Biomedical Applications of Electrets: Recent Advance and Future Perspectives
Abstract
1. Introduction
2. Classification and Fabrication
2.1. Inorganics
2.1.1. Silicon Dioxide (SiO2)
2.1.2. Hydroxyapatite (HA)
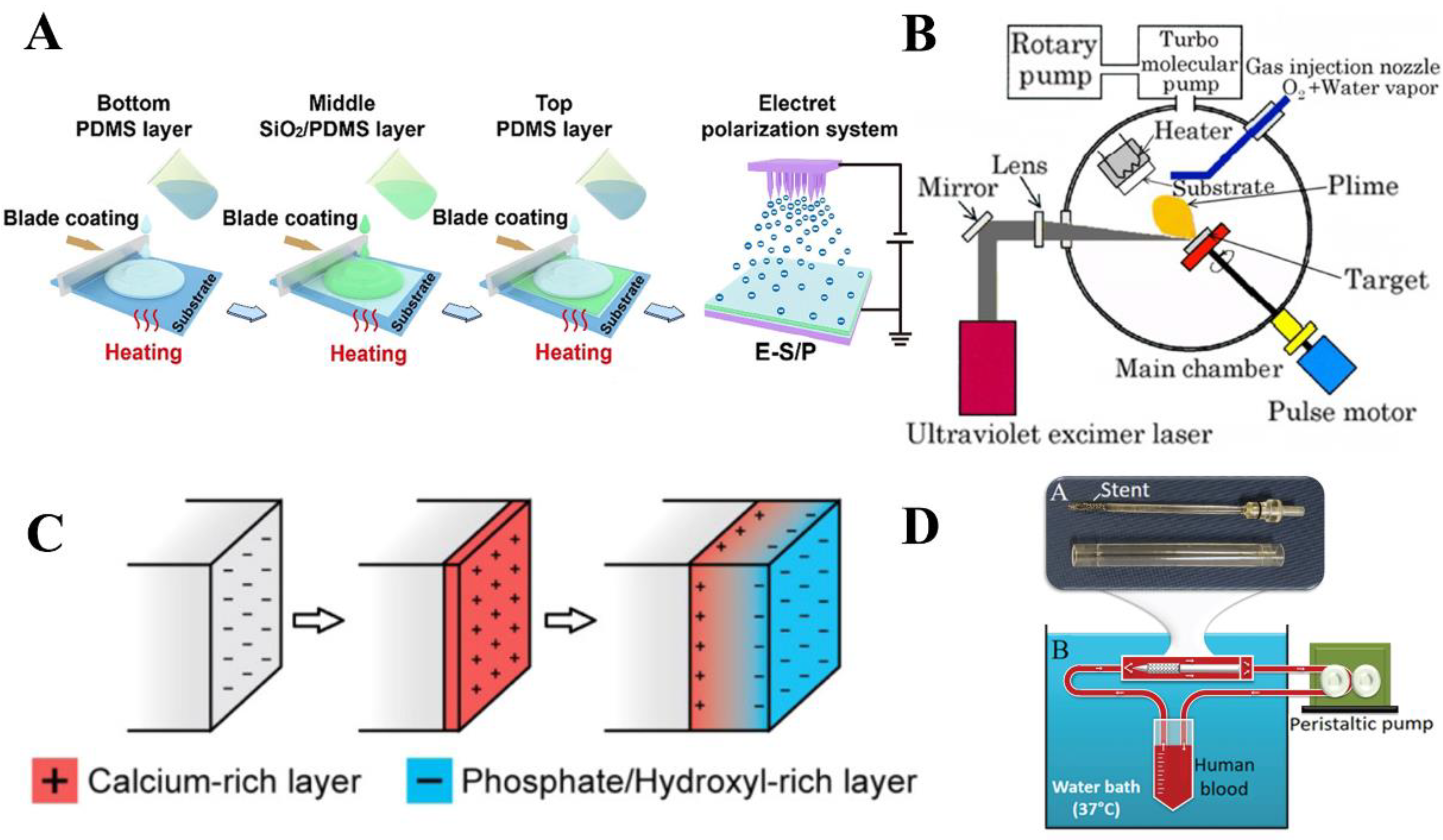
2.1.3. Tantalum Pentoxide (Ta2O5)
2.1.4. Titanium Dioxide (TiO2)
2.2. Macromolecules
2.2.1. Polyethylene (PE)
2.2.2. Polypropylene (PP)
2.2.3. Polytetrafluoroethylene (PTFE)
2.2.4. Polyvinylidene Fluoride (PVDF)
2.2.5. Fluorinated Ethylene Propylene (FEP)
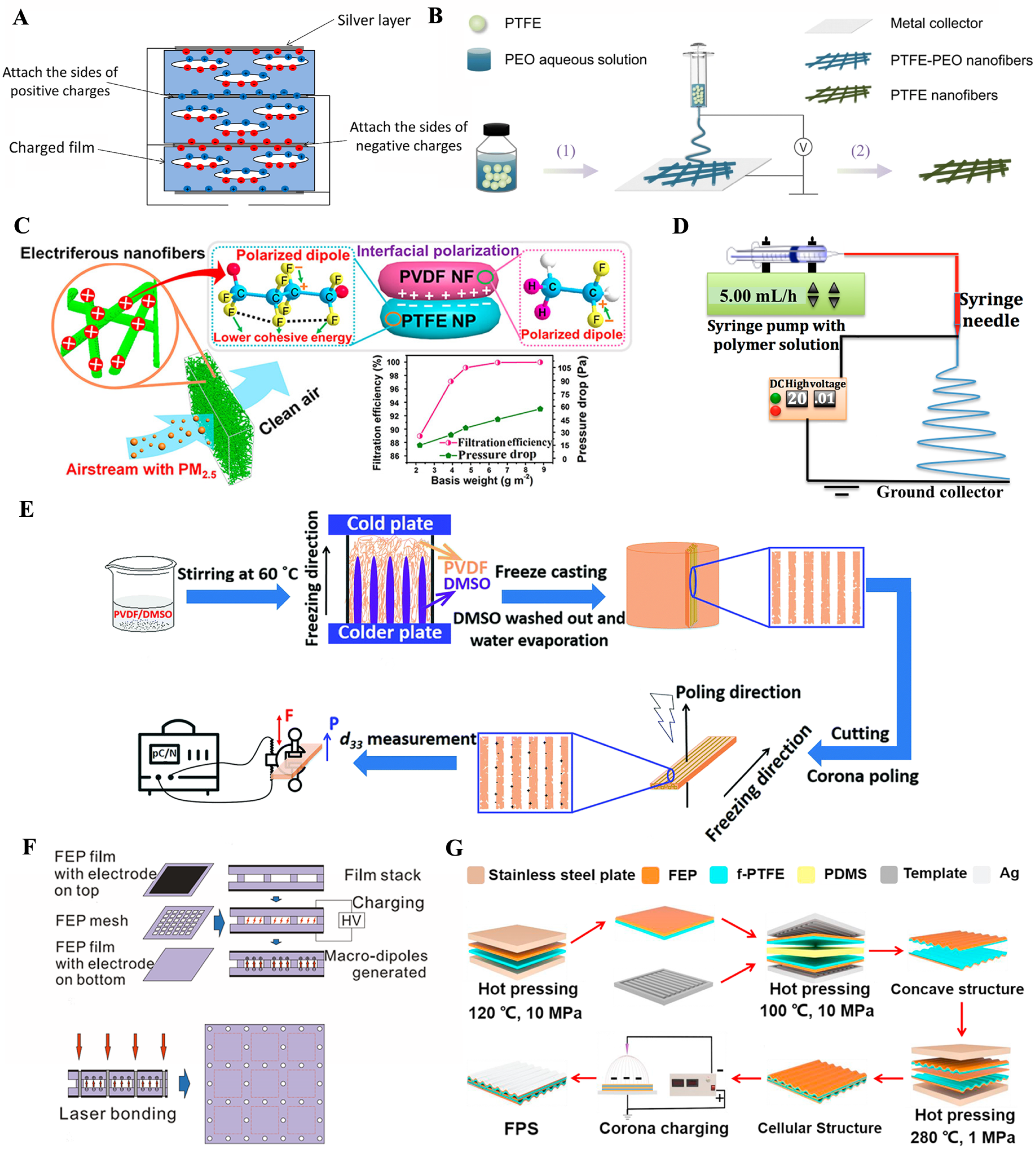
2.2.6. Chitosan (CS)
2.2.7. Collagen and Amino Acid
2.2.8. Others
3. Biomedical Applications
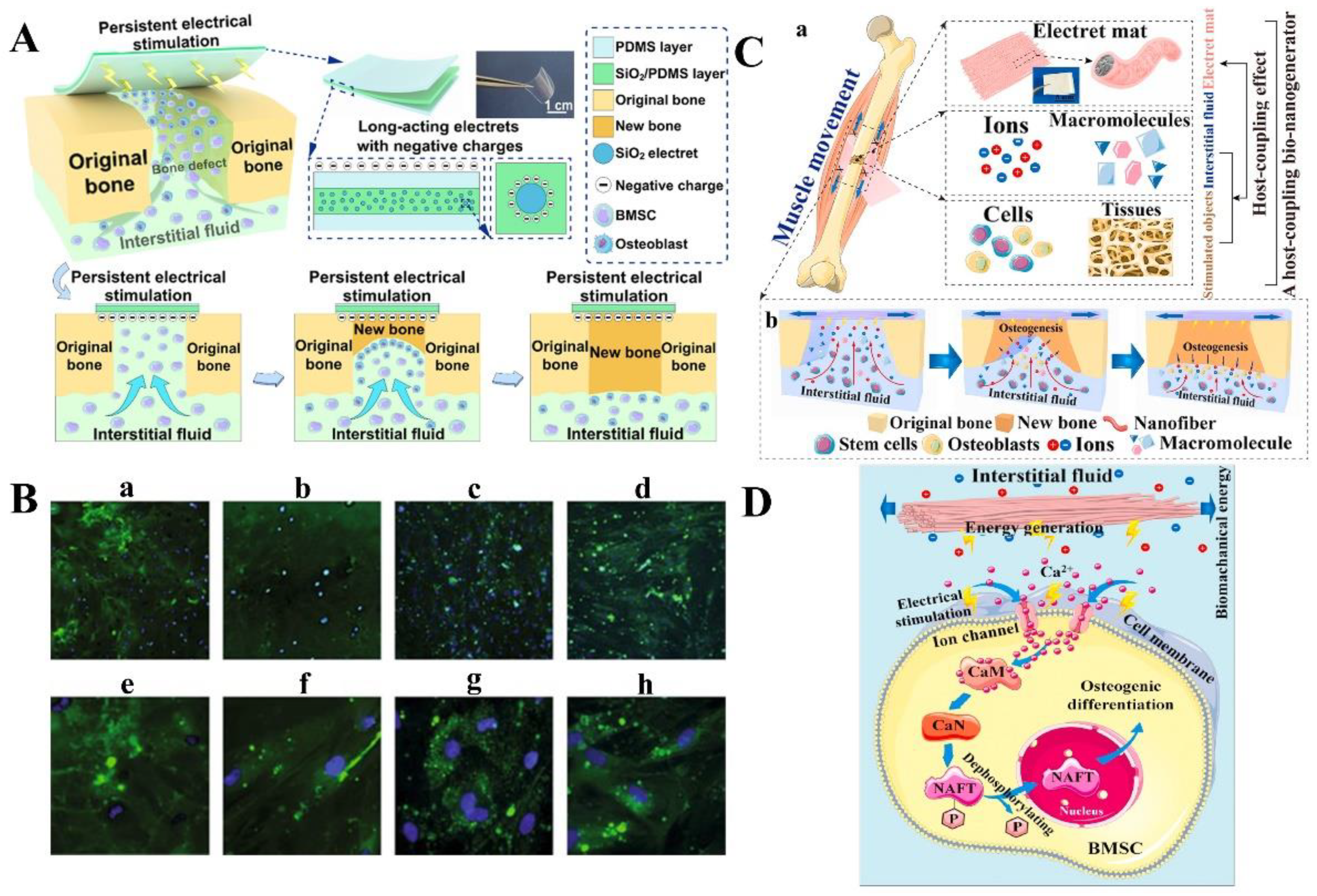
3.1. Bone Regeneration
3.2. Wound Healing
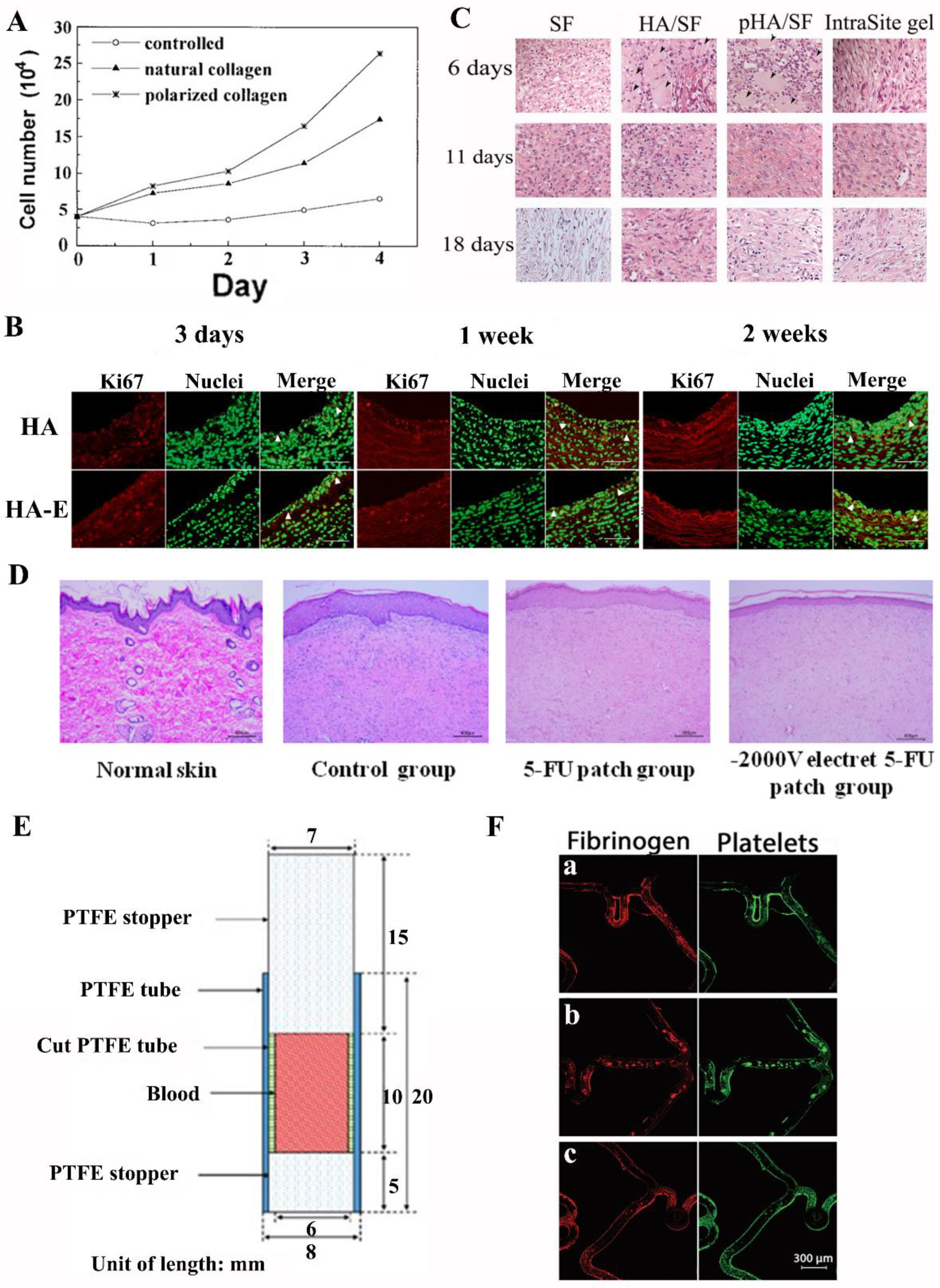
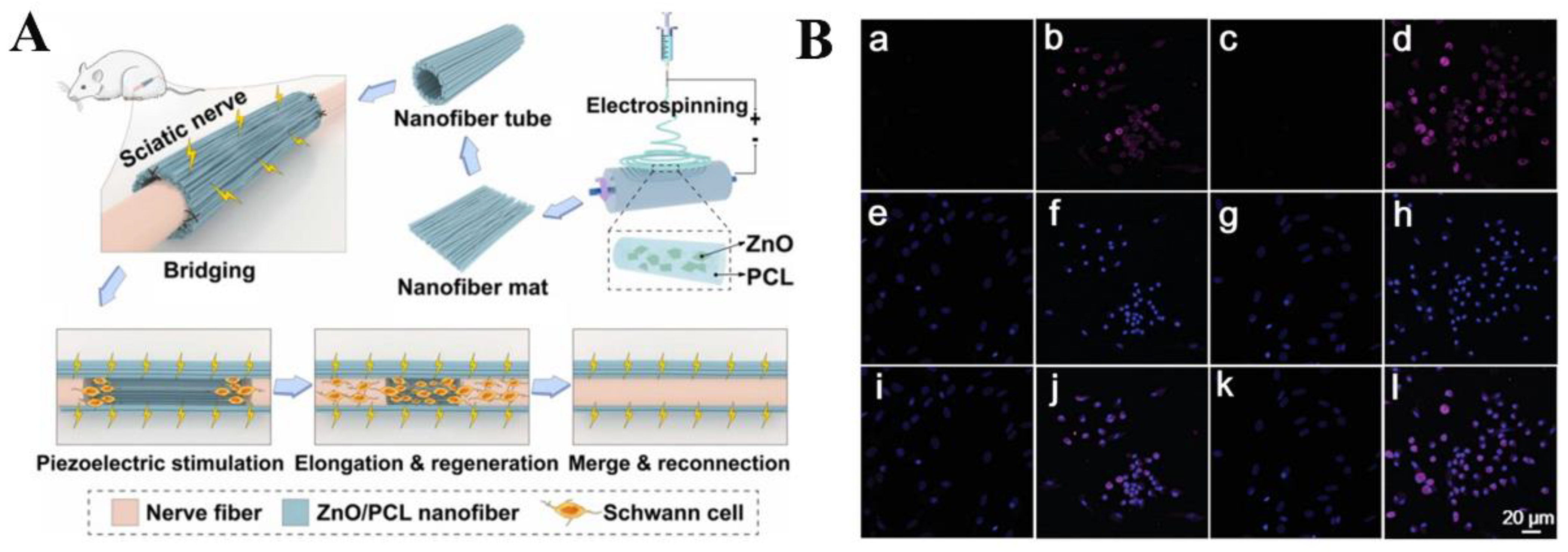
3.3. Nerve Regeneration
3.4. Drug Delivery
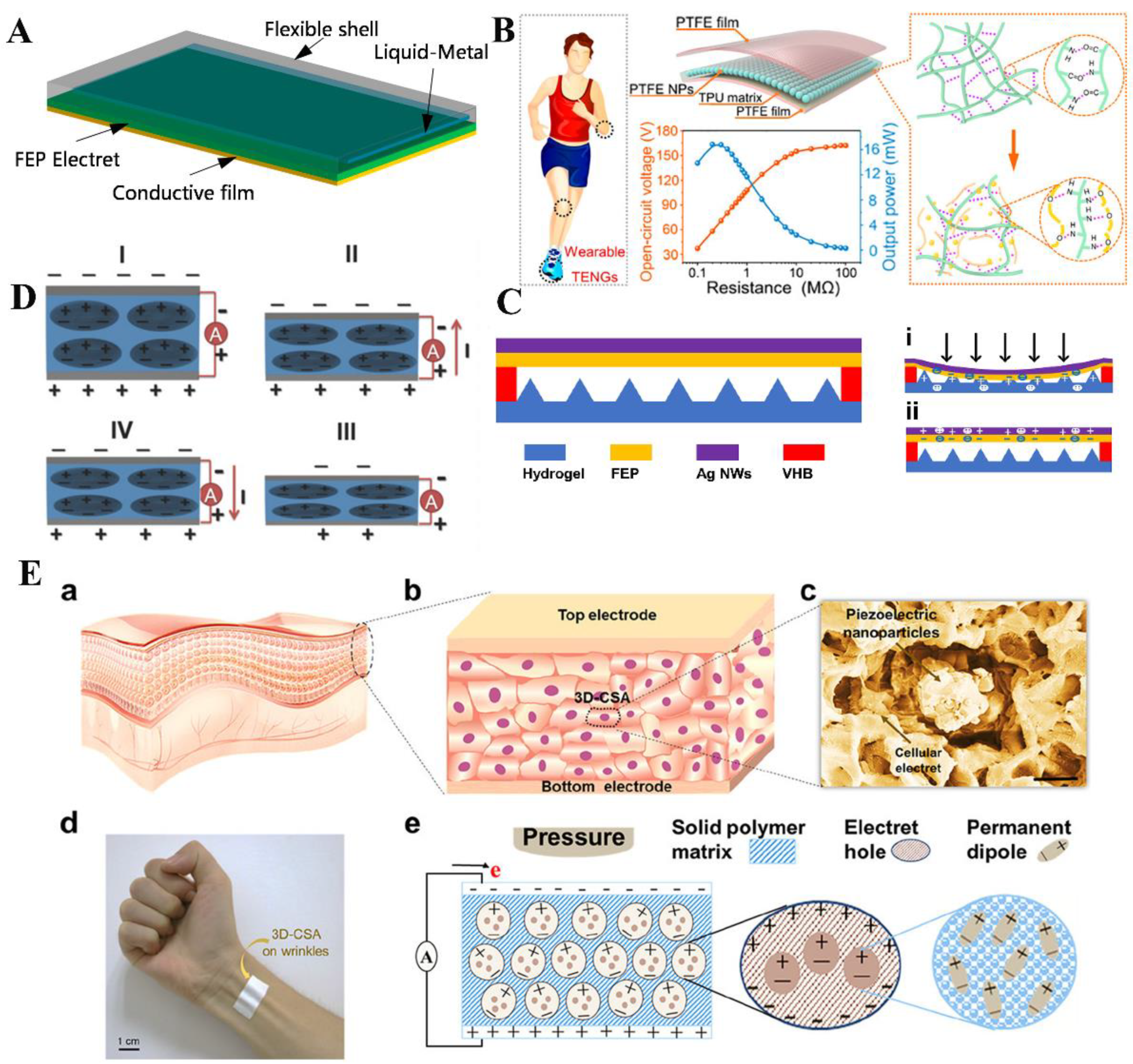
3.5. Wearable Electronics
4. Summary and Perspectives
- (1)
- Simplifying the charging method
- (2)
- Enhancing the charge density
- (3)
- Developing degradable platforms
