Solid Lipid–Polymer Hybrid Nanoplatform for Topical Delivery of siRNA: In Vitro Biological Activity and Permeation Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SLPHNs Preparation
2.3. Characterization of SLPHNs
2.3.1. Particle Size, Zeta Potential, Nanoparticle Concentration, and Morphology
2.3.2. Physical Stability of SLPHNs
2.4. Electrophoretic Mobility Shift Assay
2.4.1. Evaluation of siRNA Binding and Polyanion Competition
2.4.2. Serum Stability Study
2.5. In Vitro Skin Penetration Study
2.6. Cellular Studies
2.6.1. Cell Culture Conditions
2.6.2. Cellular Viability
2.6.3. Cellular Internalization by Flow Cytometry
2.6.4. Intracellular Localization of SLPHNs by Confocal Microscopy
2.6.5. In Vitro Silencing Efficiency of siRNA–SLPHNs
2.7. Statistics
3. Results and Discussion
3.1. Preparation and DLS Characterization of SLPHNs
3.1.1. SLPHNs Preparation
3.1.2. Effect of Compritol and Poloxamer on Nanoparticle Composition
3.1.3. Influence of Branched PEI-Coated Nanoparticles
3.2. Nanoparticle Tracking Analysis (NTA) and Morphology of SLPHN
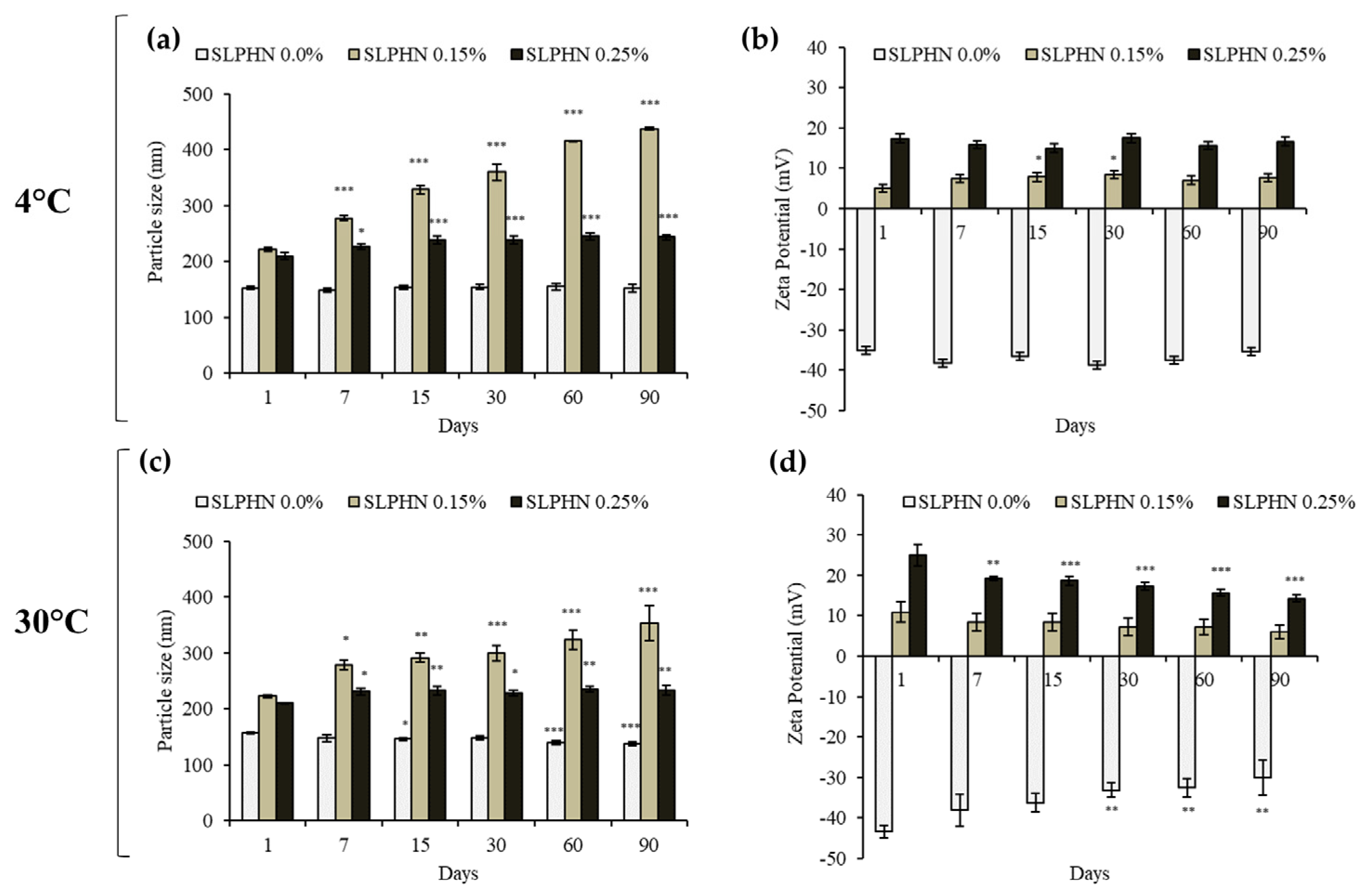
3.3. Physical Stability Studies
3.4. siRNA Binding and Stability of the SLPHNs–siRNA Complex
3.5. Protection of siRNA in SLPHN from Serum Degradation
3.6. In Vitro Skin Penetration Study
3.7. Cellular Studies
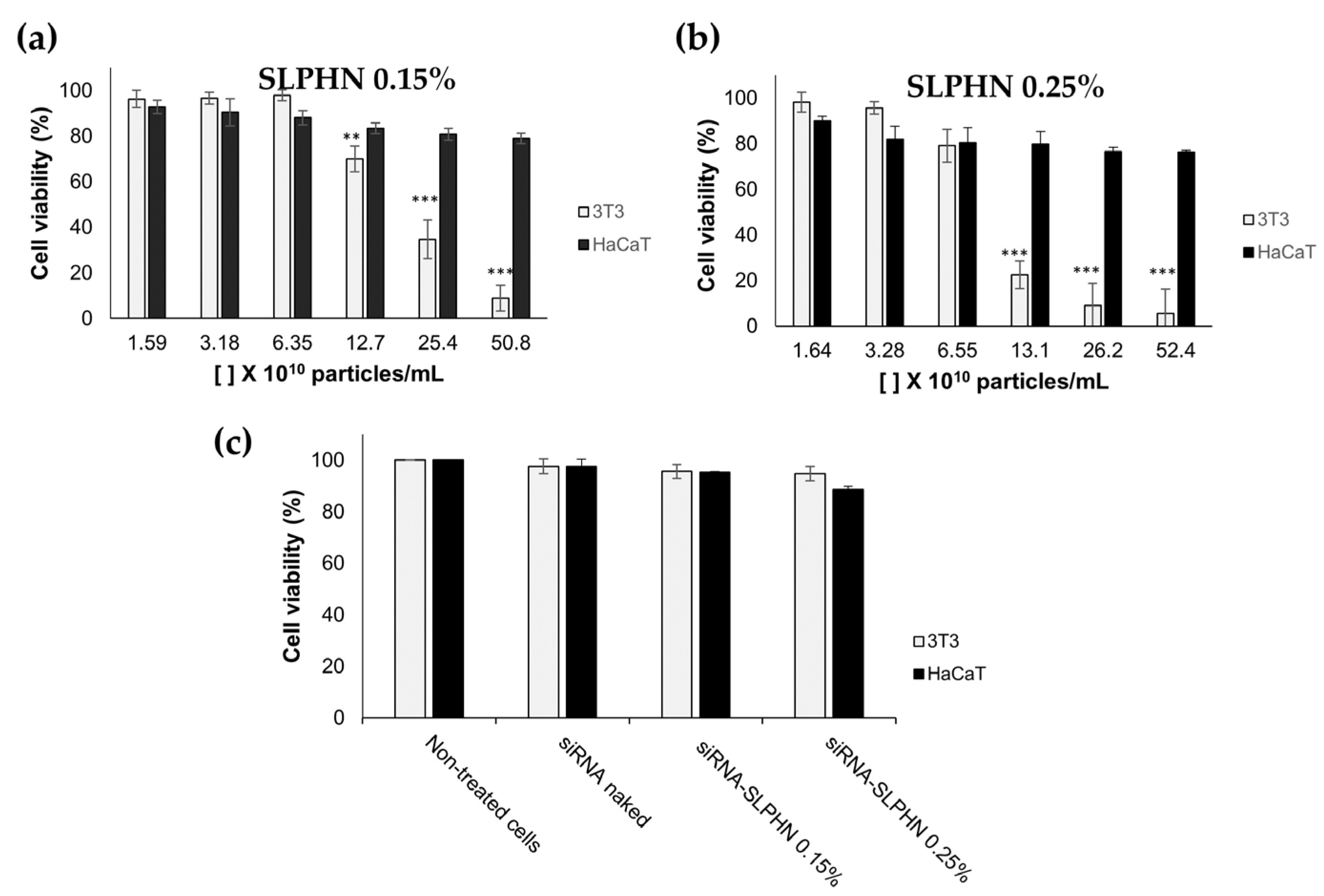
3.7.1. Cell Viability Study
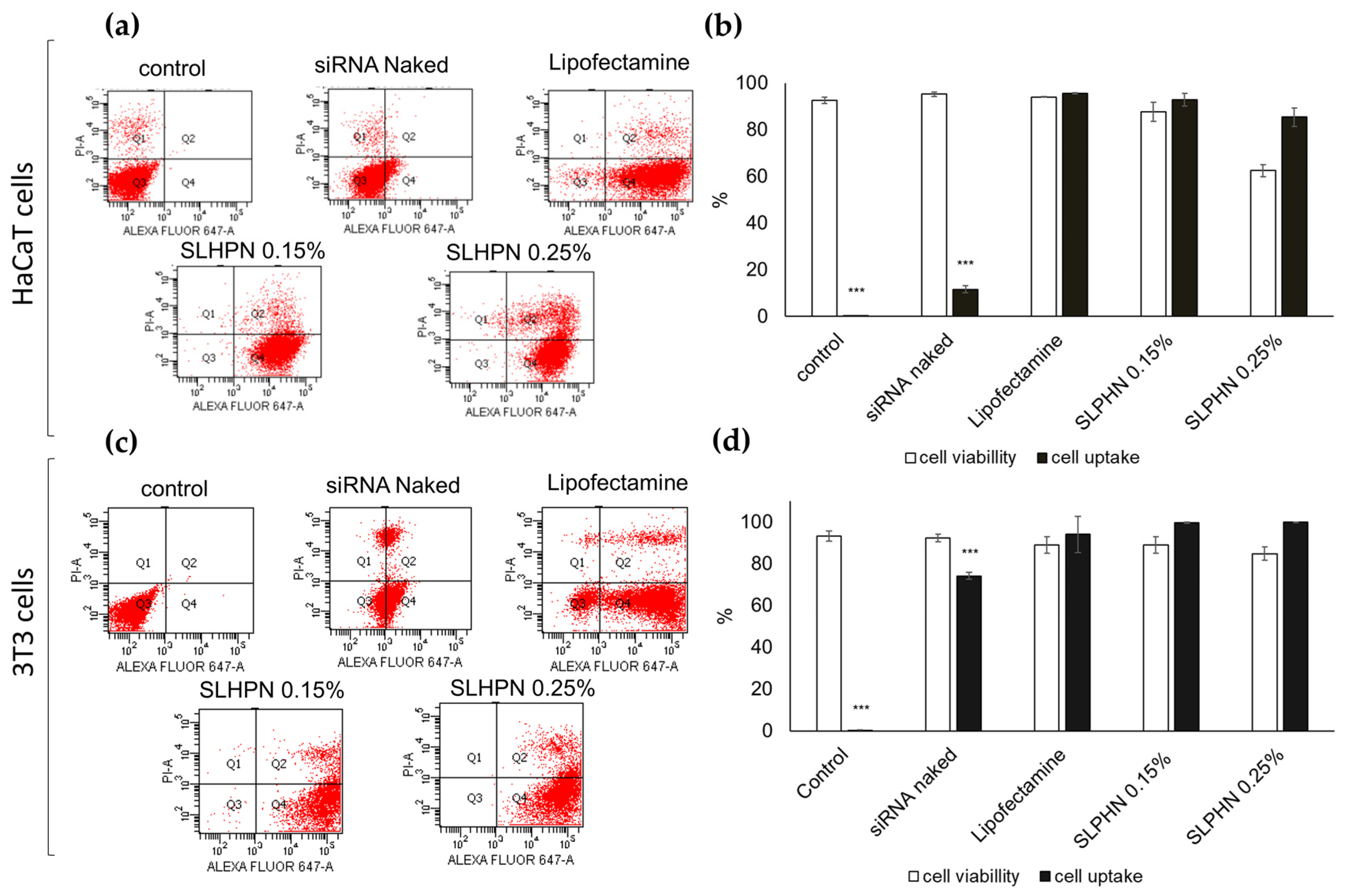
3.7.2. Cellular Uptake
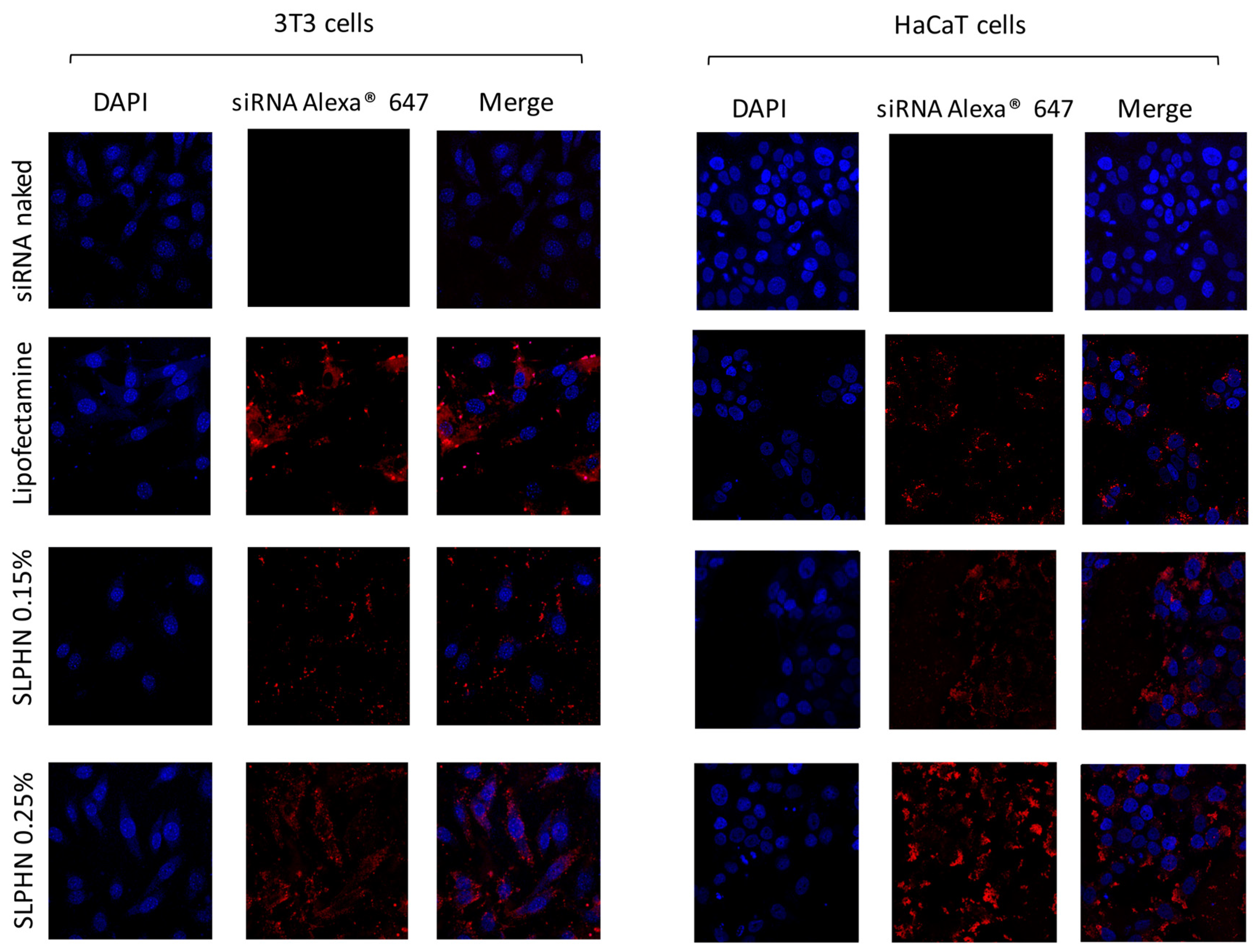
3.7.3. Intracellular Localization by Confocal Microscopy
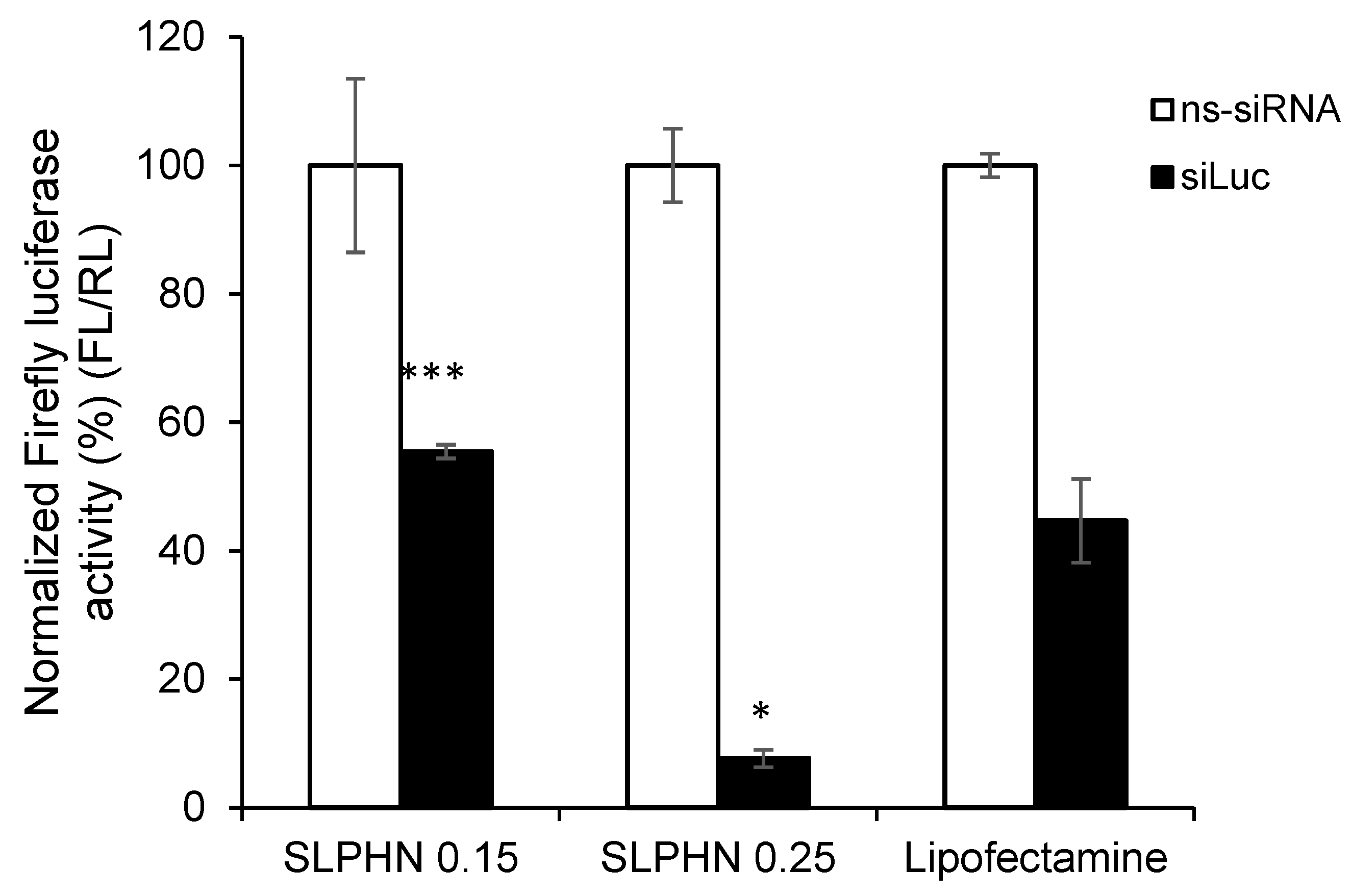
3.7.4. In Vitro Silencing Efficiency of siRNA–SLPHN
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sajid, M.I.; Moazzam, M.; Kato, S.; Cho, K.Y.; Tiwari, R.K. Overcoming Barriers for siRNA Therapeutics: From Bench to Bedside. Pharmaceuticals 2020, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.; Suzuki, I.; Kravicz, M.; Caron, A.; Pupo, A.V.; Praça, F.G.; Bentley, M.V. Current Non-viral siRNA Delivery Systems as a Promising Treatment of Skin Diseases. Curr. Pharm. Des. 2018, 24, 2644–2663. [Google Scholar] [CrossRef] [PubMed]
