Use of Dichlorodimethylsilane to Produce Polydimethylsiloxane as a Substitute for Vitreous Humour: Characteristics and In Vitro Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
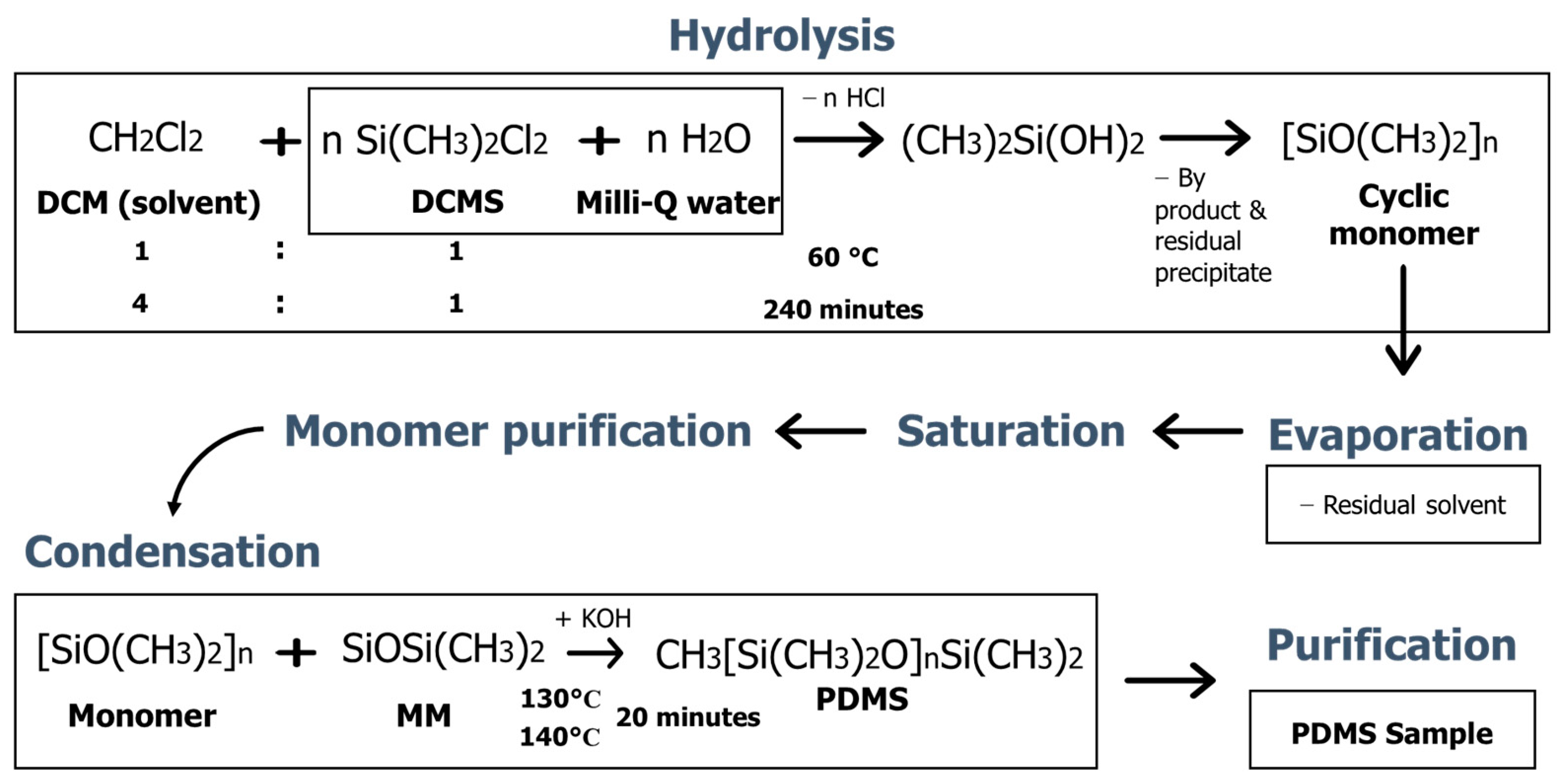
2.2. Synthesis Procedure
2.3. Characterization
2.4. In Vitro HET–CAM Test
3. Results
3.1. Synthesis Result of PDMS
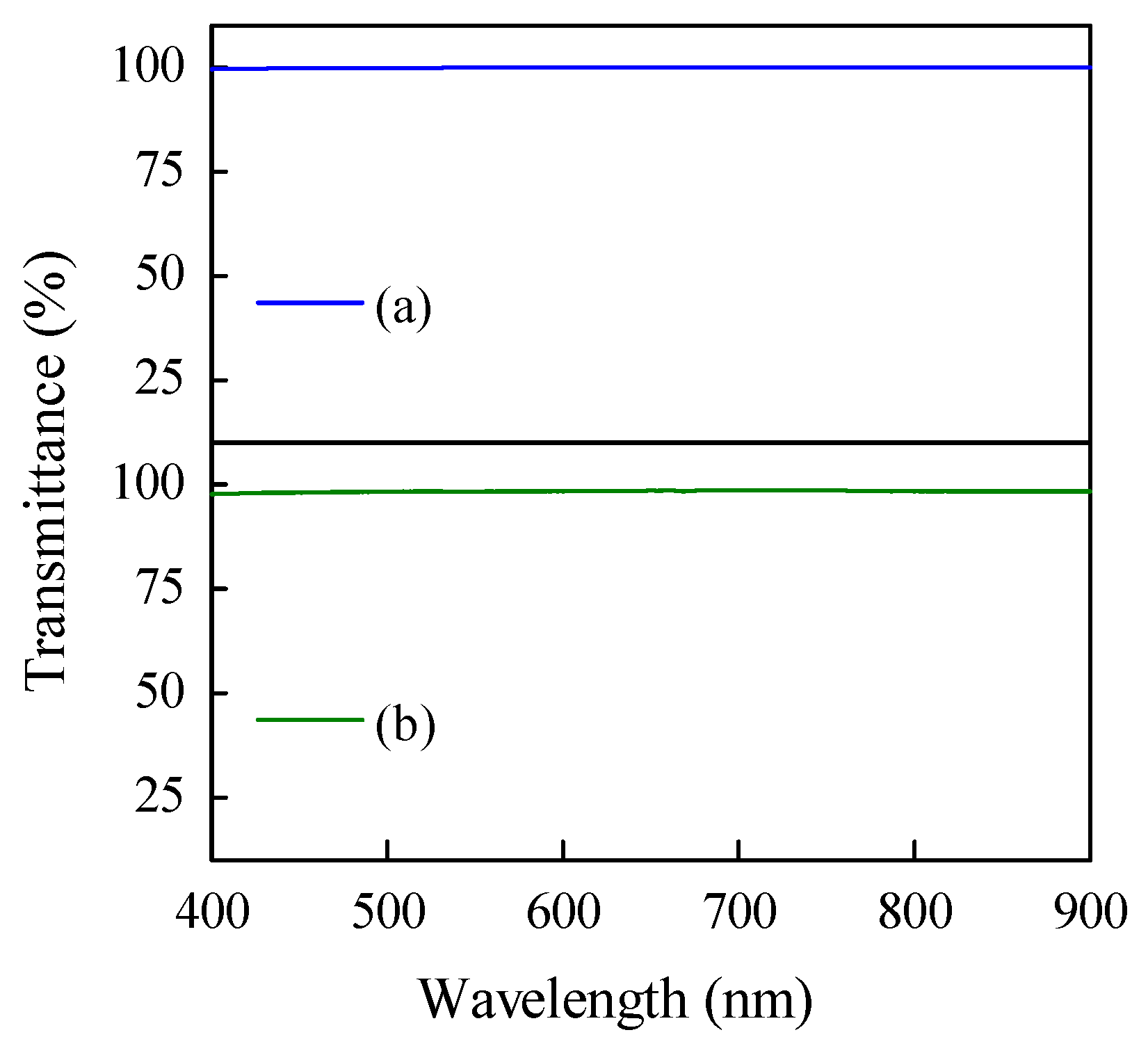
3.2. Characteristics of PDMS
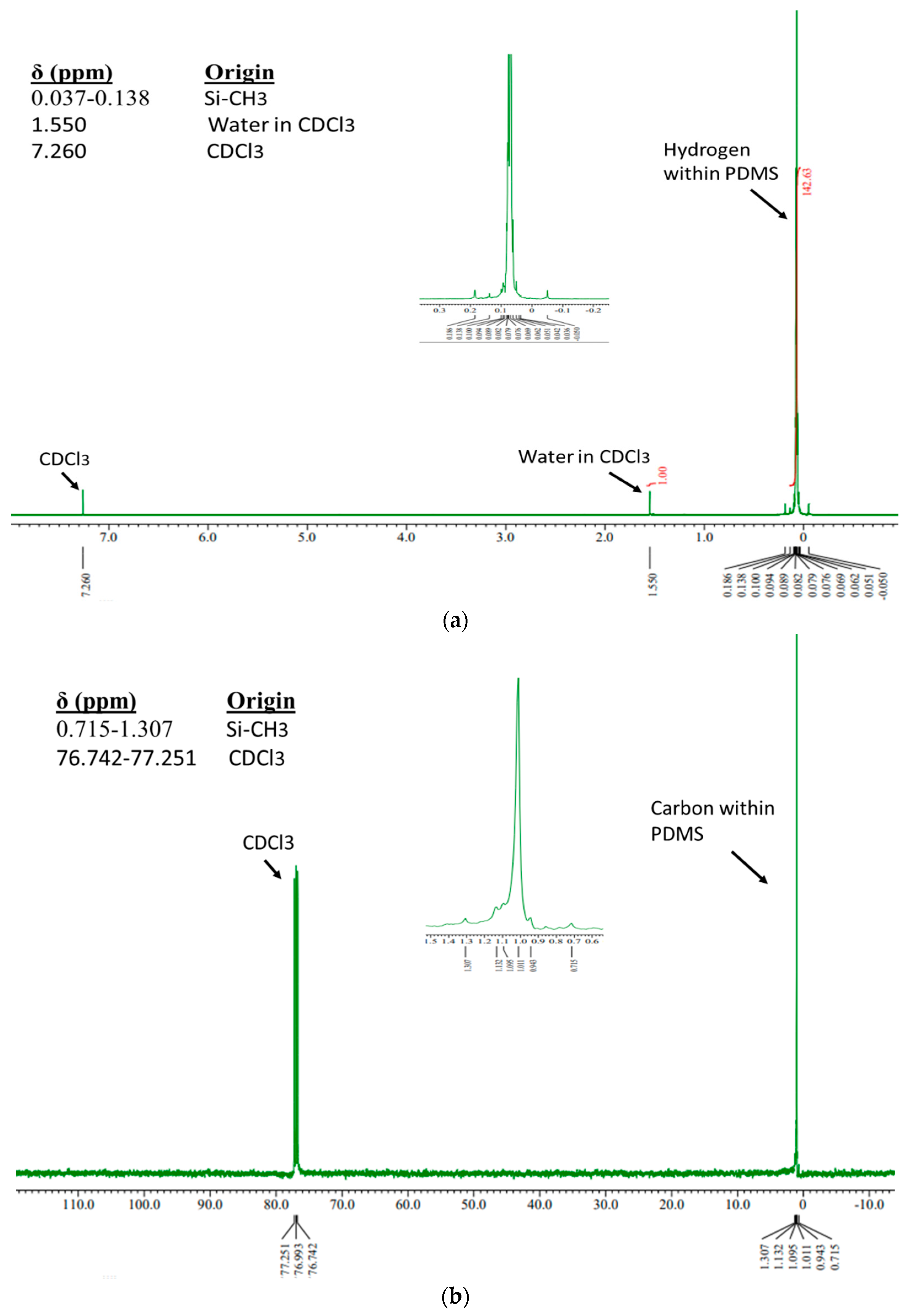
Characterization via 1H-NMR and 13C-NMR
3.3. In Vitro Toxicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thacker, M.; Tseng, C.-L.; Lin, F.-H. Substitutes and Colloidal System for Vitreous Replacement and Drug Delivery: Recent Progress and Future Prospective. Polymers 2021, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Alovisi, C.; Panico, C.; de Sanctis, U.; Eandi, C.M. Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery. J. Ophthalmol. 2017, 2017, 3172138. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. The Use of Polymers in the Treatment of Retinal Detachment: Current Trends and Future Perspectives. Polymers 2010, 2, 286–322. [Google Scholar] [CrossRef]
- Khurana, A.K. Comprehensive Ophtalmology, 6rd, ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2015. [Google Scholar]
- Barca, F.; Caporossi, T.; Rizzo, S. Silicone Oil: Different Physical Proprieties and Clinical Applications. Bio. Med. Res. Int. 2014, 2014, e502143. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Schickhardt, S.; Munro, D.J.; Scheuerle, A.; Mayer, C.S.; Auffarth, G.U. Physicochemical Properties of Explanted Silicone Oil After Use as an Intraocular Tamponade. Transl. Vis. Sci. Technol. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Kartasasmita, A.; Kusdiono, W.; Virgana, R.; Boesorie, S. In Vivo Emulsification Analysis of 1000 cs and 5000 cs Silicone Oil after Rhegmatogenous Retinal Detachment Vitrectomy Surgery. Open J. Ophthalmol. 2017, 7, 4. [Google Scholar] [CrossRef][Green Version]
