Bioengineered Lacrimal Gland Organ Regeneration in Vivo
Abstract
:1. Introduction
2. Physiological Function of the Lacrimal Glands
3. Dry Eye Disease
4. Organogenesis of the Lacrimal Glands
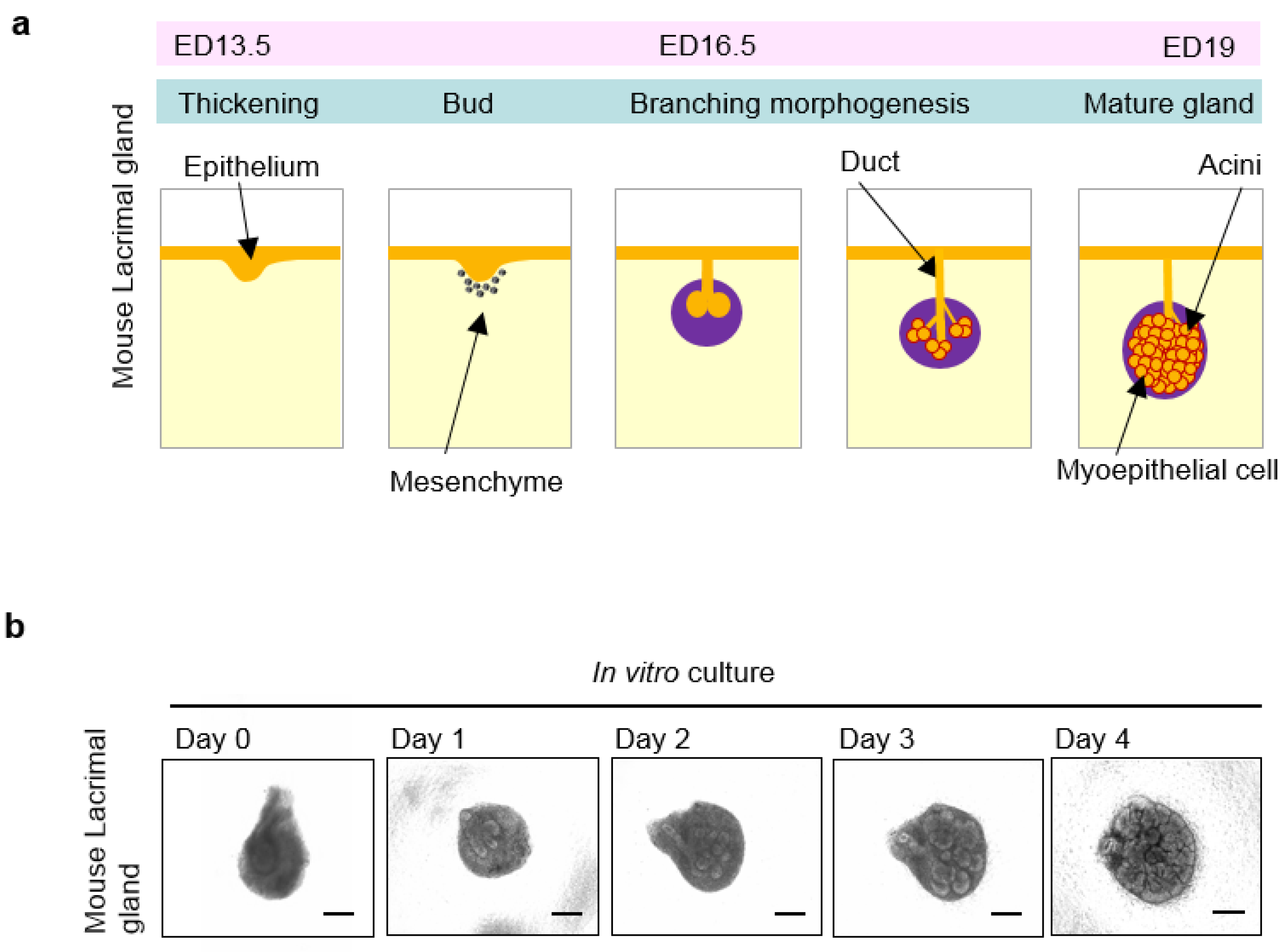
5. Tissue Stem Cells in the Lacrimal Gland
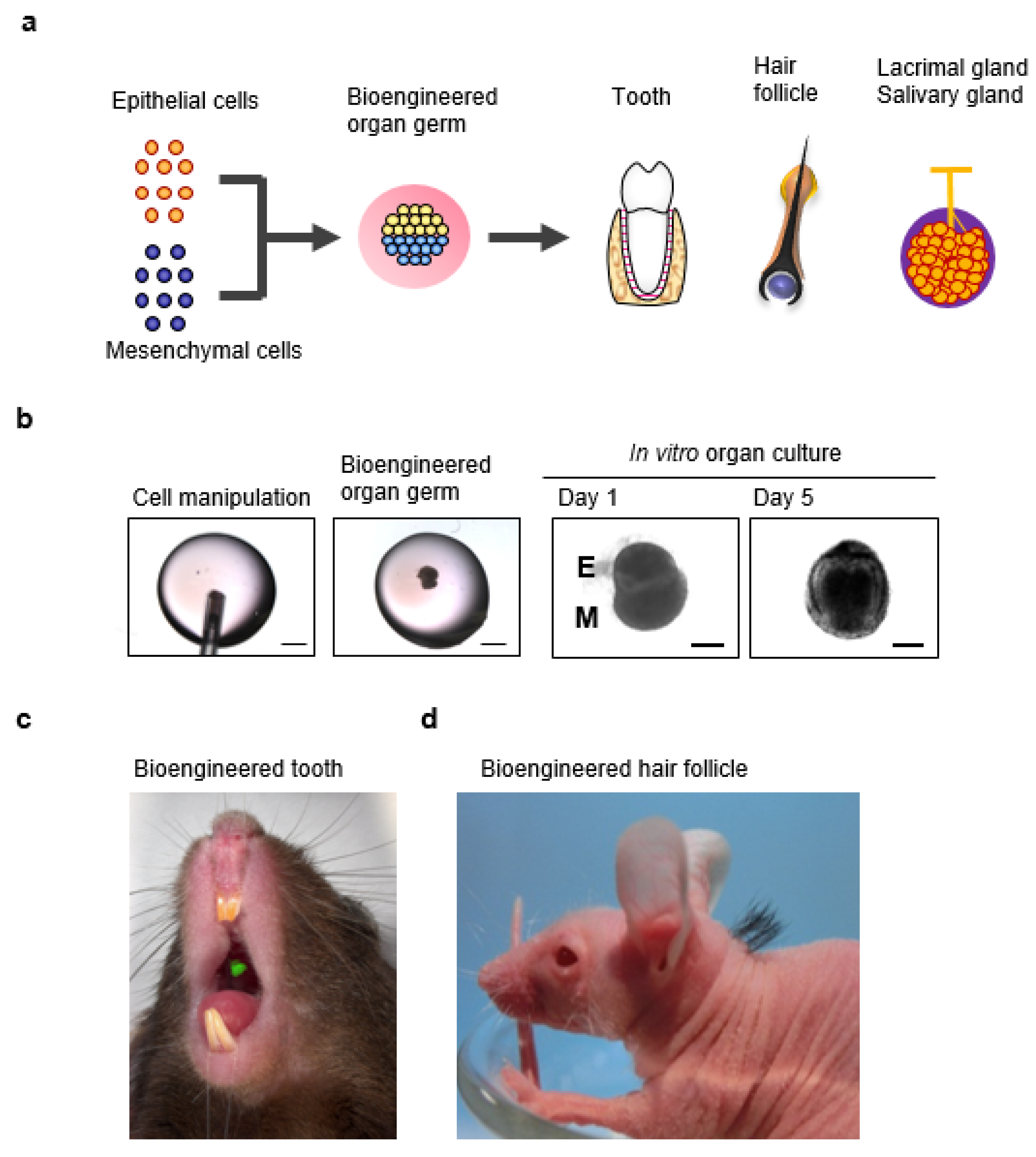
6. A Novel Three-Dimensional Cell Manipulation Method Termed the Organ Germ Method
7. Fully Functional Lacrimal Gland Organ Regeneration
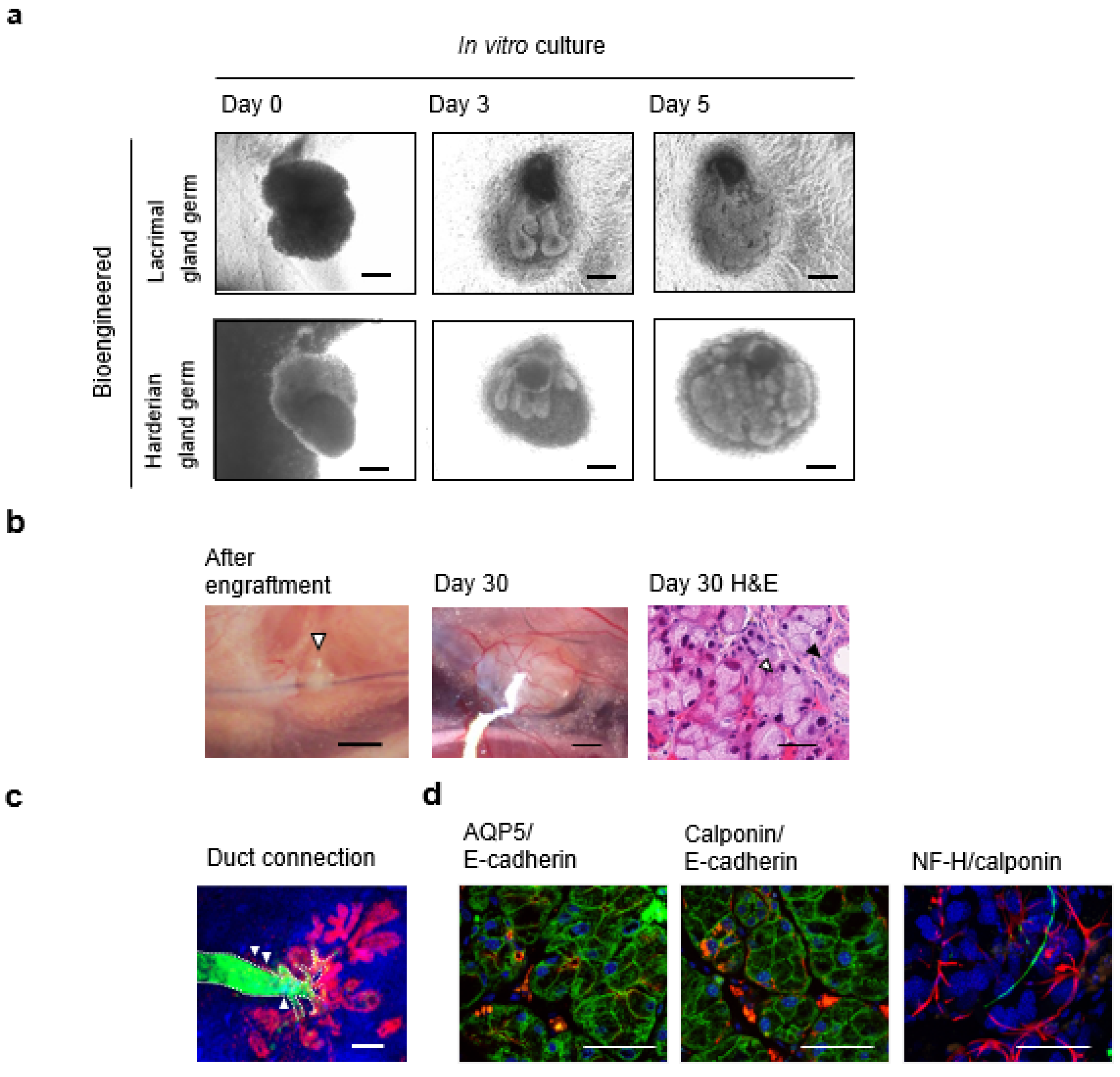
7.1. Engraftment of Bioengineered Lacrimal Gland Germ with Duct Association
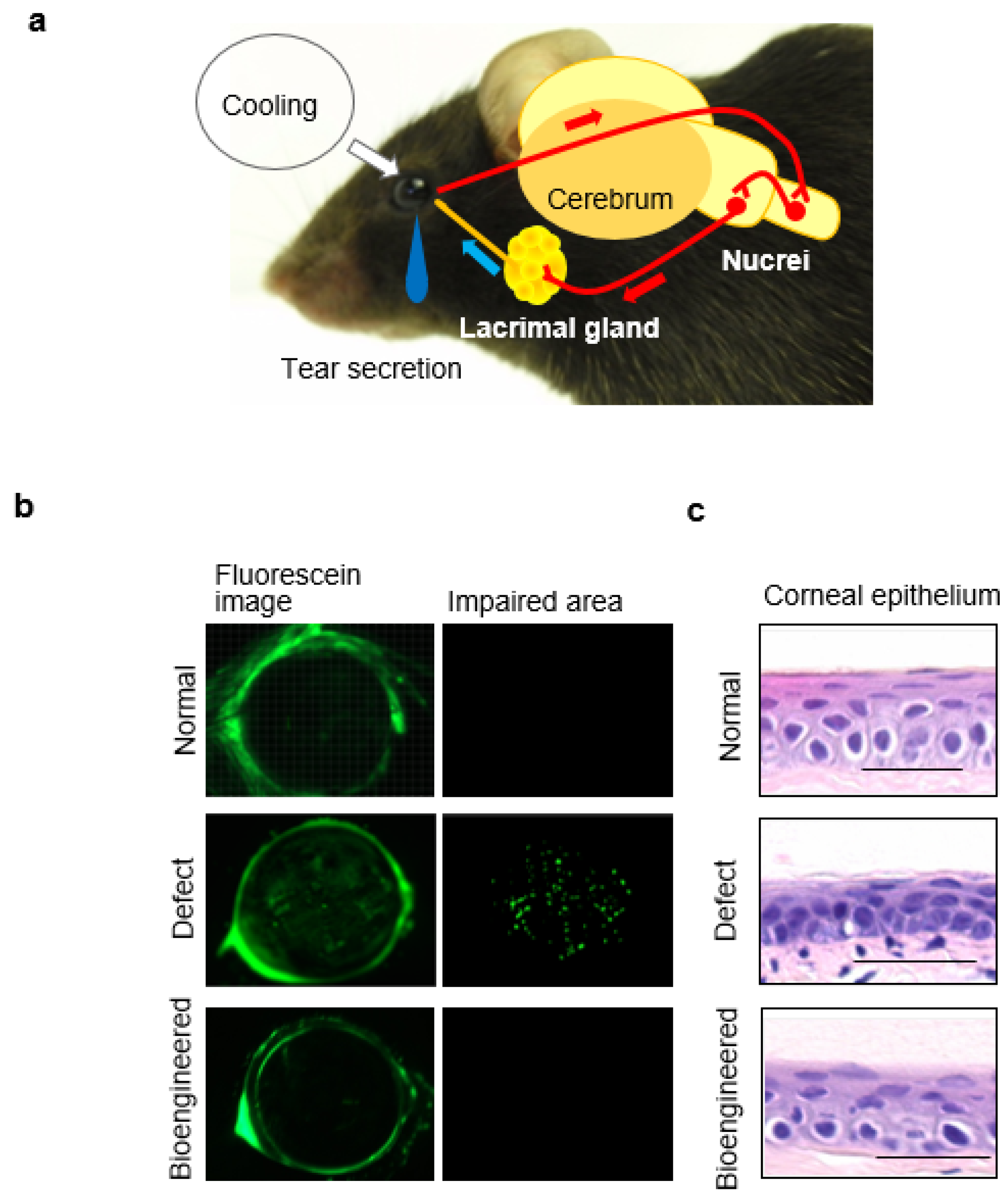
7.2. Tear Secretion Ability of the Bioengineered Lacrimal Gland
7.3. Ocular Surface Protection Effect by the Bioengineered Lacrimal Gland
8. Conclusions and Future Directions
Acknowledgements
Conflicts of Interest
References
- Okano, H.; Yamanaka, S. iPS cell technologies: Significance and applications to CNS regeneration and disease. Mol. Brain. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sekine, H.; Shimizu, T.; Yang, J.; Kobayashi, E.; Okano, T. Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation 2006, 114, I87–I93. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R. The current status of hematopoietic cell transplantation. Annu. Rev. Med. 2003, 54, 491–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Auerbach, J.M.; Rodriguez-Gomez, J.A.; Velasco, I.; Gavin, D.; Lumelsky, N.; Lee, S.H.; Nguyen, J.; Sanchez-Pernaute, R.; Bankiewicz, K.; et al. Dopamine neurons derived from embryonic stem cells function in an animal model of parkinson’s disease. Nature 2002, 418, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z. Stem cells for the treatment of neurological disorders. Nature 2006, 441, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Copelan, E.A. Hematopoietic stem-cell transplantation. New Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.; Lee, R.T. Stem-cell therapy for cardiac disease. Nature 2008, 451, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Foster, M.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Grompe, M. The origin and liver repopulating capacity of murine oval cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11881–11888. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Shimizu, T.; Matsuura, K.; Sekine, H.; Tanaka, N.; Tadakuma, K.; Yamato, M.; Kaneko, M.; Okano, T. Cell sheet technology for cardiac tissue engineering. Methods Mol. Biol. 2014, 1181, 139–155. [Google Scholar] [PubMed]
