Prediction of True Circulatory Decompensation in Chronic Heart Failure for Optimal Timing of Mechanical Circulatory Support: Non-Invasive Arterial-Ventricular Coupling
Abstract
:1. Background
2. Patients and Methods
2.1. Patients
| Characteristics | Group 1 (ambulatory VAD patients) N = 151 | Group 2 (patients with true decompensation) N = 11 | p | |
|---|---|---|---|---|
| 1 | Age, yrs | 47 ± 11 | 50 ± 11 | 0.48 |
| 2 | Height, cm | 175 ± 8 | 178 ± 11 | 0.2 |
| 3 | Weight, kg | 79 ± 15 | 75 ± 10 | 0.46 |
| 4 | Dilative cardiomyopathy, n | 150 | 11 | |
| 5 | Duration of heart failure symptoms (years) | 5.7 ± 3.3 | 5.9 ± 3.4 | 0.32 |
| 6 | Number of decompensations | 1.5 ± 0.06 | 1.6 ± 0.9 | 0,4 |
| 7 | Serum sodium, mmol/L | 136 ± 6.1 | 134 ± 5.8 | 0.2 |
| 8 | GOT, u/L | 15.1 ± 7.1 | 17.7 ± 8.2 | 0.2 |
| 9 | LDH, u/L | 76 ± 42 | 209 ± 42 | 0.001 |
| 10 | Serum troponin, n/L | negative | 0.54 ± 0.11 | - |
| Hemodynamic data | ||||
| 11 | PAP mean, mmHg | 27 ± 11 | 36 ± 6 | 0.003 |
| 12 | PCP, mmHg | 18 ± 10 | 27 ± 3.3 | 0.0002 |
| 13 | CI mL/min/m² | 2.4 ± 0.81 | 2.0 ± 0.3 | 0.09 |
2.2. Methods
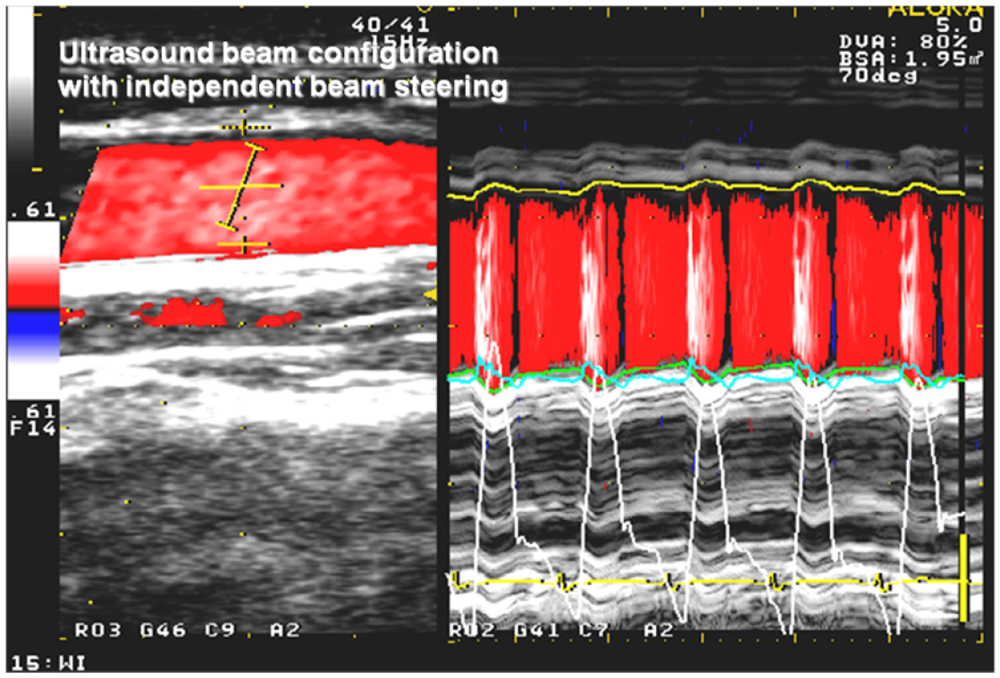
2.3. Echocardiography
2.4. Proposed Heart Failure Values for 1st and 2nd Peak
2.5. Statistical Analysis
3. Results
3.1. Clinical, Invasive and Non-Invasive Characteristics
| Echocardiography median (min-max) | Group 1 (ambulatory VAD patients) N = 151 | Group 2 (patients with true decompensation) N = 11 | p | |
|---|---|---|---|---|
| 1 | Heart rate, beats/min | 77 (50–130) | 89 (69–110) | 0.01 |
| 2 | LVEDD, mm | 72.0 (51–100) | 75.0 (60–90) | 0.43 |