- Baran-Rachwalska, P.; Torabi-Pour, N.; Sutera, F.M.; Ahmed, M.; Thomas, K.; Nesbit, M.A.; Welsh, M.; Moore, C.T.; Saffie-Siebert, S.R. Topical siRNA delivery to the cornea and anterior eye by hybrid silicon-lipid nanoparticles. J. Control. Release 2020, 326, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, L.; Hu, P.; Yang, Y.; Wei, W.; Deng, X.; Wang, L.; Tay, F.R.; Ma, J. Recent Advances in Stimulus-Responsive Nanocarriers for Gene Therapy. Adv. Sci. 2021, 8, 2100540. [Google Scholar] [CrossRef]
- Mehmood, Y.; Shahid, H.; Rashid, A.; Alhamhoom, Y.; Kazi, M. Developing of SiO2 Nanoshells Loaded with Fluticasone Propionate for Potential Nasal Drug Delivery: Determination of Pro-Inflammatory Cytokines through mRNA Expression. J. Funct. Biomater. 2022, 13, 229. [Google Scholar] [CrossRef]
- Islam, R.A.; Al-Busaidi, H.; Zaman, R.; Abidin, S.A.Z.; Othman, I.; Chowdhury, E.H. Carbonate Apatite and Hydroxyapatite Formulated with Minimal Ingredients to Deliver SiRNA into Breast Cancer Cells In Vitro and In Vivo. J. Funct. Biomater. 2020, 11, 63. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Jiang, G.; Zhang, Z.; Nör, J.E.; ElSayed, M.E.H. Silencing Bcl-2 Expression in Epithelial Cancer Cells Using “Smart” Particles. J. Funct. Biomater. 2014, 5, 167–182. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPSJ. 2021, 23, 78. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
- Ain, Q.U.; Campos, E.V.R.; Huynh, A.; Witzigmann, D.; Hedtrich, S. Gene Delivery to the Skin—How Far Have We Come? Trends Biotechnol. 2021, 39, 474–487. [Google Scholar] [CrossRef]
- Aljabbari, A.; Lokras, A.G.; Kirkensgaard, J.J.K.; Rades, T.; Franzyk, H.; Thakur, A.; Zhang, Y.; Foged, C. Elucidating the nanostructure of small interfering RNA-loaded lipidoid-polymer hybrid nanoparticles. J. Colloid Interface Sci. 2023, 633, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Depieri, L.V.; Borgheti-Cardoso, L.N.; Campos, P.M.; Otaguiri, K.K.; Vicentini, F.T.M.D.C.; Lopes, L.B.; Fonseca, M.J.V.; Bentley, M.V.L.B. RNAi mediated IL-6 in vitro knockdown in psoriasis skin model with topical siRNA delivery system based on liquid crystalline phase. Eur. J. Pharm. Biopharm. 