Immobilization of Radioiodine via an Interzeolite Transformation to Iodosodalite
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
2.3. Adsorption of CH3I
3. Results and Discussion
3.1. Interzeolite Transformation to Sodalite
3.2. Adsorption of CH3I and Immobilization of Iodine in Sod-Cages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L. Materials and Processes for the Effective Capture and Immobilization of Radioiodine: A Review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef]
- Shim, H.E.; Yang, J.E.; Jeong, S.-W.; Lee, C.H.; Song, L.; Mushtaq, S.; Choi, D.S.; Choi, Y.J.; Jeon, J. Silver Nanomaterial-Immobilized Desalination Systems for Efficient Removal of Radioactive Iodine Species in Water. Nanomaterials 2018, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- OECD/NEA. Status Report on Filtered Containment Venting; OECD/NEA: Paris, France, 2014. [Google Scholar]
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive Iodine Capture in Silver-Containing Mordenites through Nanoscale Silver Iodide Formation. J. Am. Chem. Soc. 2010, 132, 8897–8899. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Koch, A. A Combined DRIFTS and DR-UV-Vis Spectroscopic In Situ Study on the Trapping of CH3I by Silver-Exchanged Faujasite Zeolite. J. Phys. Chem. C 2016, 120, 18694–18706. [Google Scholar] [CrossRef]
