Chitosan-Based High-Intensity Modification of the Biodegradable Substitutes for Cancellous Bone
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bone Substitutes [21]
2.2. Surface Modification of Substitutes [12]
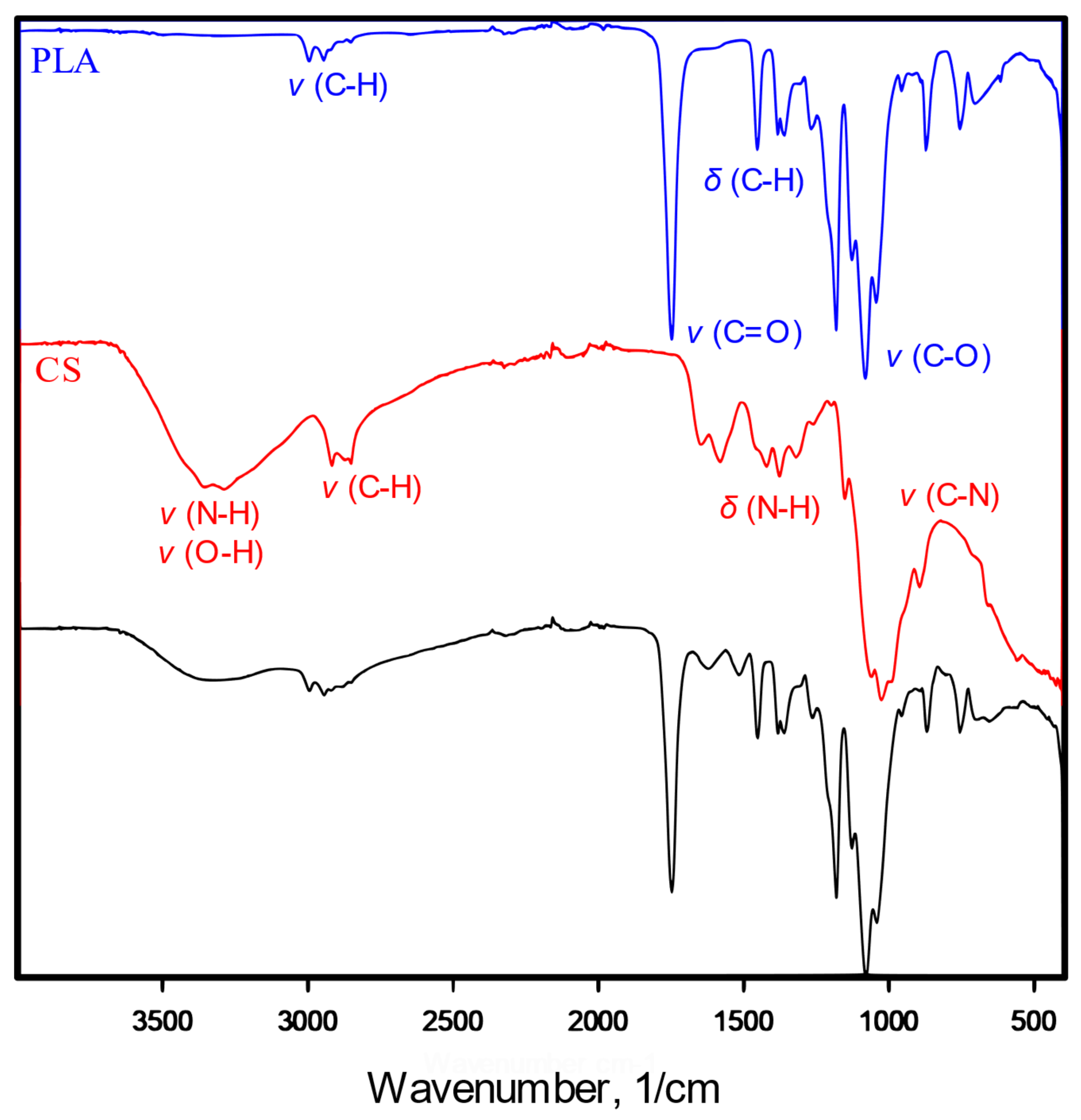
2.3. Infrared Spectroscopy
2.4. Elemental Analysis
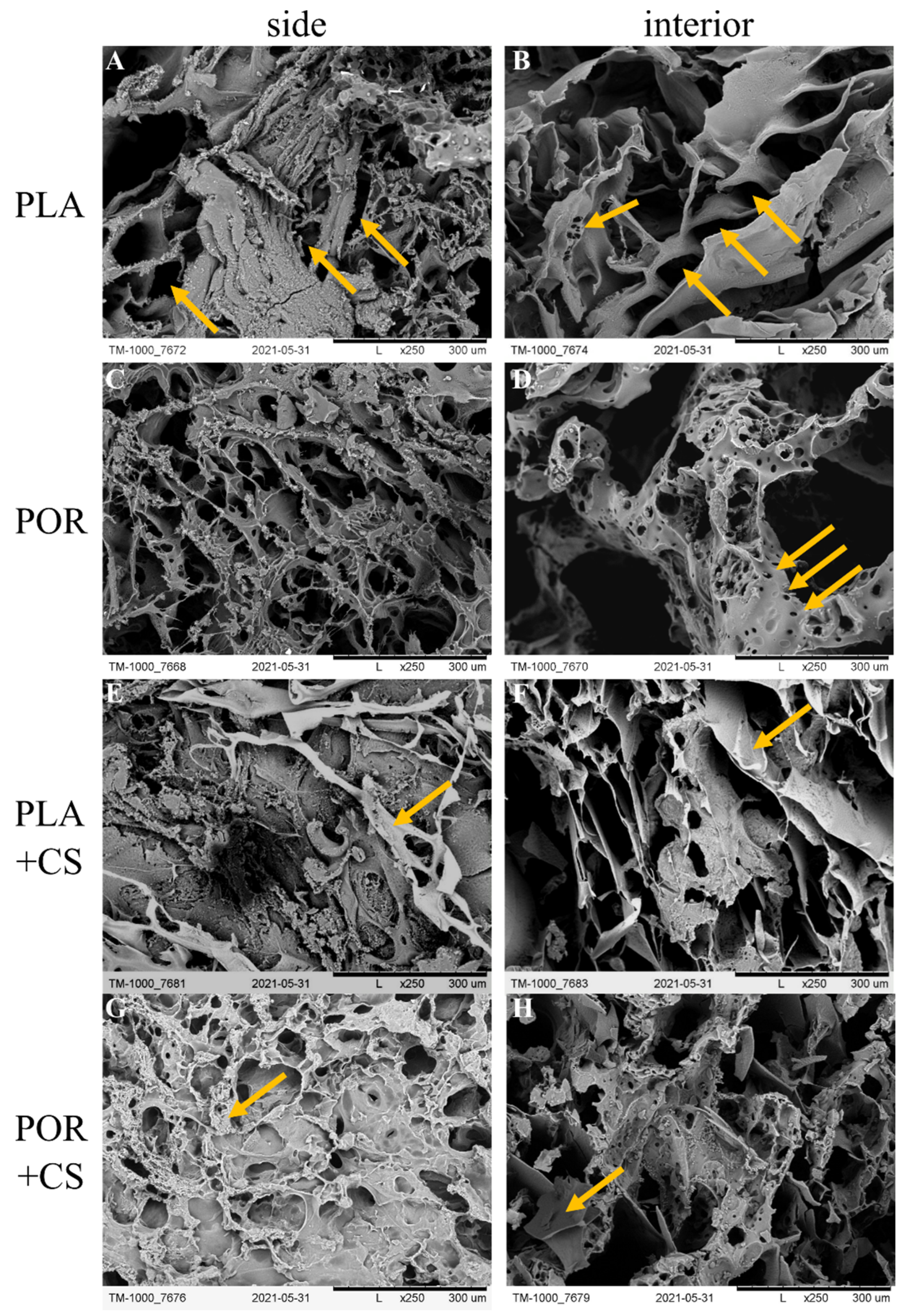
2.5. Scanning Electron Microscopy (SEM) [21]
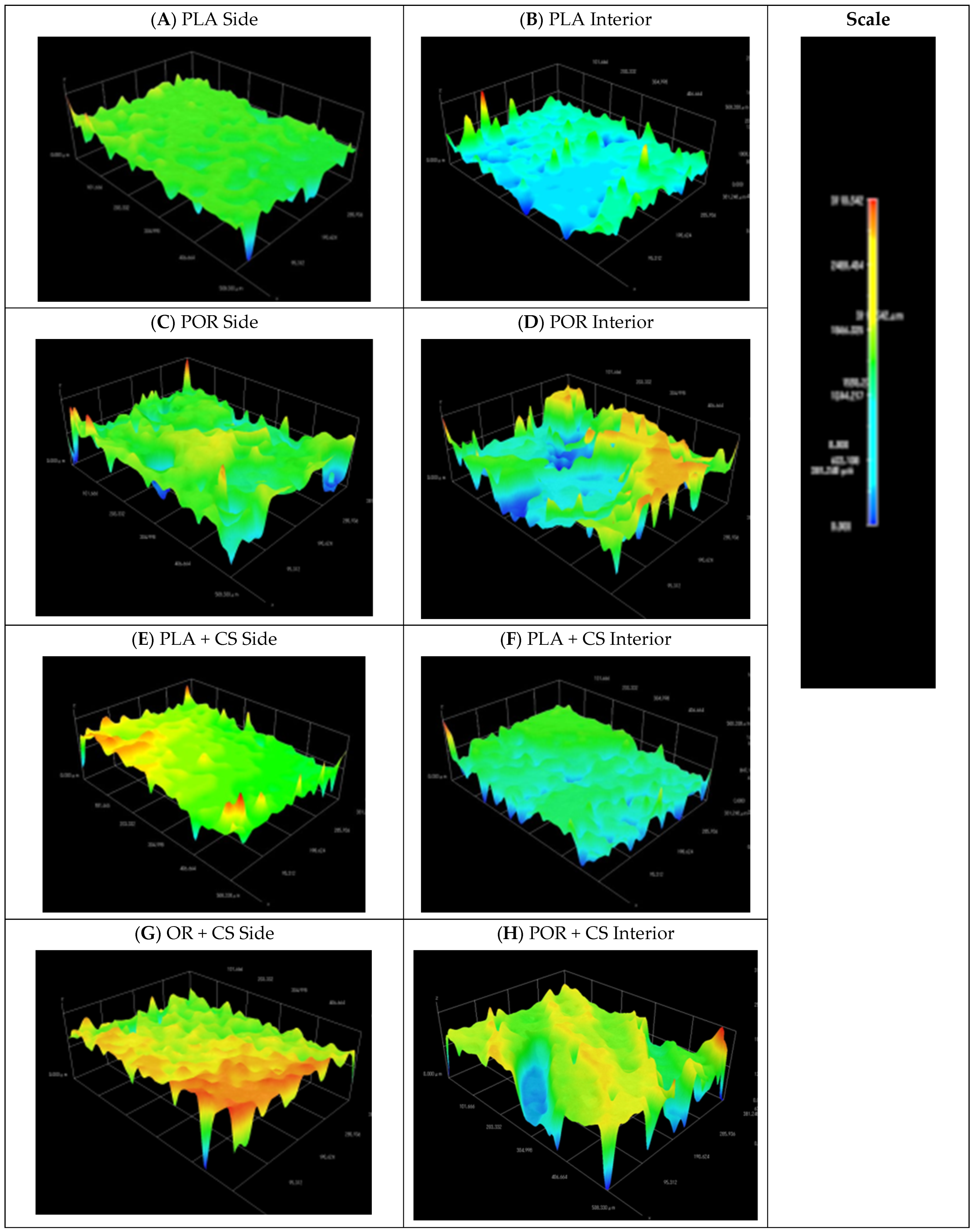
2.6. 3D Surface Modelling
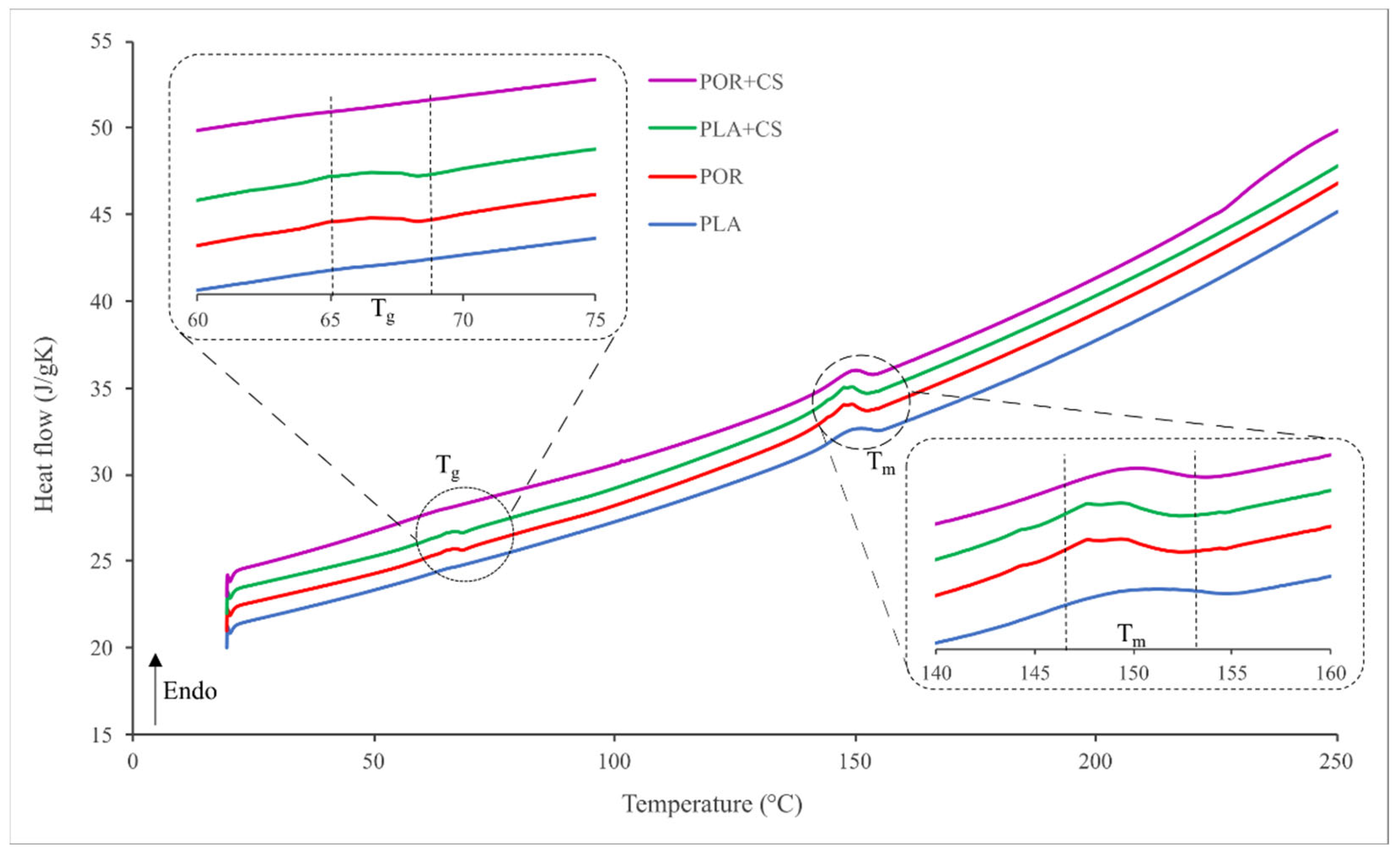
2.7. Differential Scanning Calorimetry (DSC)
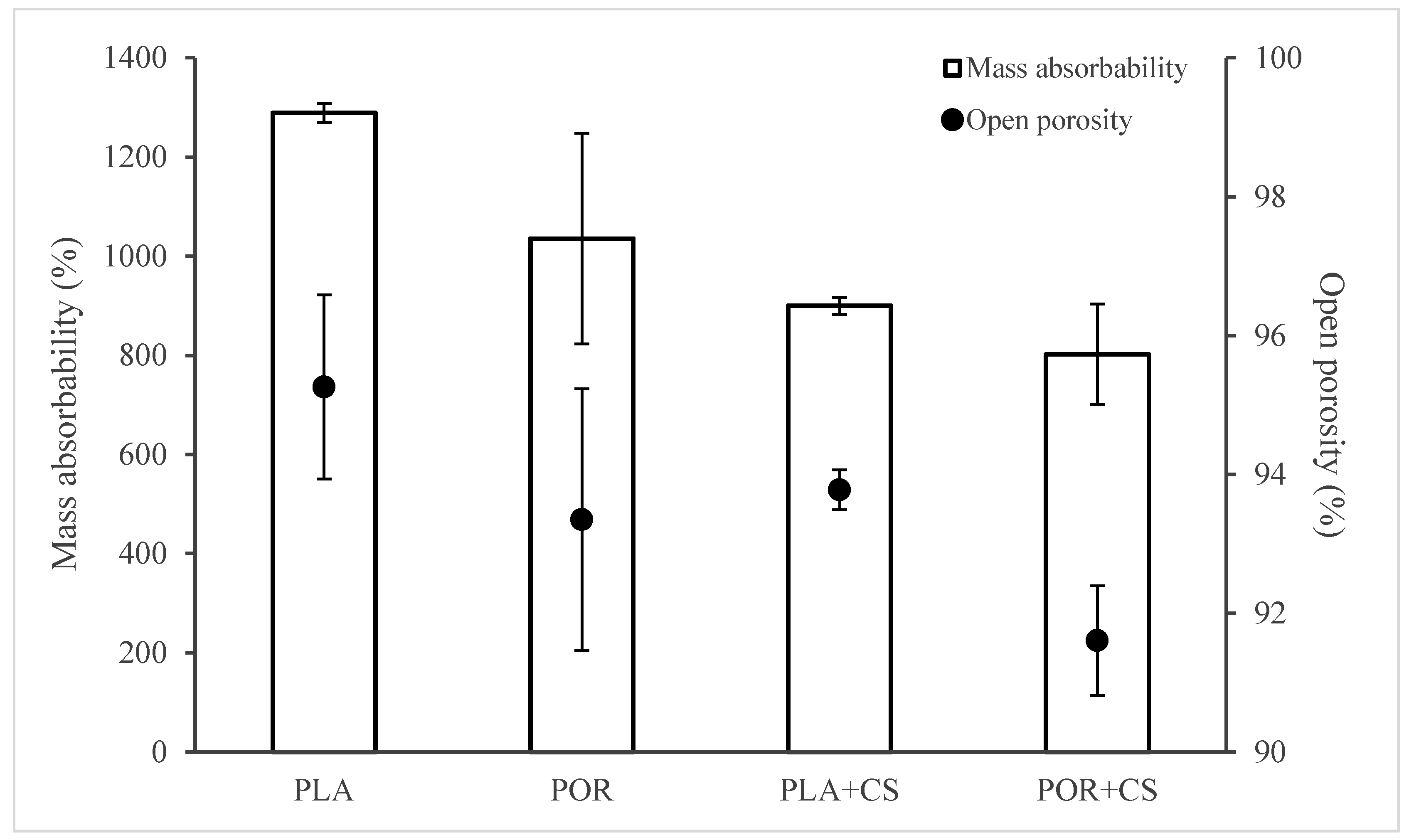
2.8. Open Porosity (Po) and Mass Absorbability (Am) [22,23]
2.9. Elasticity
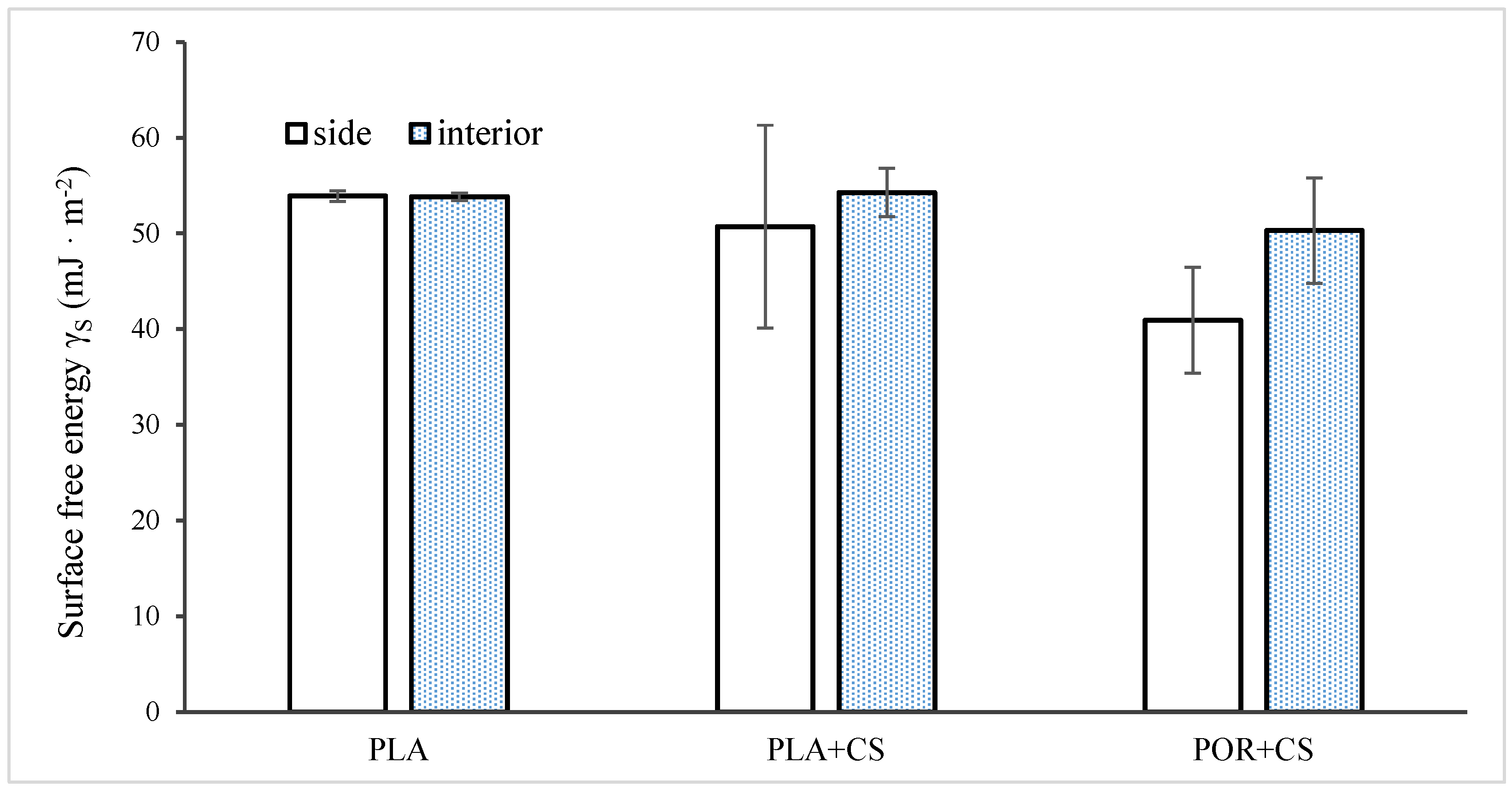
2.10. Surface Free Energy Determination [24]
- —the SFE of diiodomethane (50.80 mJ/m2);
- —the polar component of diiodomethane SFE (0.00 mJ/m2);
- —the dispersive component of diiodomethane SFE (50.80 mJ/m2);
- —the measured contact angle of diiodomethane;
- —the SFE of water (72.80 mJ/m2):
- —the polar component of water SFE (51.00 mJ/m2);
- —the dispersive component of water SFE (21.80 mJ/m2);
- —the measured contact angle of water.
2.11. Cytotoxicity Analysis [21]
3. Results
3.1. Preparation of Substitutes
3.2. Surface Modification
