Newly Developed Resorbable Magnesium Biomaterials for Orbital Floor Reconstruction in Caprine and Ovine Animal Models—A Prototype Design and Proof-of-Principle Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
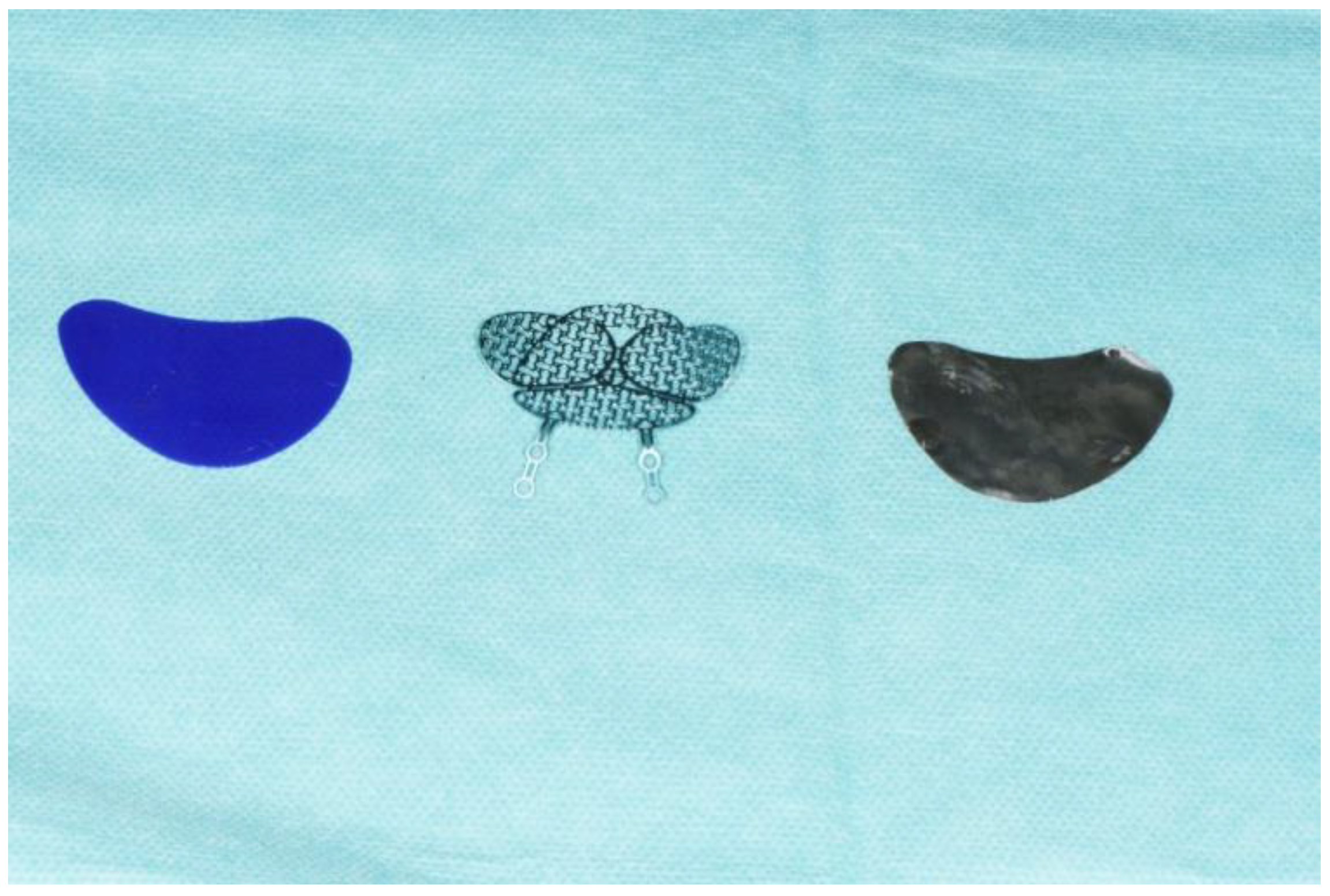
2.2. Three Implants
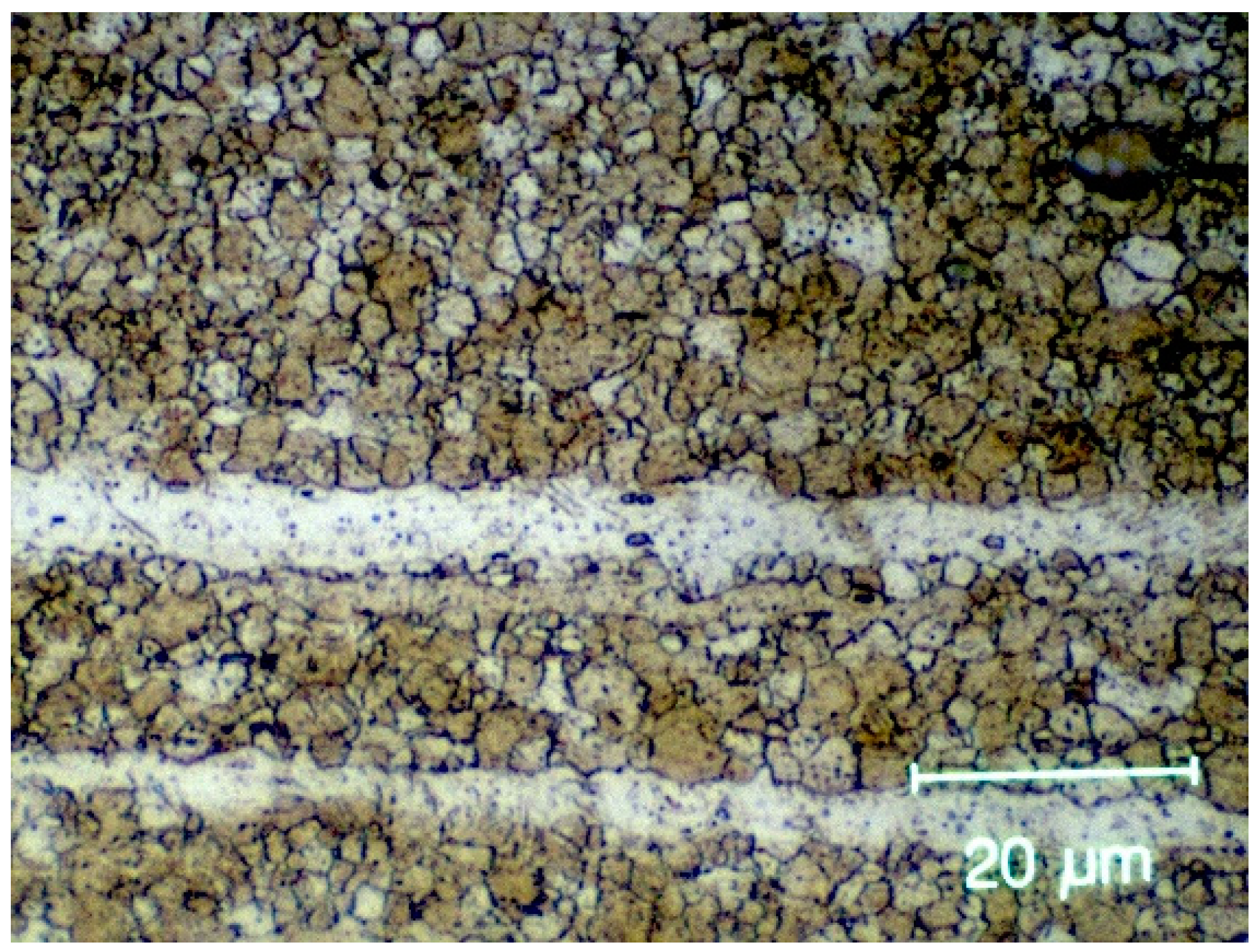
2.2.1. Magnesium Implant
- Magnesium (Mg): 98.1%.
- Zinc (Zn): 0.45%.
- Calcium (Ca): 0.45%.
- Other trace elements: <0.1%.
- Billet preparation: the alloy was first cast into billets of appropriate size and shape.
- Preheating: the billets were heated to a temperature of around 350–400 °C to make them soft and ductile.