- Azambre, B.; Chebbi, M. Evaluation of Silver Zeolites Sorbents toward Their Ability to Pomote Stable CH3I Stroage as AgI Pecipitates. ACS Appl. Mater. Interfaces 2017, 9, 25194–25203. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, M.; Chibani, S.; Paul, J.F.; Cantrel, L.; Badawi, M. Evaluation of Volatile Iodine Trapping in Presence of Contaminants: A Periodic DFT Study on Cation Exchanged-Faujasite. Microporous Mesoporous Mater. 2017, 239, 111–122. [Google Scholar] [CrossRef]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Huvé, M.; Albiol, T. Influence of Structural, Textural and Chemical Parameters of Silver Zeolites on the Retention of Methyl Iodide. Microporous Mesoporous Mater. 2017, 244, 137–150. [Google Scholar] [CrossRef]
- Bučko, T.; Chibani, S.; Paul, J.-F.; Cantrel, L.; Badawi, M. Dissociative Iodomethane Adsorption on Ag-MOR and the Formation of AgI Clusters: Ab Initio Molecular Dynamics Study. Phys. Chem. Chem. Phys. 2017, 19, 27530–27543. [Google Scholar] [CrossRef]
- Park, S.; An, H.; Park, M.B.; Lee, J. Adsorption Behavior of Methyl Iodide on a Silver Ion-Exchanged ZSM-5. Microporous Mesoporous Mater. 2020, 294, 109842. [Google Scholar] [CrossRef]
- Azambre, B.; Chebbi, M.; Hijazi, A. Effects of the Cation and Si/Al Ratio on CH3I Adsorption by Faujasite Zeolites. Chem. Eng. J. 2020, 379, 122308. [Google Scholar] [CrossRef]
- Chong, S.; Peterson, J.; Nam, J.; Riley, B.; McCloy, J. Synthesis and Characterization of Iodosodalite. J. Am. Ceram. Soc. 2017, 100, 2273–2284. [Google Scholar] [CrossRef]
- Lepry, W.C.; Riley, B.J.; Crum, J.V.; Rodriguez, C.P.; Pierce, D.A. Solution-Based Approaches for Making High-Density Sodalite Waste Forms to Immobilize Spent Electrochemical Salts. J. Nucl. Mater. 2013, 442, 350–359. [Google Scholar] [CrossRef]