2016, 105, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.L.; de Araujo, M.M.; Bagnato, V.S.; Bentley, M.V.L.B. TNFα siRNA delivery by nanoparticles and photochemical internalization for psoriasis topical therapy. J. Control. Release 2021, 338, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Ciampo, J.O.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Praça, F.S.G.; Del Ciampo, J.O.; Fantini, M.A.C.; Fonseca, M.J.V.; Bentley, M.V.L.B. Liquid Crystalline Nanodispersions Functionalized with Cell-Penetrating Peptides for Topical Delivery of Short-Interfering RNAs: A Proposal for Silencing a Pro-Inflammatory Cytokine in Cutaneous Diseases. J. Biomed. Nanotechnol. 2016, 12, 1063–1075. [Google Scholar] [CrossRef]
- Silvestrini, A.V.P.; Praça, F.G.; Leite, M.N.; Fantini, M.C.d.A.; Frade, M.A.C.; Bentley, M.V.L.B. Liquid crystalline nanoparticles enable a multifunctional approach for topical psoriasis therapy by co-delivering triptolide and siRNAs. Int. J. Pharm. 2023, 640, 123019. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Farahani, M.V.; Sharifzadeh, S.O.; Hamzehlou, S.; et al. Biomedical application of chitosan-based nanoscale delivery systems: Potential usefulness in siRNA delivery for cancer therapy. Carbohydr. Polym. 2021, 260, 117809. [Google Scholar] [CrossRef]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Zade, A.K.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef]
- Li, R.; Wei, F.; Wu, X.; Zhou, P.; Chen, Q.; Cen, Y.; Xu, G.; Cheng, X.; Zhang, A.; Hu, Q. PEI modified orange emissive carbon dots with excitation-independent fluorescence emission for cellular imaging and siRNA delivery. Carbon 2021, 177, 403–411. [Google Scholar] [CrossRef]
- Djemaa SBen Munnier, E.; Chourpa, I.; David, S. Versatile electrostatically assembled polymeric siRNA nanovectors: Can they overcome the limits of siRNA tumor delivery ? Int. J. Pharm. 2019, 567, 118432. [Google Scholar] [CrossRef]