- Barać, J.; Katusić, D.; Ivancić, D.; Sisljagić, V.; Bradvica, M. Effect of intraocular silicone oil on ocular tissue. Coll. Antropol. 2005, 29, 51–54. [Google Scholar]
- Jiesheng, L.; Ke, L.; Lian, X.; Xiang, H. Synthesis of Polysiloxanes in Microemulsion Via ring opening of D4. Nanochemistry Res. 2016, 1, 229–236. [Google Scholar] [CrossRef]
- Fitrilawati, F.; Fauza, A.N.; Ardi, A.; Novianti, R.M.; Syakir, N.; Kartasasmita, A.S. Effect of KOH concentration on characteristics of polydimethylsiloxane synthesized by ring opening polymerization method. J. Phys. Conf. Ser. 2018, 1080, 012016. [Google Scholar] [CrossRef]
- Setiadji, S.; Fitrilawati, F.; Fauza, A.N.; Ardi, A.; Novianti, R.; Syakir, N.; Risdiana, R. Optimization of Polydimethylsiloxane Synthesized Parameters as Vitreous Humour Substitutes. Mater. Sci. Forum 2019, 966, 189–193. [Google Scholar] [CrossRef]
- Auliya, D.G.; Setiadji, S.; Agasa, Z.M.; Fitrilawati, F.; Syakir, N.; Risdiana, R. Synthesis of Low Viscosity Polydimethylsiloxane Using Low Grade of Octamethylcyclotetrasiloxane. Mater. Sci. Forum 2021, 1028, 365–370. [Google Scholar] [CrossRef]
- Setiadji, S.; Agasa, Z.M.; Auliya, D.G.; Fitrilawati, F.; Syakir, N.; Noviyanti, A.R.; Rahayu, I.; Supriadin, A.; Risdiana, R. Synthesis and Characterization of Polydimethylsiloxane (PDMS) with Medium Viscosity via Ring-Opening Polymerization. Mater. Sci. Forum 2021, 1028, 346–351. [Google Scholar] [CrossRef]