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zimmermann, J.; Budde, K.; Arbeiter, N.; Molina, F.; Storch, A.; Uhrmacher, A.M.; Rienen, U.V. Using a digital twin of an electrical stimulation device to monitor and control the electrical stimulation of cells in vitro. Front. Bioeng. Biotech. 2021, 9, 765516–765536. [Google Scholar] [CrossRef]
- Zhao, S.; Mehta, A.S.; Zhao, M. Biomedical applications of electrical stimulation. Cell. Mol. Life Sci. 2020, 77, 2681–2699. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-J.; Oh, J.Y.; Kim, Y.-J.; Bhang, S.H.; Jang, H.-K.; Han, J.; Yoon, J.-K.; Kwon, S.-M.; Lee, T.I.; Kim, B.-S. Therapeutic angiogenesis via solar cell-facilitated electrical stimulation. ACS Appl. Mater. Interfaces 2017, 9, 38344–38355. [Google Scholar] [CrossRef] [PubMed]
- Marsudi, M.A.; Ariski, R.T.; Wibowo, A.; Cooper, G.; Rachmantyo, R.; Bartolo, P.J.D.S. Conductive polymeric-based electroactive scaffolds for tissue engineering applications: Current progress and challenges from biomaterials and manufacturing perspectives. Int. J. Mol. Sci. 2021, 22, 11543. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Verdes, M.; Mace, K.; Margetts, L.; Cartmell, S. Status and challenges of electrical stimulation use in chronic wound healing. Curr. Opin. Biotech. 2022, 75, 102710. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Wang, B.; Zhu, Q.; Zeng, L.; Wen, Z.; Yang, C.; Pan, Y. Triboelectric nanogenerators for cellular bioelectrical stimulation. Adv. Funct. Mater. 2022, 32, 2203029. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J. Side effects of whole-body electro-myo-stimulation. Wien. Med. Wochenschr. 2019, 169, 173–180. [Google Scholar] [CrossRef]
- Qiao, Z.; Lian, M.; Liu, X.; Zhang, X.; Han, Y.; Ni, B.; Xu, R.; Yu, B.; Xu, Q.; Dai, K. Electreted sandwich membranes with persistent electrical stimulation for enhanced bone regeneration. ACS Appl. Mater. Interfaces 2022, 14, 31655–31666. [Google Scholar] [CrossRef]
- Guo, Z.; Patil, Y.; Shinohara, A.; Nagura, K.; Yoshida, M.; Nakanishi, T. Organic molecular and polymeric electrets toward soft electronics. Mol. Syst. Des. Eng. 2022, 7, 537–552. [Google Scholar] [CrossRef]
