The Effect of Surface Modification of Gold Nanotriangles for Surface-Enhanced Raman Scattering Performance
Abstract
1. Introduction
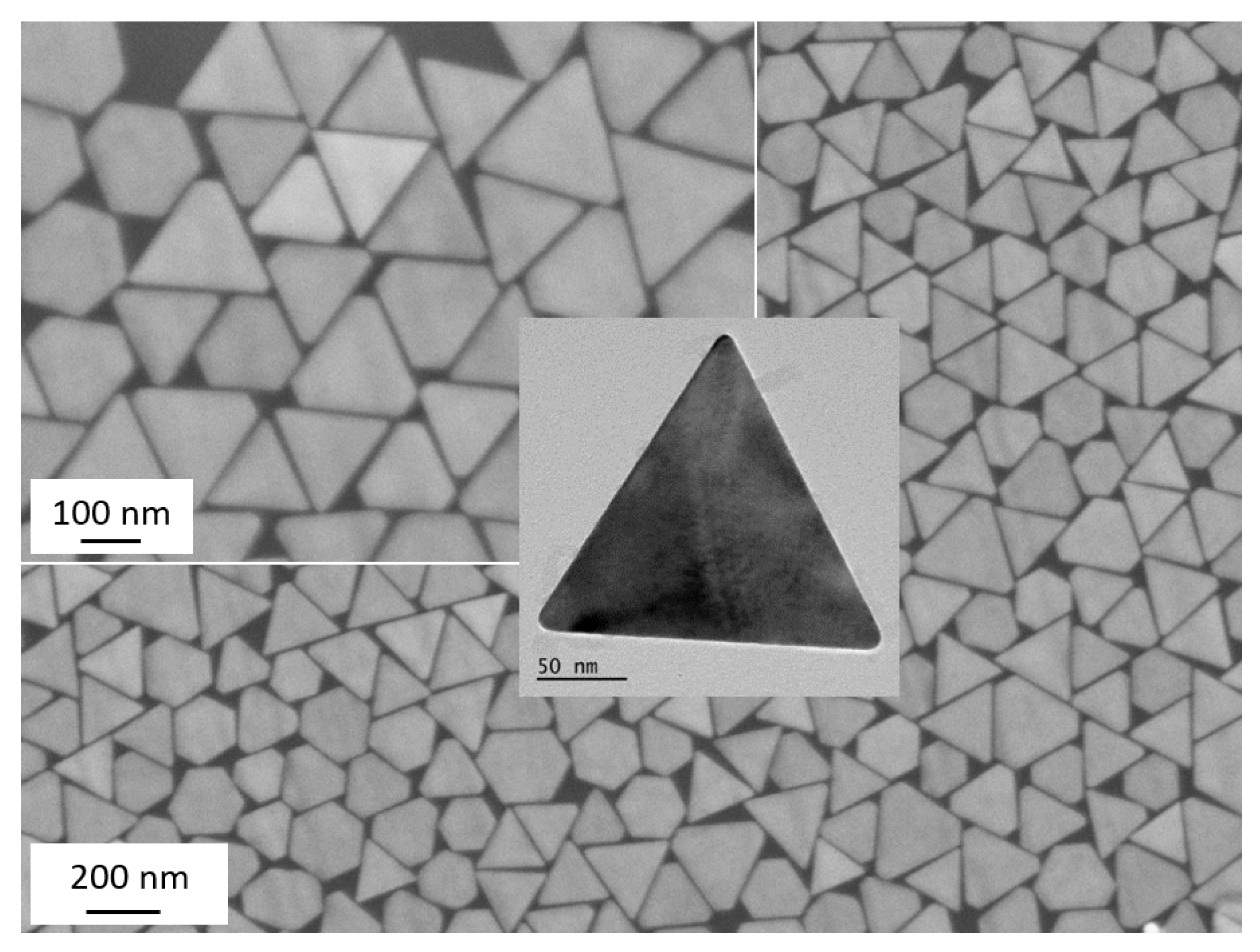
2. Smooth (Bare) Dioctyl Sodium