3.3. Properties of Substitutes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Sampath, S.K.; Doble, M. Design of Biocomposite Materials for Bone Tissue Regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Alvarez Primo, F.; Anil Kumar, S.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone Tissue Engineering Techniques, Advances, and Scaffolds for Treatment of Bone Defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Wells, J.A.; Gibbs, D.M.R.; Marshall, K.M.; Tang, D.K.O.; Oreffo, R.O.C. Bone Tissue Engineering and Bone Regeneration. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 917–935. ISBN 9780128184226. [Google Scholar]
- Chandra, P.K.; Soker, S.; Atala, A. Tissue Engineering: Current Status and Future Perspectives. In Principles of Tissue Engineering; INC: New York, NY, USA, 2020; ISBN 9780128184226. [Google Scholar]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan Based Polymer/Bioglass Composites for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 96, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered Three-Dimensional Scaffolds for Enhanced Bone Regeneration in Osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Biswal, T. Biopolymers for Tissue Engineering Applications: A Review. Mater. Today Proc. 2019, 41, 397–402. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen-Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Attia, S.; Narberhaus, C.; Schaaf, H.; Streckbein, P.; Pons-kühnemann, J.; Schmitt, C.; Neukam, F.W.; Howaldt, H.P.; Böttger, S. Long-Term Influence of Platelet-Rich Plasma (Prp) on Dental Implants after Maxillary Augmentation: Retrospective Clinical and Radiological Outcomes of a Randomized Controlled Clinical Trial. J. Clin. Med. 2020, 9, 355. [Google Scholar] [CrossRef]
