Gold Nanoparticle-Based Colorimetric and Fluorescent Dual-Mode Lateral Flow Immunoassay for SARS-CoV-2 Detection
Abstract
1. Introduction
2. Experiments and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of DMNPs and the Control Nanoprobes
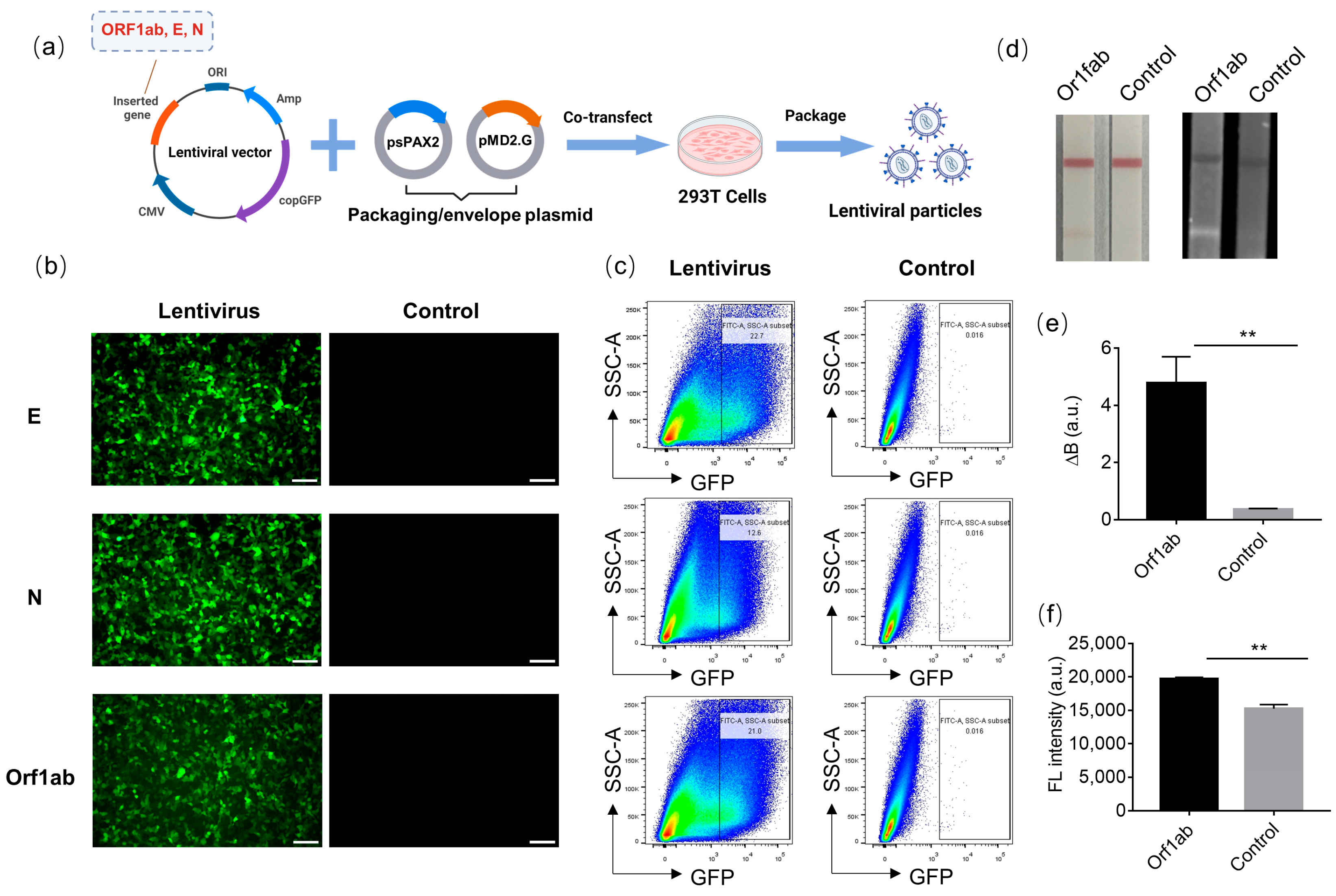
2.3. Production of Lentiviral Particles Carrying Three Different SARS-CoV-2 RNA Sequences
2.4. Determination of the Transduction Titer of Lentiviral Particles
2.5. The LFIA’s Fabrication
2.6. Dual-Mode Detection of Lentiviral Particles Carrying Three Different SARS-CoV-2 RNA Sequences
2.7. Statistical Analysis
3. Results and Discussion
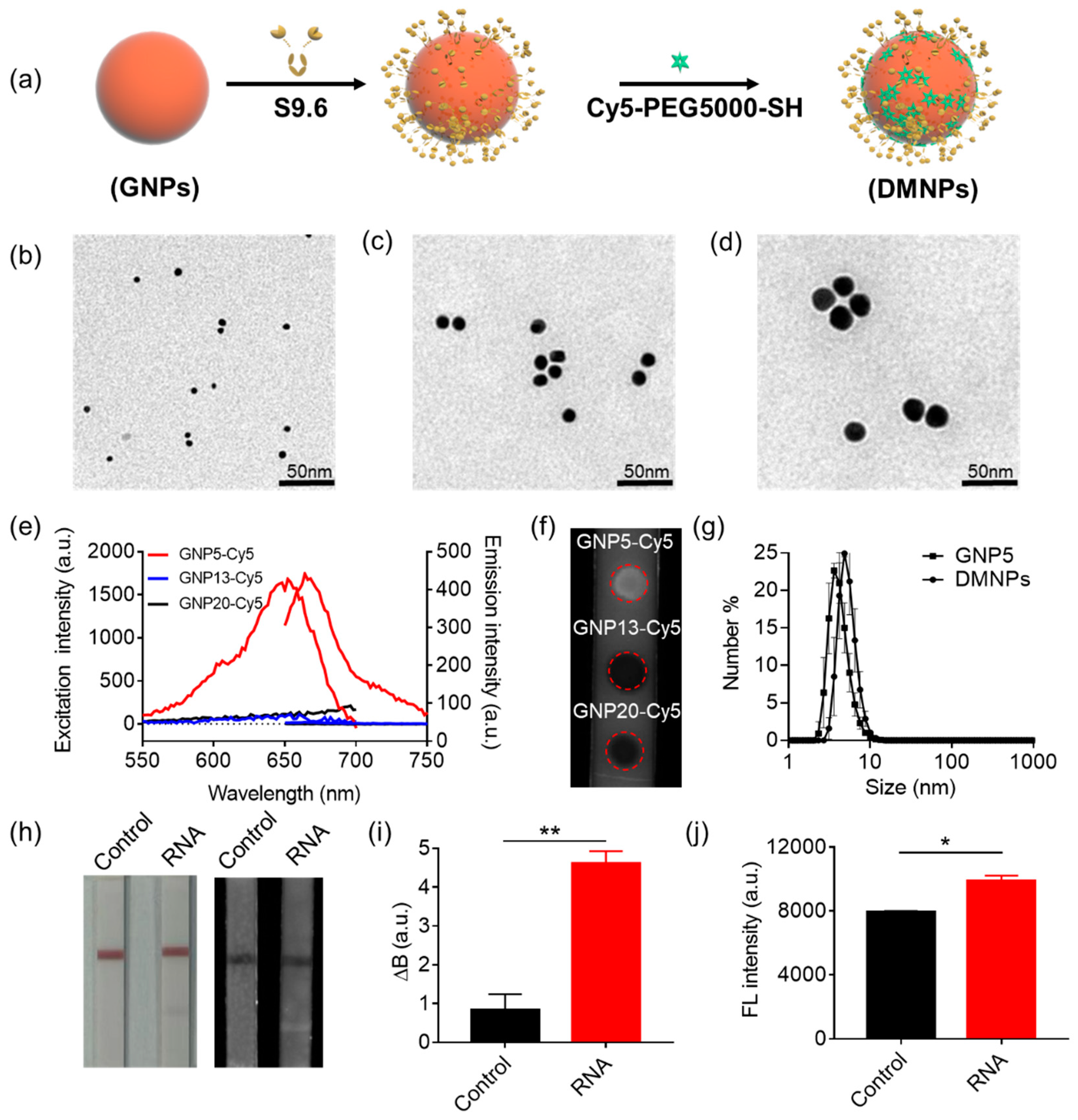
3.1. The Design and Workflow of LFIA
3.2. Validation of the Performance of the Synthesized DMNPs in RNA Detection
3.3. SARS-CoV-2 RNA-Carrying Lentiviral Particle Titer Determination and the Feasibility of LFIA for Sensing Lentiviral Particles
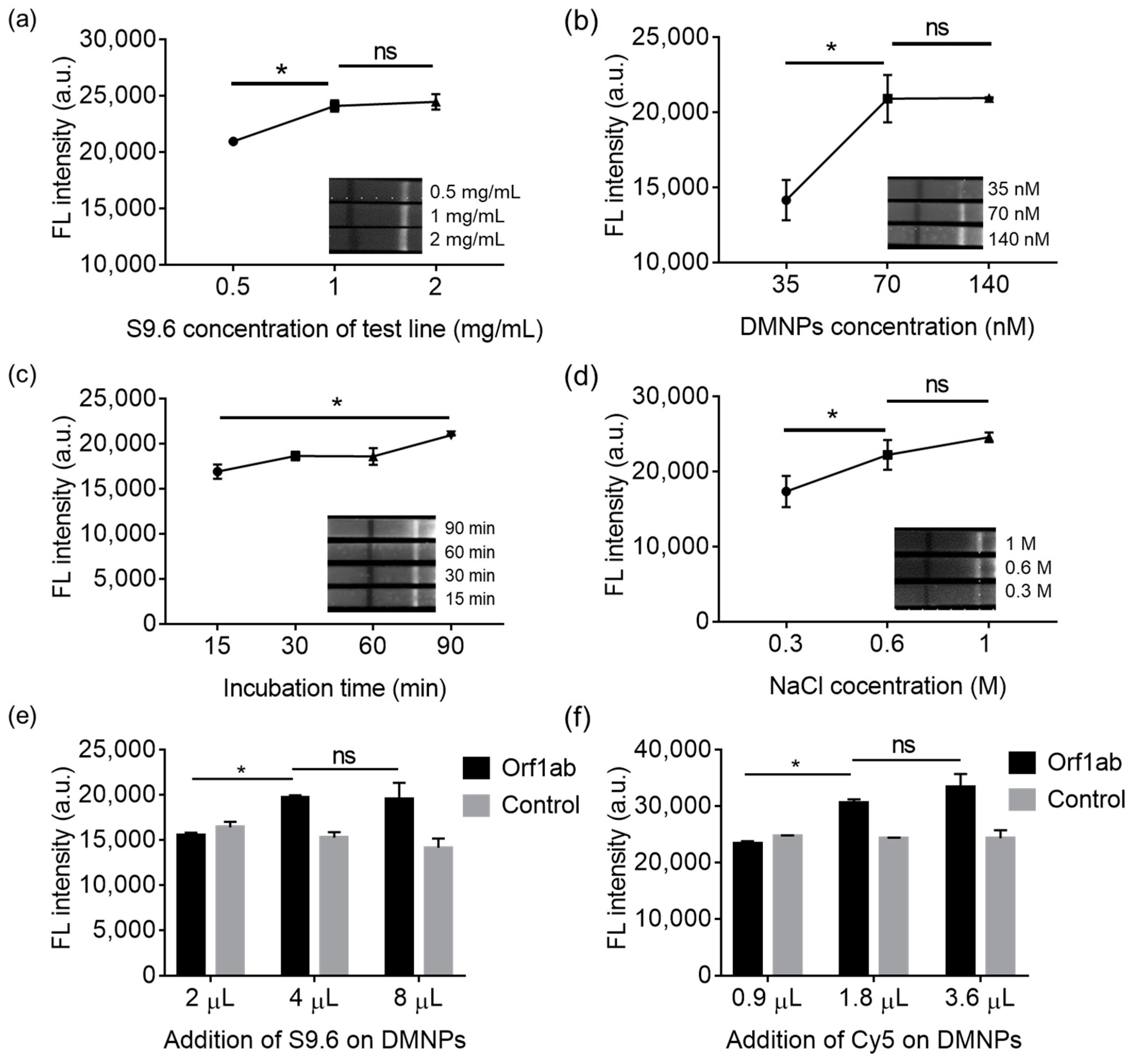
3.4. Optimization of the LFIA’s Fabrication and Operation Conditions for Sensing Lentiviral RNA
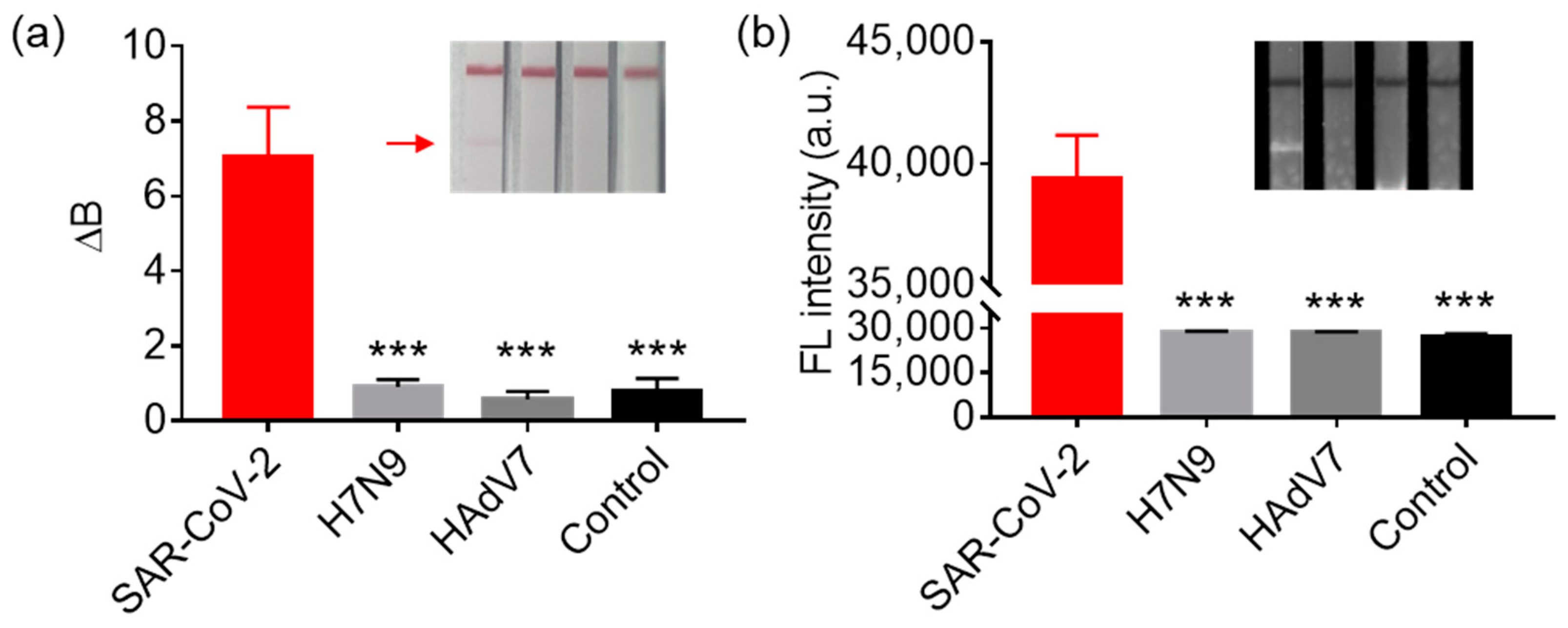
3.5. The Sensitivity and Selectivity of LFIA for SARS-CoV-2 Detection
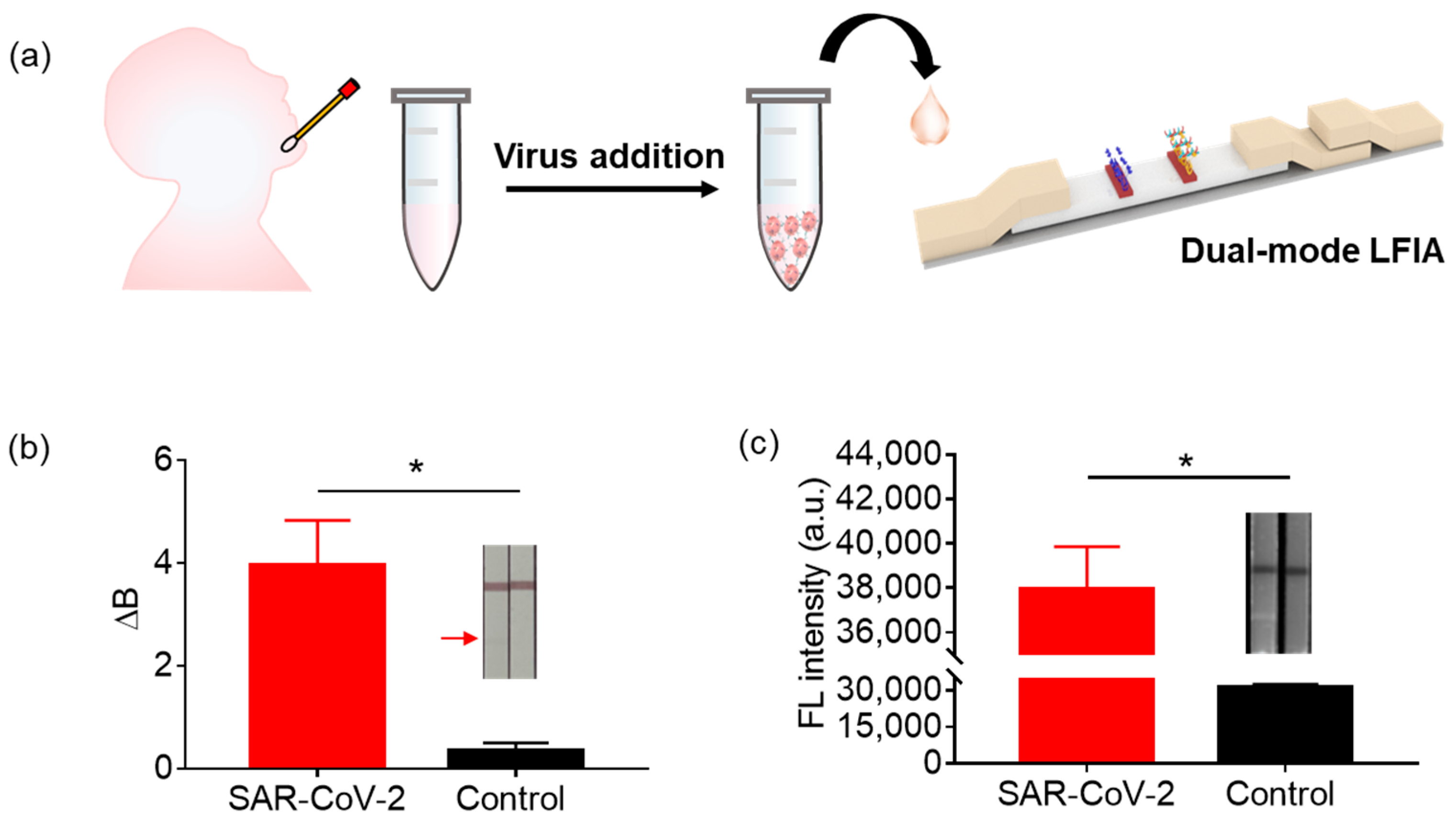
3.6. The Performance of LFIA in SARS-CoV-2 Detection in Saliva Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265. [Google Scholar] [CrossRef]