Sulfosuccinate (AOT)-Coated Gold Nanotriangles (AuNTs)
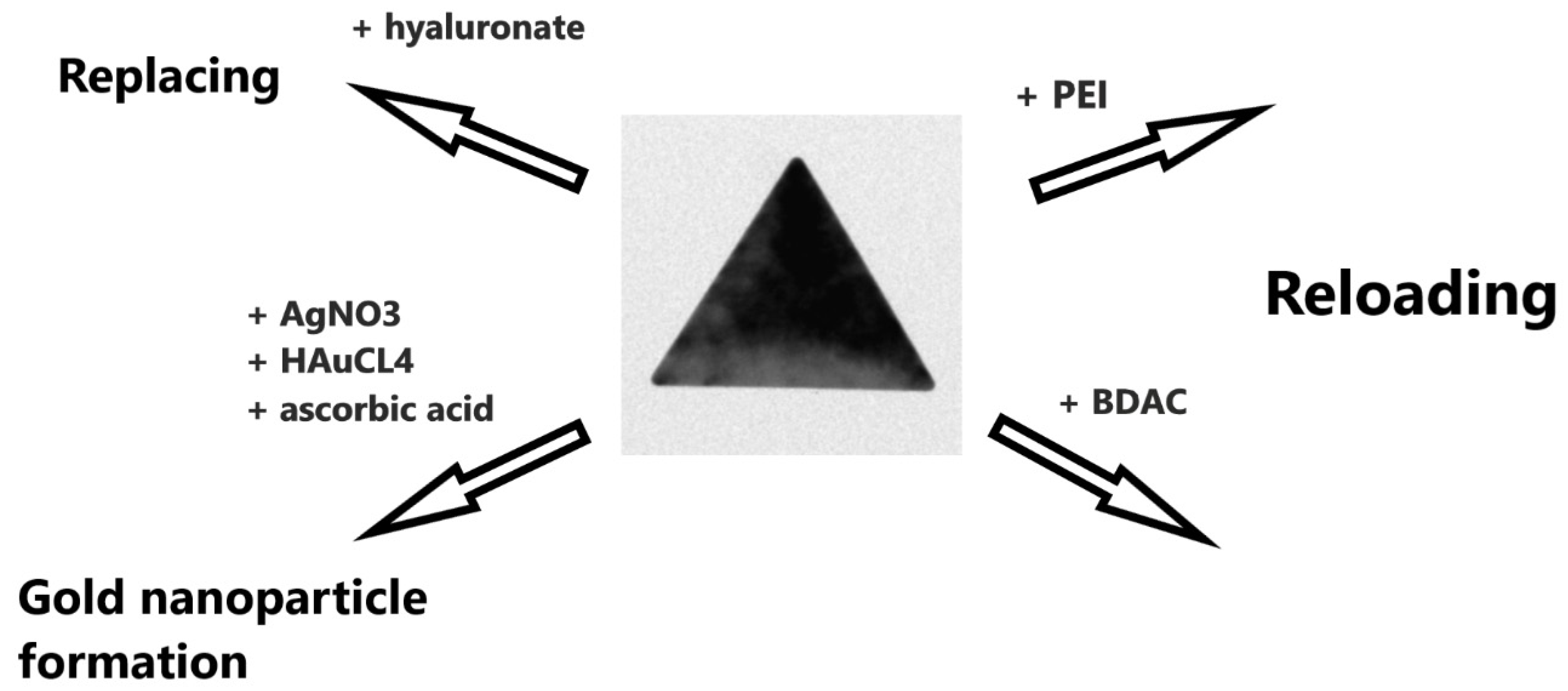
3. Surface Modification of Smooth AOT-Coated AuNTs
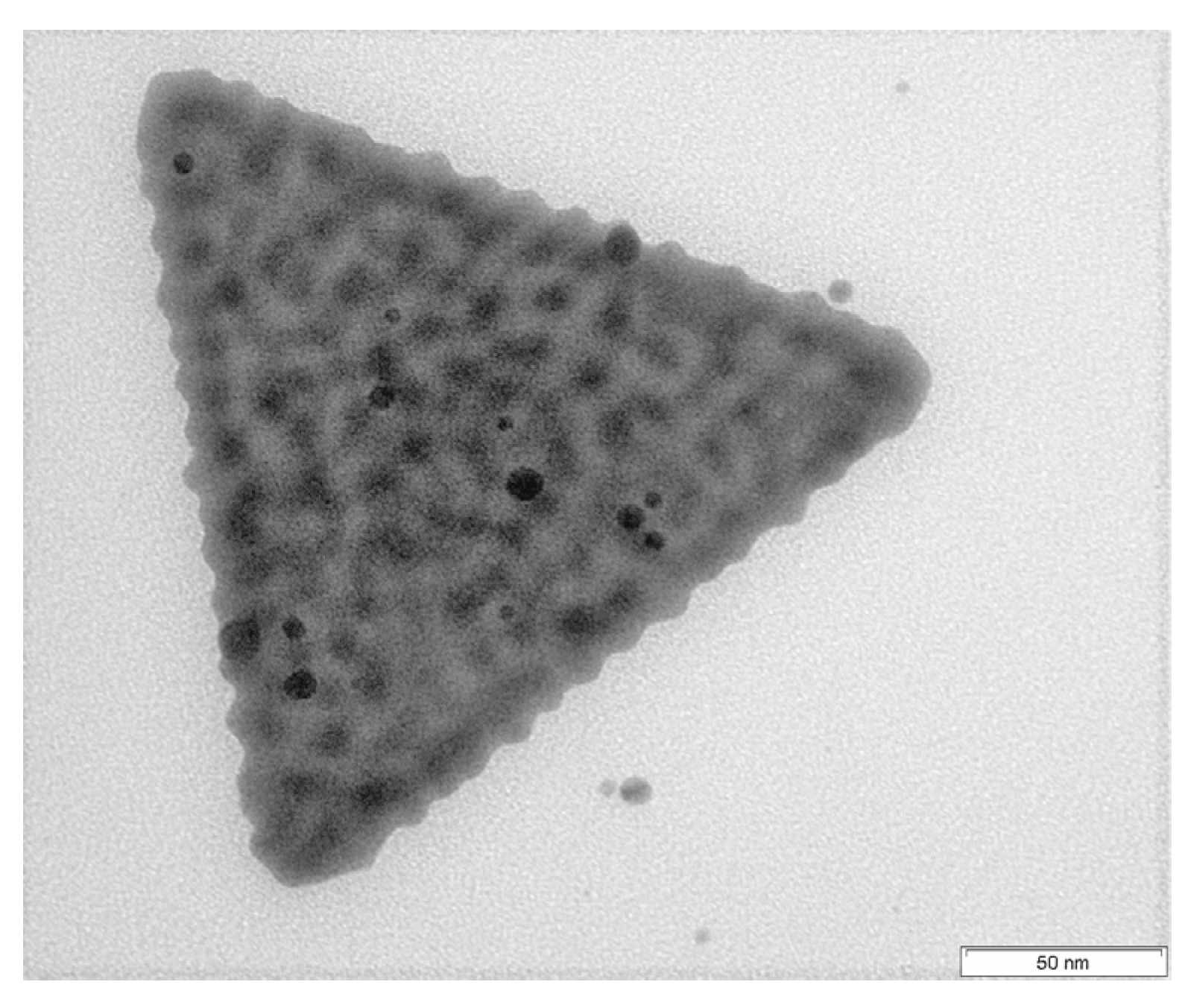
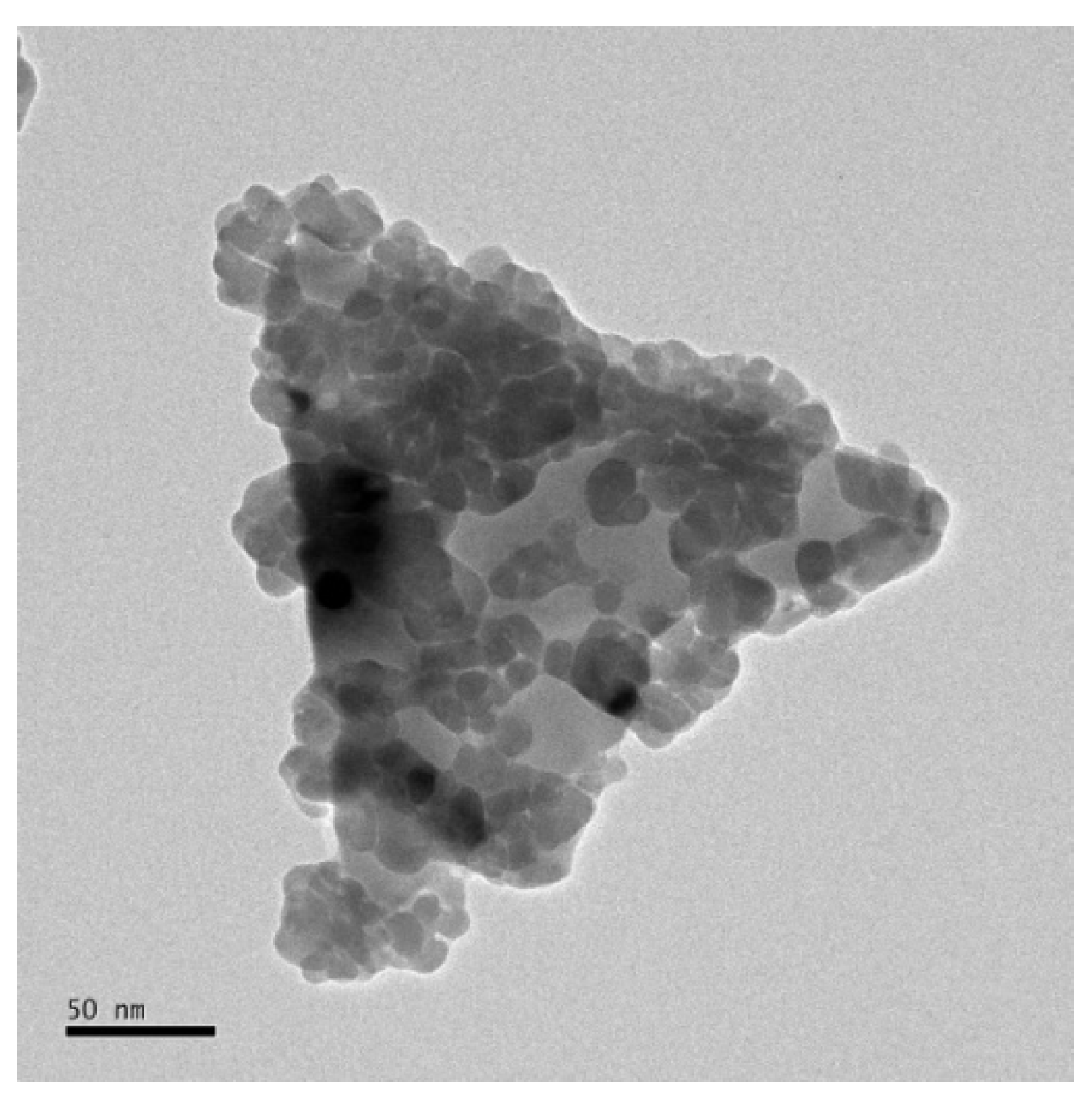