- Maddrell, E.; Gandy, A.; Stennett, M. The Durability of Iodide Sodalite. J. Nucl. Mater. 2014, 449, 168–172. [Google Scholar] [CrossRef]
- Maddrell, E.R.; Vance, E.R.; Gregg, D.J. Capture of Iodine From the Vapour Phase and Immobilisation as Sodalite. J. Nucl. Mater. 2015, 467, 271–279. [Google Scholar] [CrossRef]
- Vance, E.R.; Gregg, D.J.; Grant, C.; Stopic, A.; Maddrell, E.R. Silver Iodide Sodalite for 129I Immobilisation. J. Nucl. Mater. 2016, 480, 177–181. [Google Scholar] [CrossRef]
- Chong, S.; Peterson, J.A.; Riley, B.J.; Tabada, D.; Wall, D.; Corkhill, C.L.; McCloy, J.S. Glass-Bonded Iodosodalite Waste Form for Immobilization of 129I. J. Nucl. Mater. 2018, 504, 109–121. [Google Scholar] [CrossRef]
- Dwyer, F.G.; Chu, P. ZSM-4 Crystallization via Faujasite Metamorphosis. J. Catal. 1979, 59, 263–271. [Google Scholar] [CrossRef]
- Subotić, B.; Škrtić, D.; Šmit, I.; Sekovanić, L. Transformation of Zeolite A into Hydroxysodalite. J. Cryst. Growth 1980, 50, 498–508. [Google Scholar] [CrossRef]
- Chiyoda, O.; Davis, M.E. Hydrothermal Conversion of Y-Zeolite Using Alkaline Earth Cations. U.S. Patent 6,436,364, 13 February 2001. [Google Scholar]
- Wang, Y.; Li, X.; Xue, Z.; Dai, L.; Xie, S.; Li, Q. Preparation of Zeolite ANA Crystal from Zeolite Y by In Situ Solid Phase Iso-Structure Transformation. J. Phys. Chem. B 2010, 114, 5747–5754. [Google Scholar] [CrossRef]
