Peculiarities of Low-Temperature Behavior of Liquids Confined in Nanostructured Silicon-Based Material
Abstract
1. Introduction
2. Experimental
2.1. Formation of Por-Si
2.2. Characterization of Por-Si
2.3. Differential Scanning Calorimetry
3. Results
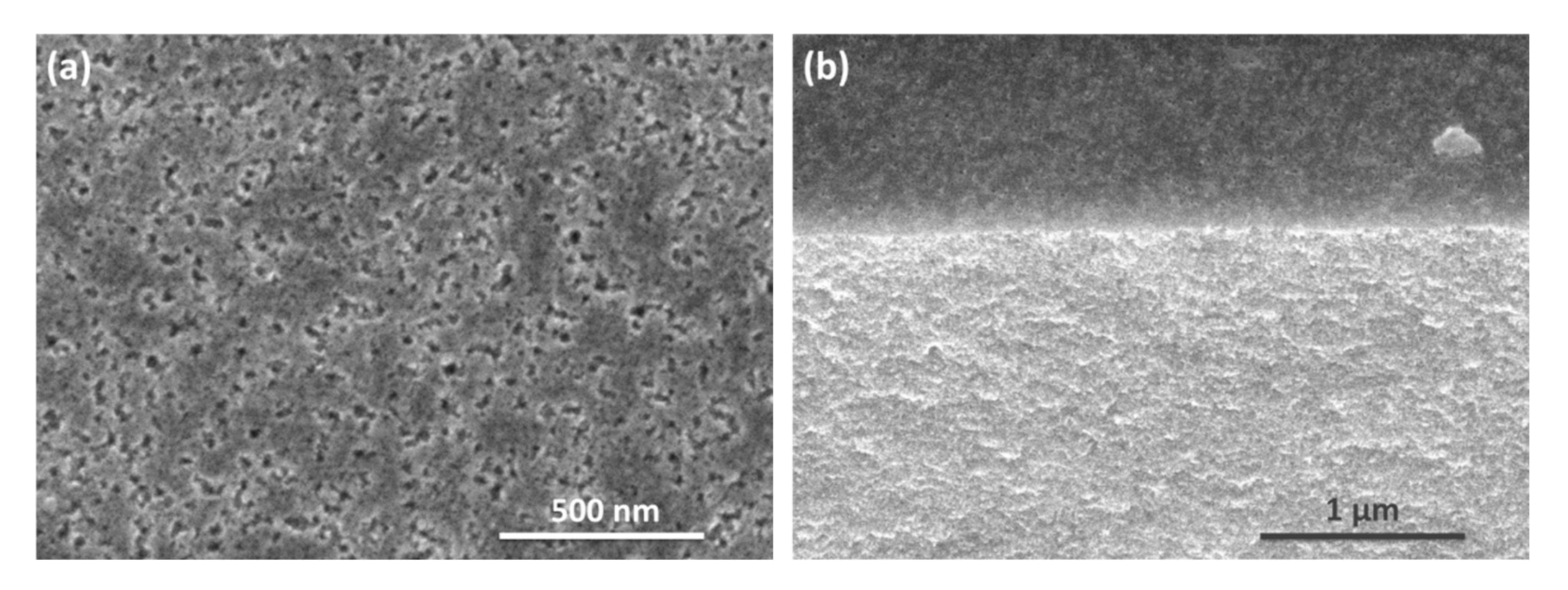
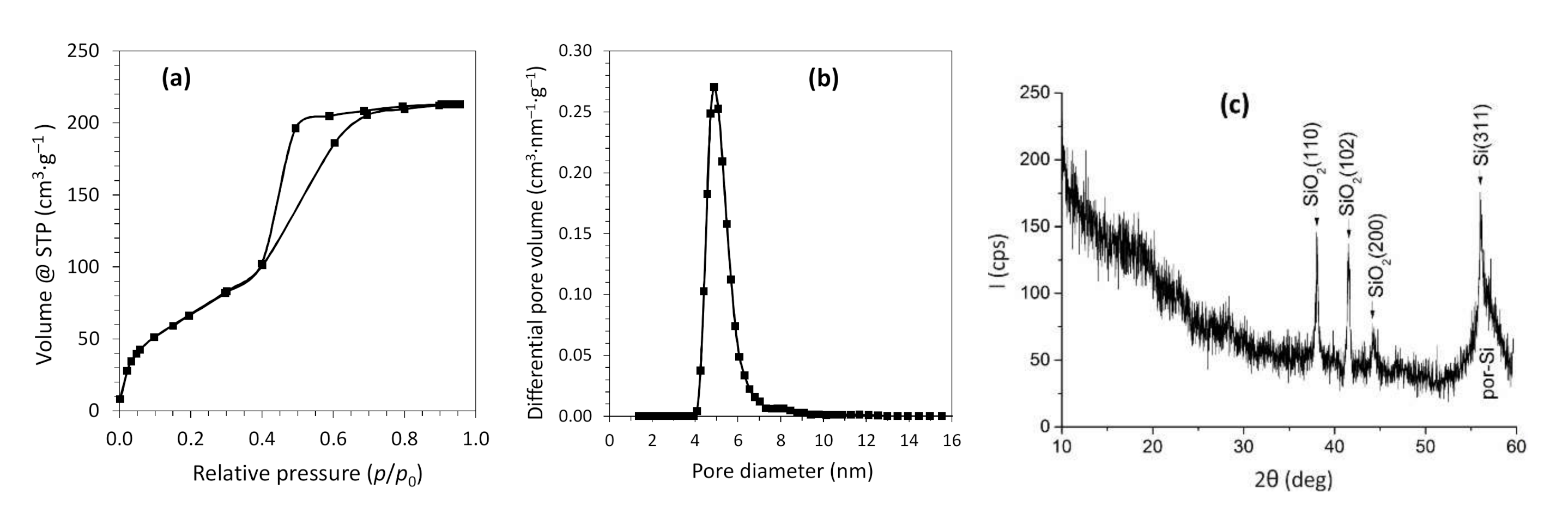
3.1. Structural Properties of the Nanostructured Si Matrices
3.2. DSC Investigations
4. Model
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, C.M.; Kim, J.H.; Kim, H.; Sohn, H.J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Luo, W.; Wang, L.; Jiang, W.; Yang, J. Silicon: Toward eco-friendly reduction techniques for lithium-ion battery applications. J. Mater. Chem. A 2019, 7, 24715–24737. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Ma, T.; Rong, J.; Zhang, R.; Shaffer, J.; An, Y.; Liu, Q.; Wei, B.; Jiang, H. Silicon thin films as anodes for high-performance lithium-ion batteries with effective stress relaxation. Adv. Energy Mater. 2012, 2, 68–73. [Google Scholar] [CrossRef]
- Takamura, T.; Ohara, S.; Uehara, M.; Suzuki, J.; Sekine, K. A vacuum deposited Si film having a Li extraction capacity over 2000 mAh/g with a long cycle life. J. Power Sources 2004, 129, 96–100. [Google Scholar] [CrossRef]
- Liang, K.; Yang, H.; Guo, W.; Du, J.; Tian, L.; Wen, X. Facile preparation of nanoscale silicon as an anode material for lithium ion batteries by a mild temperature metathesis route. J. Alloy Compd. 2018, 735, 441–444. [Google Scholar] [CrossRef]
- Song, T.; Xia, J.; Lee, J.H.; Lee, D.H.; Kwon, M.S.; Choi, J.M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.I.; et al. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef]
- Tian, H.; Tan, X.; Xin, F.; Wang, C.; Han, W. Micro-sized nano-porous Si/C anodes for lithium ion batteries. Nano Energy 2015, 11, 490–499. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L.; Zhou, G.; Zhang, Z.; Yu, D.; Mo, Y.J.; Pei, N. The crystal structural evolution of nano-Si anode caused by lithium insertion and extraction at room temperature. Solid State Ion. 2000, 135, 181–191. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-based anode materials for Li-ion batteries: A mini review. Nano-Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef]