- Zhong, J.X.; Tang, J.G.; Ye, C.; Dong, L.L. The immunology of COVID-19: Is immune modulation an option for treatment? Lancet Rheumatol. 2020, 2, e428. [Google Scholar] [CrossRef]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World health organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.P.; Song, Y.T.; Chen, Y.; Wu, N.P.; Xu, J.L.; Sun, C.J.; Zhang, J.X.; Weng, T.H.; Zhang, Z.Y.; Wu, Z.G.; et al. Molecular architecture of the SARS-CoV-2 virus. Cell 2020, 183, 730. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Yuan, S.F.; Yuen, K.S.; Fung, S.Y.; Chan, C.P.; Jin, D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Wang, C.; Zeng, B.; Li, Z.; Li, X.; Li, H. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur. J. Radiol. 2020, 126, 108961. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Shetti, N.P.; Jagannath, S.; Aminabhavi, T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022, 430, 132966. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhou, M.X.; Ma, H.Q.; Gong, J.M.; Fung, S.Y.; Huang, X.; Yang, H. Silver nano-reporter enables simple and ultrasensitive profiling of microRNAs on a nanoflower-like microelectrode array on glass. J. Nanobiotechnol. 2022, 20, 456. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47. [Google Scholar] [CrossRef] [PubMed]
- Ao, L.; Liao, T.; Huang, L.; Lin, S.; Xu, K.; Ma, J.; Qiu, S.; Wang, X.; Zhang, Q. Sensitive and simultaneous detection of multi-index lung cancer biomarkers by an NIR-Ⅱ fluorescence lateral-flow immunoassay platform. Chem. Eng. J. 2022, 436, 135204. [Google Scholar] [CrossRef]