- Tsubota, K.; Satake, Y.; Kaido, M.; Shinozaki, N.; Shimmura, S.; Bissen-Miyajima, H.; Shimazaki, J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N. Engl. J. Med. 1999, 340, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Satake, Y.; Shimazaki, J. Treatment of severe dry eye. Lancet 1996, 348, 123. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. New Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Hirami, Y.; Takahashi, M. Stem cell biology and cell transplantation therapy in the retina. Biotechnol. Genet. Eng. Rev. 2010, 26, 297–334. [Google Scholar] [CrossRef] [PubMed]
- Abouna, G.M. Organ shortage crisis: Problems and possible solutions. Transplant. Proc. 2008, 40, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Inoue, K.; Nakajima, K.; Tachikawa, T.; Yamazaki, H.; Isobe, T.; Sugawara, A.; Ogawa, M.; Tanaka, C.; Saito, M.; et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.V. The artificial kidney. Science 1952, 115, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.G.; Smith, R.G.; Arabia, F.A.; Nolan, P.E.; Sethi, G.K.; Tsau, P.H.; McClellan, D.; Slepian, M.J. Cardiac replacement with a total artificial heart as a bridge to transplantation. New Engl. J. Med. 2004, 351, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Fort, A.; Fort, N.; Ricordi, C.; Stabler, C.L. Biohybrid devices and encapsulation technologies for engineering a bioartificial pancreas. Cell Transplant. 2008, 17, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Tsuji, T. Growing bioengineered teeth from single cells: Potential for dental regenerative medicine. Expert Opin. Boil. Ther. 2008, 8, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Oshima, M.; Tsuji, T. Development and prospects of organ replacement regenerative therapy. Cornea 2013, 32, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Ogawa, M.; Oshima, M.; Sekine, Y.; Ishida, K.; Yamashita, K.; Ikeda, K.; Shimmura, S.; Kawakita, T.; Tsubota, K.; et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Dilly, P.N. Structure and function of the tear film. Adv. Exp. Med. Boil. 1994, 350, 239–247. [Google Scholar]
- Schechter, J.E.; Warren, D.W.; Mircheff, A.K. A lacrimal gland is a lacrimal gland, but rodent’s and rabbit’s are not human. Ocul. Surf. 2010, 8, 111–134. [Google Scholar] [CrossRef]
- Holly, F.J. Formation and stability of the tear film. Int. Ophthalmol. Clin. 1973, 13, 73–96. [Google Scholar] [PubMed]
- Schoenwald, R.D.; Vidvauns, S.; Wurster, D.E.; Barfknecht, C.F. The role of tear proteins in tear film stability in the dry eye patient and in the rabbit. Adv. Exp. Med. Boil. 1998, 438, 391–400. [Google Scholar]
- Sweeney, D.F.; Millar, T.J.; Raju, S.R. Tear film stability: A review. Exp. Eye. Res. 2013, 117, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S. Some physiological aspects of the precorneal tear film. Arch. Ophthalmol. 1965, 73, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M.; Winter, N.; Bliss, G. Tear film stability and tear surface tension. Curr. Eye Res. 1989, 8, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Broekhuyse, R.M. Tear lactoferrin: A bacteriostatic and complexing protein. Invest. Ophthalmol. 1974, 13, 550–554. [Google Scholar] [PubMed]
