The Effect of RANKL/OPG Balance on Reducing Implant Complications
Abstract
:1. Introduction—The Clinical Need
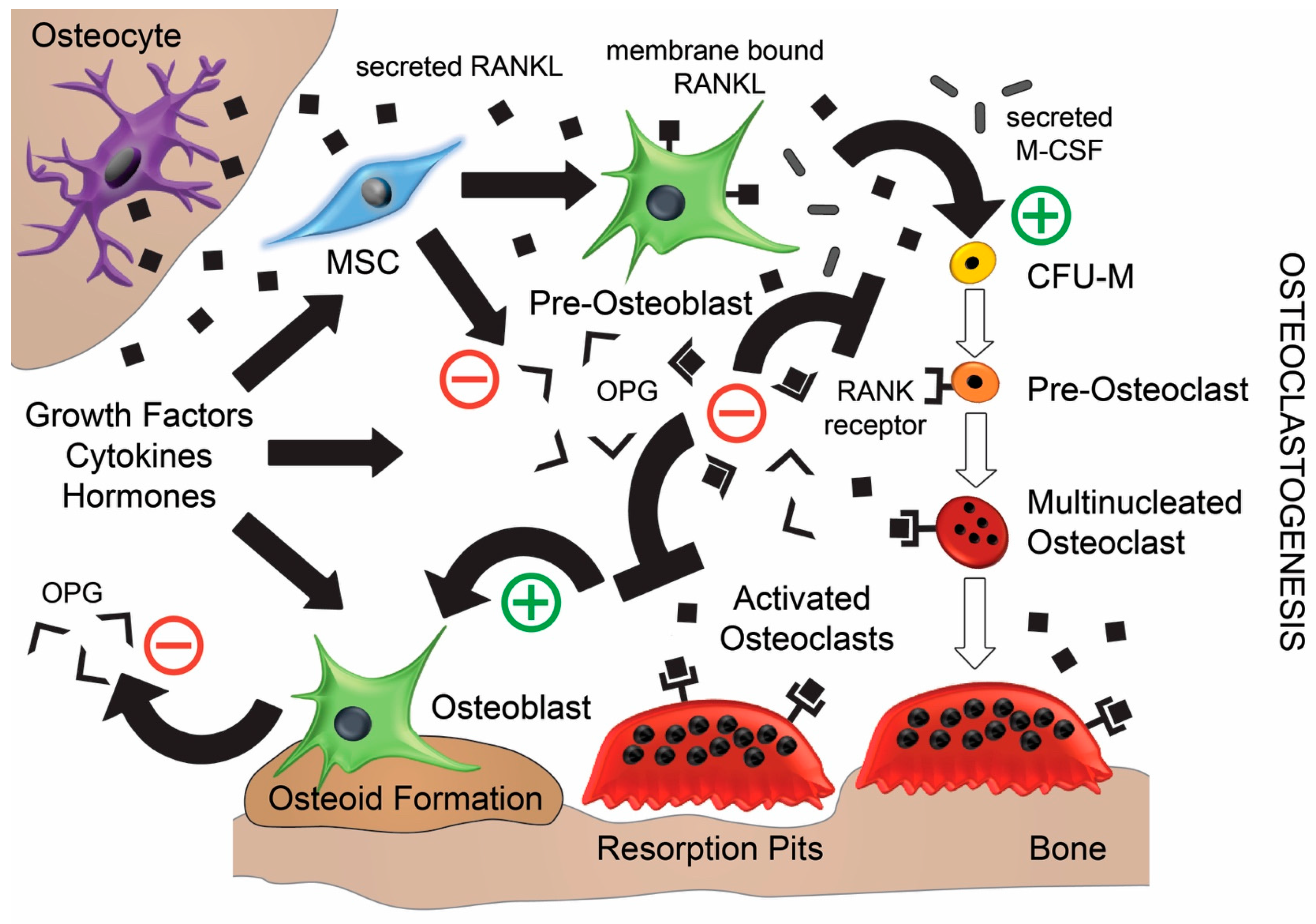
2. The Roles of RANK, RANKL, and OPG in Bone Remodelling
3. The Bone Repair Cascade Post-Implantation
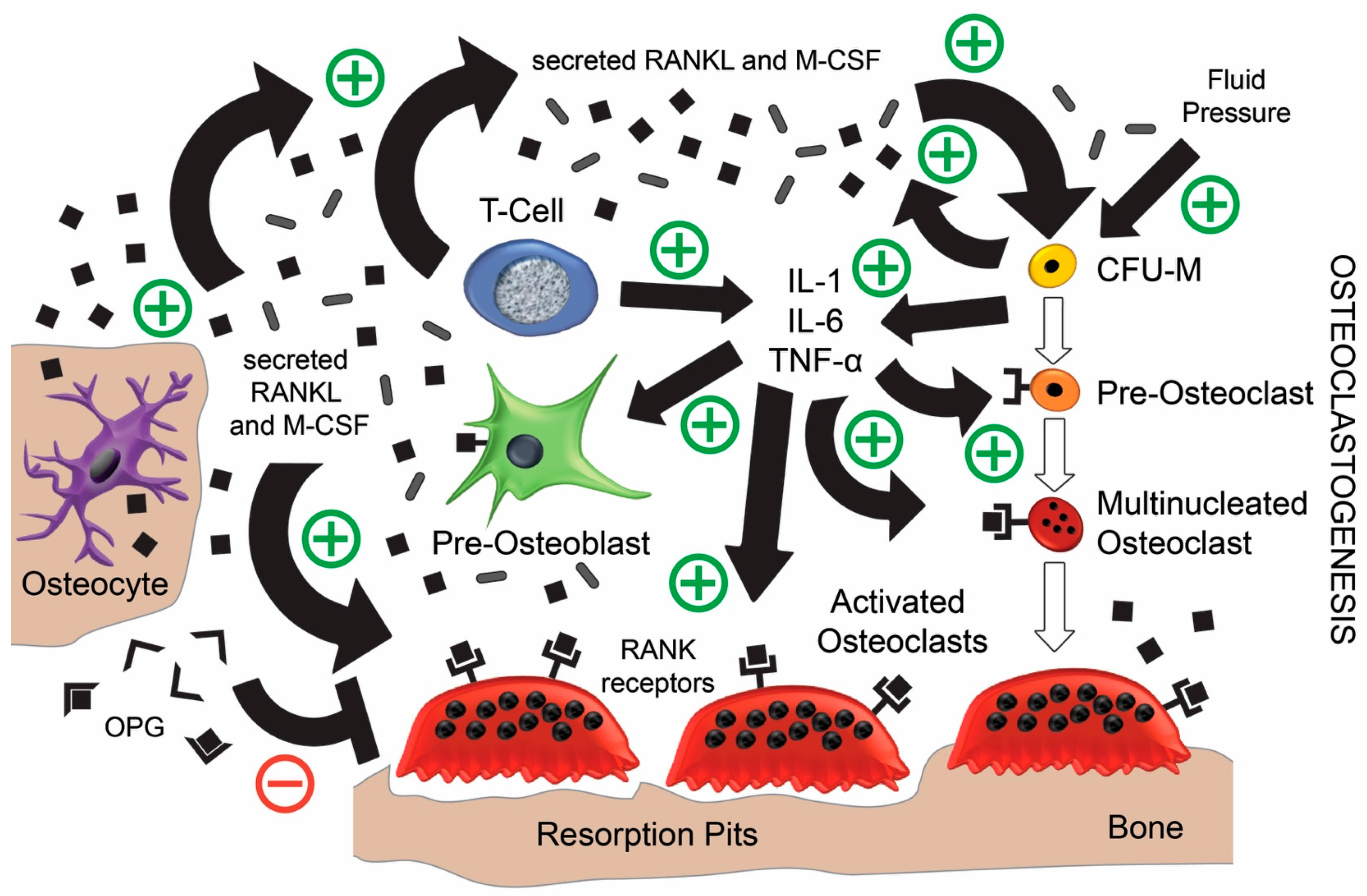
4. Post-Implantation Complications
5. Current Clinical Solutions to Correct the RANKL/OPG Balance
5.1. Correcting the Intrinsic RANKL/OPG Balance Internally
5.2. Enhancing the RANKL/OPG Ratio Extrinsically
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haynes, D.R. Bone lysis and inflammation. Inflamm. Res. 2004, 53, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Clough, J. The Cleveland Clinic Guide to Arthritis; Kaplan Publishing: New York, NY, USA, 2009; p. 245. [Google Scholar]
- Gwinnutt, J.M.; Symmons, D.P.M.; MacGregor, A.J.; Chipping, J.R.; Lapraik, C.; Marshall, T.; Lunt, M.; Verstappen, S.M.M. Predictors of and outcomes following orthopaedic joint surgery in patients with early rheumatoid arthritis followed for 20 years. Rheumatology 2017, 56, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.; Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Crotti, T.N.; Dharmapatni, A.A.; Alias, E.; Haynes, D.R. Osteoimmunology: Major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. J. Immunol. Res. 2015, 2015, 281287. Available online: https://www.hindawi.com/journals/jir/2015/281287/ (accessed on 20 September 2017). [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9, S1. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Bonnelye, E. Osteoprotegerin and its ligand: A new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporos. Int. 2000, 11, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Kostenuik, P.J. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr. Opin. Pharmacol. 2005, 5, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.; McCarthy, H.S.; Middleton, J.; Marshall, M.J. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr. Rev. Musculoskelet. Med. 2009, 2, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Verborgt, O.; Gibson, G.J.; Schaffler, M.B. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J. Bone Miner. Res. 2000, 15, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Colopy, S.; Benz-Dean, J.; Barrett, J.; Sample, S.; Lu, Y.; Danova, N.; Kalscheur, V.; Vanderby, R.; Markel, M.; Muir, P. Response of the osteocyte syncytium adjacent to and distant from linear microcracks during adaptation to cyclic fatigue loading. Bone 2004, 35, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Fazzalari, N.L. Bone fracture and bone fracture repair. Osteoporos. Int. 2011, 22, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Sharaf-Eldin, W.E.; Abu-Shahba, N.; Mahmoud, M.; El-Badri, N. The modulatory effects of mesenchymal stem cells on osteoclastogenesis. Stem Cells Int. 2016, 2016, 1908365. Available online: https://www.hindawi.com/journals/sci/2016/1908365/ (accessed on 20 September 2017). [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica 2013, 2013, 125705. Available online: https://www.hindawi.com/journals/scientifica/2013/125705/ (accessed on 20 September 2017). [CrossRef] [PubMed]
- Kong, Y.-Y.; Boyle, W.J.; Penninger, J.M. Osteoprotegerin ligand: A regulator of immune responses and bone physiology. Immunol. Today 2000, 21, 495–502. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Penninger, J.M. RANK/RANKl: Regulators of immune responses and bone physiology. Ann. N. Y. Acad. Sci. 2008, 1143, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Ai-Aql, Z.; Alagl, A.; Graves, D.; Gerstenfeld, L.; Einhorn, T. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Cho, T.J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of nf-κb ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, H.; Shu, B.; Mira, R.R.; Chen, D. Chondrocytes-specific expression of osteoprotegerin modulates osteoclast formation in metaphyseal bone. Sci. Rep. 2015, 5, 13667. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.R.; Crotti, T.N.; Zreiqat, H. Regulation of osteoclast activity in peri-implant tissues. Biomaterials 2004, 25, 4877–4885. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.R.; Crotti, T.N.; Potter, A.E.; Loric, M.; Atkins, G.J.; Howie, D.W.; Findlay, D.M. The osteoclastogenic molecules RANKL and rank are associated with periprosthetic osteolysis. J. Bone Jt. Surg. Br. 2001, 83, 902–911. [Google Scholar] [CrossRef]
- Crotti, T.N.; Smith, M.D.; Findlay, D.M.; Zreiqat, H.; Ahern, M.J.; Weedon, H.; Hatzinikolous, G.; Capone, M.; Holding, C.; Haynes, D.R. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: Expression of receptor activator nfκb, rank ligand and osteoprotegerin. Biomaterials 2004, 25, 565–573. [Google Scholar] [CrossRef]
- Masui, T.; Sakano, S.; Hasegawa, Y.; Warashina, H.; Ishiguro, N. Expression of inflammatory cytokines, RANKL and OPG induced by titanium, cobalt-chromium and polyethylene particles. Biomaterials 2005, 26, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Holding, C.A.; Findlay, D.M.; Stamenkov, R.; Neale, S.D.; Lucas, H.; Dharmapatni, A.; Callary, S.A.; Shrestha, K.R.; Atkins, G.J.; Howie, D.W. The correlation of RANK, RANKL and TNF-α expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials 2006, 27, 5212–5219. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Lin, Y.-T.; Chiang, B.