- Bedell, M.L.; Guo, J.L.; Xie, V.Y.; Navara, A.M.; Mikos, A.G. Polymer Scaffold Fabrication. In Principles of Tissue Engineering; INC: New York, NY, USA, 2020; ISBN 9780128184226. [Google Scholar]
- Gadomska-Gajadhur, A.; Ruśkowski, P.; Synoradzki, L.; Trzaskowska, J.; Kruk, A.; Budnicka, M. Sposób Wytwarzania Dynamicznego Substytutu Kości Gąbczastej. Patent PL 236111, 2018. [Google Scholar]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast Adhesion on Biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.L.; Skirtach, A.G.; Mohan, M.K. Surface Functionalization of Chitosan as a Coating Material for Orthopaedic Applications: A Comprehensive Review. Carbohydr. Polym. 2021, 255, 117487. [Google Scholar] [CrossRef]
- Budnicka, M.; Szymaniak, M.; Gadomska-Gajadhur, A.A. Metody Modyfikacji Powierzchni Implantów Polimerowych Do Regeneracji Tkanki Kostnej. In Wybrane Rozwiązania Technologiczne w Medycynie; Wydawnictwo Naukowe TYGIEL: Lublin, Poland, 2018; pp. 79–96. [Google Scholar]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications—A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Halim, A.S.; Keong, L.C.; Zainol, I.; Rashid, A.H.A. Biocompatibility and Biodegradation of Chitosan and Derivatives. In Chitosan-Based