results in changing the type of adsorbed oxygen molecules from O2− to O− (O− is more reactive than O2−) and helps the target gas to react very easily with O−.
- Kinetic energy of the target gas is provided by high temperature, which speeds up the abstraction of the target gas on the surface of the ZnGa2O4 gas sensor.
5.2.3. Gas Selectivity
5.3. Phosphors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dazai, T.; Yasui, S.; Taniyama, T.; Itoh, M. Cation-Deficiency-Induced Crystal-Site Engineering for ZnGa2O4: Mn2+ Thin Film. Lnorg. Chem. 2020, 59, 8744–8748. [Google Scholar] [CrossRef]
- Da Silva, M.N.; de Carvalho, J.M.; de Abreu Fantini, M.C.; Chiavacci, L.A.; Bourgaux, C. Nanosized ZnGa2O4: Cr3+ Spinels as Highly Luminescent Materials for Bioimaging. ACS Appl. Nano Mater. 2019, 2, 6918–6927. [Google Scholar] [CrossRef]
- Omata, T.; Ueda, N.; Ueda, K.; Kawazoe, H. New ultraviolet-transport electroconductive oxide, ZnGa2O4 spinel. Appl. Phys. Lett. 1994, 64, 1077–1078. [Google Scholar] [CrossRef]
- Dixit, H.; Tandon, N.; Cottenier, S.; Saniz, R.; Lamoen, D.; Partoens, B.; Van Speybroeck, V.; Waroquier, M. Electronic structure and band gap of zinc spinel oxides beyond LDA: ZnAl2O4, ZnGa2O4 and ZnIn2O4. New J. Phys. 2011, 13, 063002. [Google Scholar] [CrossRef]
- López, I.; Nogales, E.; Méndez, B.; Piqueras, J.; Castaldini, A.; Cavallini, A. Hierarchical ZnGa2O4 and Cr doped Zn1-xMnxGa2O4 nanostructures for room temperature light-emitting devices. Mater. Res. Express. 2014, 1, 025017. [Google Scholar] [CrossRef]
- Jang, Y.; Hong, S.; Seo, J.; Cho, H.; Char, K.; Galazka, Z. Thin film transistors based on ultra-wide bandgap spinel ZnGa2O4. Appl. Phys. Lett. 2020, 116, 202104. [Google Scholar] [CrossRef]
- Chikoidze, E.; Sartel, C.; Madaci, I.; Mohamed, H.; Vilar, C.; Ballesteros, B.; Belarre, F.; Del Corro, E.; Vales-Castro, P.; Sauthier, G.; et al. P-Type Ultrawide-Band-Gap Spinel ZnGa2O4: New Perspectives for Energy Electronics. Cryst. Growth Des. 2020, 20, 2535–2546. [Google Scholar] [CrossRef]
- van den Boom, H.; Henning, J.C.M.; Damen, J.P.M. Electron spin resonance on chromium doped ZnGa2O4. Solid State Commun. 1970, 8, 717–719. [Google Scholar] [CrossRef]
- Kahan, H.M.; Macfarlane, R.M. Optical and microwave spectra of Cr3+ in the spinel ZnGa2O4. J. Chem. Phys. 1971, 54, 5197–5205. [Google Scholar] [CrossRef]
- Henning, J.C.M.; Den Boef, J.H.; Van Gorkom, G.G.P. Electron-spin-resonance spectra of nearest-neighbor Cr3+ pairs in the spinel ZnGa2O4. Phys. Rev. B 1973, 7, 1825–1833. [Google Scholar] [CrossRef]
- Van Gorkom, G.G.P.; Henning, J.C.M.; Van Stapele, R.P. Optical spectra of Cr3+ Pairs in the spinel ZnGa2O4. Phys. Rev. B 1973, 8, 955–973. [Google Scholar] [CrossRef]
- Available online: https://apps.webofknowledge.com/CitationReport.do?product=UA&search_mode=CitationReport&SID=D3cLBJqf9ChwAOSuxfc&page=1&cr_pqid=3&viewType=summary (accessed on 22 October 2020).
- Hussen, M.K.; Dejene, F.B. Effect of Cr3+ doping on structural and optical property of ZnGa2O4 synthesized by sol gel method. Optik 2019, 181, 514–523. [Google Scholar] [CrossRef]
- Gil-Rostra, J.; Yubero Valencia, F.; González-Elipe, A.R. Thin film electroluminescent device based on magnetron sputtered Tb doped ZnGa2O4 layers. J. Lumin. 2020, 228, 117617. [Google Scholar] [CrossRef]
- Minami, T.; Maeno, T.; Kuroi, Y.; Takata, S. High-luminance green-emitting thin-film electroluminescent devices using ZnGa2O4: Mn phosphor. Jpn. J. Appl. Phys. 1995, 34, L684–L687. [Google Scholar] [CrossRef]
- Matsui, H.; Xu, C.N.; Akiyama, M.; Watanabe, T. Strong mechanoluminescence from UV-irradiated spinels of ZnGa2O4: Mn and MgGa2O4: Mn. Jpn. J. Appl. Phys. Part 1 Regul. Papers Short Notes 2000, 39, 6582–6586. [Google Scholar] [CrossRef]