3.1. Undulated AuNTs after Gold Nanoparticle Formation in a Polyethyleneimine (PEI) Shell
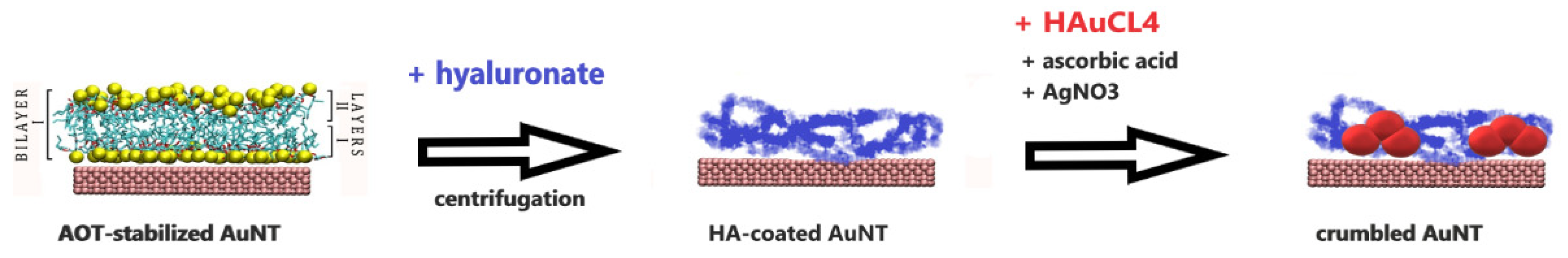
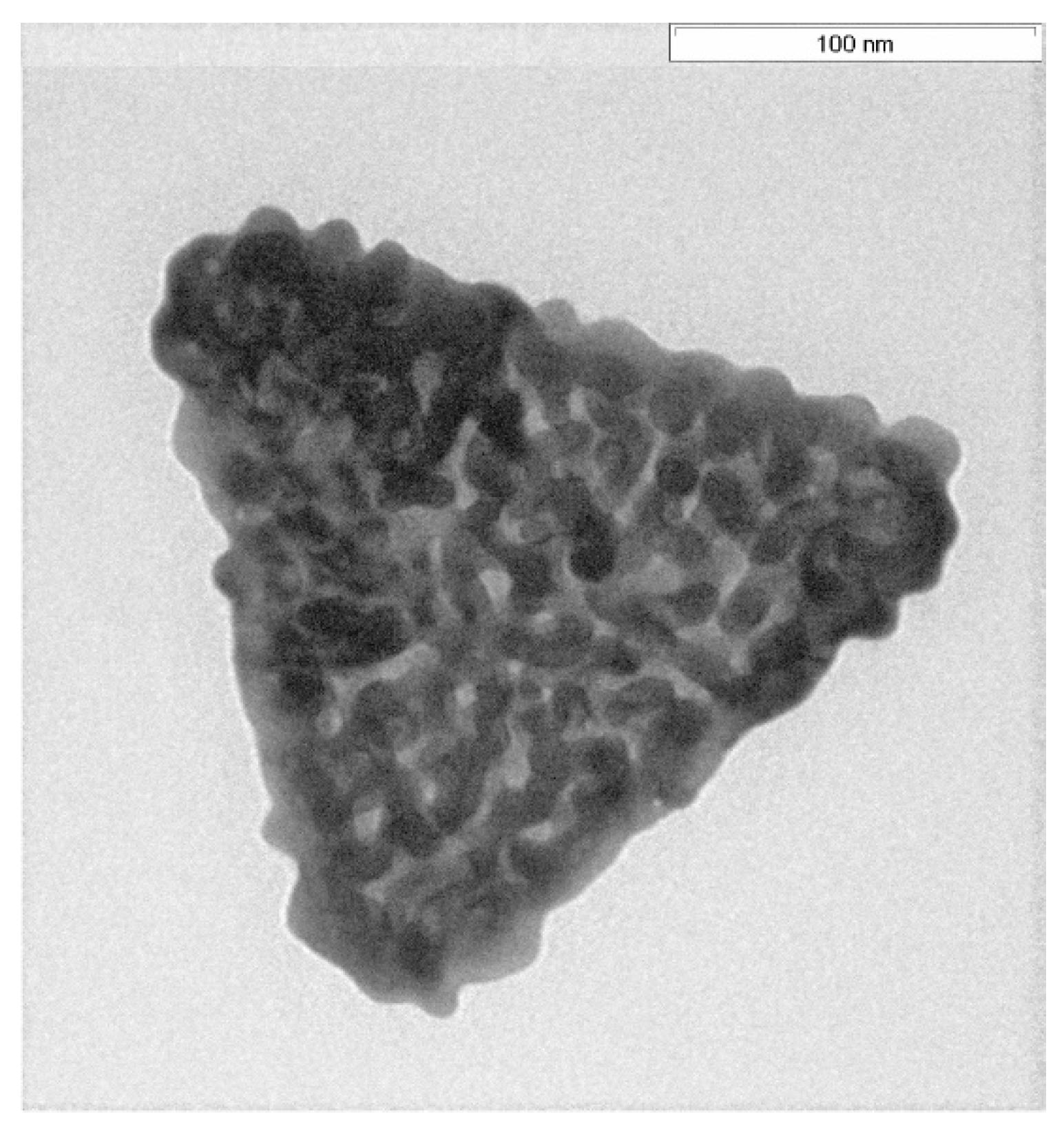
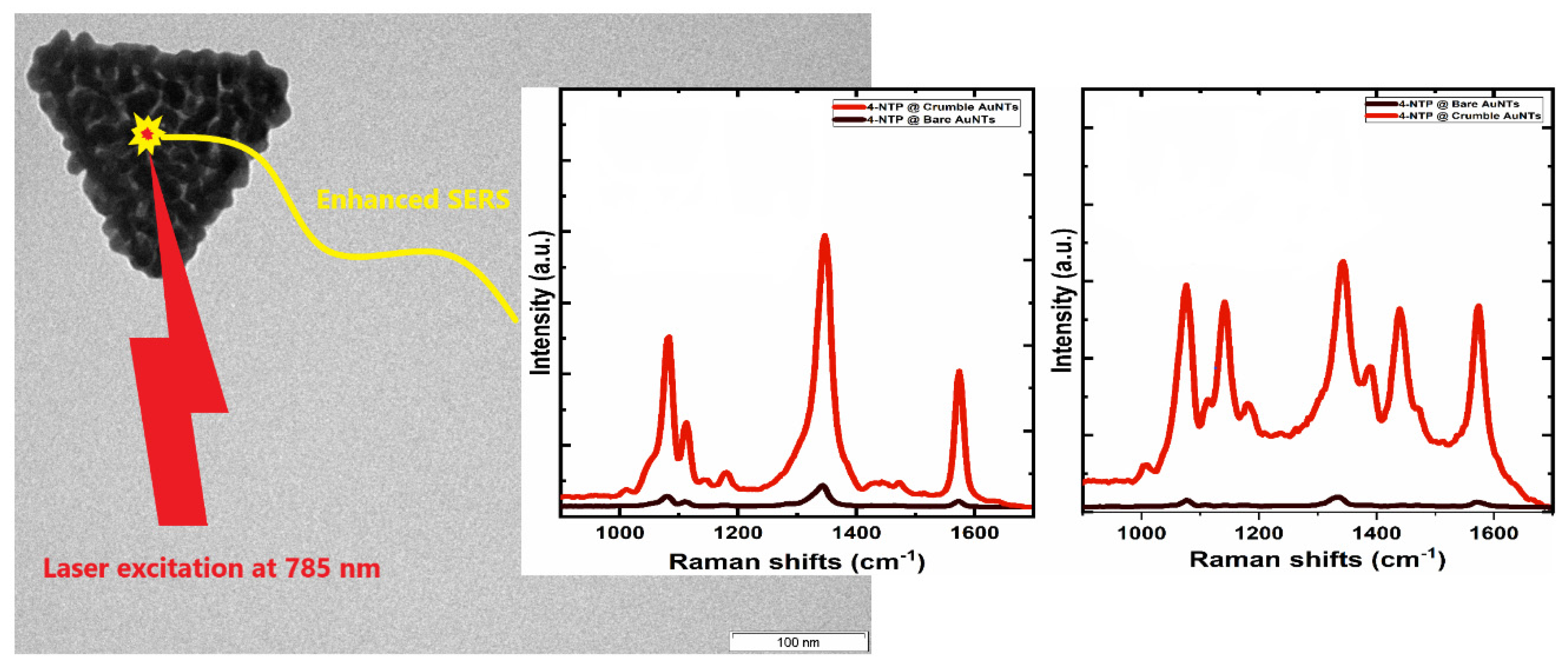