- Lin, S.; Xu, Z.; Wang, S.; Cao, J.; Zhong, J.; Li, G.; Fang, P. Multiplying the stable electrostatic field of electret based on the heterocharge-synergy and superposition effect. Adv. Sci. 2022, 9, 2203150. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, D.; Tan, K.; Deng, Q.; Shen, S. Flexoelectret: An electret with a tunable flexoelectriclike response. Phys. Rev. Lett. 2019, 122, 148001–148006. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Dong, C.; Liu, L.; Gao, C. Applications of tannic acid in membrane technologies: A review. Adv. Colloid Interface Sci. 2020, 284, 102267. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, F. The electret. Rev. Mod. Phys. 1948, 20, 457–472. [Google Scholar] [CrossRef]
- Moreira, M.M.A.C.; Soares, I.N.; Assagra, Y.A.O.; Sousa, F.S.I.; Nordi, T.M.; Dourado, D.M.; Gounella, R.H.; Carmo, J.P.; Altafim, R.A.C.; Altafim, R.A.P. Piezoelectrets: A brief introduction. IEEE Sens. J. 2021, 21, 22317–22328. [Google Scholar] [CrossRef]
- Mascarenhas, S. Bioelectrets: Electrets in biomaterials and biopolymers. Top. Appl. Phys. 2005, 33, 321–346. [Google Scholar]