- Minami, T.; Kuroi, Y.; Miyata, T.; Yamada, H.; Takata, S. ZnGa2O4 as host material for multicolor-emitting phosphor layer of electroluminescent devices. J. Lumin. 1997, 72, 997–998. [Google Scholar] [CrossRef]
- Akazawa, H.; Shinojima, H. Efficient optical activation of Eu3+ ions doped in ZnGa2O4 thin films: Correlation between crystalline phase and photoluminescence. J. Phys. Chem. Solids 2018, 117, 60–69. [Google Scholar] [CrossRef]
- Akazawa, H. Structure-specific photoluminescence of ZnGa2O4: Eu3+ thin films deposited on sapphire c-plane substrates. J. Vac. Sci. Technol. B 2019, 37, 011205. [Google Scholar] [CrossRef]
- Huang, W.-L.; Li, C.-H.; Chang, S.-P.; Chang, S.-J. The Effect of Oxygen Partial Pressure and Annealing Process on the Characteristics of ZnGa2O4 MSM UV Photodetector. ECS J. Solid State Sci. Technol. 2019, 8, Q3213–Q3216. [Google Scholar] [CrossRef]
- Tian, Q.; Ren, S.; Cai, Z.; Chen, C.; Zheng, Y.; Zhuang, J. Glucose-Mediated Synthesis of Hierarchical Porous ZnGa2O4 Microspheres for Effective Photocatalytic Removal of Aromatic and Arsenic Pollutants. Catalysts 2019, 9, 828. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Ivanova, T.; Hamelmann, F.; Strijkova, V.; Gesheva, K. Study of Sputtered ZnO: Ga2O3 Films for Energy Harvesting Applications. Coatings 2020, 10, 650. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, J.; Cai, C.; Cong, L.; Wang, T. Synthesis and photoluminescent properties of Eu3+-doped ZnGa2O4 nanophosphors. Mater. Sci. Eng. B 2008, 149, 82–86. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, K. Color modification of ZnGa2O4: Yb3+, Er3+, Tm3+ upconversion phosphors with the doping of Sn4+ and Ge4+ ions. Appl. Opt. 2020, 59, 7313–7320. [Google Scholar] [CrossRef]
- Abritta, T.; Blak, F.H. Luminescence study of ZnGa2O4: Co2+. J. Lumin. 1991, 48, 558–560. [Google Scholar] [CrossRef]
- Sosman, L.P.; Abritta, T.; Pereira, A.C.; Vargas, H. Photoacoustic spectroscopy of Co2+ in ZnGa2O4 and MgGa2O4. Chem. Phys. Lett. 1994, 227, 485–489. [Google Scholar] [CrossRef]
- Shea, L.E. Photoluminescence of Mn2+-Activated ZnGa2O4. J. Electrochem. Soc. 1994, 141, 1950. [Google Scholar] [CrossRef]
- Choi, H.W.; Hong, B.J.; Lee, S.K.; Kim, K.H.; Park, Y.S. Cathode luminescence characteristics of ZnGa2O4 phosphor thin films with the doped activator. J. Lumin. 2007, 126, 359–364. [Google Scholar] [CrossRef]
- Bessière, A.; Jacquart, S.; Priolkar, K.; Lecointre, A.; Viana, B.; Gourier, D. ZnGa2O4: Cr3+: A new red long-lasting phosphor with high brightness. Opt. Express 2011, 19, 10131. [Google Scholar] [CrossRef] [PubMed]
- Allix, M.; Chenu, S.; Véron, E.; Poumeyrol, T.; Kouadri-Boudjelthia, E.A.; Alahraché, S.; Porcher, F.; Massiot, D.; Fayon, F. Considerable improvement of long-persistent luminescence in germanium and tin substituted ZnGa2O4. Chem. Mater. 2013, 25, 1600–1606. [Google Scholar] [CrossRef]
- Uheda, K.; Maruyama, T.; Takizawa, H.; Endo, T. Synthesis and long-period phosphorescence of ZnGa2O4: Mn2+ spinel. J. Alloys Compd. 1997, 262, 60–64. [Google Scholar] [CrossRef]
- Jung, H.K.; Park, D.S.; Park, Y.C. Preparation and characterization of ZnGa2O4: Mn phosphors by multistage precipitation method. Mater. Res. Bull. 1999, 34, 43–51. [Google Scholar] [CrossRef]
- Hsieh, I.J.; Chu, K.T.; Yu, C.F.; Feng, M.S. Cathodoluminescent characteristics of ZnGa2O4 phosphor grown by radio frequency magnetron sputtering. J. Appl. Phys. 1994, 76, 3735–3739. [Google Scholar] [CrossRef]
- Itoh, S. The ZnGa2O4 Phosphor for Low-Voltage Blue Cathodoluminescence. J. Electrochem. Soc. 1991, 138, 1509. [Google Scholar] [CrossRef]
- Hsieh, I.J. Growth of ZnGa2O4 Phosphor by Radio Frequency Magnetron Sputtering. J. Electrochem. Soc. 1994, 141, 1617. [Google Scholar] [CrossRef]