3.2. Crumble AuNTs after Replacing the AOT Bilayer by Hyaluronic Acid
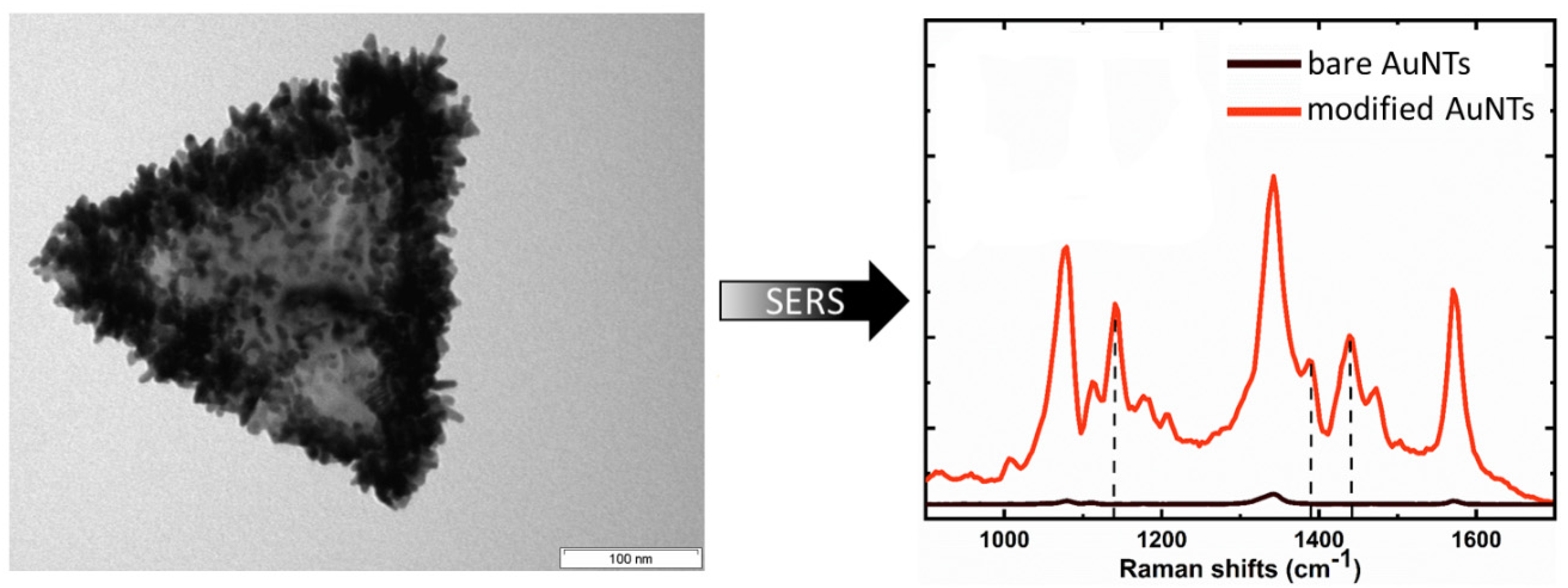
3.3. Spiked AuNTs Formed in the AOT Bilayer
3.4. Silver-Decorated AuNTs Formed in an