- Dunn, J.R.; Hudec, P.P. Water, Clay and Rock Soundness. Ohio J. Sci. 1966, 66, 153–168. [Google Scholar]
- Gillott, J.E. Properties of aggregates affecting concrete in North America. Q. J. Eng. Geol. Hydrogeol. 1980, 13, 289–303. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.C. Structure of confined water. J. Phys. Condens. Matter 2001, 13, 9165. [Google Scholar] [CrossRef]
- Dutta, D.; Sachdeva, A.; Pujari, P.K. Positron Annihilation Studies on the Phase Transition of Benzene and Reactivity of Nitrobenzene in Confined Framework of ZSM-5 Zeolite. Chem. Phys. Lett. 2006, 432, 116–121. [Google Scholar] [CrossRef]
- Lee, J.A.; Rosner, H.; Corrigan, J.F.; Huang, Y. Phase transitions of naphthalene and its derivatives confined in mesoporous silicas. J. Phys. Chem. C 2011, 115, 4738–4748. [Google Scholar] [CrossRef]
- Nwaka, D.; Tahmasebi, A.; Tian, L.; Yu, J. The effects of pore structure on the behavior of water in lignite coal and activated carbon. J. Colloid Interf. Sci 2016, 477, 138–147. [Google Scholar] [CrossRef]
- Domin, K.; Chan, K.Y.; Yung, H.; Gubbins, K.E.; Jarek, M.; Sterczynska, A.; Sliwinska-Bartkowiak, M. Structure of ice in confinement: Water in mesoporous carbons. J. Chem. Eng. Data 2016, 61, 4252–4260. [Google Scholar] [CrossRef]
- Alba-Simionesco, C.; Coasne, B.; Dosseh, G.; Dudziak, G.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Effects of confinement on freezing and melting. J. Phys. Condens. Matter 2006, 18, R15–R68. [Google Scholar] [CrossRef]
- Maniwa, Y.; Kataura, H.; Abe, M.; Suzuki, S.; Achiba, Y.; Kira, H.; Matsuda, K. Phase transition in confined water inside carbon nanotubes. J. Phys. Soc. Jpn. 2002, 71, 2863–2866. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Majid, A.A.; Martínez-Escandell, M.; Daemen, L.L.; Missyul, A.; Koh, C.; Silvestre-Albero, J. Freezing/melting of water in the confined nanospace of carbon materials: Effect of an external stimulus. Carbon 2020, 158, 346–355. [Google Scholar] [CrossRef]
- Jähnert, S.; Chávez, F.V.; Schaumann, G.E.; Schreiber, A.; Schönhoff, M.; Findenegg, G.H. Melting and freezing of water in cylindrical silica nanopores. Phys. Chem. Chem. Phys. 2008, 10, 6039–6051. [Google Scholar] [CrossRef]