- Danjo, Y.; Lee, M.; Horimoto, K.; Hamano, T. Ocular surface damage and tear lactoferrin in dry eye syndrome. Acta Ophthalmol. 1994, 72, 433–437. [Google Scholar] [CrossRef]
- Ohashi, Y.; Dogru, M.; Tsubota, K. Laboratory findings in tear fluid analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2006, 369, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Seal, D.V.; McGill, J.I.; Mackie, I.A.; Liakos, G.M.; Jacobs, P.; Goulding, N.J. Bacteriology and tear protein profiles of the dry eye. Br. J. Ophthalmol. 1986, 70, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Delaire, A.; Lassagne, H.; Gachon, A.M. New members of the lipocalin family in human tear fluid. Exp. Eye Res. 1992, 55, 645–647. [Google Scholar] [CrossRef]
- Ahn, J.M.; Lee, S.H.; Rim, T.H.; Park, R.J.; Yang, H.S.; Kim, T.I.; Yoon, K.C.; Seo, K.Y. Prevalence of and risk factors associated with dry eye: The Korea national health and nutrition examination survey 2010–2011. Am. J. Ophthalmol. 2014, 158, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.H.; Klein, B.E.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Carter, D.; Pouyeh, B.; Prunty, W.J.; Perez, V.L. Prevalence and risk factors of dry eye syndrome in a united states veterans affairs population. Am. J. Ophthalmol. 2011, 152, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Shoja, M.R.; Besharati, M.R. Dry eye after lasik for myopia: Incidence and risk factors. Eur. J. Ophthalmol. 2007, 17, 1–6. [Google Scholar] [PubMed]
- De Paiva, C.S.; Chen, Z.; Koch, D.D.; Hamill, M.B.; Manuel, F.K.; Hassan, S.S.; Wilhelmus, K.R.; Pflugfelder, S.C. The incidence and risk factors for developing dry eye after myopic lasik. Am. J. Ophthalmol. 2006, 141, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Lee, J.; Saw, S.M.; Gazzard, G.; Koh, D.; Widjaja, D.; Tan, D.T. Prevalence and risk factors associated with dry eye symptoms: A population based study in indonesia. Br. J. Ophthalmol. 2002, 86, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Fonseca, E.C.; Alves, M.F.; Malki, L.T.; Arruda, G.V.; Reinach, P.S.; Rocha, E.M. Dry eye disease treatment: A systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul. Surf. 2013, 11, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Massaro-Giordano, M.; Macchi, I.; Lambiase, A.; Bonini, S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J. Cell. Physiol. 2013, 228, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Toda, I.; Asano-Kato, N.; Hori-Komai, Y.; Tsubota, K. Ocular surface treatment before laser in situ keratomileusis in patients with severe dry eye. J. Refract. Surg. 2004, 20, 270–275. [Google Scholar] [PubMed]