AOT/Benzylhexadecyldimethylammonium Chloride (BDAC) Shell
4. Conclusions
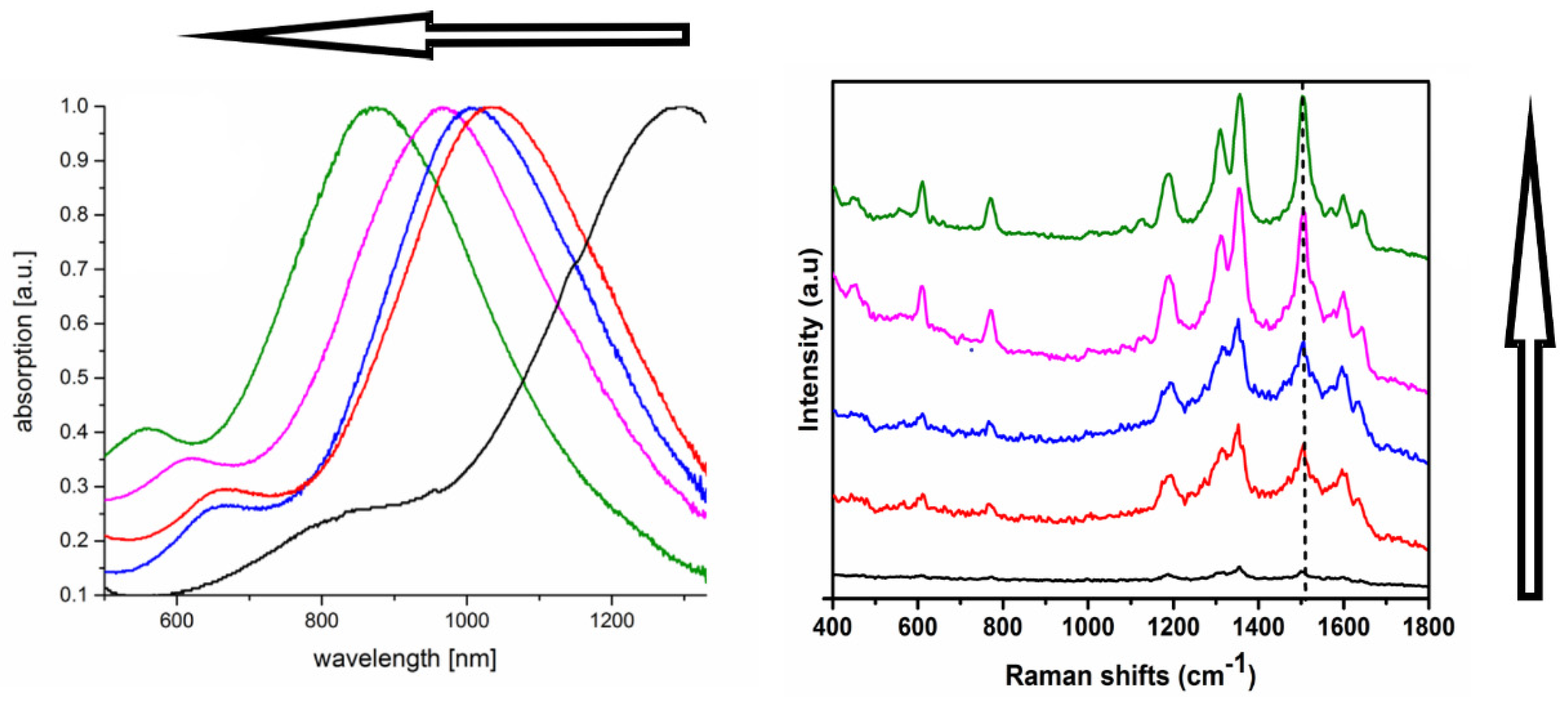
- by a factor of 2 by a decoration with gold half spheres after reloading the AOT shell with PEI [31].