- Alsaad, A.A.A.; Hussien, A.A.; Gareeb, M.M. Solid Lipid Nanoparticles (SLN) as a Novel Drug Delivery System: A Theoretical Review. Syst. Rev. Pharm. 2020, 11, 259–273. [Google Scholar]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid Nanoparticles and Their Hydrogel Composites for Drug Delivery: A Review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, V.K.; Sharma, S.; Roy, A.; Alexander, A.; Singhvi, G. Solid lipid nanocarriers embedded hydrogel for topical delivery of apremilast: In-vitro, ex-vivo, dermatopharmacokinetic and anti-psoriatic evaluation. J. Drug Deliv. Sci. Technol. 2021, 63, 102442. [Google Scholar] [CrossRef]
- EMEA. ICH Topic Q 1 A (R2). Stability Testing of New Drug Substances and Products Step; European Medicines Agency: Amsterdam, The Netherlands, 2003; pp. 1–20. [Google Scholar]
- Borgheti-Cardoso, L.N.; Depieri, L.V.; Kooijmans, S.A.; Diniz, H.; Calzzani, R.A.; Vicentini, F.T.; van der Meel, R.; Fantini, M.C.; Iyomasa, M.M.; Schiffelers, R.M.; et al. In situ gelling liquid crystalline system based on monoglycerides and polyethylenimine for local delivery of siRNAs. Eur. J. Pharm. Sci. 2015, 74, 103–117. [Google Scholar] [CrossRef]
- Borgheti-Cardoso, L.N.; Depieri, L.V.; Diniz, H.; Calzzani, R.A.; de Abreu Fantini, M.C.; Iyomasa, M.M.; Moura de Carvalho Vicentini, F.T.; Bentley, M.V. Self-assembling gelling formulation based on a crystalline-phase liquid as a non-viral vector for siRNA delivery. Eur. J. Pharm. Sci. 2014, 58, 72–82. [Google Scholar] [CrossRef]
- Borgheti-Cardoso, L.N.; Kooijmans, S.A.A.; Fens, M.H.A.M.; van der Meel, R.; Vicentini, F.T.M.C.; Fantini, M.C.A.; Bentley, M.V.L.B.; Schiffelers, R.M. In Situ Gelling Liquid Crystalline System as Local siRNA Delivery System. Mol. Pharm. 2017, 14, 1681–1690. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for the Testing of Chemicals. Skin Absorption: In Vitro Method. Test; OECD: Paris, France, 2004; Volume 428, pp. 1–8. [Google Scholar]
- Praça, F.S.G.; Medina, W.S.G.; Eloy, J.O.; Petrilli, R.; Campos, P.M.; Ascenso, A.; Bentley, M.V.L. Evaluation of critical parameters for in vitro skin permeation and penetration studies using animal skin models. Eur. J. Pharm. Sci. 2017, 111, 121–132. [Google Scholar] [CrossRef]