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and risk factors of dry eye disease in japan: Koumi study. Ophthalmology 2011, 118, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Nakamori, K. Dry eyes and video display terminals. N. Engl. J. Med. 1993, 328, 584. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Kawashima, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The osaka study. Am. J. Ophthalmol. 2013, 156, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kaido, M.; Ishida, R.; Dogru, M.; Tsubota, K. Visual function changes after punctal occlusion with the treatment of short but type of dry eye. Cornea 2012, 31, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Gadaria-Rathod, N.; Lee, K.I.; Asbell, P.A. Emerging drugs for the treatment of dry eye disease. Expert Opin. Emerging Drugs 2013, 18, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Ishida, R.; Dogru, M.; Goto, E.; Matsumoto, Y.; Kaido, M.; Tsubota, K. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: A prospective randomized case-control study. Am. J. Ophthalmol. 2005, 139, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Deutsch. Arztebl. Int. 2015, 112, 71–81. [Google Scholar]
- Kojima, T.; Higuchi, A.; Goto, E.; Matsumoto, Y.; Dogru, M.; Tsubota, K. Autologous serum eye drops for the treatment of dry eye diseases. Cornea 2008, 27, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Brockes, J.P.; Kumar, A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science 2005, 310, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Pispa, J.; Thesleff, I. Mechanisms of ectodermal organogenesis. Dev. Biol. 2003, 262, 195–205. [Google Scholar] [CrossRef]
- Sakai, T. Epithelial branching morphogenesis of salivary gland: Exploration of new functional regulators. J. Med. Invest. JMI 2009, 56, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Carbe, C.; Powers, A.; Zhang, E.E.; Esko, J.D.; Grobe, K.; Feng, G.S.; Zhang, X. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development 2008, 135, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Makarenkova, H.P.; Ito, M.; Govindarajan, V.; Faber, S.C.; Sun, L.; McMahon, G.; Overbeek, P.A.; Lang, R.A. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development 2000, 127, 2563–2572. [Google Scholar] [PubMed]
- Dean, C.; Ito, M.; Makarenkova, H.P.; Faber, S.C.; Lang, R.A. Bmp7 regulates branching morphogenesis of the lacrimal gland by promoting mesenchymal proliferation and condensation. Development 2004, 131, 4155–4165. [Google Scholar] [CrossRef] [PubMed]