-L.; Lee, S.-S.; Hou, S.-M. Over-expression of receptor activator of nuclear factor-κb ligand (RANKL), inflammatory cytokines, and chemokines in periprosthetic osteolysis of loosened total hip arthroplasty. Biomaterials 2010, 31, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Cenni, E.; Savarino, L.; Ciapetti, G.; Forbicini, G.; Vancini, M.; Maini, C.; Baldini, N.; Giunti, A. Bone cement extracts modulate the osteoprotegerin/osteoprotegerin-ligand expression in MG63 osteoblast-like cells. Biomaterials 2002, 23, 2359–2365. [Google Scholar] [CrossRef]
- Tao, K.; Zeng, H.; Xiao, D.M.; Xiong, A.; Weng, J.; Kang, B. Influences of IL-6R antibody on PMMA bone cement-mediated expression of OPG and RANKL in synovial fibroblasts. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.-T.A.; Nguyen, H.; Gao, X.; Kong, Y.-Y.; Gorczynski, R.M.; Singh, B.; Ellen, R.P.; Penninger, J.M. Functional human t-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 2000, 106, R59–R67. [Google Scholar] [CrossRef] [PubMed]
- Pioletti, D.P.; Kottelat, A. The influence of wear particles in the expression of osteoclastogenesis factors by osteoblasts. Biomaterials 2004, 25, 5803–5808. [Google Scholar] [CrossRef] [PubMed]
- Lader, C.; Flanagan, A. Prostaglandin e2, interleukin 1α, and tumor necrosis factor-α increase human osteoclast formation and bone resorption in vitro. Endocrinology 1998, 139, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Sabokbar, A.; Kudo, O.; Athanasou, N. Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J. Orthop. Res. 2003, 21, 73–80. [Google Scholar] [CrossRef]
- Neale, S.D.; Sabokbar, A.; Howie, D.W.; Murray, D.W.; Athanasou, N.A. Macrophage colony-stimulating factor and interleukin-6 release by periprosthetic cells stimulates osteoclast formation and bone resorption. J. Orthop. Res. 1999, 17, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, P.E.; Chang, E.; Preston, C.F.; Liu, C.-J. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J. Bone Jt. Surg. 2005, 87, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Otogoto, J.; Ota, N.; Togari, A. Differential expression of rankl and osteoprotegerin in gingival crevicular fluid of patients with periodontitis. J. Dent. Res. 2004, 83, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Skripitz, R.; Aspenberg, P. Pressure-induced periprosthetic osteolysis: A rat model. J. Orthop. Res. 2000, 18, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [PubMed]
- Stadelmann, V.A.; Terrier, A.; Pioletti, D.P. Microstimulation at the bone–implant interface upregulates osteoclast activation pathways. Bone 2008, 42, 358–364. [Google Scholar] [CrossRef] [PubMed]