- Turov, V.V.; Gun’ko, V.M.; Zarko, V.I.; Goncharuk, O.V.; Krupska, T.V.; Turov, A.V.; Leboda, R.; Skubiszewska-Zieba, J. Interfacial behavior of n-decane bound to weakly hydrated silica gel and nanosilica over a broad temperature range. Langmuir 2013, 29, 4303–4314. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Agrawal, K.V.; O’Mahony, M.; Drahushuk, L.W.; Manohar, N.; Myerson, A.S.; Strano, M.S. Understanding and analyzing freezing-point transitions of confined fluids within nanopores. Langmuir 2015, 31, 10113–10118. [Google Scholar] [CrossRef] [PubMed]
- Maloney, T.C. Thermoporosimetry of hard (silica) and soft (cellulosic) materials by isothermal step melting. J. Therm. Anal. Calorim. 2015, 121, 7–17. [Google Scholar] [CrossRef]
- Charmas, B.; Skubiszewska-Zięba, J. Application of differential scanning calorimetry to study porous structure of hydrothermally modified silicas. J. Therm. Anal. Calorim. 2017, 129, 23–32. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Shilyaeva, Y.I.; Bardushkin, V.V.; Gavrilov, S.A.; Silibin, M.V.; Yakovlev, V.B.; Pyatilova, O.V. Bulk density of the energy of deformation in an anodic aluminum oxide with pores filled by threadlike metal nanocrystals. Russ. J. Phys. Chem. A 2013, 87, 1870–1874. [Google Scholar] [CrossRef]
- Shilyaeva, Y.I.; Bardushkin, V.V.; Gavrilov, S.A.; Silibin, M.V.; Yakovlev, V.B.; Borgardt, N.I.; Volkov, R.L.; Smirnov, D.I.; Zheludkevich, M.L. Melting temperature of metal polycrystalline nanowires electrochemically deposited into the pores of anodic aluminum oxide. Phys. Chem. Chem. Phys. 2014, 16, 19394–19401. [Google Scholar] [CrossRef] [PubMed]
- Solanki, C.S.; Bilyalov, R.R.; Poortmans, J.; Celis, J.P.; Nijs, J.; Mertens, R. Self-standing porous silicon films by one-step anodizing. J. Electrochem. Soc. 2004, 151, C307. [Google Scholar] [CrossRef]
- Volovlikova, O.V.; Gavrilov, S.A.; Silakov, G.O.; Zheleznyakova, A.V.; Dudin, A.A. Preparation of Hydrophobic Porous Silicon by Metal-Assisted Etching with Pd-Catalyst. Russ. J. Electrochem. 2019, 55, 1186–1195. [Google Scholar] [CrossRef]
- Shilyaeva, Y.; Gavrilov, S.; Dudin, A.; Matyna, L.; Shulyat’ev, A.; Volkova, A.; Zheleznyakova, A. Anodic aluminium oxide templates for synthesis and study of thermal behaviour of metallic nanowires. Surf. Interface Anal. 2016, 48, 934–938. [Google Scholar] [CrossRef]