Systems for Biopharmaceuticals; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 57–73. [Google Scholar]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone Tissue Engineering: Scaffold Preparation Using Chitosan and Other Biomaterials with Different Design and Fabrication Techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in Developing of Chitosan—Based Polyelectrolyte Complexes as a Platform for Mucosal and Skin Drug Delivery. Eur. Polym. J. 2020, 140, 110020–110034. [Google Scholar] [CrossRef]
- Budnicka, M.; Szymaniak, M.; Kołbuk, D.; Ruśkowski, P.; Gadomska-Gajadhur, A. Biomineralization of Poly-l-Lactide Spongy Bone Scaffolds Obtained by Freeze-Extraction Method. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 108, 868–879. [Google Scholar] [CrossRef]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and physico-chemical properties of highly porous PLA/HA scaffolds for bone reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef]
- Mazur, K.; Singh, R.; Friedrich, R.P.; Genç, H.; Unterweger, H.; Sałasińska, K.; Bogucki, R.; Kuciel, S.; Cicha, I. The effect of antibacterial particle incorporation on the mechanical properties, biodegradability, and biocompatibility of PLA and PHBV composites. Macromol. Mater. Eng. 2020, 305, 2000244. [Google Scholar] [CrossRef]
- Młotek, M.; Gadomska-Gajadhur, A.; Sobczak, A.; Kruk, A.; Perron, M.; Krawczyk, K. Modification of PLA scaffold surface for medical applications. Appl. Sci. 2021, 11, 1815. [Google Scholar] [CrossRef]