- Extrusion: The preheated billets were then loaded into an extrusion press and forced through a die of the desired shape and dimensions. The die was heated to prevent the alloy from sticking to it.
- Cooling: the extruded profile was then cooled and straightened to the desired length.
- Aging: the extruded profile was then aged at a temperature of around 175–200 °C for several hours to improve its strength and hardness.
2.2.2. Titanium Mesh
2.2.3. PDO
2.3. Animal Models
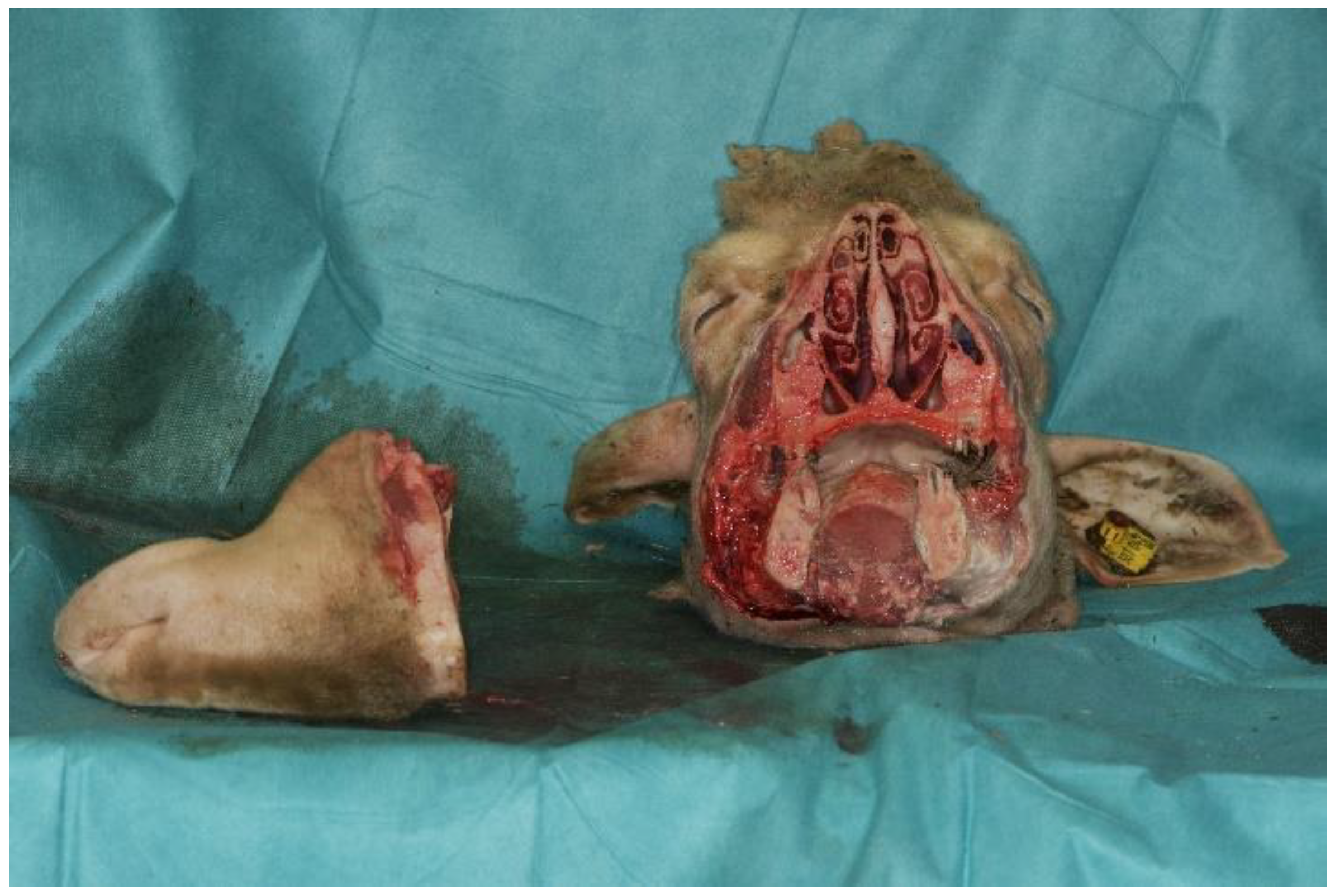
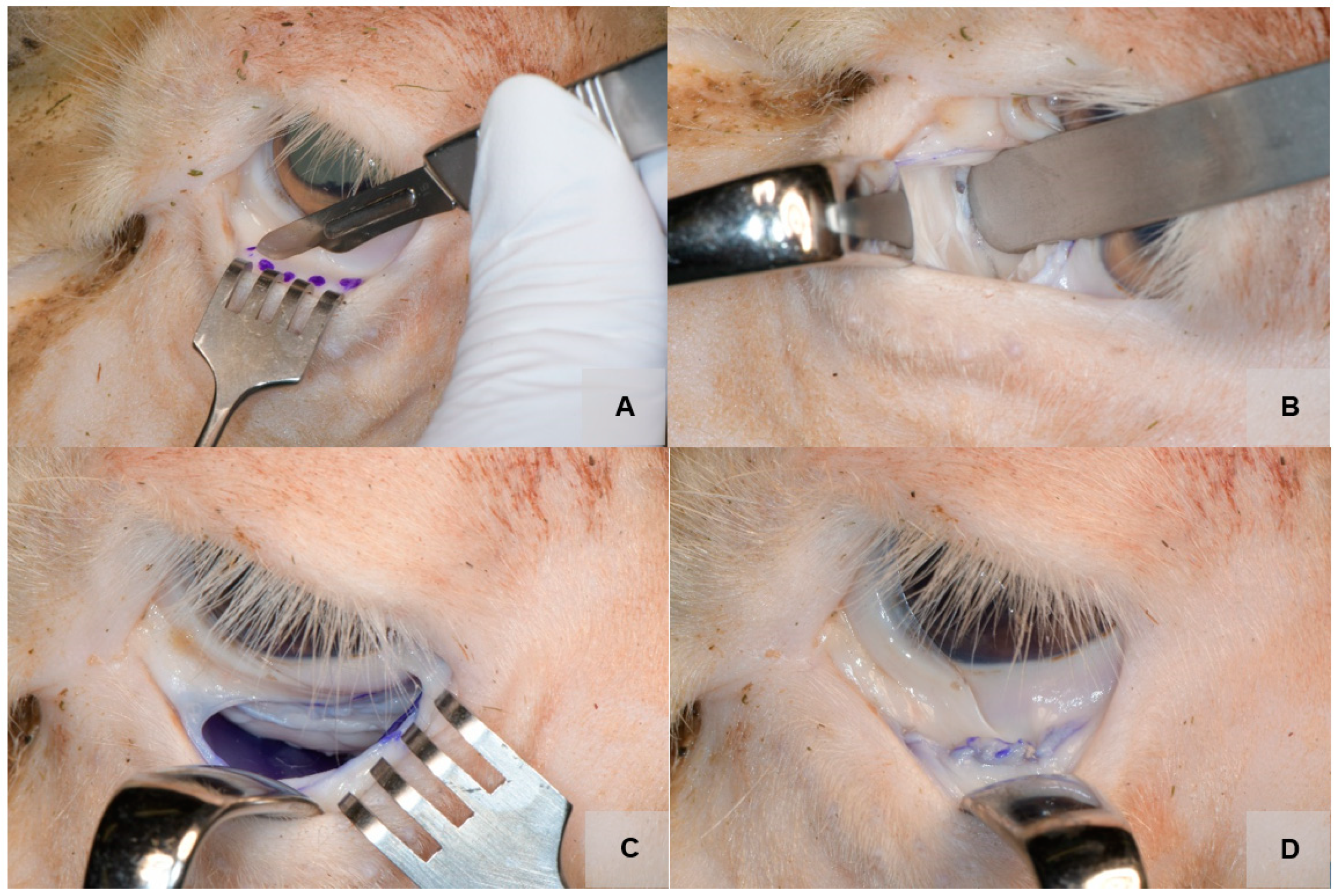
2.4. Surgical Approach
2.5. Study Outcomes
- Sufficient stability of the orbital plate against fracture and torsion during implantation.