- Yang, M.; Tang, Y.; Qi, L.; Zhang, S.; Liu, Y.; Lu, B.; Yu, J.; Zhu, K.; Li, B.; Du, Y. SARS-CoV-2 point-of-care (POC) diagnosis based on commercial pregnancy test strips and a palm-size microfluidic device. Anal. Chem. 2021, 93, 11956. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Su, F.; Zhang, R.; Jiang, X.; Xiao, H.; Yan, X.; Yang, C.; Fan, X.; Wu, G. Rapid point-of-care testing for SARS-CoV-2 virus nucleic acid detection by an isothermal and nonenzymatic Signal amplification system coupled with a lateral flow immunoassay strip. Sens. Actuator B—Chem. 2021, 342, 129899. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.L.; Gong, J.S.; Ma, M.; Ji, Y.X.; Tian, P.L.; Mei, X.M.; Gu, N.; Zhang, Y. Tri-primer-enhanced strand exchange amplification combined with rapid lateral flow fluorescence immunoassay to detect SARS-CoV-2. Analyst 2021, 146, 6650. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; He, S.G.; Wang, X.H.; Yan, Y.Q.; Liu, J.Z.; Wu, S.M.; Liu, S.G.; Lei, Y.; Chen, M.; Li, L.; et al. Rapid lateral flow immunoassay for the fluorescence detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1150. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS based lateral flow immunoassay for point-of-care detection of SARS-CoV-2 in clinical samples. ACS Appl. Bio Mater. 2021, 4, 2974. [Google Scholar] [CrossRef]
- Chen, S.; Meng, L.; Wang, L.; Huang, X.; Ali, S.; Chen, X.; Yu, M.; Yi, M.; Li, L.; Chen, X.; et al. SERS-based lateral flow immunoassay for sensitive and simultaneous detection of anti-SARS-CoV-2 IgM and IgG antibodies by using gap-enhanced Raman nanotags. Sens. Actuator B—Chem. 2021, 348, 130706. [Google Scholar] [CrossRef]