- by a factor of 20 by a decoration with gold crumbles after replacing the AOT shell by a hyaluronate shell [32].
- by a factor of 20 by a decoration with silver nanoparticles after reloading the AOT shell with BDAC [42].
- by a factor of 75 by a decoration with gold spikes after a direct formation in the AOT shell [41].
5. Experimental Section
5.1. Self-Assembled AuNT Monolayer Formation
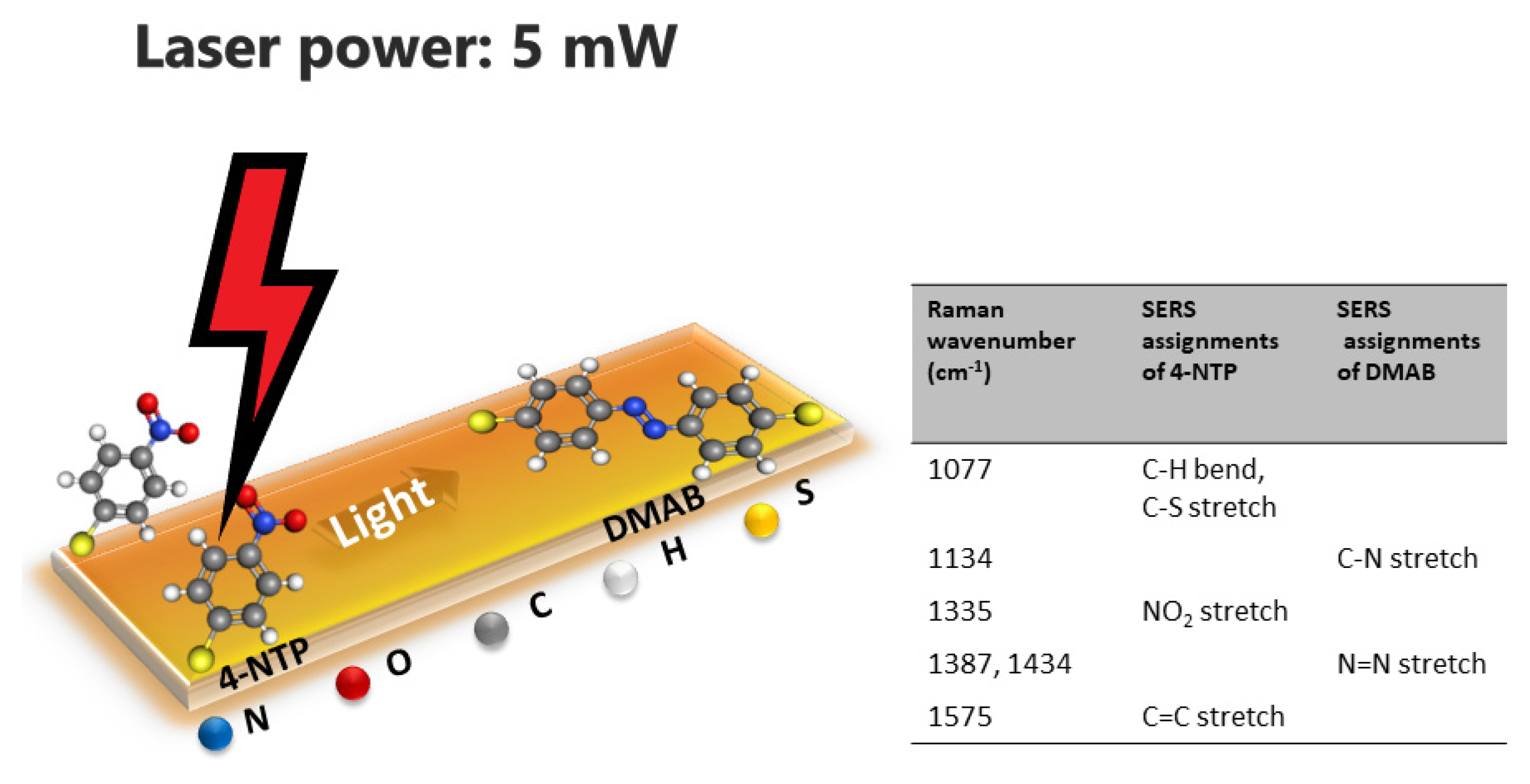
5.2. Surface-Enhanced Raman Scattering (SERS) Performance
Funding
Conflicts of Interest
References
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiao, Y.; Chai, Q.; Yu, X. Gold Nanoparticles: Synthesis and Biological Applications. Nano Life 2015, 5, 1542007. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronada, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Moores, A.; Goettmann, F. The Plasmon Band in Noble Metal Nanoparticles: An Introduction to Theory and Applications. New J. Chem. 2006, 30, 1121–1132. [Google Scholar] [CrossRef]
- Odom, T.; Nehl, C.L. How Gold Nanoparticles Have Stayed in the Light: The 3M´s Principle. ACS Nano 2008, 2, 612–616. [Google Scholar] [CrossRef]
- Anderson, L.J.E.; Zhen, Y.R.; Payne, C.M.; Nordlander, P.; Hafner, J.H. Gold Nanobelts as High Confinement Plasmonic Waveguides. Nano Lett. 2013, 13, 6256–6261. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second Window for in vivo Imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–1178. [Google Scholar] [CrossRef] [PubMed]
- Bryant, G.W.; Gracia-de Abajo, F.J.; Aizpurua, J. Mapping the Plasmon Resonances of Metallic Nanoantennas. Nano Lett. 2008, 8, 631–636. [Google Scholar] [CrossRef]
- Tebbe, M.; Kuttner, C.; Mayer, M.; Maennel, M.; Pazos-Perez, N.; König, T.A.F.; Fery, A. Silver-Overgrowth-Induced Changes in Intrinsic Optical Properties of Gold Nanorods: From Noninvasive Monitoring of Growth Kinetics to Tailoring Internal Mirror Changes. J. Phys. Chem. C 2015, 119, 9513–9523. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Prietzel, C.; Reinecke, A.; Koetz, J. “Green” Gold Nanotriangles: Synthesis, Purification by Polyelectrolyte/Micelle Depletion Flocculation and Performance in Surface-Enhanced Raman Scattering. RSC Adv. 2016, 6, 33561–33568. [Google Scholar] [CrossRef]
- Nelaya, J.; Kociak, M.; Stephan, O.; Gracia-de Abajo, F.J.; Henrard, L.; Taverna, D.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Colliex, C. Mapping Surface Plasmons on a Single Metallic Nanoparticle. Nat. Phys. 2007, 3, 348–353. [Google Scholar] [CrossRef]
- Scarabelli, L.; Coronado-Puchau, M.; Giner-Casares, J.J.; Langer, J.; Liz-Marzan, L.M. Monodisperse Gold Nanotriangles: Size Control, Large-Scale Self-Assembly, and Performance in Surface-Enhanced Raman Scattering. ACS Nano 2014, 8, 5833–5842. [Google Scholar] [CrossRef]
- Kuttner, C.; Mayer, M.; Dulle, M.; Moscoso, A.; Lopez-Romero, J.M.; Förster, S.; Fery, A.; Perez-Juste, J.; Contreras-Caceres, R. Seeded Growth Synthesis of Gold Nanotriangles: Size Control, SAXS Analysis, and SERS Performance. ACS Appl. Mater. Interfaces 2018, 10, 11152–11163. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Sander, M.; Koopman, W.; Schütz, R.; Bargheer, M.; Koetz, J. Deposition of Gold Nanotriangles in Large Scale Close-Packed Monolayers for X-ray-Based Temperature Calibration and SERS Monitoring of Plasmon-Driven Catalytic Reactions. ACS Appl. Mater. Interfaces 2017, 9, 20247–20253. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, C.K.; Tan, B.; Tan, J.M.R.; Phang, I.Y.; Ling, X.Y. Using the Langmuir-Schaefer Technique to Fabricate Large-Area Dense SERS-Active Au Nanoprism Monolayer Films. Nanoscale 2013, 5, 6404–6412. [Google Scholar] [CrossRef] [PubMed]