- Shea, L.E. Low Voltage Cathodoluminescence of Mn2+-Activated ZnGa2O4. J. Electrochem. Soc. 1994, 141, 2198. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Galazka, Z.; Ganschow, S.; Schewski, R.; Irmscher, K.; Klimm, D.; Kwasniewski, A.; Pietsch, M.; Fiedler, A.; Schulze-Jonack, I.; Albrecht, M.; et al. Ultra-wide bandgap, conductive, high mobility, and high quality melt-grown bulk ZnGa2O4 single crystals. APL Mater. 2019, 7, 022512. [Google Scholar] [CrossRef]
- Xue, J.; Wu, S.; Li, J. Synthesis, microstructure, and microwave dielectric properties of spinel ZnGa2O4 ceramics. J. Am. Ceram. Soc. 2013, 96, 2481–2485. [Google Scholar] [CrossRef]
- Boy, J.; Handwerg, M.; Mitdank, R.; Galazka, Z.; Fischer, S.F. Charge carrier density, mobility and Seebeck coefficient of melt-grown bulk ZnGa2O4 single crystals. AIP Adv. 2020, 10, 55005. [Google Scholar] [CrossRef]
- Grimes, R.W.; Anderson, A.B.; Heuer, A.H. Predictions of Cation Distributions in AB2O4 Spinels from Normalized Ion Energies. J. Am. Chem. Soc. 1989, 111, 1–7. [Google Scholar] [CrossRef]
- Zerarga, F.; Bouhemadou, A.; Khenata, R.; Bin-Omran, S. Structural, electronic and optical properties of spinel oxides ZnAl2O4, ZnGa2O4 and ZnIn2O4. Solid State Sci. 2011, 13, 1638–1648. [Google Scholar] [CrossRef]
- Kushwaha, A.K. Vibrational, elastic properties and sound velocities of ZnGa2O4 spinel. Comput. Mater. Sci. 2014, 85, 259–263. [Google Scholar] [CrossRef]
- De Vos, A.; Lejaeghere, K.; Vanpoucke, D.E.P.; Joos, J.J.; Smet, P.F.; Hemelsoet, K. First-Principles Study of Antisite Defect Configurations in ZnGa2O4: Cr Persistent Phosphors. Lnorg. Chem. 2016, 55, 2402–2412. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, G.F.; Zhao, F.; Gong, Z.Z. First-principles study of the structural, mechanical and electronic properties of ZnX2O4 (X = Al, Cr and Ga). Chin. Phys. B 2011, 20, 047102. [Google Scholar] [CrossRef]
- López-Moreno, S.; Rodríguez-Hernández, P.; Muñoz, A.; Romero, A.H.; Manjón, F.J.; Errandonea, D.; Rusu, E.; Ursaki, V.V. Lattice dynamics of ZnAl2O4 and ZnGa2O4 under high pressure. Ann. Phys. (Leipzig) 2011, 523, 157–167. [Google Scholar] [CrossRef]
- Geselbracht, M.J.; Erickson, A.S.; Rogge, M.P.; Greedan, J.E.; Walton, R.I.; Stoltzfus, M.W.; Eng, H.W.; Woodward, P.M. Structure property relationships in the ATi2O4 (A = Na, Ca) family of reduced titanates. J. Solid State Chem. 2006, 179, 3489–3499. [Google Scholar] [CrossRef]
- Van Gorkom, G.G.P.; Haanstra, J.H.; vd Boom, H. Infrared and Raman spectra of the spinel ZnGa2O4. J. Raman Spectrosc. 1973, 1, 513–519. [Google Scholar] [CrossRef]
- Lopez-Moreno, S.; Romero, A.H.; Rodriguez-Hernandez, P.; Munoz, A. Ab initio study of the high-pressure phases and dynamical properties of ZnAl2O4 and ZnGa2O4. High Press. Res. 2009, 29, 573–577. [Google Scholar] [CrossRef]
- Bouhemadou, A.; Khenata, R. Pseudo-potential calculations of structural and elastic properties of spinel oxides ZnX2O4 (X = Al, Ga, In) under pressure effect. Phys. Lett. A 2006, 360, 339–343. [Google Scholar] [CrossRef]
- Brik, M.G. First-principles calculations of electronic, optical and elastic properties of ZnAl2S4 and ZnGa2O4. J Phys. Chem. Solids 2010, 71, 1435–1442. [Google Scholar] [CrossRef]
- Sampath, S.K.; Cordaro, J.F. Optical Properties of Zinc Aluminate, Zinc Gallate, and Zinc Aluminogallate Spinels. J. Am. Ceram. Soc. 2005, 81, 649–654. [Google Scholar] [CrossRef]
- Sampath, S.K.; Kanhere, D.G.; Pandey, R. Electronic structure of spinel oxides: Zinc aluminate and zinc gallate. J. Phys. Condens. Matter 1999, 11, 3635–3644. [Google Scholar] [CrossRef]