- Budyanto, L.; Goh, Y.Q.; Ooi, C.P. Fabrication of Porous Poly(L-Lactide) (PLLA) Scaffolds for Tissue Engineering Using Liquid-Liquid Phase Separation and Freeze Extraction. J. Mater. Sci. Mater. Med. 2009, 20, 105–111. [Google Scholar] [CrossRef]
- Budnicka, M.; Kołbuk, D.; Ruśkowski, P.; Gadomska-Gajadhur, A. Poly-L-Lactide Scaffolds with Super Pores Obtained by Freeze-Extraction Method. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 108, 3162–3173. [Google Scholar] [CrossRef]
- Budnicka, M.; Trzaskowska, J.; Gadomska-Gajadhur, A.; Ruśkowski, P.; Synoradzki, L. Preparation of Polylactide Scaffolds for Cancellous Bone Regeneration—Preliminary Investigation and Optimization of the Process. Pure Appl. Chem. 2019, 91, 1509–1519. [Google Scholar] [CrossRef]
- Gadomska-Gajadhur, A.; Łojek, K.; Szymaniak, M.; Gadomska, A. Materiały Porowate Do Regeneracji Tkanki Chrzęstnej i Kostnej. Wyr. Med. 2018, 3, 51–58. [Google Scholar]
- Deb, P.; Deoghare, A.B.; Borah, A.; Barua, E.; Das Lala, S. Scaffold Development Using Biomaterials: A Review. Mater. Today Proc. 2018, 5, 12909–12919. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Kochel, M.; Depprich, R.; Kübler, N.R.; Würzler, K.K. Influence of Platelet-Rich Plasma (PRP) on Osteogenic Differentiation of Rat Bone Marrow Stromal Cells. An in Vitro Study. Int. J. Oral Maxillofac. Surg. 2004, 33, 60–70. [Google Scholar] [CrossRef]