- Fauziah, U.; Sandi, D.; Arini, V.F.; Aulia, D.G.; Setiadji, S.; Fitrilawati, F.; Risdiana, R. Synthesis of Polydimethylsiloxane with hydrolysis and condensation methods using monomer of Dichlorodimethylsilane as vitreous humour substitute. J. Phys. Conf. Ser. 2022, 2165, 012026. [Google Scholar] [CrossRef]
- Setiadji, S.; Sumiyanto, E.; Fitrilawati, F.; Syakir, N.; Noviyanti, A.R.; Rahayu, I.; Risdiana, R. Synthesis of Polydimethylsiloxane and its Monomer from Hydrolysis of Dichlorodimethylsilane. Key Eng. Mater. 2020, 860, 234–238. [Google Scholar] [CrossRef]
- Wang, Y. Synthesis, Characterization and Surface Modification of Polydimethylsiloxane and Its Composites; University of Massachusetts: Amherst, MS, USA, 2015. [Google Scholar]
- Rutnakornpituk, M. Synthesis of Silicone Magnetic Fluids for Use in Eye Surgery; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2002. [Google Scholar]
- Caramoy, A.; Hagedorn, N.; Fauser, S.; Kugler, W.; Groß, T.; Kirchhof, B. Development of Emulsification-Resistant Silicone Oils: Can We Go Beyond 2000 mPas Silicone Oil? Invest. Ophthalmol. Vis. Sci. 2011, 52, 5432–5436. [Google Scholar] [CrossRef]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop® and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Jorge Murillo, G.; Perez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Martínez, D.T.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Valdes, T.I.; Kreutzer, D.; Moussy, F. The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J. Biomed. Mater. Res. 2002, 62, 273–282. [Google Scholar] [CrossRef]