- Eguchi, M. On the permanent electret. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1925, 49, 178–192. [Google Scholar] [CrossRef]
- Eguchi, M. On dielectric polarisation. Proc. Phys.-Math. Soc. Jpn. 1919, 1, 326–331. [Google Scholar]
- Gross, B. Electret research—Stages in its development. IEEE Trans. Electr. Insul. 1986, 3, 249–269. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Yao, X.; Floch, P.L.; Yang, X.; Liu, J.; Suo, Z. Stretchable electrets: Nanoparticle-elastomer composites. Nano Lett. 2020, 20, 4580–4587. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, Y.; Li, D.; Wei, P.; Lu, W.; Bu, L.; Lu, G. Light-assisted charge injection and depletion of insulator electrets for organic field-effect transistors. J. Mater. Chem. C 2019, 7, 12862–12868. [Google Scholar] [CrossRef]
- Mo, X.; Zhou, H.; Li, W.; Xu, Z.; Duan, J.; Huang, L.; Hu, B.; Zhou, J. Piezoelectrets for wearable energy harvesters and sensors. Nano Energy 2019, 65, 104033. [Google Scholar] [CrossRef]
- Eisenmenger, W.; Schmidt, H.; Dehlen, B. Space charge and dipoles in polyvinylidenefluoride. Braz. J. Phys. 1999, 29, 295–305. [Google Scholar] [CrossRef]