- Good position of the implant on the orbital floor.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cornelius, C.P.; Stiebler, T.; Mayer, P.; Smolka, W.; Kunz, C.; Hammer, B.; Jaquiéry, C.C.; Buitrago-Téllez, C.; Leiggener, C.S.; Metzger, M.C.; et al. Prediction of surface area size in orbital floor and medial orbital wall fractures based on topographical subregions. J. Cranio-Maxillofacial Surg. 2021, 49, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Gart, M.S.; Gosain, A.K. Evidence-based medicine: Orbital floor fractures. Plast. Reconstr. Surg. 2014, 134, 1345e–1355e. [Google Scholar] [CrossRef] [PubMed]
- Holweg, P.; Berger, L.; Cihova, M.; Donohue, N.; Clement, B.; Schwarze, U.; Sommer, N.G.; Hohenberger, G.; van den Beucken, J.J.J.P.; Seibert, F.; et al. A lean magnesium–zinc–calcium alloy ZX00 used for bone fracture stabilization in a large growing-animal model. Acta Biomater. 2020, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Analysis of a Pure Magnesium Membrane Degradation Process and Its Functionality When Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 3106. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium fixation screw for barrier membranes used in guided bone regeneration. Bioact. Mater. 2022, 14, 15. [Google Scholar] [CrossRef]
- Jung, O.; Porchetta, D.; Schroeder, M.L.; Klein, M.; Wegner, N.; Walther, F.; Feyerabend, F.; Barbeck, M.; Kopp, A. In Vivo Simulation of Magnesium Degradability Using a New Fluid Dynamic Bench Testing Approach. Int. J. Mol. Sci. 2019, 20, 4859. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Gabryelczak, I.; Bielecki-Kowalski, B. Clinical Evaluation of Magnesium Alloy Osteosynthesis in the Mandibular Head. Materials 2022, 15, 711. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Bode, K.; Jang, Y.S.; Kwon, T.Y.; Jeon, M.H.; Lee, M.H. Gas formation and biological effects of biodegradable magnesium in a preclinical and clinical observation. Sci. Technol. Adv. Mater. 2018, 19, 324–335. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef]
- Gander, T.; Essig, H.; Metzler, P.; Lindhorst, D.; Dubois, L.; Rücker, M.; Schumann, P. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J. Cranio-Maxillofacial Surg. 2015, 43, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.H.; Nunery, W.R. Orbital adherence syndrome secondary to titanium implant material. Ophthal. Plast. Reconstr. Surg. 2009, 25, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Waelti, S.L.; Markart, S.; Willems, E.P.; Fischer, T.; Dietrich, T.J.; Ditchfield, M.; Matissek, C.; Krebs, T. Radiographic features of magnesium-based bioabsorbable screw resorption in paediatric fractures. Pediatr. Radiol. 2022, 52, 2368–2376. [Google Scholar] [CrossRef]
- Grün, N.G.; Holweg, P.; Tangl, S.; Eichler, J.; Berger, L.; van den Beucken, J.J.J.P.; Löffler, J.F.; Klestil, T.; Weinberg, A.M. Comparison of a resorbable magnesium implant in small and large growing-animal models. Acta Biomater. 2018, 78, 378–386. [Google Scholar] [CrossRef]
- Kruber, D.; Hierl, T.; Doerfler, H.M.; Huempfner-Hierl, H.; Krause, M. Preforming of polydioxanone sheets for orbital wall fractures—A technical note. J. Cranio-Maxillofacial Surg. 2018, 46, 1159–1161. [Google Scholar] [CrossRef]
- Jank, S.; Emshoff, R.; Schuchter, B.; Strobl, H.; Brandlmaier, I.; Norer, B. Orbital floor reconstruction with flexible Ethisorb patches: A retrospective long-term follow-up study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 16–22. [Google Scholar] [CrossRef]