- Tsau, C.; Ito, M.; Gromova, A.; Hoffman, M.P.; Meech, R.; Makarenkova, H.P. Barx2 and Fgf10 regulate ocular glands branching morphogenesis by controlling extracellular matrix remodeling. Development 2011, 138, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.; Ito, M.; Makarenkova, H.P.; Lang, R.A.; Overbeek, P.A. Endogenous and ectopic gland induction by Fgf-10. Dev. Biol. 2000, 225, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.M. The ocular secretory immune system: A review. Curr. Eye Res. 1989, 8, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Tan, Y.; Satoh, Y.; Ono, K. Morphological changes of myoepithelial cells of mouse lacrimal glands during postnatal development. Histol. Histopathol. 1995, 10, 821–827. [Google Scholar] [PubMed]
- Payne, A.P. The harderian gland: A tercentennial review. J. Anat. 1994, 185, 1–49. [Google Scholar] [PubMed]
- Satoh, Y.; Gesase, A.P.; Habara, Y.; Ono, K.; Kanno, T. Lipid secretory mechanisms in the mammalian harderian gland. Microsc. Res. Tech. 1996, 34, 104–110. [Google Scholar] [CrossRef]
- Sant’ Anna, A.E.; Hazarbassanov, R.M.; de Freitas, D.; Gomes, J.A. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with stevens-johnson syndrome. Br. J. Ophthalmol. 2012, 96, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.J.; Franca, V.P. Transplantation of labial salivary glands for severe dry eye treatment. Arq. Bras. Oftalmol. 2005, 68, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Zoukhri, D. Mechanisms involved in injury and repair of the murine lacrimal gland: Role of programmed cell death and mesenchymal stem cells. Ocul. Surf. 2010, 8, 60–69. [Google Scholar] [CrossRef]
- Walker, N.I. Ultrastructure of the rat pancreas after experimental duct ligation. I. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J. Pathol. 1987, 126, 439–451. [Google Scholar] [PubMed]
- Scoggins, C.R.; Meszoely, I.M.; Wada, M.; Means, A.L.; Yang, L.; Leach, S.D. P53-dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G827–G836. [Google Scholar] [PubMed]
- Walker, N.I.; Bennett, R.E.; Kerr, J.F. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am. J. Anat. 1989, 185, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Sumita, Y.; Liu, Y.; Khalili, S.; Maria, O.M.; Xia, D.; Key, S.; Cotrim, A.P.; Mezey, E.; Tran, S.D. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int. J. Biochem. Cell Boil. 2011, 43, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.; Brunsting, J.F.; Wierenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PloS One 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; van der Zwaag, M.; Stokman, M.A.; van Os, R.; Coppes, R.P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 92, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Schoch, E.; Walker, N.I. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int. J. Exp. Pathol. 1998, 79, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Cummins, M.; Dardick, I.; Brown, D.; Burford-Mason, A. Obstructive sialadenitis: A rat model. J. Otolaryngol. 1994, 23, 50–56. [Google Scholar] [PubMed]