- Can, M.M.; Hassnain Jaffari, G.; Aksoy, S.; Shah, S.I.; Firat, T. Synthesis and characterization of ZnGa2O4 particles prepared by solid state reaction. J. Alloys Compd. 2013, 549, 303–307. [Google Scholar] [CrossRef]
- Zou, L.; Xiang, X.; Wei, M.; Li, F.; Evans, D.G. Single-crystalline ZnGa2O4 spinel phosphor via a single-source inorganic precursor route. Lnorg. Chem. 2008, 47, 1361–1369. [Google Scholar] [CrossRef]
- Chase, A.B.; Osmer, J.A. Localized Cooling in Flux Crystal Growth. J. Am. Ceram. Soc. 1967, 50, 325–328. [Google Scholar] [CrossRef]
- Van der Straten, P.J.M.; Metselaar, R.; Jonker, H.D. Flux growth of ZnGa2O4 single crystals. J. Cryst. Growth 1978, 43, 270–272. [Google Scholar] [CrossRef][Green Version]
- Yan, Z.; Takei, H. Flux growth of single crystals of spinel ZnGa2O4 and CdGa2O4. J. Cryst. Growth 1997, 171, 131–135. [Google Scholar] [CrossRef]
- Yan, Z.; Takei, H.; Kawazoe, H. Electrical Conductivity in Transparent ZnGa2O4: Reduction and Surface-Layer Structure Transformation. J. Am. Ceram. Soc. 2005, 81, 180–186. [Google Scholar] [CrossRef]
- Feigelson, R.S. Pulling optical fibers. J. Cryst. Growth 1986, 79, 669–680. [Google Scholar] [CrossRef]
- Gazit, D.; Feigelson, R.S. Laser-heated pedestal growth of high Tc Bi-Sr-Ca-Cu-O superconducting fibers. J. Cryst. Growth 1988, 91, 318–330. [Google Scholar] [CrossRef]
- Reyes Ardila, D.R.; Andreeta, M.R.B.; Cuffini, S.L.; Hernandes, A.C.; Andreeta, J.P.; Mascarenhas, Y.P. Laser heated pedestal growth of Sr2RuO4 single-crystal fibers from SrRuO3. J. Cryst. Growth 1997, 177, 52–56. [Google Scholar] [CrossRef]
- Wilde, J.P.; Jundt, D.H.; Galambos, L.; Hesselink, L. Growth of Sr0.61Ba0.39Nb2O6 fibers: New results regarding orientation. J. Cryst. Growth 1991, 114, 500–506. [Google Scholar] [CrossRef]
- Jia, W. Photoluminescence of Mn2+-Doped ZnGa2O4 Single-Crystal Fibers. J. Electrochem. Soc. 1995, 142, 1637. [Google Scholar] [CrossRef]
- Hirano, M. Hydrothermal synthesis and characterization of ZnGa2O4 spinel fine particles. J. Mater. Chem. 2000, 10, 469–472. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Lu, Z.; Huang, K. Hydrothermal synthesis and characterization of ZnGa2O4 phosphors. Mater. Chem. Phys. 2006, 97, 247–251. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; Cao, L.; Wu, J.; Fei, J.; Ouyang, H.; Yao, C. Influence of temperature on the morphology and photocatalytic activity of ZnGa2O4 crystallites prepared by hydrothermal method. Ceram. Int. 2013, 39, 3165–3171. [Google Scholar] [CrossRef]
- Safeera, T.A.; Johns, N.; Krishna, K.M.; Sreenivasan, P.V.; Reshmi, R.; Anila, E.I. Zinc gallate and its starting materials in solid state reaction route- A comparative study. Mater. Chem. Phys. 2016, 181, 21–25. [Google Scholar] [CrossRef]
- Hirano, M.; Imai, M.; Inagaki, M. Preparation of ZnGa2O4 Spinel Fine Particles by the Hydrothermal Method. J. Am. Ceram. Soc. 2004, 83, 977–979. [Google Scholar] [CrossRef]
- Bae, J.S.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Yi, S.S.; Kim, I.W.; Lee, J.S. Photoluminescence behaviors in ZnGa2O4 thin film phosphors deposited by a pulsed laser ablation. Thin Solid Films 2003, 424, 291–295. [Google Scholar] [CrossRef]
- Yi, S.S.; Kim, I.W.; Bae, J.S.; Moon, B.K.; Kim, S.B.; Jeong, J.H. Luminescence characteristics of ZnGa2O4 thin film phosphors grown by pulsed laser deposition. Mater. Lett. 2002, 57, 904–909. [Google Scholar] [CrossRef]
- Lee, Y.E.; Norton, D.P.; Budai, J.D. Enhanced photoluminescence in epitaxial ZnGa2O4: Mn thin-film phosphors using pulsed-laser deposition. Appl. Phys. Lett. 1999, 74, 3155–3157. [Google Scholar] [CrossRef]