- Bonilla, R.S.; Wilshaw, P.R. Potassium ions in SiO2: Electrets for silicon surface passivation. J. Phys. D Appl. Phys. 2018, 51, 025101. [Google Scholar] [CrossRef]
- Luo, A.; Xu, Y.; Zhang, Y.; Zhang, M.; Zhang, X.; Lu, Y.; Wang, F. Spray-coated electret materials with enhanced stability in a harsh environment for an MEMS energy harvesting device. Microsyst. Nanoeng. 2021, 7, 15. [Google Scholar] [CrossRef]
- Cai, R.-R.; Zhang, L.-Z.; Bao, A.-B. PM collection performance of electret filters electrospun with different dielectric materials-a numerical modeling and experimental study. Build. Environ. 2018, 131, 210–219. [Google Scholar] [CrossRef]
- Jiang, T.; Zeng, G.; Hu, C.; Meng, C.; Chen, Y. Optimization of processing parameters for particle filtration efficiency of polypropylene melt-blown fabric. Fibers Polym. 2021, 22, 957–963. [Google Scholar] [CrossRef]
- Bonacci, F.; Michele, A.D.; Capon, S.; Cottone, F.; Mattarelli, M. High charge density silica micro-electrets fabricated by electron beam. Smart Mater. Struct. 2018, 27, 075052–075062. [Google Scholar] [CrossRef]