- Błaszczyk, B.; Kaspera, W.; Ficek, K.; Kajor, M.; Binkowski, M.; Stodolak-Zych, E.; Grajoszek, A.; Stojko, J.; Bursig, H.; Ładziński, P. Effects of Polylactide Copolymer Implants and Platelet-Rich Plasma on Bone Regeneration within a Large Calvarial Defect in Sheep. Biomed Res. Int. 2018, 2018, 4120471–4120482. [Google Scholar] [CrossRef]
- Ficek, K.; Filipek, J.; Wojciechowski, P.; Kopec, K.; Ewa, S.Z.; Blazewicz, S. A Bioresorbable Polylactide Implant Used in Bone Cyst Filling. J. Mater. Sci. Mater. Med. 2016, 27, 33–41. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone Tissue Engineering: State of the Art and Future Trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. The Use of Contact Angle and the Surface Free Energy as the Surface Characteristics of the Polymers Used in Medicine. Polim. Med. 2014, 44, 29–37. [Google Scholar]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface Modification of Polylactic Acid (PLA) by Air Atmospheric Plasma Treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Gallyamov, M.O.; Chaschin, I.S.; Khokhlova, M.A.; Grigorev, T.E.; Bakuleva, N.P.; Lyutova, I.G.; Kondratenko, J.E.; Badun, G.A.; Chernysheva, M.G.; Khokhlov, A.R. Collagen Tissue Treated with Chitosan Solutions in Carbonic Acid for Improved Biological Prosthetic Heart Valves. Mater. Sci. Eng. C 2014, 37, 127–140. [Google Scholar] [CrossRef]