- Tendeloo, L.V.; Gobechiya, E.; Breynaert, E.; Martens, J.A.; Kirschhock, C.E.A. Alkaline Cations Directing the Transformation of FAU Zeolites into Five Different Framework Types. Chem. Commun. 2013, 49, 11737–11739. [Google Scholar] [CrossRef]
- Buhl, J.C. Enhanced Methods of Crystallization: The Crossover Synthesis from Gel to Melt Flow—A Case Study on Sodalites. Microporous Mesoporous Mater. 2016, 236, 13–20. [Google Scholar] [CrossRef]
- Li, C.; Moliner, M.; Corma, A. Building Zeolites from Precrystallized Units: Nanoscale Architecture. Angew. Chem. Int. Ed. 2018, 57, 15330–15353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, J.; Wakihara, T.; Okubo, T. Ultrafast Synthesis of Zeolites: Breakthrough, Progress and Perspective. Inorg. Chem. Front. 2019, 6, 14–31. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 27 October 2020).
- Trill, H.; Eckert, H.; Srdanov, V.I. Mixed Halide Sodalite Solid Solution Systems. Hydrothermal Synthesis and Structural Characterization by Solid State NMR. J. Phys. Chem. B 2003, 107, 8779–8788. [Google Scholar] [CrossRef]
- Kato, Y.; Ono, L.K.; Lee, M.V.; Wang, S.; Raga, S.R.; Qi, Y. Silver Iodide Formation in Methyl Ammonium Lead Iodide Perovskite Solar Cells with Silver Top Electrodes. Adv. Mater. Interfaces 2015, 2, 2–7. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Huang, B.; Cheng, H.; Wang, Z.; Zhan, J.; Qin, X.; Zhang, X.; Dai, Y. Controlled Synthesis of Ag2O Microcrystals with Facet-Dependent Photocatalytic Activities. J. Mater. Chem. 2012, 22, 21189–21194. [Google Scholar] [CrossRef]