- Leitner, S.; Grijalvo, S.; Solans, C.; Eritja, R.; García-Celma, M.; Calderó, G. Ethylcellulose nanoparticles as a new “in vitro” transfection tool for antisense oligonucleotide delivery. Carbohydr. Polym. 2020, 229, 115451. [Google Scholar] [CrossRef]
- Rouco, H.; García-García, P.; Évora, C.; Díaz-Rodríguez, P.; Delgado, A. Screening strategies for surface modification of lipid-polymer hybrid nanoparticles. Int. J. Pharm. 2022, 624, 121973. [Google Scholar] [CrossRef]
- Arunkumar, R.; Baskaran, V. Lutein Encapsulated in PLGA–Phospholipid Nano-Carrier Effectively Mitigates Cytokines by Inhibiting Tumor Necrosis Factor TNF-α and Nuclear Factor NF-κB in Mice Retina. J. Funct. Biomater. 2023, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Sriraman, S.K.; Navarro, G.; Biswas, S.; Dalvi, R.A.; Torchilin, V.P. Biomaterials Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials 2012, 33, 3942–3951. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.; Franz-Montan, M.; Breitkreitz, M.C.; Alcântara, A.C.; DE Castro, S.; Guilherme, V.A.; Barbosa, R.; de Paula, E. Nanostructured lipid carriers as robust systems for topical lidocaine-prilocaine release in dentistry. Eur. J. Pharm. Sci. 2016, 93, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural lipids-based NLC containing lidocaine: From pre-formulation to in vivo studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Development and characterization of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs) using central composite design. Int. J. Pharm. 2018, 548, 255–262. [Google Scholar] [CrossRef]
- Farid, R.M.; El-Salamouni, N.S.; El-Kamel, A.H.; El-Gamal, S.S. Chapter 16—Lipid-based nanocarriers for ocular drug delivery. In Nanostructures for Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 495–522. [Google Scholar]
- Shukla, T.; Upmanyu, N.; Pandey, S.P.; Gosh, D. Chapter 1. Lipid nanocarriers. In Lipid Nanocarriers for Drug Targeting; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–48. [Google Scholar]