- El-Gammal, M.Y.; El-Gammal, N.Y.; Fadhil, O.N.; Maria, O.M. Biological reactions to different dental implant surface treatments. Int. J. Contemp. Dent. Med. Rev. 2015, 2015, 051015. [Google Scholar]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 791725. Available online: https://www.hindawi.com/journals/bmri/2015/791725/ (accessed on 20 September 2017). [CrossRef] [PubMed]
- Stanford, C.M. Surface modification of biomedical and dental implants and the processes of inflammation, wound healing and bone formation. Int. J. Mol. Sci. 2010, 11, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Filgueira, L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening—Current concepts. J. Biomed. Mater. Res. A 2009, 91, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Simmen, H.P.; Filgueira, L. Bio-corrosion of stainless steel by osteoclasts—In vitro evidence. J. Orthop. Res. 2009, 27, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Cadosch, D.; Sutanto, M.; Chan, E.; Mhawi, A.; Gautschi, O.P.; von Katterfeld, B.; Simmen, H.P.; Filgueira, L. Titanium uptake, induction of RANK-L expression, and enhanced proliferation of human t-lymphocytes. J. Orthop. Res. 2010, 28, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Cadosch, D.; Al-Mushaiqri, M.S.; Gautschi, O.P.; Meagher, J.; Simmen, H.P.; Filgueira, L. Biocorrosion and uptake of titanium by human osteoclasts. J. Biomed. Mater. Res. A 2010, 95, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.; Wang, X.; Schröder, H.C.; Kolb, U.; Schloßmacher, U.; Ushijima, H.; Müller, W.E. The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials 2010, 31, 7716–7725. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Heinz, C.; Muller, W.E. Enzymatically synthesised inorganic polymers morphogenetically active bone scaffolds: Application in regenerative medicine. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Elsevier Science: Waltham, MA, USA, 2014; Volume 313, pp. 27–75. [Google Scholar]
- Schwartz, Z.; Olivares-Navarrete, R.; Wieland, M.; Cochran, D.L.; Boyan, B.D. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials 2009, 30, 3390–3396. [Google Scholar] [CrossRef] [PubMed]
- Durual, S.; Pernet, F.; Rieder, P.; Mekki, M.; Cattani-Lorente, M.; Wiskott, H. Titanium nitride oxide coating on rough titanium stimulates the proliferation of human primary osteoblasts. Clin. Oral Implants Res. 2011, 22, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Pollice, P.F.; Rosier, R.N.; Looney, R.J.; Puzas, J.E.; Schwarz, E.M.; O’Keefe, R.J. Oral pentoxifylline inhibits release of tumor necrosis factor-alpha from human peripheral blood monocytes a potential treatment for aseptic loosening of total joint components. J. Bone Jt. Surg. 2001, 83, 1057–1061. [Google Scholar] [CrossRef]