- Washburn, E.W.; Smith, E. Note on an electrical conductance method for determining liquefaction temperatures of solids. J. Res. Natl. Bur. Stand. 1929, 2, 787–791. [Google Scholar] [CrossRef]
- Shi, Z.; Wynblatt, P.; Srinivasan, S.G. Melting behavior of nanosized lead particles embedded in an aluminum matrix. Acta Mater. 2004, 52, 2305–2316. [Google Scholar] [CrossRef]
- Landry, M.R. Thermoporometry by differential scanning calorimetry: Experimental considerations and applications. Thermochim. Acta 2005, 433, 27–50. [Google Scholar] [CrossRef]
- Faivre, C.; Bellet, D.; Dolino, G. Phase transitions of fluids confined in porous silicon: A differential calorimetry investigation. Eur. Phys. J. B 1999, 7, 19–36. [Google Scholar] [CrossRef]
- Bardushkin, V.V.; Kirillov, D.A.; Shilyaeva, Y.I.; Gavrilov, S.A.; Yakovlev, V.B.; Silibin, M.V. Effect of the thermoelastic properties of components on the melting point of filamentary nanoparticles of Cu, Ag, and Au in the matrix of anodic Al2O3. Russ. J. Phys. Chem. A 2017, 91, 1099–1104. [Google Scholar] [CrossRef]
- Kolesnikov, V.I.; Yakovlev, V.B.; Bardushkin, V.V.; Sychev, A.P. O prognozirovanii raspredelenij lokal’nykh uprugikh polej v neodnorodnykh sredakh na osnove obobshhennogo singulyarnogo priblizheniya [On the prediction of local elastic fields’ distributions in non-uniform media on the basis of a generalized singular approximation]. Vestnik Yuzhnogo nauchnogo tsentra RAN Bull. South Sci. Center Russ. Acad. Sci. 2015, 11, 11–17. (In Russian) [Google Scholar]
- Shermergor, T.D. Teoriya Uprugosti Mikroneodnorodnykh Sred [Micromechanics of Inhomogeneous Medium]; Nauka: Moscow, Russia, 1977; p. 399. (In Russian) [Google Scholar]
- Kolesnikov, V.I.; Bardushkin, V.V.; Sorokin, A.I.; Sychev, A.P.; Yakovlev, V.B. Effect of thermoelastic characteristics of components, shape of non-isometric inclusions, and their orientation on average stresses in matrix structures. Phys. Mesomech. 2018, 21, 258–262. [Google Scholar] [CrossRef]
- Belosludov, V.R.; Inerbaev, T.M.; Shpakov, V.P.; Tse, D.S.; Belosludov, R.V.; Kavazoe, E. Moduli uprugosti i granitsy stabil’nosti l’dov i klatratnykh gidratov kubicheskoy struktury I [Moduli of elasticity and stability boundaries of ice and clathrate hydrates of cubic structure I]. Ros. khim. zh. (ZH. Ros. khim. ob-va im. D.I. Mendeleyeva) 2001, XLV, 45–50. (In Russian) [Google Scholar]