- Jeznach, O.; Kołbuk, D.; Marzec, M.; Bernasik, A.; Sajkiewicz, P. Aminolysis as a Surface Functionalization Method of Aliphatic Polyester Nonwovens: Impact on Material Properties and Biological Response. RSC Adv. 2022, 12, 11303–11317. [Google Scholar] [CrossRef]
- Kołbuk, D.; Ciechomska, M.; Jeznach, O.; Sajkiewicz, P. Effect of Crystallinity and Related Surface Properties on Gene Expression of Primary Fibroblasts. RSC Adv. 2022, 12, 4016–4028. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhang, Y.; Sun, S.; Li, Q.; Chen, K.; An, C.; Wang, L.; Van Den Beucken, J.J.J.P.; Wang, H. Control of Matrix Stiffness Using Methacrylate-Gelatin Hydrogels for a Macrophage-Mediated Inflammatory Response. ACS Biomater. Sci. Eng. 2020, 6, 3091–3102. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic Study of Osteoblast and Fibroblast Response to Roughness by Means of Surface-Morphology Gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of Microporosity on Scaffolds for Bone Tissue Engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Abpeikar, Z.; Milan, P.B.; Moradi, L.; Anjomshoa, M.; Asadpour, S. Influence of Pore Sizes in 3D-Scaffolds on Mechanical Properties of Scaffolds and Survival, Distribution, and Proliferation of Human Chondrocytes. Mech. Adv. Mater. Struct. 2021, 29, 4911–4922. [Google Scholar] [CrossRef]