| 3 | LVEF, % | 20.0 (10–40) | 19.0 (15–25) | 0.1 |
| Non-invasive instantaneous mean values (carotid artery)* | ||||
| 4 | Pressure max. median (min-max), mmHg | 104 (76–151) | 84 (75–114) | 0.007 |
| 5 | Pressure min. median (min-max), mmHg | 64 (36–100) | 60 (38–72) | 0.44 |
| 6 | Velocity max. median (min-max), m/s | 0.60 (0.22–1.47) | 0.47 (0.91–0.47) | 0.03 |
| 7 | Velocity min. median (min-max), m/s | 0.01 (−0.03–0.24) | 0.007 (−0.8–0.09) | 0.3 |
| 8 | 1st WI peak, mean ± SD | 5.400 ± 3.500 | 2.900 ± 1200 | <0.001 |
| 9 | 2nd WI peak, mean ± SD | 1900 ± 1200 | 1800 ± 900 | 0.25 |
| 10 | Β value, mean ± SD | 15 ± 7 | 15 ± 7 | 0.2 |
3.2. Follow-Up of Ambulatory Patients
| Group A N = 107 median (min-max) | Group B N = 44 median (min-max) | p | ||
|---|---|---|---|---|
| Invasive Data | ||||
| Heart rate, beats/min | 77 (53–127) | 75 (54–129) | 0.42 | |
| 1 | PAP mean, mmHg | 26 (8–55) | 25 (13–51) | 0.41 |
| 2 | PAP diast., mmHg | 18 (14–45) | 21 (13–53) | 0.069 |
| 3 | CI, mL/min/m² | 2.4 (1.3–5.2) | 2.2 (1.1–3.6) | 0.13 |
| 4 | SV, mL | 47.1 (19–108) | 39.5 (10–71) | 0.042 |
| 5 | PCP, mmHg | 17.0 (4–36) | 19.0 (7–40) | 0.058 |
| Echocardiography | ||||
| 1 | LVEDD, mm | 72.0 (51–100) | 77.0 (55–95) | 0.27 |
| 2 | LVEF, % | 20.0 (10–40) | 20.0 (10–35) | 0.017 |
| 3 | Mitral flow E/A | 1.24 (0.01–2.9) | 1.8 (0.1–9.0) | 0.05 |
| Exercise test | ||||
| 1 | VO2 max., mL/min | 17.6 (9–35) | 13.9 (4–31) | 0.037 |
| Non-invasive instantaneous mean values ( carotid artery)* | ||||
| 1 | Heart rate beats/min | 73.9 (64–118) | 77.2 (68–130) | 0.65 |