- Javadzadeh, Y.; Bahari, L.A. Therapeutic Nanostructures for Dermal and Transdermal Drug Delivery. In Nano- and Microscale Drug Delivery Systems; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 131–146. [Google Scholar]
- Wang, T.; Bae, M.; Lee, J.-Y.; Luo, Y. Solid lipid-polymer hybrid nanoparticles prepared with natural biomaterials: A new platform for oral delivery of lipophilic bioactives. Food Hydrocoll. 2018, 84, 581–592. [Google Scholar] [CrossRef]
- Jangde, R.; Elhassan, G.O.; Khute, S.; Singh, D.; Singh, M.; Sahu, R.K.; Khan, J. Hesperidin-Loaded Lipid Polymer Hybrid Nanoparticles for Topical Delivery of Bioactive Drugs. Pharmaceuticals 2022, 15, 211. [Google Scholar] [CrossRef]
- Carvalho, I.; Miranda, M.; Silva, L.; Chrysostomo-Massaro, T.; Paschoal, J.; Bastos, J.; Marcato, P. IN VITRO Anticancer Activity and Physicochemical Properties of SOLANUM LYCOCARPUM Alkaloidic Extract Loaded in Natural Lipid-Based Nanoparticles. Colloid Interface Sci. Commun. 2019, 28, 5–14. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; Romeijn, S.; Junginger, H.E.; Borchard, G. PLGA–PEI nanoparticles for gene delivery to pulmonary epithelium. Eur. J. Pharm. Biopharm. 2004, 58, 1–6. [Google Scholar] [CrossRef]
- Ulasov, A.V.; Khramtsov, Y.V.; Trusov, G.A.; Rosenkranz, A.A.; Sverdlov, E.D.; Sobolev, A.S. Properties of PEI-based Polyplex Nanoparticles That Correlate With Their Transfection Efficacy. Mol. Ther. 2009, 19, 103–112. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the Anticancer Activity of Sunitinib Malate in Breast Cancer through Lipid Polymer Hybrid Nanoparticles Approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug. Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Panalytical, M. NTA and DLS—Examples of Complementary Nanomaterial Characterization Techniques. AZO Mater. 2017, 1, 1–11. [Google Scholar]
- ASTM E2834-12R22; Standard Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Nanoparticle Tracking Analysis (NTA). American Society for Testing and Materials: West Conshohocken, PA, USA, 2012; Volume 9, pp. 1–11.