- Hagino, S.; Kinoshita, S.; Tani, N.; Nakamura, T.; Ono, N.; Konishi, K.; Iimura, H.; Kojima, H.; Ohno, Y. Interlaboratory validation of in vitro eye irritation tests for cosmetic ingredients. (2) Chorioallantoic membrane (CAM) test. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 1999, 13, 99–113. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Zhou, X.; Li, X.; Kappl, M.; Steffen, W.; Butt, H.J. One-Step Synthesis of a Durable and Liquid-Repellent Poly(dimethylsiloxane) Coating. Adv. Mater. 2021, 33, 2100237. [Google Scholar] [CrossRef]
- Hellström, K.; Diószegi, A.; Diaconu, L. A Broad Literature Review of Density Measurements of Liquid Cast Iron. Metals 2017, 7, 165. [Google Scholar] [CrossRef]
- Nusa, H.S.; Astuti, W.; Kartasasmita, A.S.; Virgana, R.; Syakir, N.; Bahtiar, A.; Safriani, L.; Risdiana, R. Characterization of Optical and Structure Properties of Polydimethylsiloxanes. Mater. Sci. Forum 2015, 827, 99–104. [Google Scholar] [CrossRef]
- Swindle, K.E.; Ravi, N. Recent advances in polymeric vitreous substitutes. Expert Rev. Ophthalmol. 2007, 2, 255–265. [Google Scholar] [CrossRef]
- Caramoy, A.; Schröder, S.; Fauser, S.; Kirchhof, B. In vitro emulsification assessment of new silicone oils. Br. J. Ophthalmol. 2010, 94, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Smith, G.T.; Wong, D. Refractive changes in silicone filled eyes. Eye 1990, 4, 230–234. [Google Scholar] [CrossRef]
- Stefánsson, E.; Anderson, M.M.; Landers, M.B.; Tiedeman, J.S.; McCuen, B.W. Refractive changes from use of silicone oil in vitreous surgery. Retina Phila. Pa 1988, 8, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Budai, P.; Kormos, É.; Buda, I.; Somody, G.; Lehel, J. Comparative evaluation of HET-CAM and ICE methods for objective assessment of ocular irritation caused by selected pesticide products. Toxicol. In Vitro 2021, 74, 105150. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Ferrara, M.; Gatto, C.; Giurgola, L.; Zanoni, M.; Angi, M.; Rinaldi, M.; Borgia, A.; Sorrentino, T.; Tóthová, J.D.A. Safety of silicone oils as intraocular medical device: An in vitro cytotoxicity study. Exp. Eye Res. 2020, 194, 108018. [Google Scholar] [CrossRef] [PubMed]
- Karaca, U.; Kucukevcilioglu, M.; Durukan, A.H.; Akincioglu, D. The effect of longstanding silicone oil on retina choroid and optic nerve in eyes with retinal detachment: An optical coherence tomography study. BMC Ophthalmol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Auliya, D.G.; Setiadji, S.; Fitrilawati, F.; Risdiana, R. Physical Characterization and In Vitro Toxicity Test of PDMS Synthesized from Low-Grade D4 Monomer as a Vitreous Substitute in the Human Eyes. J. Funct. Biomater. 2022, 13, 3. [Google Scholar] [CrossRef]



| Condition | P-1 | P-2 |
|---|---|---|
| Ratio of DCMS:DCM | 1:1 | 1:4 |
| Hydrolysis Temperature (°C) | 60 | 60 |
| Time of Hydrolysis (min) | 240 | 240 |
| Polymerization Temperature (°C) | 130 | 140 |
| Time of Polymerization (min) | 20 | 20 |
| Sample | ρ (g/mL) | η (Pa·s) | n | Additional Diopters (D) | γ (×10−3 N/m) |
|---|---|---|---|---|---|
| P-1 | 0.96 | 2.06 | 1.4036 | 3.406 | 21 |
| P-2 | 0.99 | 3.59 | 1.4034 | 3.396 | 21 |
| Commercial Low-Viscosity [11] | 0.97 | 1.08 | 1.4044 | 4.445 | 20 |
| Commercial High-Viscosity [11] | 0.97 | 3.55 | 1.4040 | 4.425 | 19 |
| Functional Group | Wavenumber (cm−1) | |||
|---|---|---|---|---|
| Commercial Low Viscosity PDMS [11] | P-1 | P-2 | Commercial High Viscosity PDMS [11] | |
| (1) Si-C stretching and CH3 rocking | 792.8–823.8 | 788.1; 861.0 | 784.0–862.2 | 815.1 |
| (2) Si-O-Si stretching | 1022.8; 1111.9 | 1008.0 | 1007.9 | 1099.1 |