- Wang, W.K.; Liu, K.F.; Tsai, P.C.; Xu, Y.J.; Huang, S.Y. Influence of annealing temperature on the properties of ZnGa2O4 thin films by magnetron sputtering. Coatings 2019, 9, 859. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, C.; Zhang, D.; Li, S.; Zhang, L.; Wang, W.; Zhang, J. Luminescence of Cr3+-doped ZnGa2O4 thin films deposited by pulsed laser ablation. Thin Solid Films 2012, 520, 6845–6849. [Google Scholar] [CrossRef]
- Horng, R.H.; Huang, C.Y.; Ou, S.L.; Juang, T.K.; Liu, P.L. Epitaxial Growth of ZnGa2O4: A New, Deep Ultraviolet Semiconductor Candidate. Cryst. Growth Des. 2017, 17, 6071–6078. [Google Scholar] [CrossRef]
- Reshmi, R.; Krishna, K.M.; Manoj, R.; Jayaraj, M.K. Pulsed laser deposition of ZnGa2O4 phosphor films. Surf. Coat. Technol. 2005, 198, 345–349. [Google Scholar] [CrossRef]
- Wang, W.K.; Xu, Y.J.; Huang, S.Y.; Liu, K.F.; Tsai, P.C. Structural characteristics and photoluminescence properties of sputter-deposition ZnGa2O4 thin films on sapphire and Si(100) substrates. Coatings 2019, 9, 469. [Google Scholar] [CrossRef]
- Lee, Y.E.; Norton, D.P.; Park, C.; Rouleau, C.M. Blue photoluminescence in ZnGa2O4 thin-film phosphors. J. Appl. Phys. 2001, 89, 1653–1656. [Google Scholar] [CrossRef]
- Yi, S.S.; Kim, I.W.; Park, H.L.; Bae, J.S.; Moon, B.K.; Jeong, J.H. Luminescence characteristics of pulsed laser deposited ZnGa2O4 thin film phosphors grown on various substrates. J. Cryst. Growth 2003, 247, 213–218. [Google Scholar] [CrossRef]
- Luo, S.; Harrington, G.F.; Wu, K.-T.; Lippert, T. Heteroepitaxial (111) ZnGa2O4 Thin Films Grown on (00.1) Sapphire by Pulsed Laser Deposition. Phys. Status Solidi Rapid Res. Lett. 2020, 14, 2000270. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, H.L.; Chon, C.M.; Moon, H.S.; Kim, T.W. The origin of emission color of reduced and oxidized ZnGa2O4 phosphors. Solid State Commun. 2004, 129, 163–167. [Google Scholar] [CrossRef]
- Oshima, T.; Niwa, M.; Mukai, A.; Nagami, T.; Suyama, T.; Ohtomo, A. Epitaxial growth of wide-band-gap ZnGa2O4 films by mist chemical vapor deposition. J. Cryst. Growth 2014, 386, 190–193. [Google Scholar] [CrossRef]
- Lin, X.; Chen, D.; Niu, W.; Huang, C.Y.; Horng, R.H.; Cheng, L.C.; Talwar, D.N.; Lin, H.H.; Lee, J.F.; Feng, Z.C.; et al. Evolution of the local structure and crystal phase for thin ZnGaO films grown by metal organic chemical vapor deposition. J. Cryst. Growth 2019, 520, 89–95. [Google Scholar] [CrossRef]
- Tsai, S.H.; Shen, Y.C.; Huang, C.Y.; Horng, R.H. Deep-ultraviolet Schottky photodetectors with high deep-ultraviolet/visible rejection based on a ZnGa2O4 thin film. Appl. Surf. Sci. 2019, 496, 143670. [Google Scholar] [CrossRef]
- Han, D.; Liu, K.; Hou, Q.; Chen, X.; Yang, J.; Li, B.; Zhang, Z.; Liu, L.; Shen, D. Self-powered solar-blind ZnGa2O4 UV photodetector with ultra-fast response speed. Sens. Actuators A Phys. 2020, 29, 112354. [Google Scholar] [CrossRef]
- Lee, Y.E.; Norton, D.P.; Budai, J.D.; Wei, Y. Enhanced ultraviolet photoconductivity in semiconducting ZnGa2O4 thin films. J. Appl. Phys. 2001, 90, 3863–3866. [Google Scholar] [CrossRef]
- Hetterich, J.; Bastian, G.; Gippius, N.A.; Tikhodeev, S.G.; von Plessen, G.; Lemmer, U. Optimized design of plasmonic MSM photodetector. IEEE J. Quantum Electron. 2007, 43, 855–859. [Google Scholar] [CrossRef]
- Kim, J.H.; Griem, H.T.; Friedman, R.A.; Chan, E.Y.; Ray, S. High-Performance Back-Illuminated InGaAs/InAlAs MSM Photodetector with a Record Responsivity of 0.96 A/W. IEEE Photon. Technol. Lett. 1992, 4, 1241–1244. [Google Scholar] [CrossRef]
- Chen, P.W.; Huang, S.Y.; Yuan, S.H.; Chen, Y.A.; Hsiao, P.W.; Wuu, D.S. Quasi-Single-Crystalline ZnGa2O4 Films via Solid Phase Epitaxy for Enhancing Deep-Ultraviolet Photoresponse. Adv. Mater. Interfaces 2019, 6, 1901075. [Google Scholar] [CrossRef]