- Schulson, E.M. The Structure and Mechanical Behavior of Ice. JOM 1999, 51, 21–27. [Google Scholar] [CrossRef]
- Grigor’ev, I.S.; Meilikhov, E.Z. (Eds.) Fizicheskie Velichiny: Spravochnik [Physical Quantities: Handbook]; Pod. red.; Energoatomizdat: Moscow, Russia, 1991; p. 1232. (In Russian) [Google Scholar]
- Bahloul, N.; Baba, M.; Nedelec, J.M. Universal behavior of linear alkanes in a confined medium: Toward a calibrationless use of thermoporometry. J. Phys. Chem. B 2005, 109, 16227–16229. [Google Scholar] [CrossRef] [PubMed]
- Ishikiriyama, K.; Todoki, M. Evaluation of water in silica pores using differential scanning calorimetry. Thermochim. Acta 1995, 256, 213–226. [Google Scholar] [CrossRef]





| Liquid | Tonset, K | Tpeak, K | Tpeak,∞, K | ΔT * | Tm,calc, K ** |
|---|---|---|---|---|---|
| deionized water | 247 | 256 | 274 | 18 | 247–257 |
| 0.6 M aqueous K2SO4 solution | 242 | 254 | 273 | 19 | – |
| n-decane | 214 | 224 | 243 | 19 | 194–219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardushkin, V.; Kochetygov, A.; Shilyaeva, Y.; Volovlikova, O.; Dronov, A.; Gavrilov, S. Peculiarities of Low-Temperature Behavior of Liquids Confined in Nanostructured Silicon-Based Material. Nanomaterials 2020, 10, 2151. https://doi.org/10.3390/nano10112151
Bardushkin V, Kochetygov A, Shilyaeva Y, Volovlikova O, Dronov A, Gavrilov S. Peculiarities of Low-Temperature Behavior of Liquids Confined in Nanostructured Silicon-Based Material. Nanomaterials. 2020; 10(11):2151. https://doi.org/10.3390/nano10112151
Chicago/Turabian StyleBardushkin, Vladimir, Andrey Kochetygov, Yulia Shilyaeva, Olga Volovlikova, Alexey Dronov, and Sergey Gavrilov. 2020. "Peculiarities of Low-Temperature Behavior of Liquids Confined in Nanostructured Silicon-Based Material" Nanomaterials 10, no. 11: 2151. https://doi.org/10.3390/nano10112151
APA StyleBardushkin, V., Kochetygov, A., Shilyaeva, Y., Volovlikova, O., Dronov, A., & Gavrilov, S. (2020). Peculiarities of Low-Temperature Behavior of Liquids Confined in Nanostructured Silicon-Based Material. Nanomaterials, 10(11), 2151. https://doi.org/10.3390/nano10112151