- Zhao, Y.; Low, Z.-X.; Pan, Y.; Zhong, Z.; Gao, G. Universal water disinfection by piezoelectret aluminium oxide-based electroporation and generation of reactive oxygen species. Nano Energy 2022, 92, 106749–106759. [Google Scholar] [CrossRef]
- Galikhanov, M.F.; Guzhova, A.A.; Efremova, A.A.; Nazmieva, A.I. Effect of aluminum oxide coating on structural, barrier and electret properties of polyethylene terephthalate films. IEEE Trans. Dielect Elect. Insul. 2015, 22, 1492–1496. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Song, W.; Wang, H.; Gao, M.; Cho, M.; Park, I. Zinc oxide-enhanced piezoelectret polypropylene microfiber for mechanical energy harvesting. ACS Appl. Mater. Interfaces 2018, 10, 19940–19947. [Google Scholar] [CrossRef] [PubMed]
- Sangawar, V.S.; Golchha, M.C. Thermally stimulated discharge conductivity study of zinc oxide thermoelectrets. Bull. Mater. Sci. 2014, 37, 1497–1501. [Google Scholar] [CrossRef]
- Lang, S.B. Review of ferroelectric hydroxyapatite and its application to biomedicine. Phase Transit. 2016, 89, 678–694. [Google Scholar] [CrossRef]