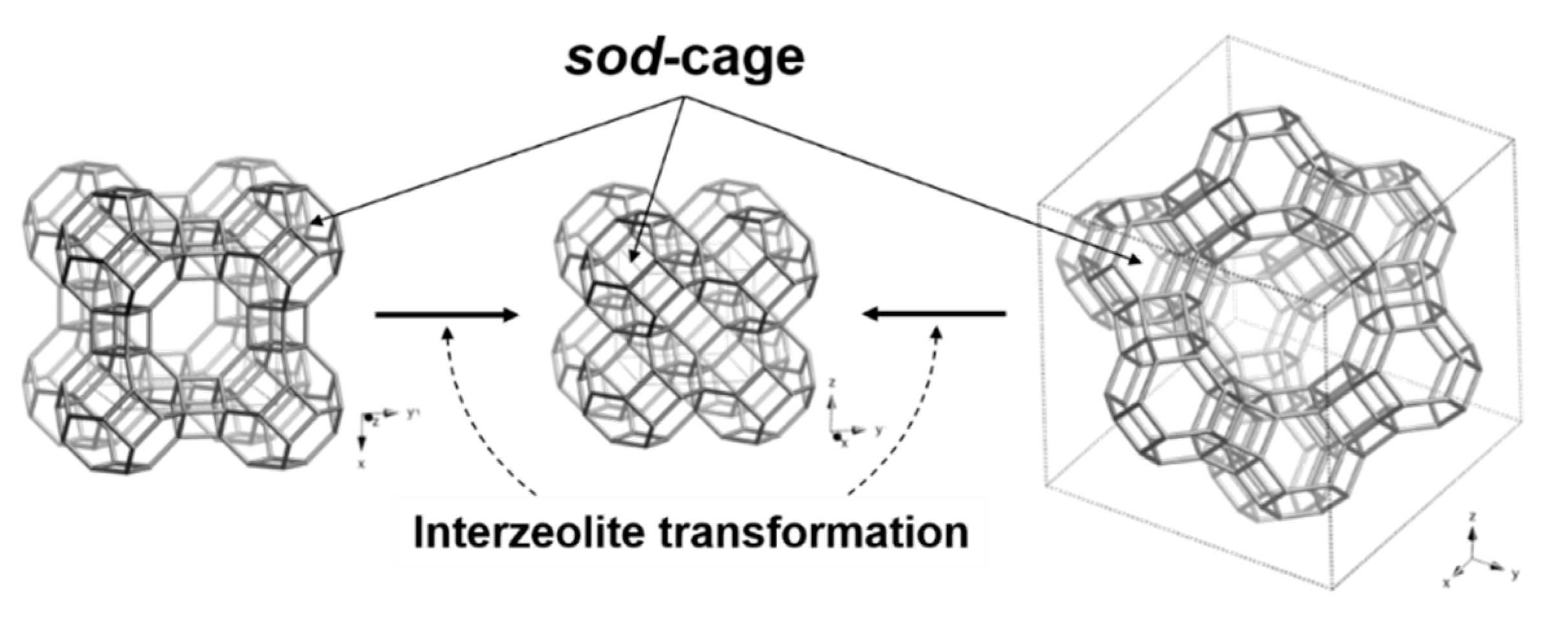


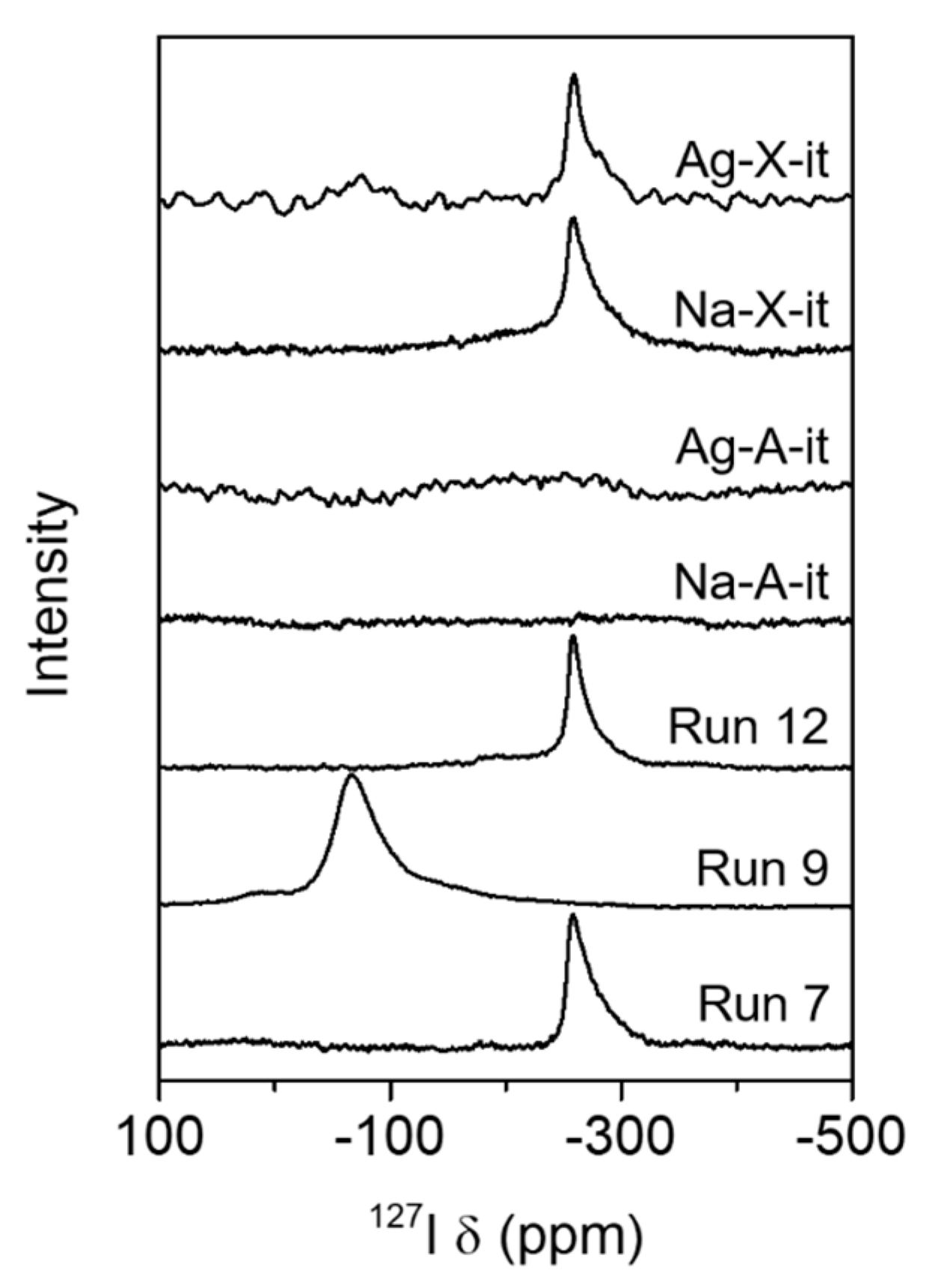

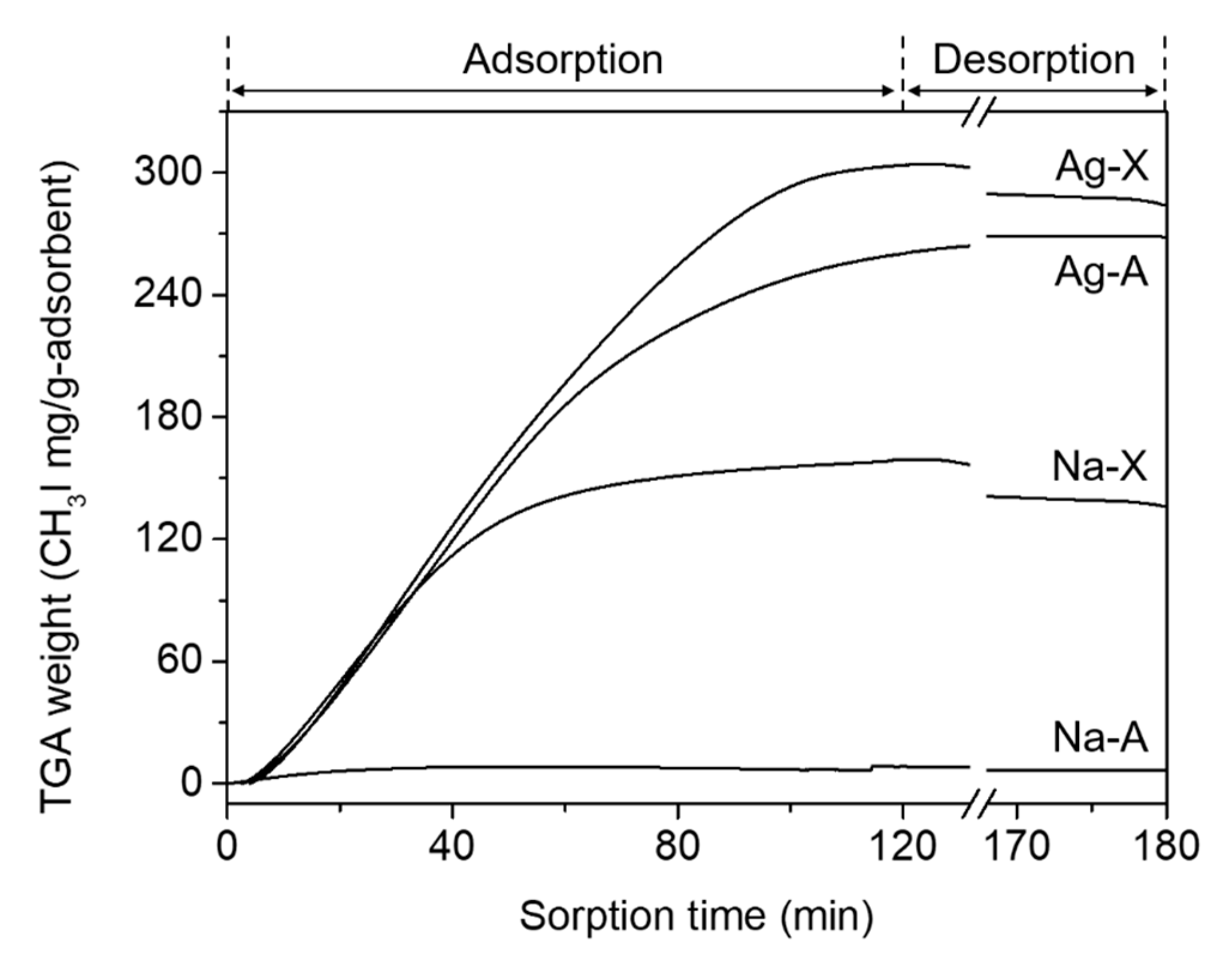
| Run No. | Parent Zeolite 2 | IZA Code | Synthesis Composition | Product Phase 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Si/Al | NaOH/Al | MI/Al | H2O/Al | |||||
| M = Na | M = Ag | |||||||
| 1 | Na-A | LTA | 1.0 | 1 | - | - | 80 | SOD + GIS 4 |
| 2 | Na-A | LTA | 1.0 | 2 | - | - | 80 | SOD |
| 3 | Na-A | LTA | 1.0 | 3 | - | - | 80 | SOD |
| 4 | Na-A | LTA | 1.0 | 4 | - | - | 80 | SOD |
| 5 | Na-A | LTA | 1.0 | 1 | 0.3 | - | 80 | SOD + GIS 4 |
| 6 | Na-A | LTA | 1.0 | 2 | 0.3 | - | 80 | SOD |
| 7 | Na-A | LTA | 1.0 | 4 | 0.3 | - | 80 | SOD |
| 8 | Na-A | LTA | 1.0 | 2 | - | 0.3 | 80 | SOD + AgI + Ag |
| 9 | Na-A | LTA | 1.0 | 4 | - | 0.3 | 80 | SOD + AgI + Ag |
| 10 | Na-A | LTA | 1.0 | 2 | - | 0.1 | 80 | SOD + AgI + Ag |
| 11 | Na-X | FAU | 1.2 | 2 | - | - | 80 | SOD + ANA + U 5 |
| 12 | Na-X | FAU | 1.2 | 2 | 0.3 | - | 80 | SOD + ANA |
| 13 | Na-X | FAU | 1.2 | 2 | - | 0.3 | 80 | SOD + AgI + Ag |
| 14 | Na-X | FAU | 1.2 | 4 | - | 0.3 | 80 | SOD + AgI + Ag |
| 15 | Na-X | FAU | 1.2 | 4 | - | 0.1 | 80 | SOD + AgI + Ag |
| 16 | Na-Y | FAU | 2.6 | 4 | 0.3 | - | 80 | ANA |
| 17 | Na-Y | FAU | 2.6 | 4 | - | 0.3 | 80 | ANA + AgI + Ag |
| 18 1 | Na-Y | FAU | 1.0 | 2 | 0.3 | - | 80 | SOD + LTA + U 5 |
| 19 | Na-A-ad | LTA | 1.0 | 4 | - | - | 80 | SOD |
| 20 | Ag-A-ad | LTA | 1.0 | 4 | - | - | 80 | SOD + Ag |
| 21 | Na-X-ad | FAU | 1.2 | 2 | - | - | 80 | SOD + ANA |