- Sitterberg, J.; Özcetin, A.; Ehrhardt, C.; Bakowsky, U. Utilising atomic force microscopy for the characterisation of nanoscale drug delivery systems. Eur. J. Pharm. Biopharm. 2010, 74, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tofani, L.B.; Depieri, L.V.; Campos, P.M.; Riul, T.B.; Antonietto, K.S.; Fantini, M.C.D.A.; Bentley, M.V.L.B. In Vitro TyRP-1 Knockdown Based on siRNA Carried by Liquid Crystalline Nanodispersions: An Alternative Approach for Topical Treatment of Vitiligo. Pharm. Res. 2018, 35, 104. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.K.; Vuddanda, P.R.; Karunanidhi, P.; Singh, S.K. Development and Evaluation of Solid Lipid Nanoparticles of Raloxifene Hydrochloride for Enhanced Bioavailability. BioMed Res. Int. 2013, 2013, 584549. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, Z.; Chi, W.; Yang, X.; Wang, W.; Zhang, B. Mono-methoxy-poly(3-hydroxybutyrate-co-4-hydroxybutyrate)-graft-hyper-branched polyethylenimine copolymers for siRNA delivery. Biomaterials 2012, 33, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Liu, S.; Chen, J.; Xiao, H.; Yan, L.; Huang, Y.; Jing, X. Biodegradable copolymers with identical cationic segments and their performance in siRNA delivery. J. Control. Release 2012, 159, 251–260. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Uludağ, H.; Lavasanifar, A. Biodegradable amphiphilic poly(ethylene oxide)-block-polyesters with grafted polyamines as supramolecular nanocarriers for efficient siRNA delivery. Biomaterials 2009, 30, 242–253. [Google Scholar] [CrossRef]
- Pauley, K.M.; Cha, S. RNAi Therapeutics in Autoimmune Disease. Pharmaceuticals 2013, 6, 287–294. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Park, M.-R.; Song, S.-C. An injectable cell penetrable nano-polyplex hydrogel for localized siRNA delivery. Biomaterials 2013, 34, 4493–4500. [Google Scholar] [CrossRef]
- Abstiens, K.; Figueroa, S.M.; Gregoritza, M.; Goepferich, A.M. Interaction of functionalized nanoparticles with serum proteins and its impact on colloidal stability and cargo leaching. Soft Matter 2019, 15, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.R.; Paller, A.S. Gene Regulation Using Spherical Nucleic Acids to Treat Skin Disorders. Pharmaceuticals 2020, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Dharamdasani, V.; Mandal, A.; Qi, Q.M.; Suzuki, I.; Bentley, M.V.L.B.; Mitragotri, S. Topical delivery of siRNA into skin using ionic liquids. J. Control. Release 2020, 323, 475–482. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem. Int. Ed. Engl. 2022, 61, e202107960. [Google Scholar] [CrossRef] [PubMed]
- Joris, F.; Manshian, B.B.; Peynshaert, K.; De Smedt, S.C.; Braeckmans, K.; Soenen, S.J. Assessing nanoparticle toxicity in cell-based assays: Influence of cell culture parameters and optimized models for bridging the in vitro–in vivo gap. Chem. Soc. Rev. 2013, 42, 8339–8359. [Google Scholar] [CrossRef]
- Khanbeigi, R.A.; Kumar, A.; Sadouki, F.; Lorenz, C.; Forbes, B.; Dailey, L.A.; Collins, H. The delivered dose: Applying particokinetics to in vitro investigations of nanoparticle internalization by macrophages. J. Control. Release 2012, 162, 259–266. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, E.; Lee, S. Comparison of Sensitivity Between Balb/c 3T3 Cell and HaCaT Cell by NRU Assay to Predict Skin Phototoxicity Potential. Toxicol. Public Health 2002, 18, 227–232. [Google Scholar]
- Gürcan, S.; Tsapis, N.; Reynaud, F.; Denis, S.; Vergnaud, J.; Özer, Ö.; Fattal, E. Combining dexamethasone and TNF-α siRNA within the same nanoparticles to enhance anti-inflammatory effect. Int. J. Pharm. 2021, 598, 120381. [Google Scholar] [CrossRef]