- Tsai, S.H.; Basu, S.; Huang, C.Y.; Hsu, L.C.; Lin, Y.G.; Horng, R.H. Deep-Ultraviolet Photodetectors Based on Epitaxial ZnGa2O4 Thin Films. Sci. Rep. 2018, 8, 14056. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Giubileo, F.; Grillo, A.; Luongo, G.; Iemmo, L.; Urban, F.; Lozzi, L.; Capista, D.; Nardone, M.; Passacantando, M. Bias Tunable Photocurrent in Metal-Insulator-Semiconductor Heterostructures with Photoresponse Enhanced by Carbon Nanotubes. Nanomaterials 2019, 9, 1598. [Google Scholar] [CrossRef]
- Di Bartolomeo, A. Graphene Schottky diodes: An experimental review of the rectifying graphene/semiconductor heterojunction. Phys. Rep. 2016, 606, 1–58. [Google Scholar] [CrossRef]
- Wu, M.R.; Li, W.Z.; Tung, C.Y.; Huang, C.Y.; Chiang, Y.H.; Liu, P.L.; Horng, R.H. NO gas sensor based on ZnGa2O4 epilayer grown by metalorganic chemical vapor deposition. Sci. Rep. 2019, 9, 7459. [Google Scholar] [CrossRef]
- An, S.; Park, S.; Ko, H.; Jin, C.; Lee, C. NO2 gas sensing properties of multiple networked ZnGa2O4 nanorods coated with TiO2. J. Nanosci. Nanotechnol. 2015, 15, 433–438. [Google Scholar] [CrossRef]
- Satyanarayana, L.; Gopal Reddy, C.V.; Manorama, S.V.; Rao, V.J. Liquid-petroleum-gas sensor based on a spinel semiconductor, ZnGa2O4. Sens. Actuators B Chem. 1998, 46, 1–7. [Google Scholar] [CrossRef]
- Jiao, Z.; Ye, G.; Chen, F.; Li, M.; Liu, J. The preparation of ZnGa2O4 nano crystals by spray coprecipitation and its gas sensitive characteristics. Sensors 2002, 2, 71–78. [Google Scholar] [CrossRef]
- Chen, I.C.; Lin, S.S.; Lin, T.J.; Hsu, C.L.; Hsueh, T.J.; Shieh, T.Y. The assessment for sensitivity of a NO2 gas sensor with ZnGa2O4/ZnO core-shell nanowires-a novel approach. Sensors 2010, 10, 3057–3072. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, G.; Liu, Y. Synthesis of ZnGa2O4 assisted by high-energy ball milling and its gas-sensing characteristics. Powder Technol. 2015, 281, 7–11. [Google Scholar] [CrossRef]
- Itoh, S. Degradation Mechanism for Low Voltage Cathodoluminescence of Sulfide Phosphors. J. Electrochem. Soc. 1989, 136, 1819. [Google Scholar] [CrossRef]
- Dazai, T.; Yasui, S.; Taniyama, T.; Itoh, M. Epitaxial strain engineering of luminescent properties in ZnGa2O4: Mn thin films. Appl. Phys. Express 2020, 13, 082004. [Google Scholar] [CrossRef]
- Dutta, D.P.; Ghildiyal, R.; Tyagi, A.K. Luminescent properties of doped zinc aluminate and zinc gallate white light emitting nanophosphors prepared via sonochemical method. J. Phys. Chem. C 2009, 113, 16954–16961. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, F.; Li, X.; Howe, J.; Xu, J.; Zhao, Y.; Pan, Z. Red, green, and blue luminescence from ZnGa2O4 nanowire arrays. J. Phys. Chem. Lett. 2010, 1, 354–357. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xu, K.; Li, L.; Hu, Z. Persistently luminescent and photocatalytic properties of ZnGa2O4 phosphors. Mater. Res. Express 2015, 2, 046202. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.J.; Yoo, K.; Kim, B.W.; Lee, J.C.; Park, S. Characteristics of nano-sized ZnGa2O4 phosphor prepared by solution combustion method and solid state reaction method. J. Eur. Ceram. Soc. 2007, 27, 965–968. [Google Scholar] [CrossRef]
- Duan, X.; Yu, F.; Wu, Y. Synthesis and luminescence properties of ZnGa2O4 spinel doped with Co2+ and Eu3+ ions. Appl. Surf. Sci. 2012, 261, 830–834. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Wu, M.-R.; Huang, C.-Y.; Juang, T.-K.; Liu, P.-L.; Horng, R.-H. Effect of Defects on the Properties of ZnGa2O4 Thin-Film Transistors. ACS Appl. Electron. Mater. 2019, 1, 253–259. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, D.; Zhou, Y.; Su, H.; Wang, R.; Zhang, C.; Yan, S.; Xiao, M.; Zou, Z. Single-crystalline, ultrathin ZnGa2O4 nanosheet scaffolds to promote photocatalytic activity in CO2 reduction into methane. ACS Appl. Mater. Interfaces 2014, 6, 2356–2361. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced room-temperature gas sensing of ZnGa2O4 nanowires functionalized with Au catalyst nanoparticles. Appl. Phys. A 2014, 114, 903–910. [Google Scholar] [CrossRef]