| Experimental Element Content (%) | Calculated Element Content (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | O | N | C | H | O | N | |
| PLA | 50.1 | 5.6 | 44.3 | 0.0 | 50.0 | 5.6 | 44.4 | 0.0 |
| PLA + CS | 45.8 | 5.8 | 45.4 | 3.0 | 45.1 | 6.5 | 39.9 | 8.5 |
| POR + CS | 47.8 | 5.8 | 43.7 | 2.7 | 45.1 | 6.5 | 39.9 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołakowska, A.; Kołbuk, D.; Chwojnowski, A.; Rafalski, A.; Gadomska-Gajadhur, A. Chitosan-Based High-Intensity Modification of the Biodegradable Substitutes for Cancellous Bone. J. Funct. Biomater. 2023, 14, 410. https://doi.org/10.3390/jfb14080410
Kołakowska A, Kołbuk D, Chwojnowski A, Rafalski A, Gadomska-Gajadhur A. Chitosan-Based High-Intensity Modification of the Biodegradable Substitutes for Cancellous Bone. Journal of Functional Biomaterials. 2023; 14(8):410. https://doi.org/10.3390/jfb14080410
Chicago/Turabian StyleKołakowska, Anna, Dorota Kołbuk, Andrzej Chwojnowski, Andrzej Rafalski, and Agnieszka Gadomska-Gajadhur. 2023. "Chitosan-Based High-Intensity Modification of the Biodegradable Substitutes for Cancellous Bone" Journal of Functional Biomaterials 14, no. 8: 410. https://doi.org/10.3390/jfb14080410
APA StyleKołakowska, A., Kołbuk, D., Chwojnowski, A., Rafalski, A., & Gadomska-Gajadhur, A. (2023). Chitosan-Based High-Intensity Modification of the Biodegradable Substitutes for Cancellous Bone. Journal of Functional Biomaterials, 14(8), 410. https://doi.org/10.3390/jfb14080410