- Karim, M.E.; Chowdhury, E.H. PEGylated Strontium Sulfite Nanoparticles with Spontaneously Formed Surface-Embedded Protein Corona Restrict Off-Target Distribution and Accelerate Breast Tumour-Selective Delivery of siRNA. J. Funct. Biomater. 2022, 13, 211. [Google Scholar] [CrossRef]
- Suñé-Pou, M.; Prieto-Sánchez, S.; El Yousfi, Y.; Boyero-Corral, S.; Nardi-Ricart, A.; Nofrerias-Roig, I.; Pérez-Lozano, P.; García-Montoya, E.; Miñarro-Carmona, M.; Ticó, J.R.; et al. Cholesteryl oleate-loaded cationic solid lipid nanoparticles as carriers for efficient gene-silencing therapy. Int. J. Nanomed. 2018, 13, 3223–3233. [Google Scholar] [CrossRef]
- Liang, W.; Gong, H.; Yin, D.; Lu, S.; Fu, Q. High-Molecular-Weight Polyethyleneimine Conjuncted Pluronic for Gene Transfer Agents. Chem. Pharm. Bull. 2011, 59, 1094–1101. [Google Scholar] [CrossRef]



| Samples | PEI % | Compritol® 888 ATO % | Poloxamer 188% | Aqueous Phase % | Size ± SD (nm) | PdI 1 ± SD 3 | ZP 2 ± SD 3 (mV) |
|---|---|---|---|---|---|---|---|
| F1 | 0.00 | 2.00 | 1.50 | 96.50 | 143.20 ± 20.79 | 0.34 ± 0.03 | −8.33 ± 7.88 |
| F2 | 1.00 | 1.00 | 1.50 | 96.50 | 182.80 ± 13.45 | 0.37 ± 0.03 | 38.75 ± 2.14 |
| F3 | 1.00 | 2.00 | 1.50 | 95.50 | 219.35 ± 8.84 | 0.42 ± 0.00 | 36.60 ± 0.28 |
| F4 | 1.00 | 3.00 | 1.50 | 94.50 | 236.35 ± 14.24 | 0.43 ± 0.09 | 36.83 ± 1.56 |
| F5 | 1.00 | 4.00 | 1.50 | 93.50 | 250.95 ± 59.25 | 0.45 ± 0.07 | 37.18 ± 1.74 |
| F6 | 1.00 | 5.00 | 1.50 | 92.50 | 267.55 ± 43.18 | 0.44 ± 0.02 | 38.63 ± 3.21 |
| F7 | 1.00 | 2.00 | 0.50 | 96.50 | 977.05 ± 67.81 | 0.39 ± 0.08 | 36.70 ± 0.85 |
| F8 | 1.00 | 2.00 | 3.00 | 94.00 | 201.90 ± 0.42 | 0.36 ± 0.03 | 30.85 ± 0.78 |
| F9 | 1.00 | 2.00 | 5.00 | 92.00 | 111.30 ± 15.41 | 0.37 ± 0.01 | 30.13 ± 0.66 |
| F10 | 0.10 | 2.00 | 1.50 | 96.40 | 174.20 ± 1.68 | 0.23 ± 0.02 | 4.46 ± 0.75 |
| F11 | 0.15 | 2.00 | 1.50 | 96.35 | 164.03 ± 4.74 | 0.27 ± 0.05 | 11.50 ± 1.15 |
| F12 | 0.20 | 2.00 | 1.50 | 96.30 | 162.70 ± 11.01 | 0.38 ± 0.04 | 20.90 ± 2.87 |
| F13 | 0.25 | 2.00 | 1.50 | 96.25 | 175.15 ± 17.71 | 0.29 ± 0.12 | 27.18 ± 1.55 |
| F14 | 0.50 | 2.00 | 1.50 | 96.00 | 180.93 ± 5.85 | 0.48 ± 0.05 | 34.10 ± 3.55 |
| F15 | 0.75 | 2.00 | 1.50 | 95.75 | 212.68 ± 15.85 | 0.46 ± 0.02 | 32.38 ± 3.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araujo, M.M.; Borgheti-Cardoso, L.N.; Praça, F.G.; Marcato, P.D.; Bentley, M.V.L.B. Solid Lipid–Polymer Hybrid Nanoplatform for Topical Delivery of siRNA: In Vitro Biological Activity and Permeation Studies. J. Funct. Biomater. 2023, 14, 374. https://doi.org/10.3390/jfb14070374
de Araujo MM, Borgheti-Cardoso LN, Praça FG, Marcato PD, Bentley MVLB. Solid Lipid–Polymer Hybrid Nanoplatform for Topical Delivery of siRNA: In Vitro Biological Activity and Permeation Studies. Journal of Functional Biomaterials. 2023; 14(7):374. https://doi.org/10.3390/jfb14070374
Chicago/Turabian Stylede Araujo, Margarete Moreno, Livia Neves Borgheti-Cardoso, Fabíola Garcia Praça, Priscyla Daniely Marcato, and Maria Vitória Lopes Badra Bentley. 2023. "Solid Lipid–Polymer Hybrid Nanoplatform for Topical Delivery of siRNA: In Vitro Biological Activity and Permeation Studies" Journal of Functional Biomaterials 14, no. 7: 374. https://doi.org/10.3390/jfb14070374
APA Stylede Araujo, M. M., Borgheti-Cardoso, L. N., Praça, F. G., Marcato, P. D., & Bentley, M. V. L. B. (2023). Solid Lipid–Polymer Hybrid Nanoplatform for Topical Delivery of siRNA: In Vitro Biological Activity and Permeation Studies. Journal of Functional Biomaterials, 14(7), 374. https://doi.org/10.3390/jfb14070374