- Burgess, K.L.; Dardick, I.; Cummins, M.M.; Burford-Mason, A.P.; Bassett, R.; Brown, D.H. Myoepithelial cells actively proliferate during atrophy of rat parotid gland. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 674–680. [Google Scholar] [CrossRef]
- Takahashi, S.; Shinzato, K.; Domon, T.; Yamamoto, T.; Wakita, M. Mitotic proliferation of myoepithelial cells during regeneration of atrophied rat submandibular glands after duct ligation. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2004, 33, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Shinzato, K.; Nakamura, S.; Domon, T.; Yamamoto, T.; Wakita, M. The roles of apoptosis and mitosis in atrophy of the rat sublingual gland. Tissue Cell 2002, 34, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Nakamura, S.; Suzuki, R.; Islam, N.; Domon, T.; Yamamoto, T.; Wakita, M. Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell 2000, 32, 457–463. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kawakita, T.; Kawashima, M.; Okada, N.; Mishima, K.; Saito, I.; Ito, M.; Shimmura, S.; Tsubota, K. Characterization of cultivated murine lacrimal gland epithelial cells. Mol. Vision 2012, 18, 1271–1277. [Google Scholar]
- You, S.; Avidan, O.; Tariq, A.; Ahluwalia, I.; Stark, P.C.; Kublin, C.L.; Zoukhri, D. Role of epithelial-mesenchymal transition in repair of the lacrimal gland after experimentally induced injury. Inv. Ophthalmol. Vis. Sci. 2012, 53, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zoukhri, D.; Fix, A.; Alroy, J.; Kublin, C.L. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4399–4406. [Google Scholar] [CrossRef] [PubMed]
- Zoukhri, D.; Macari, E.; Kublin, C.L. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp. Eye Res. 2007, 84, 894–904. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Kublin, C.L.; Avidan, O.; Miyasaki, D.; Zoukhri, D. Isolation and propagation of mesenchymal stem cells from the lacrimal gland. Invest. Ophthalmol. Vis. Sci. 2011, 52, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Ali, M.J.; Vemuganti, G.K. Human lacrimal gland regeneration: Perspectives and review of literature. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2014, 28, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Ali, M.J.; Balla, M.M.; Naik, M.N.; Honavar, S.G.; Reddy, V.A.; Vemuganti, G.K. Establishing human lacrimal gland cultures with secretory function. PloS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Purnell, B. New release: The complete guide to organ repair. Science 2008, 322. [Google Scholar] [CrossRef] [PubMed]