- Han, H.; Wang, C.; Yang, X.; Zheng, S.; Cheng, X.; Liu, Z.; Zhao, B.; Xiao, R. Rapid field determination of SARS-CoV-2 by a colorimetric and fluorescent dual-functional lateral flow immunoassay biosensor. Sens. Actuator B—Chem. 2022, 351, 130897. [Google Scholar] [CrossRef]
- Lu, M.; Joung, Y.; Jeon, C.S.; Kim, S.; Yong, D.; Jang, H.; Pyun, S.H.; Kang, T.; Choo, J. Dual-mode SERS-based lateral flow assay strips for simultaneous diagnosis of SARS-CoV-2 and influenza a virus. Nano Converg. 2022, 9, 39. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Gu, B.; Liu, H.; Zhou, Z.; Shi, L.; Cheng, X.; Wang, S. Sensitive and simultaneous detection of SARS-CoV-2-specific IgM/IgG using lateral flow immunoassay based on dual-mode quantum dot nanobeads. Anal. Chem. 2020, 92, 15542. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, X.; Liu, L.; Zhang, X.; Yang, X.; Zheng, S.; Rong, Z.; Wang, S. Ultrasensitive and simultaneous detection of two specific SARS-CoV-2 antigens in human specimens using direct/enrichment dual-mode fluorescence lateral flow immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 40342. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021, 20, 593. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Mostafa, A.; Berger, J.; Aydin, M.Y.; Sun, F.; de Ramirez, S.A.S.; Valera, E.; Cunningham, B.T.; King, W.P.; Bashir, R. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 22727. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes. Infect. 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Duan, C.; Xue, L.; Liu, Y.; Sun, W.; Xiang, Y. DNA nanoscaffold-based SARS-CoV-2 detection for COVID-19 diagnosis. Biosens. Bioelectron. 2020, 167, 112479. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.-Y.; Chen, L.; Wang, M. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020, 323, 1406. [Google Scholar] [CrossRef] [PubMed]