| 2 | BP max., mmHg | 106 (76–145) | 104 (71–141) | 0.23 |
| 3 | BP min., mmHg | 64 (36–100) | 63.5 (42–86) | 0.3 |
| 4 | Velocity max., m/s | 0.626 (0.230–1.469) | 0.524 (0.216–1.1215) | 0.057 |
| 5 | Velocity min., m/s | 0.013 (−0.303–0.235) | 0.023 (−0.85–0.153) | 0.2 |
| 6 | 1st peak WI mean ± SD | 6400 ± 3.300 | 2.900 ± 1200 | <0.001 |
| 7 | 2nd peak WI mean ± SD | 2000 ± 900 | 1400 ± 800 | 0.15 |
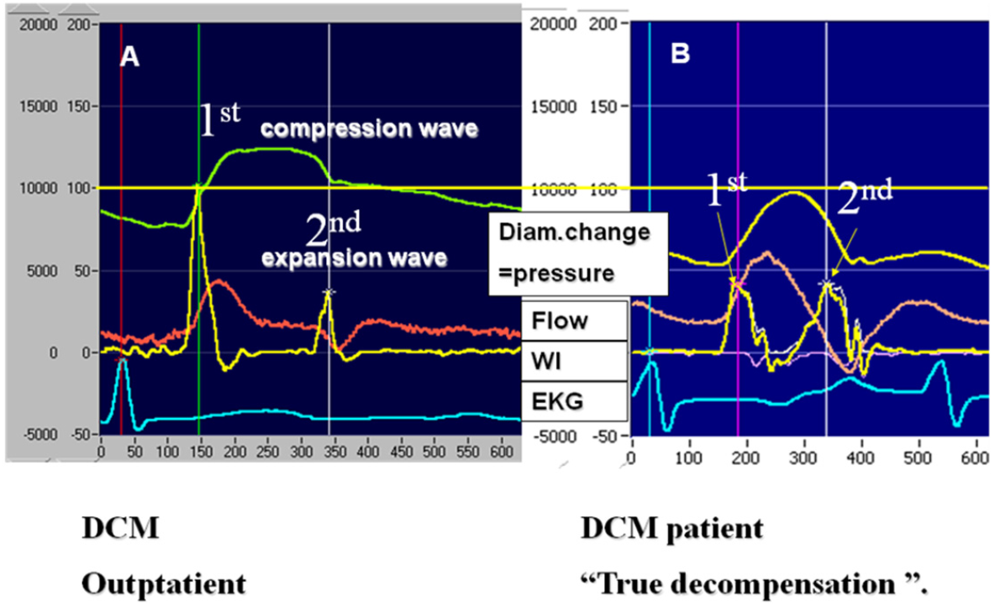
3.3. Wave Intensity Study
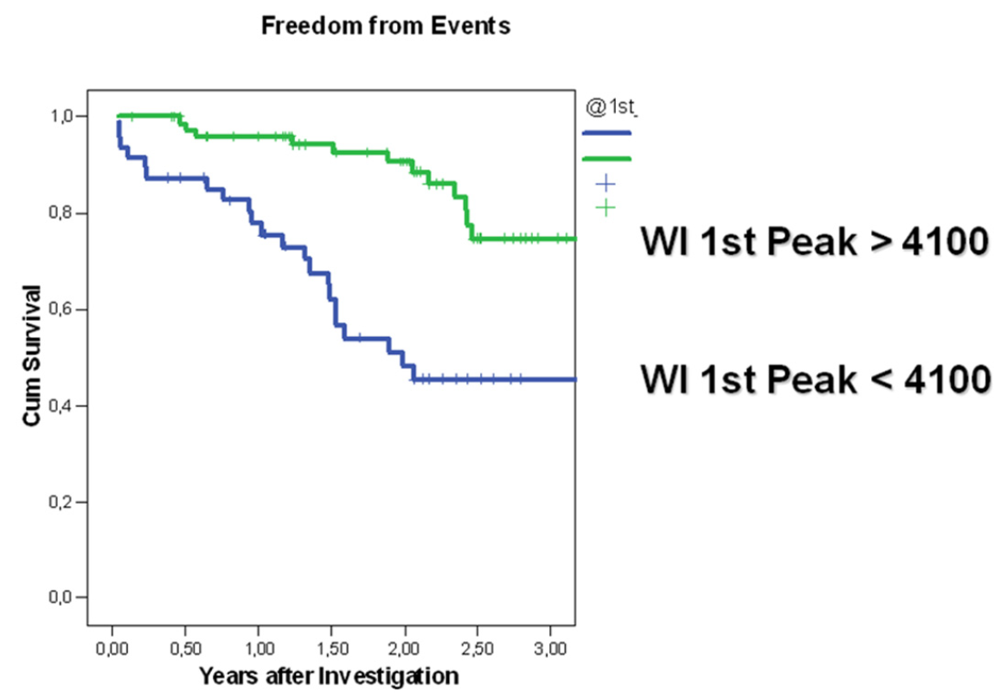
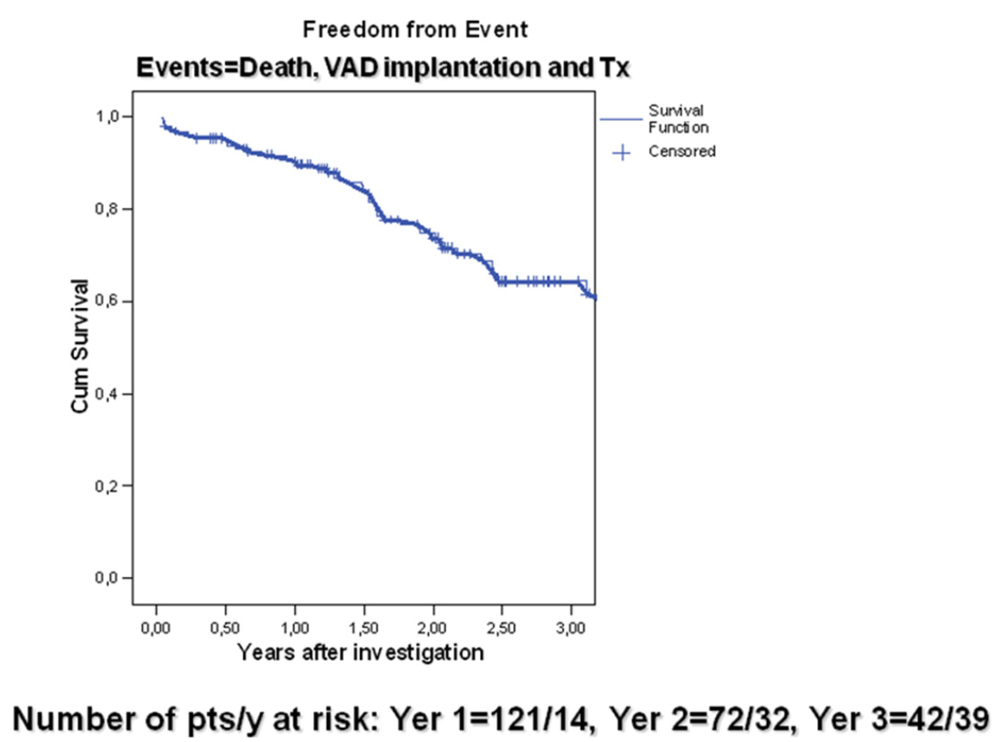
4. Discussion
4.1. Measurement of WI Values for Decompensation Evaluation
4.2. Wave Intensity Parameters as Predictors of Hemodynamic Deterioration
5. Critical Remarks and Conclusion
Acknowledgements
Conflict of Interest Statement
References
- Hunt, S.A.; Baker, D.W.; Chin, M.H.; Cinquegrani, M.P.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Goldstein, S.; Gregoratos, G.; Jessup, M.L.; et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: Executive summary: A report of the American College of Cardiology/American heart association task force on practice guidelines committee to revise the 1995 guidelines for the evaluation and management of heart failure. J. Am. Coll. Cardiol. 2001, 38, 2101–2113. [Google Scholar] [CrossRef]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. ACC/AHA 2005 update for the diagnosis and management of chronic heart failure in the adult: Summary article. Circulation 2005, 112, e154–e235. [Google Scholar] [CrossRef]
- Wilson, J.R.; Rayos, G.; Yeoh, T.K.; Gothard, P.; Bak, K. Dissociation between exertional symptoms and circulatory function in patients with heart failure. Circulation 1995, 92, 47–53. [Google Scholar]