| 22 | Ag-X-ad | FAU | 1.2 | 2 | - | - | 80 | SOD + ANA + Ag |
| Product | Na/Al | I/Al 2 | Na/I | I−/Sod-Cage 3 |
|---|---|---|---|---|
| Run 7 | 1.1 | 0.16 (0.01) | 7.2 | 0.5 |
| Run 12 | 0.60 | 0.34 (0.00) | 1.8 | 1.0 |
| Na-X-it | 0.57 | 0.26 (0.06) | 2.2 | 0.8 |
| Ag-X-it | 0.63 | 0.13 (0.04) | 4.7 | 0.4 |
| Adsorbent | Si/Al 3 | Na/Al | Ag/Al | CH3I/Adsorbent (mg/g) | I/Adsorbent (mg/g) |
|---|---|---|---|---|---|
| Na-A | 1.0 | 0.89 | - | 6 | 5 |
| Ag-A | - | - | 0.48 | 260 | 230 |
| Na-X | 1.2 | 0.43 | - | 140 | 120 |
| Ag-X | - | - | 0.43 | 280 | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.; Kweon, S.; Park, S.; Lee, J.; Min, H.-K.; Park, M.B. Immobilization of Radioiodine via an Interzeolite Transformation to Iodosodalite. Nanomaterials 2020, 10, 2157. https://doi.org/10.3390/nano10112157
An H, Kweon S, Park S, Lee J, Min H-K, Park MB. Immobilization of Radioiodine via an Interzeolite Transformation to Iodosodalite. Nanomaterials. 2020; 10(11):2157. https://doi.org/10.3390/nano10112157
Chicago/Turabian StyleAn, Hyejin, Sungjoon Kweon, Sanggil Park, Jaeyoung Lee, Hyung-Ki Min, and Min Bum Park. 2020. "Immobilization of Radioiodine via an Interzeolite Transformation to Iodosodalite" Nanomaterials 10, no. 11: 2157. https://doi.org/10.3390/nano10112157
APA StyleAn, H., Kweon, S., Park, S., Lee, J., Min, H.-K., & Park, M. B. (2020). Immobilization of Radioiodine via an Interzeolite Transformation to Iodosodalite. Nanomaterials, 10(11), 2157. https://doi.org/10.3390/nano10112157