| (3) CH3 symmetric deformation of Si-CH3 | 1263.0 | 1256.8 | 1256.6 | 1257.9 |
| (4) CH3 asymmetric deformation of Si-CH3 | 1412.3 | 1410.5 | 1416.4 | 1412.3 |
| (5) CH stretching of CH3 | 2905.5; 2971.7 | 2903.8; 2961.9 | 2905.5; 2961.7 | 2905.6; 2969.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auliya, D.G.; Fauziah, U.; Arini, V.F.; Setiadji, S.; Fitrilawati, F.; Kartasasmita, A.S.; Risdiana, R. Use of Dichlorodimethylsilane to Produce Polydimethylsiloxane as a Substitute for Vitreous Humour: Characteristics and In Vitro Toxicity. J. Funct. Biomater. 2023, 14, 425. https://doi.org/10.3390/jfb14080425
Auliya DG, Fauziah U, Arini VF, Setiadji S, Fitrilawati F, Kartasasmita AS, Risdiana R. Use of Dichlorodimethylsilane to Produce Polydimethylsiloxane as a Substitute for Vitreous Humour: Characteristics and In Vitro Toxicity. Journal of Functional Biomaterials. 2023; 14(8):425. https://doi.org/10.3390/jfb14080425
Chicago/Turabian StyleAuliya, Diba Grace, Ulfa Fauziah, Vira Fuji Arini, Soni Setiadji, Fitrilawati Fitrilawati, Arief Sjamsulaksan Kartasasmita, and Risdiana Risdiana. 2023. "Use of Dichlorodimethylsilane to Produce Polydimethylsiloxane as a Substitute for Vitreous Humour: Characteristics and In Vitro Toxicity" Journal of Functional Biomaterials 14, no. 8: 425. https://doi.org/10.3390/jfb14080425
APA StyleAuliya, D. G., Fauziah, U., Arini, V. F., Setiadji, S., Fitrilawati, F., Kartasasmita, A. S., & Risdiana, R. (2023). Use of Dichlorodimethylsilane to Produce Polydimethylsiloxane as a Substitute for Vitreous Humour: Characteristics and In Vitro Toxicity. Journal of Functional Biomaterials, 14(8), 425. https://doi.org/10.3390/jfb14080425