- Molès, J.-P.; Griez, A.; Guilhou, J.-J.; Girard, C.; Nagot, N.; Van de Perre, P.; Dujols, P. Cytosolic RNA:DNA duplexes generated by endogenous reverse transcriptase activity as autonomous inducers of skin inflammation in psoriasis. PLoS ONE 2017, 12, e0169879. [Google Scholar] [CrossRef] [PubMed]
- Nava, G.M.; Grasso, L.; Sertic, S.; Pellicioli, A.; Muzi Falconi, M.; Lazzaro, F. One, no one, and one hundred thousand: The many forms of ribonucleotides in DNA. Int. J. Mol. Sci. 2020, 21, 1706. [Google Scholar] [CrossRef]
- Sun, L.Y.; Liu, Y.; Liu, X.L.; Wang, R.; Gong, J.M.; Saferali, A.; Gao, W.; Ma, A.Y.; Ma, H.Q.; Turvey, S.E.; et al. Nano-enabled reposition of proton pump inhibitors for TLR inhibition: Toward a new targeted nanotherapy for acute lung injury. Adv. Sci. 2022, 9, e2104051. [Google Scholar] [CrossRef]
- Gao, W.; Wang, L.; Wang, K.; Sun, L.; Rao, Y.; Ma, A.; Zhang, M.; Li, Q.; Yang, H. Enhanced anti-inflammatory activity of peptide-gold nanoparticle hybrids upon cigarette smoke extract modification through TLR inhibition and autophagy induction. ACS Appl. Mater. Interfaces 2019, 11, 32706. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, F.; Chang, R.; Xie, Z.; Peng, C.; Zhang, Y.; Wang, Z.; Liu, G. A versatile strategy of designing gold nanoparticle–cDNA nanoprobes in developing aptamer-based lateral flow assay for small-molecule detection. Langmuir 2023, 39, 8690. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, T.; Shi, F.-J.; Zeng, X.-Y.; Jiao, Y.-J. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 2020, 5, 12550. [Google Scholar] [CrossRef] [PubMed]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence quenching of dye molecules near gold nanoparticles: Radiative and nonradiative effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Guo, W.; Zhang, J.; Wu, Y.; Fu, W.; Liu, T.; Wu, X.; Wang, H.; Gong, X.; Liang, X.-j.; et al. Reverse fluorescence enhancement and colorimetric bimodal signal readout immunochromatography test strip for ultrasensitive large-scale screening and postoperative monitoring. ACS Appl. Mater. Interfaces 2016, 8, 22963. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Sharma, J.; Wang, H.N.; Zou, S.L.; Lin, S.; Yan, H.; Lindsay, S.; Liu, Y. Distance-dependent interactions between gold nanoparticles and fluorescent molecules with DNA as tunable spacers. Nanotechnology 2009, 20, 485201. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, M.; Yano, K.; Ikebukuro, K.; Nakayama, H.; Masuda, Y.; Karube, I. Optimization of the rate of DNA hybridization and rapid detection of methicillin resistant Staphylococcus aureus DNA using fluorescence polarization. J. Biotechnol. 1996, 48, 201. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Millat-Martinez, P.; Ouchi, D.; Roberts, C.H.; Alemany, A.; Corbacho-Monné, M.; Ubals, M.; Tobias, A.; Tebé, C.; Ballana, E.; et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: A cohort study. Lancet Infect. Dis. 2021, 21, 629. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, P.; Wang, S.; Melnykov, A.; Jiang, Q.S.; Seth, A.; Wang, Z.Y.; Morrissey, J.J.; George, I.; Gandra, S.; et al. Ultrasensitive lateral-flow assays via plasmonically active antibody-conjugated fluorescent nanoparticles. Nat. Biomed. Eng. 2023, 7, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Nishibata, Y.; Koshimoto, S.; Ogaki, K.; Ishikawa, E.; Wada, K.; Yoshinari, M.; Tamura, Y.; Uozumi, R.; Masuda, S.; Tomaru, U.; et al. RNase in the saliva can affect the detection of severe acute respiratory syndrome coronavirus 2 by real-time one-step polymerase chain reaction using saliva samples. Pathol. Res. Pract. 2021, 220, 153381. [Google Scholar] [CrossRef] [PubMed]


| DNA Probe | Sequence (5′-3′) |
|---|---|
| ORF1ab probe | “AACGATTGTGCATCAGCTGACTGAAGCATGGGTTCGCGGAGTTGATCACAACTACAGCCATAACCTTTCCACATACCGCAGACGGTACAGACTGTGTTTTTAAGTGTAAAACCCACAG” |
| E probe | “CGAAGCGCAGTAAGGATGGCTAGTGTAACTAGCAAGAATACCACGAAAGCAAGAAAAAGAAGTACGCTATTAACTATTAACGTACCTGT” |
| N probe | “CAGACATTTTGCTCTCAAGCTGGTTCAATCTGTCAAGCAGCAGCAAAGCAAGAGCAGCATCACCGCCATTGCCAGCCATTCTAGCAGGAGAAGTTCCCC” |
| Expression Plasmid | Sequence (5′-3′) |
|---|---|
| pCDH-ORF1ab | “GCAACAGTTCAAGAAATTCAACTCCAGGCAGCAGTAGGGGAACTTCTCCTGCTAGAATGGCTGGCAATGGCGGTGATGCTGCTCTTGCTTTGCTGCTGCTTGACAGATTGAACCAGCTTGAGAGCAAAATGTCTGGTAAAGGCCAACAACAACAAGGCCAAACTGTCACTAAGA” |
| pCDH-E154 | “CGAACTTATGTACTCATTCGTTTCGGAAGAGACAGGTACGTTAATAGTTAATAGCGTACTTCTTTTTCTTGCTTTCGTGGTATTCTTGCTAGTTACACTAGCCATCCTTACTGCGCTTCGATTGTGTGCGTACTGCTGCAATATTGTTAACGTG” |