- Stevenson, W.G.; Stevenson, L.W.; Middlekauff, H.R.; Fonarow, G.C.; Hamilton, M.A.; Woo, M.A.; Saxon, L.A.; Natterson, P.D.; Steimle, A.; Walden, J.A.; Tillish, J.H. Improving survival for patients with advanced heart failure: A study of 737 consecutive patients. J. Am. Coll. Cardiol. 1955, 26, 1417–1423. [Google Scholar]
- Deng, M.C.; de Meester, J.M.; Smits, J.M.; Heinecke, J.; Scheld, H.H. Effect of receiving a heart transplant: Analysis of a national cohort entered on to a waiting list, stratified by heart failure severity. BMJ 2000, 321, 540–545. [Google Scholar]
- Alpert, J.S. The effect of right ventricular dysfunction on left ventricular form and function. Chest 2001, 119, 1632–1633. [Google Scholar] [CrossRef]
- Salvo, T.G.; Mathier, M.; Semigran, M.J.; Dec, G.W. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J. Am. Coll. Cardiol. 1955, 25, 1143–1153. [Google Scholar]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef]
- Siniawski, H.; Heizmann, P.; Lehmkuhl, H.B.; Dandel, M.; Hoffmann, M.; Knosalla, C.; Pasic, M.; Weng, Y.; Stein, J.; Hetzer, R. Echocardiographic features of early graft dysfunction after heart transplantation. In Proceedings of the 2006 World Transplant Congress, Boston, MA, USA, 22–27 July 2006; Volume 6, p. 557.
- Harada, A.; Okada, T.; Sugawara, M.; Niki, K. Development of a non-invasive real-time measurement system of wave intensity. In Proceedings of IEEE Ultrasonics Symposium, San Juan, Puerto Rico, 22–25 October 2000; Volume 2, pp. 1517–1520.