- Im, G.-I.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 2004, 25, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Ferrari, S.; Russell, R.G.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Calderon, S.A.; Colman, M.W.; Raskin, K.A.; Hornicek, F.J.; Gebhardt, M. Use of bisphosphonates in orthopedic surgery. Orthop. Clin. 2014, 45, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.K.; Kronenberg, H.M. Bone development and remodeling. In Endocrinology: Adult and Pediatric, 7th ed.; DeGroot, L.J., Jameson, J.L., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2016; pp. 1038–1062. [Google Scholar]
- Bekker, P.J.; Holloway, D.; Nakanishi, A.; Arrighi, M.; Leese, P.T.; Dunstan, C.R. The effect of a single dose of osteoprotegerin in postmenopausal women. J. Bone Miner. Res. 2001, 16, 348–360. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R. Denosumab for the treatment of osteoporosis. Osteoporos. Sarcopenia 2017, 3, 8–17. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor κb ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.H.; Middlefell, L.S.; Mizen, K.D. Osteonecrosis of the jaws induced by anti-rank ligand therapy. Br. J. Oral Maxillofac. Surg. 2010, 48, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Diz, P.; López-Cedrún, J.L.; Arenaz, J.; Scully, C. Denosumab-related osteonecrosis of the jaw. J. Am. Dent. Assoc. 2012, 143, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-X.; Tang, L.-N.; He, A.-N.; Yao, Y.; Shen, Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: A meta-analysis of seven randomized controlled trials. Int. J. Clin. Oncol. 2014, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapasa, E.R.; Giannoudis, P.V.; Jia, X.; Hatton, P.V.; Yang, X.B. The Effect of RANKL/OPG Balance on Reducing Implant Complications. J. Funct. Biomater. 2017, 8, 42. https://doi.org/10.3390/jfb8040042
Kapasa ER, Giannoudis PV, Jia X, Hatton PV, Yang XB. The Effect of RANKL/OPG Balance on Reducing Implant Complications. Journal of Functional Biomaterials. 2017; 8(4):42. https://doi.org/10.3390/jfb8040042
Chicago/Turabian StyleKapasa, Elizabeth R., Peter V. Giannoudis, Xiaodong Jia, Paul V. Hatton, and Xuebin B. Yang. 2017. "The Effect of RANKL/OPG Balance on Reducing Implant Complications" Journal of Functional Biomaterials 8, no. 4: 42. https://doi.org/10.3390/jfb8040042
APA StyleKapasa, E. R., Giannoudis, P. V., Jia, X., Hatton, P. V., & Yang, X. B. (2017). The Effect of RANKL/OPG Balance on Reducing Implant Complications. Journal of Functional Biomaterials, 8(4), 42. https://doi.org/10.3390/jfb8040042