| pCDH-N283 | “AATTACAAAAATTCAAAATTTTCGGGTTTATTACAGGGACAGCAGAGATCCAGTTTATCGATGCAACAGTTCAAGAAATTCAACTCCAGGCAGCAGTAGGGGAACTTCTCCTGCTAGAATGGCTGGCAATGGCGGTGATGCTGCTCTTGCTTTGCTGCTGCTTGACAGATTGAACCAGCTTGAGAGCAAAATGTCTGGTAAAGGCCAACAACAACAAGGCCAAACTGTCACTAAGAATCGATACTAGTAAGGATCTGCGATCGCTCCGGTGCCCGTCAGTGGG” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Zhang, H.; Liu, J.; He, F.; Li, F.; Li, A.; Xing, M.; Zhou, D.; Fung, S.-Y.; Yang, H. Gold Nanoparticle-Based Colorimetric and Fluorescent Dual-Mode Lateral Flow Immunoassay for SARS-CoV-2 Detection. J. Funct. Biomater. 2024, 15, 58. https://doi.org/10.3390/jfb15030058
Gan Y, Zhang H, Liu J, He F, Li F, Li A, Xing M, Zhou D, Fung S-Y, Yang H. Gold Nanoparticle-Based Colorimetric and Fluorescent Dual-Mode Lateral Flow Immunoassay for SARS-CoV-2 Detection. Journal of Functional Biomaterials. 2024; 15(3):58. https://doi.org/10.3390/jfb15030058
Chicago/Turabian StyleGan, Ying, Hefan Zhang, Jing Liu, Fuqin He, Fengheng Li, Ao Li, Man Xing, Dongming Zhou, Shan-Yu Fung, and Hong Yang. 2024. "Gold Nanoparticle-Based Colorimetric and Fluorescent Dual-Mode Lateral Flow Immunoassay for SARS-CoV-2 Detection" Journal of Functional Biomaterials 15, no. 3: 58. https://doi.org/10.3390/jfb15030058
APA StyleGan, Y., Zhang, H., Liu, J., He, F., Li, F., Li, A., Xing, M., Zhou, D., Fung, S.-Y., & Yang, H. (2024). Gold Nanoparticle-Based Colorimetric and Fluorescent Dual-Mode Lateral Flow Immunoassay for SARS-CoV-2 Detection. Journal of Functional Biomaterials, 15(3), 58. https://doi.org/10.3390/jfb15030058