- Nkenke, E.; Vairaktaris, E.; Spitzer, M.; Kramer, M.; Stamminger, M.; Holbach, L.; Knipfer, C.; Stelzle, F. Secondary reconstruction of posttraumatic enophthalmos: Prefabricated implants vs titanium mesh. Arch. Facial Plast. Surg. 2011, 13, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Holweg, P.; Herber, V.; Ornig, M.; Hohenberger, G.; Donohue, N.; Puchwein, P.; Leithner, A.; Seibert, F. A lean bioabsorbable magnesium-zinc- calcium alloy ZX00 used for operative treatment of medial malleolus fractures. Bone Jt. Res. 2020, 9, 477–483. [Google Scholar] [CrossRef]
- Jing, X.; Ding, Q.; Wu, Q.; Su, W.; Yu, K.; Su, Y.; Ye, B.; Gao, Q.; Sun, T.; Guo, X.; et al. Magnesium-based materials in orthopaedics: Material properties and animal models. Biomater. Transl. 2021, 2, 197. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Thaller, S.R.; Ellis, E. Subciliary vs. transconjunctival approach for the management of orbital floor and periorbital fractures: A systematic review and meta-analysis. J. Cranio-Maxillofacial Surg. 2017, 45, 1647–1654. [Google Scholar] [CrossRef]
- Doll, C.; Thieme, N.; Schönmuth, S.; Voss, J.O.; Nahles, S.; Beck-Broichsitter, B.; Heiland, M.; Raguse, J.D. Enhanced radiographic visualization of resorbable foils for orbital floor reconstruction: A proof of principle. J. Cranio-Maxillofacial Surg. 2018, 46, 1533–1538. [Google Scholar] [CrossRef]
- Bartoli, D.; Fadda, M.T.; Battisti, A.; Cassoni, A.; Pagnoni, M.; Riccardi, E.; Sanzi, M.; Valentini, V. Retrospective analysis of 301 patients with orbital floor fracture. J. Cranio-Maxillofacial Surg. 2015, 43, 244–247. [Google Scholar] [CrossRef]
- Birkenfeld, F.; Behrens, E.; Kern, M.; Gassling, V.; Wiltfang, J. Mechanical properties of collagen membranes: Are they sufficient for orbital floor reconstructions? J. Cranio-Maxillofacial Surg. 2015, 43, 260–263. [Google Scholar] [CrossRef]
- Wittenberg, J.M.; Wittenberg, R.H.; Hipp, J.A. Biomechanical properties of resorbable poly-L-lactide plates and screws: A comparison with traditional systems. J. Oral Maxillofac. Surg. 1991, 49, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Bostman, O.M.; Paivarinta, U.; Partio, E.; Manninen, M.; Vasenius, J.; Majola, A.; Rokkanen, P. The tissue-implant interface during degradation of absorbable polyglycolide fracture fixation screws in the rabbit femur. Clin. Orthop. Relat. Res. 1992, 285, 263–272. [Google Scholar] [CrossRef]
- van Leeuwen, A.C.; Yuan, H.; Passanisi, G.; van der Meer, J.W.; de Bruijn, J.D.; van Kooten, T.G.; Grijpma, D.W.; Bos, R.R.M. Poly(trimethylene carbonate) and biphasic calcium phosphate composites for orbital floor reconstruction: A feasibility study in sheep. Eur. Cell. Mater. 2014, 27, 81–97. [Google Scholar] [CrossRef]
- Avashia, Y.J.; Sastry, A.; Fan, K.L.; Mir, H.S.; Thaller, S.R. Materials used for reconstruction after orbital floor fracture. J. Craniofac. Surg. 2012, 23, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- de Roche, R.; Kuhn, A.; de Roche-Weber, P.; Gogolewski, S.; Printzen, G.; Geissmann, A.; De Jager, M.; Hammer, B.; Prein, J.; Rahn, B. Experimental reconstruction of the sheep orbit with biodegradable implants. Mund. Kiefer. Gesichtschir. 1998, 2 (Suppl. S1), S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Šiniković, B.; Kramer, F.J.; Swennen, G.; Lübbers, H.T.; Dempf, R. Reconstruction of orbital wall defects with calcium phosphate cement: Clinical and histological findings in a sheep model. Int. J. Oral Maxillofac. Surg. 2007, 36, 54–61. [Google Scholar] [CrossRef]