- Kagami, H.; Wang, S.; Hai, B. Restoring the function of salivary glands. Oral Dis. 2008, 14, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PloS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Toyoshima, K.E.; Toki, H.; Ishibashi, N.; Asakawa, K.; Iwadate, A.; Kanayama, T.; Tobe, H.; Takeda, A.; Tsuji, T. Single follicular unit transplantation reconstructs arrector pili muscle and nerve connections and restores functional hair follicle piloerection. J. Dermatol. 2012, 39, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, K.; Toyoshima, K.E.; Ishibashi, N.; Tobe, H.; Iwadate, A.; Kanayama, T.; Hasegawa, T.; Nakao, K.; Toki, H.; Noguchi, S.; et al. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci. Rep. 2012, 2, 424. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, K.E.; Asakawa, K.; Ishibashi, N.; Toki, H.; Ogawa, M.; Hasegawa, T.; Irie, T.; Tachikawa, T.; Sato, A.; Takeda, A.; et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Tsuji, T. Functional tooth regenerative therapy: Tooth tissue regeneration and whole-tooth replacement. Odontol. Soc. Nippon. Dent. Univ. 2014, 102, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Tsuji, T. Fully functional salivary gland regeneration as a next-generation regenerative therapy. Jpn J. Clin. Immunol. 2015, 38, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Oshima, M.; Imamura, A.; Sekine, Y.; Ishida, K.; Yamashita, K.; Nakajima, K.; Hirayama, M.; Tachikawa, T.; Tsuji, T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Hogan, B.L. Developmental biology. Branching takes nerve. Science 2010, 329, 1610–1611. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Brockes, J.P. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012, 35, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.D.; Horikawa, Y.; Chen, L.L.; Kublin, C.L.; Hodges, R.R.; Dartt, D.A.; Zoukhri, D. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp. Eye Res. 2005, 80, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.; Kurose, M.; Winterson, B.J.; Meng, I.D. Menthol activation of corneal cool cells induces TRPM8-mediated lacrimation but not nociceptive responses in rodents. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7034–7042. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Sakata, M.; Tsubota, K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 1995, 113, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Goto, E.; Ono, M.; Shimmura, S.; Tsubota, K. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology 1998, 105, 1485–1488. [Google Scholar] [CrossRef]
- Driver, P.J.; Lemp, M.A. Meibomian gland dysfunction. Surv. Ophthalmol. 1996, 40, 343–367. [Google Scholar] [CrossRef]
- Mathers, W.D.; Lane, J.A. Meibomian gland lipids, evaporation, and tear film stability. Adv. Exp. Med. Boil. 1998, 438, 349–360. [Google Scholar]