- Swarnapali, A.; Indrasekara, D.S.; Thomas, R.; Fabris, L. Plasmonic Properties of Regiospecific Core-Satellite Assemblies of Gold Nanostars and Nanospheres. Phys. Chem. Chem. Phys. 2015, 17, 21133–21142. [Google Scholar]
- Rodriguez-Lorenzo, L.; Alvarez-Puebla, R.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stephan, O.; Kociak, M.; Gracia-de Abajo, F.J.; Liz-Marzan, L.M. Zeptomol Detection Through Controlled Ultrasensitive Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Beetz, M.; Fabris, L. Understanding the Role of AgNO3 Concentration and Seed Morphology in the Achievement of Tunable Shape Control in Gold Nanostars. Nanoscale 2019, 11, 2946–2958. [Google Scholar] [CrossRef] [PubMed]
- Sheen Mers, S.V.; Umadevi, S.; Ganesh, V. Controlled Growth of Gold Nanostars: Effect of Spike Length on SERS Signal Enhancement. ChemPhysChem 2017, 18, 1358–1369. [Google Scholar] [CrossRef]
- Aldenueva-Potel, P.; Carbo-Argibay, E.; Pazos-Perez, N.; Barbosa, S.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Spiked Gold Beads as Substrates for Single-Particle SERS. ChemPhysChem 2012, 13, 2561–2565. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Gao, C.; Guo, L.; Chi, M.; Ijiro, K.; Maeda, M.; Yin, Y. Island Growth in the Seed-Mediated Overgrowth of Monometallic Colloidal Nanostructures. Chem 2017, 3, 678–690. [Google Scholar] [CrossRef]
- Lohse, S.E.; Burrows, N.D.; Scarabelli, L.; Liz-Marzán, L.M.; Murphy, C.J. Anisotropic Noble Metal Nanocrystal Growth: The Role of Halides. Chem. Mater. 2014, 26, 34–43. [Google Scholar] [CrossRef]
- Chen, L.; Ji, F.; He, L.; Mi, Y.; Sun, B.; Zhang, X.; Zhang, Q. High-Yield Seedless Synthesis of Triangular Gold Nanoplates through Oxidative Etching. Nano Lett. 2014, 14, 7201–7206. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Miller, N.R.; Korgel, B.A. Iodide in CTAB Prevents Gold Nanorod Formation. Langmuir 2009, 25, 9518–9524. [Google Scholar] [CrossRef]
- Robertson, D.; Tiersch, B.; Kosmella, S.; Koetz, J. Preparation of Crystalline Gold Nanoparticles at the Surface of Mixed Phosphatidylcholine-Ionic Surfactant Vesicles. J. Colloid Interface Sci. 2007, 305, 345–351. [Google Scholar] [CrossRef]
- Liebig, F.; Thünemann, A.F.; Koetz, J. Ostwald Ripening Growth Mechanism of Gold Nanotriangles in Vesicular Template Phase. Langmuir 2016, 32, 10928–10935. [Google Scholar] [CrossRef]
- Poghosyan, A.; Adamyan, M.P.; Shahinyan, A.A.; Koetz, J. AOT Bilayer Adsorption on Gold Surfaces: A Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 948–953. [Google Scholar] [CrossRef]
- Note, C.; Kosmella, S.; Koetz, J. Poly(ethyleneimine) as Reducing and Stabilizing Agent for the Formation of Gold Nanoparticles in w/o Microemulsions. Colloids Surf. A 2006, 290, 150–156. [Google Scholar] [CrossRef]
- Sun, X.; Dong, S.; Wang, E. One-Step Preparation of Highly Concentrated Well-Stable Gold Colloids by Direct Mix of Polyelectrolyte and HAuCL4 Aqueous Solutions at Room Temperature. J. Colloid Interface Sci. 2005, 288, 301–303. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Prietzel, C.; Bargheer, M.; Koetz, J. Undulated Gold Nanoplatelet Superstructures: In Situ Growth of Hemispherical Gold Nanoparticles onto the Surface of Gold Nanotriangles. Langmuir 2018, 34, 4584–4594. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Schmitt, C.N.Z.; Thünemann, A.F.; Prietzel, C.; Bargheer, M.; Koetz, J. Gold Nanotriangles with Crumble Topping and Their Influence on Catalysis and Surface-Enhanced Raman Spectroscopy. ChemPlusChem 2020, 85, 519–526. [Google Scholar] [CrossRef]
- Kumar, P.S.; Pastoriza-Santos, I.; Rodriguez-Gonzales, B.; Garcia de Abajo, F.J.; Liz-Marzan, L.M. High-Yield Synthesis and Optical Response of Gold Nanostars. Nanotechnology 2008, 19, 015606. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.G.; Vo-Dinh, T. Gold Nanostars for Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef]