- Rack, P.D.; Peterson, J.J.; Potter, M.D.; Park, W. Eu+3 and Cr+3 doping for red cathodoluminescence in ZnGa2O4. J. Mater. Res. 2001, 16, 1429–1433. [Google Scholar] [CrossRef]
- Shen, Y.S.; Wang, W.K.; Horng, R.H. Characterizations of Metal-Oxide-Semiconductor Field-Effect Transistors of ZnGaO Grown on Sapphire Substrate. IEEE J. Electron Devices Soc. 2017, 5, 112–116. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Yang, W.; Zhu, M.; Shi, J. The improvement of coralline-like ZnGa2O4 by cocatalysts for the photocatalytic degradation of rhodamine B. Catalysts 2020, 10, 221. [Google Scholar] [CrossRef]
- Yang, S.H. Electrophoretic Prepared ZnGa2O4 Phosphor Film for FED. J. Electrochem. Soc. 2003, 150, H250. [Google Scholar] [CrossRef]
- Garcia, C.R.; Oliva, J.; Diaz-Torres, L.A.; Montes, E.; Hirata, G.; Bernal-Alvarado, J.; Gomez-Solis, C. Controlling the white phosphorescence ZnGa2O4 phosphors by surface defects. Ceram. Int. 2019, 45, 4972–4979. [Google Scholar] [CrossRef]
- Huo, Q.; Tu, W.; Guo, L. Enhanced photoluminescence property and broad color emission of ZnGa2O4 phosphor due to the synergistic role of Eu3+ and carbon dots. Opt. Mater. 2017, 72, 305–312. [Google Scholar] [CrossRef]
- Tran, T.K.; Park, W.; Tomm, J.W.; Wagner, B.K.; Jacobsen, S.M.; Summers, C.J.; Yocom, P.N.; McClelland, S.K. Photoluminescence properties of ZnGa2O4: Mn powder phosphors. J. Appl. Phys. 1995, 78, 5691–5695. [Google Scholar] [CrossRef]
- Yu, C.F.; Lin, P. Manganese-activated luminescence in ZnGa2O4. J. Appl. Phys. 1996, 79, 7191–7197. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Chen, Z.; Wang, T.; Zheng, S. Spectrum designation and effect of Al substitution on the luminescence of Cr3+ doped ZnGa2O4 nano-sized phosphors. J. Lumin. 2010, 130, 1738–1743. [Google Scholar] [CrossRef]
- Han, N.; Chen, D.; Pang, Y.; Han, Z.; Xia, Y.; Jiao, X. Structural regulation of ZnGa2O4 nanocubes for achieving high capacity and stable rate capability as an anode material of lithium ion batteries. Electrochim. Acta 2017, 235, 295–303. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, J.Y.; Choi, H.W. Characteristics of ZnGa2O4 coated solar cells and their functions as UV-C sensors. Jpn. J. Appl. Phys. 2020, 59, SGGE07. [Google Scholar] [CrossRef]
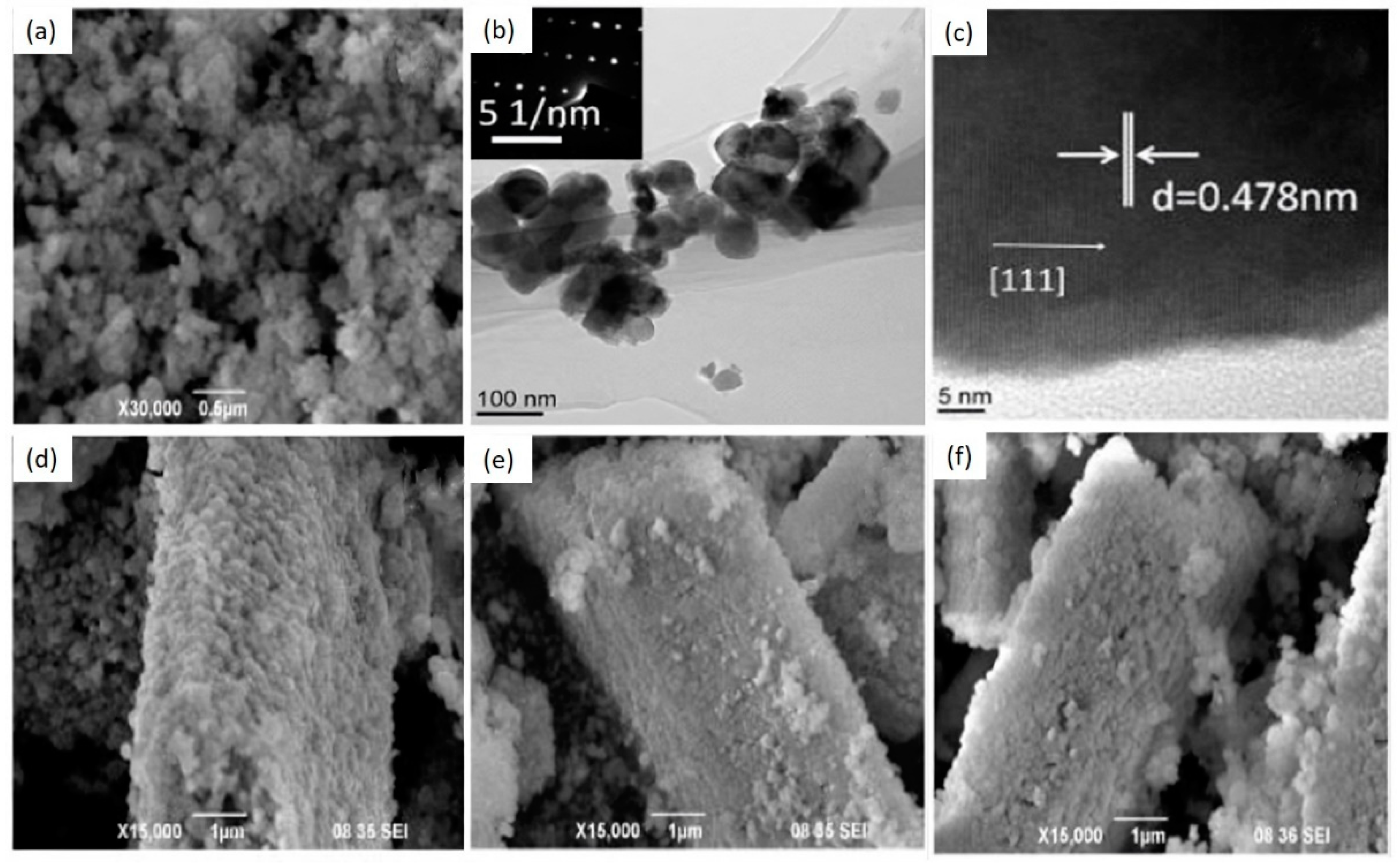
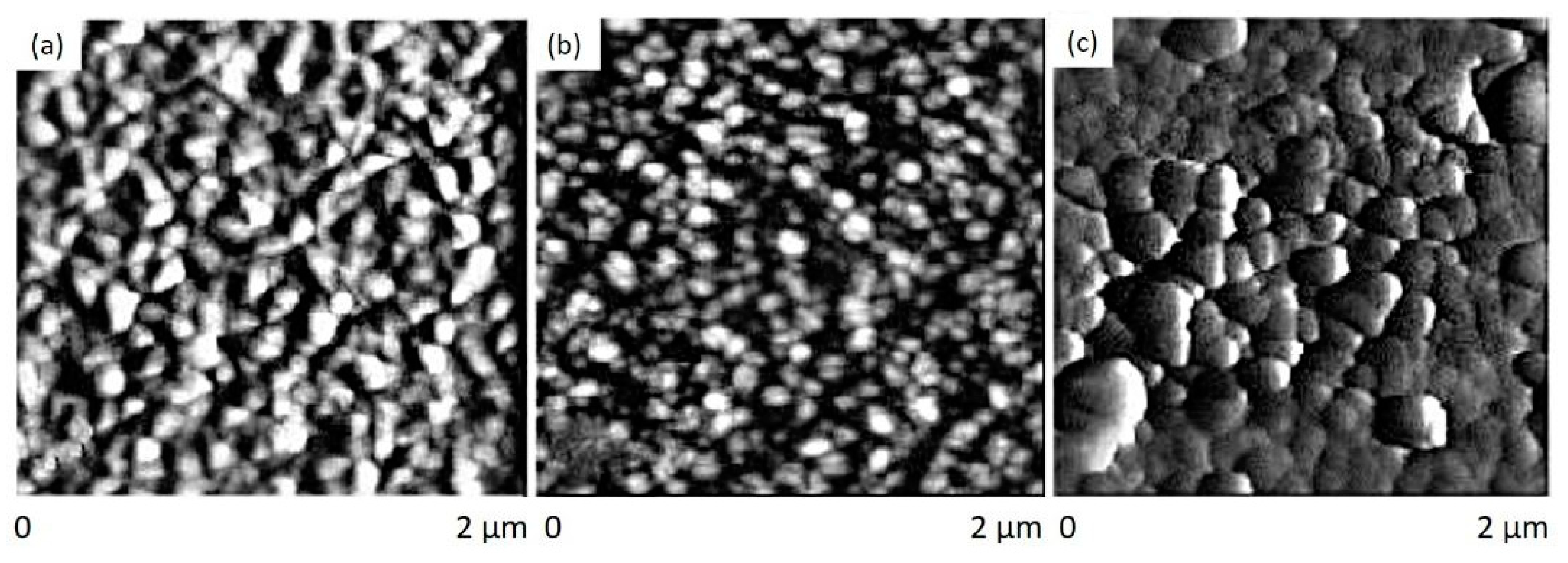




| 4H-SiC | GaN | β-Ga2O3 | ZnGa2O4 | |
|---|---|---|---|---|
| Bandgap, Eg (eV) | 3.25 | 3.4 | 4.85 | 4.325 (indirect) 4.570 (direct) [38] |
| Dielectric constant, ε | 9.7 | 9 | 10 | 10.4 [39] |
| Breakdown field, EC (MV/cm) | 2.5 | 3.3 | 8 | - |
| Electron mobility, μ (cm2/V·s) | 1000 | 1250 | 300 | 100 [6,40] |
| Saturation velocity, vs (107 cm/s) | 2 | 2.5 | 1.8–2 | - |
| Thermal conductivity λ (W/cm·K) | 4.9 | 2.3 | 0.1–0.3 | 0.22 [38] |
| Method | Solid State | Flux grown | Czochralski | LHPG | Hydrothermal |
| Raw material | ZnO and Ga2O3 | ZnSO4.10H2O and Ga2O3 | |||
| Morphology | Porous natured rods | Spinel | Fibers | Cuboids | |
| Crystal dimension | 1–5 µm | 3–10 mm | 5 mm | - | 35–60 nm |
| Temperature (°C) | 1000 | 1000–1500 | 600–800 | 1100 | 160–200 |
| Bandgap (eV) | 4.74 | 4.0 | 4.6 | - | - |
| Lattice constant (Å) | 8.37 | 8.332 | 8.333 | - | - |
| Reference | [69] | [57,58] | [38] | [64] | [70] |
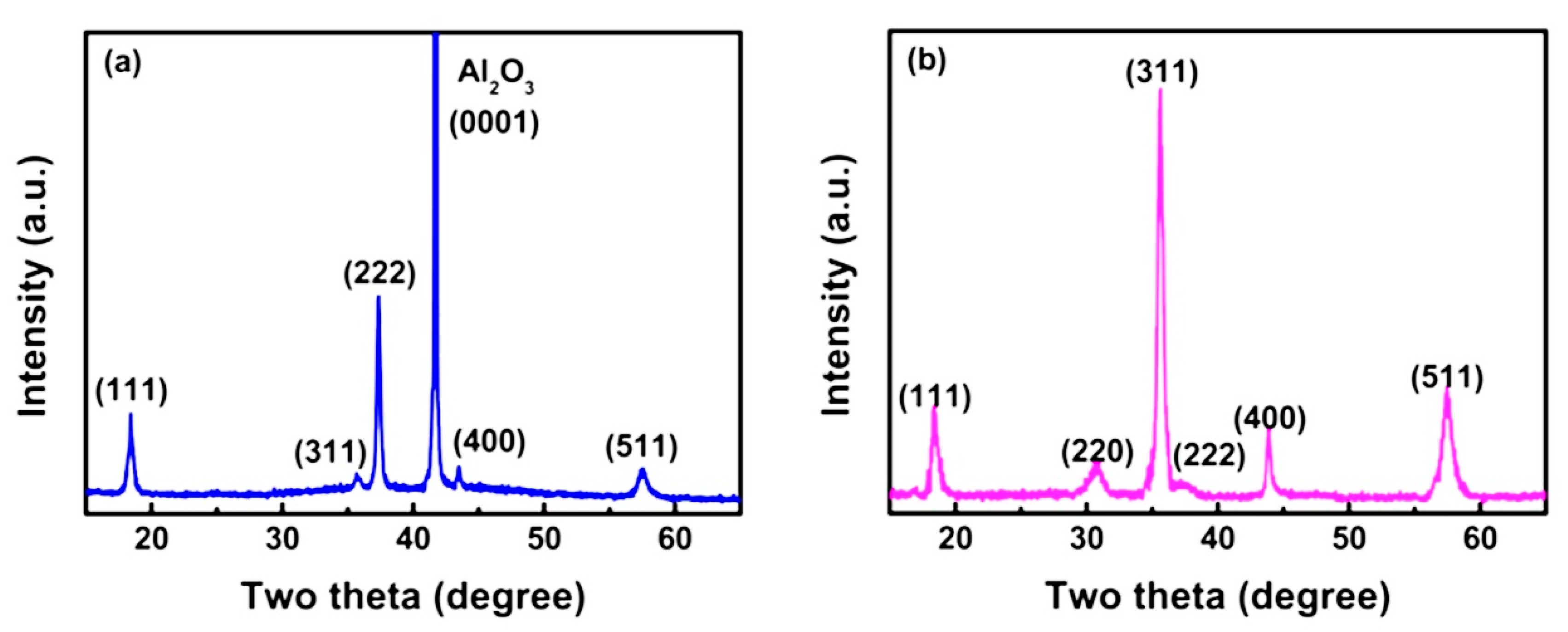
| Substrate | c-plane sapphire | Si(100) | c-plane sapphire | Si(100) |
| Working Chamber pressure (Torr) | 5 × 10−3 | 5 × 10−3 | 4 × 10−3 | |