- Guillaume, O.; Geven, M.A.; Varjas, V.; Varga, P.; Gehweiler, D.; Stadelmann, V.A.; Smidt, T.; Zeiter, S.; Sprecher, C.; Bos, R.R.M.; et al. Orbital floor repair using patient specific osteoinductive implant made by stereolithography. Biomaterials 2020, 233, 119721. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Böse, D.; Eggebrecht, H.; Haude, M.; Schmermund, A.; Erbel, R. First absorbable metal stent implantation in human coronary arteries. Am. Heart Hosp. J. 2006, 4, 128–130. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.M. Bone-implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.O. Biodegradable implants in orthopaedic surgery-A review on the state-of-the-art. Clin. Mater. 1992, 10, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.D.; Khan, S.; Lashgari, Y.; Ziegler, A. Hallux valgus correction utilising a modified short scarf osteotomy with a magnesium biodegradable or titanium compression screws—A comparative study of clinical outcomes. BMC Musculoskelet. Disord. 2019, 20, 334. [Google Scholar] [CrossRef] [PubMed]
- Grün, N.G.; Holweg, P.L.; Donohue, N.; Klestil, T.; Weinberg, A.M. Resorbable implants in pediatric fracture treatment. Innov. Surg. Sci. 2018, 3, 119–125. [Google Scholar] [CrossRef]
- Waizy, H.; Diekmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J. Biomater. Appl. 2014, 28, 667–675. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, H.; Sun, J. Long-term in vivo evolution of high-purity Mg screw degradation—Local and systemic effects of Mg degradation products. Acta Biomater. 2018, 71, 215–224. [Google Scholar] [CrossRef]
- Leigheb, M.; Veneziano, M.; Tortia, R.; Bosetti, M.; Cochis, A.; Rimondini, L.; Grassi, F.A. Osteosynthesis devices in absorbable Magnesium alloy in comparison to standard ones: A Systematic Review on effectiveness and safety. Acta Biomed. 2021, 92, 11757. [Google Scholar] [CrossRef]
- Bontzos, G.; Mazonakis, M.; Papadaki, E.; Maris, T.G.; Blazaki, S.; Drakonaki, E.E.; Detorakis, E.T. Orbital volume measurements from magnetic resonance images using the techniques of manual planimetry and stereology. Natl. J. Maxillofac. Surg. 2020, 11, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hasmat, S.; McPherson, S.; Suaning, G.J.; Lovell, N.H.; Low, T.H.H.; Clark, J.R. Recreation of eyelid mechanics using the sling concept. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 942–950. [Google Scholar] [CrossRef] [PubMed]

| Procedure | Advantages | Disadvantages | Applicable for Simulation |
|---|---|---|---|
| Transconjunctival approach—orbital floor |
|
| Yes |
| Orbital floor reconstruction |
|
| Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomic, J.; Wiederstein-Grasser, I.; Schanbacher, M.; Weinberg, A.M. Newly Developed Resorbable Magnesium Biomaterials for Orbital Floor Reconstruction in Caprine and Ovine Animal Models—A Prototype Design and Proof-of-Principle Study. J. Funct. Biomater. 2023, 14, 339. https://doi.org/10.3390/jfb14070339
Tomic J, Wiederstein-Grasser I, Schanbacher M, Weinberg AM. Newly Developed Resorbable Magnesium Biomaterials for Orbital Floor Reconstruction in Caprine and Ovine Animal Models—A Prototype Design and Proof-of-Principle Study. Journal of Functional Biomaterials. 2023; 14(7):339. https://doi.org/10.3390/jfb14070339
Chicago/Turabian StyleTomic, Josip, Iris Wiederstein-Grasser, Monika Schanbacher, and Annelie Martina Weinberg. 2023. "Newly Developed Resorbable Magnesium Biomaterials for Orbital Floor Reconstruction in Caprine and Ovine Animal Models—A Prototype Design and Proof-of-Principle Study" Journal of Functional Biomaterials 14, no. 7: 339. https://doi.org/10.3390/jfb14070339
APA StyleTomic, J., Wiederstein-Grasser, I., Schanbacher, M., & Weinberg, A. M. (2023). Newly Developed Resorbable Magnesium Biomaterials for Orbital Floor Reconstruction in Caprine and Ovine Animal Models—A Prototype Design and Proof-of-Principle Study. Journal of Functional Biomaterials, 14(7), 339. https://doi.org/10.3390/jfb14070339