- Mathers, W.D.; Billborough, M. Meibomian gland function and giant papillary conjunctivitis. Am. J. Ophthalmol. 1992, 114, 188–192. [Google Scholar] [CrossRef]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Shimmura, S.; Ueno, R.; Matsumoto, Y.; Goto, E.; Higuchi, A.; Shimazaki, J.; Tsubota, K. Albumin as a tear supplement in the treatment of severe dry eye. Br. J. Ophthalmol. 2003, 87, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Goto, E.; Fujita, H.; Ono, M.; Inoue, H.; Saito, I.; Shimmura, S. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br. J. Ophthalmol. 1999, 83, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Okamoto, S.; Mori, T.; Yamada, M.; Mashima, Y.; Watanabe, R.; Kuwana, M.; Tsubota, K.; Ikeda, Y.; Oguchi, Y. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003, 31, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Chen, S.X. Autologous serum in the management of recalcitrant dry eye syndrome. Clin. Exp. Ophthalmol. 2008, 36, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Angelina, A.; Zambrano, A.; Marrone, M.; Stark, W.J.; Heflin, T.; Tang, L.; Akpek, E.K. Autologous serum eye drops for dry eye. Cochrane Database Syst. Rev. 2013, 8. [Google Scholar] [CrossRef]
- Ward, K.W. Superficial punctate fluorescein staining of the ocular surface. Optom. Vis. Sci. 2008, 85, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Herman, J.P.; Finnemore, V.M.; Exford, J.M.; Blackie, C.A. An evaluation of the efficacy of fluorescein, rose bengal, lissamine green, and a new dye mixture for ocular surface staining. Eye Contact Lens 2008, 34, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Chen, D.; Liu, J.; Liu, B.; Li, N.; Zhou, Y.; Liang, X.; Ma, P.; Ye, C.; Ge, J.; et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Nyunt, A.K.; Ishida, Y.; Yu, Y.; Shimada, S. Topical apolipoprotein A-1 may have a beneficial effect on the corneal epithelium in a mouse model of dry eye: A pilot study. Eye Contact Lens 2008, 34, 287–292. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirayama, M.; Tsubota, K.; Tsuji, T. Bioengineered Lacrimal Gland Organ Regeneration in Vivo. J. Funct. Biomater. 2015, 6, 634-649. https://doi.org/10.3390/jfb6030634
Hirayama M, Tsubota K, Tsuji T. Bioengineered Lacrimal Gland Organ Regeneration in Vivo. Journal of Functional Biomaterials. 2015; 6(3):634-649. https://doi.org/10.3390/jfb6030634
Chicago/Turabian StyleHirayama, Masatoshi, Kazuo Tsubota, and Takashi Tsuji. 2015. "Bioengineered Lacrimal Gland Organ Regeneration in Vivo" Journal of Functional Biomaterials 6, no. 3: 634-649. https://doi.org/10.3390/jfb6030634
APA StyleHirayama, M., Tsubota, K., & Tsuji, T. (2015). Bioengineered Lacrimal Gland Organ Regeneration in Vivo. Journal of Functional Biomaterials, 6(3), 634-649. https://doi.org/10.3390/jfb6030634