| RF power (W) | 150 | |||
| Substrate temperature (°C) | From 200 to 600 | 400 | 200–600 | |
| Annealing temperature (°C) | - | 500–900 | 700–900 | |
| Characteristic Peaks in XRD Pattern | (111), (311), (222), (400), (511) | (111), (220), (311), (222), (400), (511) | (220), (311), (222), (400), (511), (440) | (222), (220), (311) |
| Luminescent Peak (nm) | 340, 417, 512 | 512 | 340, 520 | 470–360 |
| Reference | [78] | [74] | [33] | |
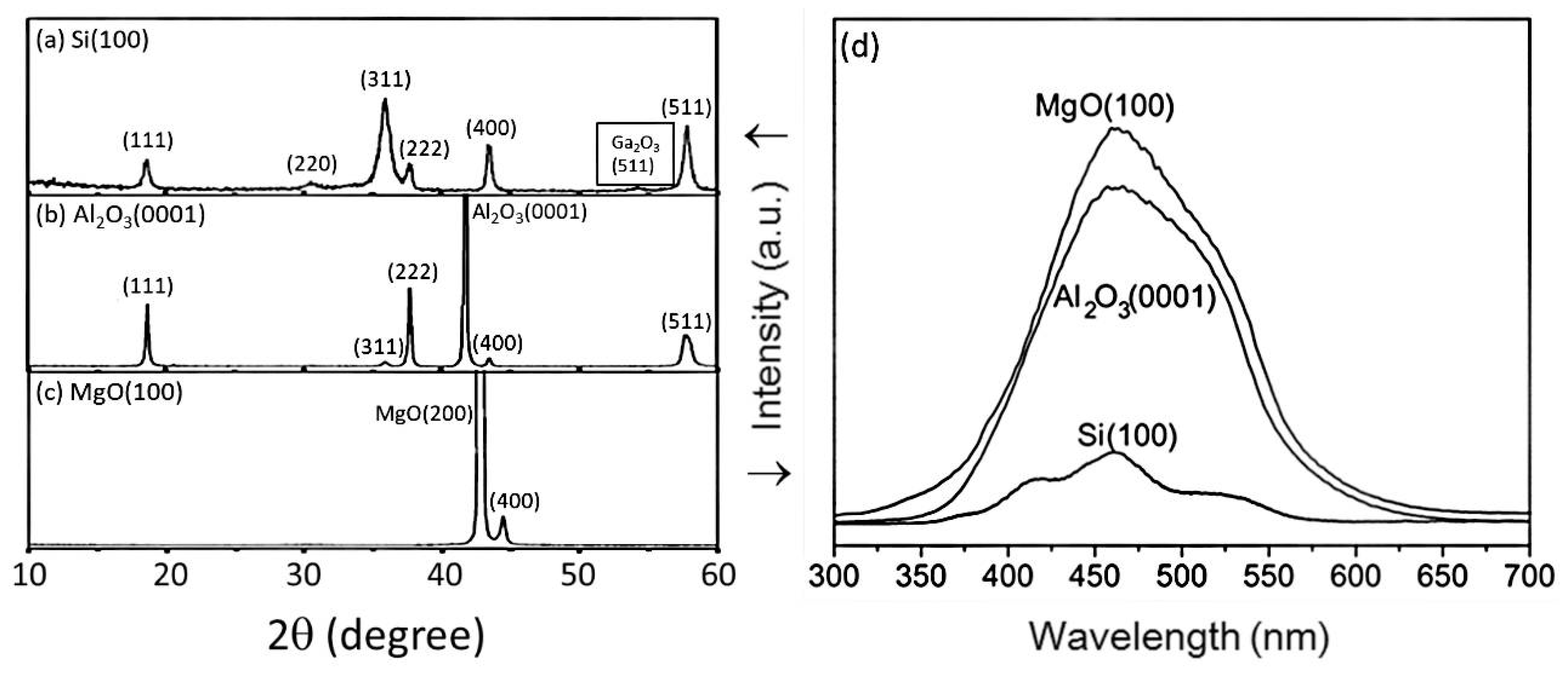
| Substrate | Si(100) | MgO (100) | Al2O3 (0001) | Si(100), Al2O3(0001), MgO(100) | (00.1) Sapphire |
| Substrate temperature (°C) | 550 | 650–730 | 450, 550, and 650 | 650, 700, 750, 850 | |
| Oxygen pressure | 50–300 mTorr | 0–130 mTorr | 0.1, 0.2, 0.3 Torr | 100 mTorr | 1.6 Pa |
| Annealed temperature (°C) | From 550 to 700 | - | - | - | |
| Characteristic Peaks in XRD Pattern | (111), (220), (311), (222), (400) | (400) | Si(100): (111), (220), (311), (222), (400), (511) Al2O3(0001): (111), (311), (222), (400), (511) MgO(100): (400) | (111), (222), (333), (444) | |
| PL peak (nm) | From 460 to 370 | 479 | 460 | - | |
| Reference | [72] | [79] | [71] | [80] | [81] |
| Process Method | Mist CVD | MOCVD |
| Substrate | (100)MgAl2O4 | c-plane (002)sapphire |
| Precursors | Concentrations: 0 ≤ (Zn)/(Ga) ≤ 10.0 (Zn) and (Ga): zinc and gallium Acetylacetonate | Flow rates: TEGa: 50 sccm O2: 200 sccm DEZn: 10, 30, 40, 50, 60 sccm |
| Grown temperature | From 400 to 800 °C | - |
| Crystalline orientation of ZnGa2O4 | (400), (800) | (111), (222), (333) |
| Crystalline orientation of Ga2O3 | (400), (800) | (-201), (-402), (-603) |
| CL peak | 365, 428 and 495 nm | Main: 332 nm Weak: 236, 499 nm |
| Reference | [84] | [76] |
| DEZn Flow Rate | Ga (Atom %) | Zn (Atom %) | O (Atom %) | C (Atom %) | Ga/Zn | O/(Zn + Ga) |
|---|---|---|---|---|---|---|
| 10 sccm | 46.4 | 2.0 | 50.7 | 0.9 | 23.2 | 1.05 |
| 30 sccm | 39.2 | 8.1 | 51.3 | 1.4 | 4.84 | 1.08 |
| 40 sccm | 38.7 | 10.9 | 49.5 | 0.9 | 3.55 | 0.99 |
| 50 sccm | 31.5 | 10.5 | 54.3 | 3.7 | 3 | 1.29 |
| 60 sccm | 26.9 | 10.0 | 44.2 | 18.9 | 2.69 | 1.20 |
| Method | MOCVD | sputtering | ||
| Substrate | c-plane (0001) sapphire | quartz | c-plane sapphire | |
| Substrate temperature (°C) | 650 | - | From RT to 600 | |
| Precursors | DEZn:40 sccm, TEGa:50 sccm, O2(99.999%): 200 sccm | - | ||
| Annealing temperature (°C) | 800 | 700, 800, 900 | 100, 200, 300 | From 500 to 900 |
| Reference | [87] | [92] | [20] | [93] |
| Light Irradiation (nm) | 230 | 260 | 240 | |
| Applied bias (V) | 20 | 5 | 10 | 5 |
| Annealed temperature (°C) | - | 800 | 200 | 700 |
| Responsivity (A/W) | 5.77 | 86.3 | 0.203 | 2.53 |
| Photo/dark current ratio | 4.68 × 104 | ~107 | ~109 | 3.77 × 104 |
| Rise time, decay time (s) | 0.96, 0.34 | <1 | 13, 2 | 4.5, 0.2 |
| Reference | [87] | [92] | [20] | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-I.; Singh, A.K.; Chiang, J.-L.; Horng, R.-H.; Wuu, D.-S. Zinc Gallium Oxide—A Review from Synthesis to Applications. Nanomaterials 2020, 10, 2208. https://doi.org/10.3390/nano10112208
Chen M-I, Singh AK, Chiang J-L, Horng R-H, Wuu D-S. Zinc Gallium Oxide—A Review from Synthesis to Applications. Nanomaterials. 2020; 10(11):2208. https://doi.org/10.3390/nano10112208
Chicago/Turabian StyleChen, Mu-I, Anoop Kumar Singh, Jung-Lung Chiang, Ray-Hua Horng, and Dong-Sing Wuu. 2020. "Zinc Gallium Oxide—A Review from Synthesis to Applications" Nanomaterials 10, no. 11: 2208. https://doi.org/10.3390/nano10112208
APA StyleChen, M.-I., Singh, A. K., Chiang, J.-L., Horng, R.-H., & Wuu, D.-S. (2020). Zinc Gallium Oxide—A Review from Synthesis to Applications. Nanomaterials, 10(11), 2208. https://doi.org/10.3390/nano10112208