- Liebig, F.; Henning, R.; Sarhan, R.M.; Prietzel, C.; Bargheer, M.; Koetz, J. A New Route to Gold Nanoflowers. Nanotechnology 2018, 29, 185603. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.K.; Raj, C.R. Seedless, Surfactantless Room Temperature Synthesis of Single Crystalline Fluorescent Gold Nanoflowers with Pronounced SERS and Electrocatalytic Activity. Chem. Mater. 2008, 20, 3546–3548. [Google Scholar] [CrossRef]
- Wu, H.L.; Chen, C.H.; Huang, M. Seed-Mediated Synthesis of Branched Gold Nanocrystals Derived from the Side Growth of Pentagonal Bipyramides and the Formation of Gold Nanostars. Chem. Mater. 2009, 21, 110–114. [Google Scholar] [CrossRef]
- Liebig, F.; Henning, R.; Sarhan, R.M.; Prietzel, C.; Schmitt, C.N.Z.; Bargheer, M.; Koetz, J. A Simple One-Step Procedure to Gold Nanostars in Concentrated Surfactant Solutions. RSC Adv. 2019, 9, 23633. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Ma, Z. Synthesis of Gold Nanostars with Tunable Morphology and their Electrochemical Application for Hydrogen Peroxide Sensing. Electrochim. Acta 2013, 108, 435–440. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Prietzel, C.; Bargheer, M.; Schmitt, C.N.Z.; Poghosyan, A.; Shahinyan, A.A.; Koetz, J. Spiked Gold Nanotriangles: Formation, Characterization and Applications in Surface-Enhanced Raman Spectroscopy and Plasmon Enhanced Catalysis. RSC Adv. 2020, 10, 8152–8160. [Google Scholar] [CrossRef]
- Liebig, F.; Sarhan, R.M.; Prietzel, C.; Schmitt, C.N.Z.; Bargheer, M.; Koetz, J. Tuned Surface-Enhanced Raman Scattering Performance of Undulated Au@Ag Triangles. ACS Appl. Nano Mater. 2018, 1, 1995–2003. [Google Scholar] [CrossRef]
- Poghosyan, A.; Shahinyan, A.A.; Koetz, J. Catanionic AOT/BDAC Micelles on Gold {111} Surfaces. Colloids Polym. Sci. A 2018, 296, 1301–1306. [Google Scholar] [CrossRef]
- Sarhan, R.M.; Koopman, W.; Schuetz, R.; Schmid, T.; Liebig, F.; Koetz, J.; Bargheer, M. The Importance of Plasmonic Heating for the Plasmon-Driven Photopolymerization of 4-Nitrothiophenol. Sci. Rep. 2019, 9, 3060. [Google Scholar] [CrossRef]
- Von Reppert, A.; Sarhan, R.M.; Stete, F.; Pudell, J.; Del Fatti, N.; Crut, A.; Koetz, J.; Liebig, F.; Prietzel, C.; Bargheer, M. Watching the Vibration and Cooling of Ultrathin Gold Nanotriangles by Ultrafast X-ray Diffraction. J. Phys. Chem. C 2016, 120, 28894–28899. [Google Scholar] [CrossRef]
- Sarhan, R.M.; Koopman, W.; Pudell, J.; Stete, F.; Rössle, M.; Herzog, M.; Schmitt, C.N.Z.; Liebig, F.; Koetz, J.; Bargheer, M. Scaling Up Nanoplasmon Catalysis: The Role of Heat Dissipation. J. Phys. Chem. C 2019, 123, 9352–9357. [Google Scholar] [CrossRef]
- Liebig, F.; Moreno, S.; Thünemann, A.F.; Temme, A.; Appelhans, D.; Koetz, J. Toxicological Investigations of “Naked” and Polymer-Entrapped AOT-Based Gold Nanotriangles. Colloids Surf. B 2018, 167, 560–567. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koetz, J. The Effect of Surface Modification of Gold Nanotriangles for Surface-Enhanced Raman Scattering Performance. Nanomaterials 2020, 10, 2187. https://doi.org/10.3390/nano10112187
Koetz J. The Effect of Surface Modification of Gold Nanotriangles for Surface-Enhanced Raman Scattering Performance. Nanomaterials. 2020; 10(11):2187. https://doi.org/10.3390/nano10112187
Chicago/Turabian StyleKoetz, Joachim. 2020. "The Effect of Surface Modification of Gold Nanotriangles for Surface-Enhanced Raman Scattering Performance" Nanomaterials 10, no. 11: 2187. https://doi.org/10.3390/nano10112187
APA StyleKoetz, J. (2020). The Effect of Surface Modification of Gold Nanotriangles for Surface-Enhanced Raman Scattering Performance. Nanomaterials, 10(11), 2187. https://doi.org/10.3390/nano10112187