- Parker, K.H.; Jones, C.J.H. Forward and backward running waves in the arteries: Analysis using the method of characteristics. J. Biomech. Eng. 1990, 112, 322–326. [Google Scholar] [CrossRef]
- Sugawara, M.; Uchida, K.; Kondoh, Y.; Magosaki, N.; Niki, K.; Jones, C.J.; Sugimachi, M.; Sunagawa, K. Aortic blood momentum the more the better for the ejecting heart in vivo? Cardiovasc. Res. 1997, 33, 433–446. [Google Scholar] [CrossRef]
- Sugawara, M.; Niki, K.; Furuhata, H.; Ohnishi, S.; Suzuki, S. Relationship between pressure and diameter of the carotid artery in humans. Heart Vessel. 2000, 15, 49–51. [Google Scholar] [CrossRef]
- Dandel, M.; Weng, Y.; Siniawski, H.; Potapov, E.; Lehmkuhl, H.; Hetzer, R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation 2005, 112, 37–45. [Google Scholar]
- Vasan, S.R.; Levy, D. Defining diastolic heart failure. A call for standardized diagnostic criteria. Circulation 2000, 101, 2118–2121. [Google Scholar] [CrossRef]
- Working Group Report. How to diagnose diastolic heart failure: European Study Group on Diastolic Heart Failure. Eur. Heart J. 1998, 19, 990–1003. [CrossRef]
- Petrie, C.J.; Robertson, M.; Voors, A.J.; van Veldhuisen, A.D.; Dargie, H.J. Abstract 2615: A low pulse pressure predicts mortality in patients with left ventricular dysfunction post myocardial infarction, but only in those with signs and symptoms of heart failure. Circulation 2007, 116 (Suppl. 16), II–579. [Google Scholar]
- Burkhoff, D.; Maurer, M.S.; Packer, M. Heart failure with a normal ejection fraction: Is it really a disorder of diastolic function? Circulation 2003, 107, 656–658. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; Sutton, M.S.J.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; Kueffer, F.J.; Bourge, R.C. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008, 118, 1433–1441. [Google Scholar]
- Jones, C.J.H.; Sugawara, M.; Kondoh, Y.; Uchida, K.; Parker, K.H. Compression and expansion wavefront travel in canine ascending aortic flow: Wave intensity analysis. Heart Vessel. 2002, 16, 91–98. [Google Scholar] [CrossRef]
- van Vliet, B.N.; Montaini, J.P. Baroreflex stabilization of the double product. Am. J. Physiol. 1999, 277, H1679–H1689. [Google Scholar]
- Niki, K.; Sugawara, M.; Chang, D.; Harada, A.; Okada, T.; Sakai, R.; Uchida, K.; Tanaka, R.; Mumford, C.E. A new noninvasive measurement system for wave intensity: Evaluation of carotid arterial wave intensity and reproducibility. Heart Vessel. 2002, 17, 12–21. [Google Scholar] [CrossRef]
- Shishido, T.; Sugimachi, M.; Kawaguchi, O.; Miyano, H.; Kawada, T.; Matsuura, W.; Ikeda, Y.; Sunagawa, K. Novel method to estimate ventricular contractility using intraventricular pulse wave velocity. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H2409–H2415. [Google Scholar]
- Wang, J.J.; Parker, K.H.; Tyberg, J.V. Left ventricular wave speed. J. Appl. Physiol. 2001, 91, 2531–2536. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siniawski, H.; Lehmkuhl, H.; Dandel, M.; Unbehaun, A.; Kemper, D.; Weng, Y.; Hetzer, R. Prediction of True Circulatory Decompensation in Chronic Heart Failure for Optimal Timing of Mechanical Circulatory Support: Non-Invasive Arterial-Ventricular Coupling. J. Funct. Biomater. 2012, 3, 100-113. https://doi.org/10.3390/jfb3010100
Siniawski H, Lehmkuhl H, Dandel M, Unbehaun A, Kemper D, Weng Y, Hetzer R. Prediction of True Circulatory Decompensation in Chronic Heart Failure for Optimal Timing of Mechanical Circulatory Support: Non-Invasive Arterial-Ventricular Coupling. Journal of Functional Biomaterials. 2012; 3(1):100-113. https://doi.org/10.3390/jfb3010100
Chicago/Turabian StyleSiniawski, Henryk, Hans Lehmkuhl, Michael Dandel, Axel Unbehaun, Dagmar Kemper, Yuguo Weng, and Roland Hetzer. 2012. "Prediction of True Circulatory Decompensation in Chronic Heart Failure for Optimal Timing of Mechanical Circulatory Support: Non-Invasive Arterial-Ventricular Coupling" Journal of Functional Biomaterials 3, no. 1: 100-113. https://doi.org/10.3390/jfb3010100
APA StyleSiniawski, H., Lehmkuhl, H., Dandel, M., Unbehaun, A., Kemper, D., Weng, Y., & Hetzer, R. (2012). Prediction of True Circulatory Decompensation in Chronic Heart Failure for Optimal Timing of Mechanical Circulatory Support: Non-Invasive Arterial-Ventricular Coupling. Journal of Functional Biomaterials, 3(1), 100-113. https://doi.org/10.3390/jfb3010100