- Tanaka, Y.; Iwasaki, T.; Nakamura, M.; Nagai, A.; Katayama, K.; Yamashita, K. Polarization and microstructural effects of ceramic hydroxyapatite electrets. J. Appl. Phys. 2010, 107, 014107–014117. [Google Scholar] [CrossRef]
- Nakayama, M.; Uenaka, Y.; Kataoka, S.; Oda, Y.; Yamamoto, K.; Tajitsu, Y. Piezoelectricity of ferroelectret porous polyethylene thin film. Jpn. J. Appl. Phys. 2009, 48, 09ke05. [Google Scholar] [CrossRef]
- Sengupta, P.; Surwase, S.S.; Prasad, B.L. Modification of porous polyethylene scaffolds for cell attachment and proliferation. Int. J. Nanomed. 2018, 13, 87–90. [Google Scholar] [CrossRef]
- Li, W.; Zhao, S.; Wu, N.; Zhong, J.; Wang, B.; Lin, S.; Chen, S.; Yuan, F.; Jiang, H.; Xiao, Y.; et al. Sensitivity-enhanced wearable active voiceprint sensor based on cellular polypropylene piezoelectret. ACS Appl. Mater. Interfaces 2017, 9, 23716–23722. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Xue, G.-A.; Yao, T.-P.; Hu, C.-Y.; Huang, P. Enhanced electron evacuation performance of zinc oxide nanocomposites for sustainable energy storage technology. J. Clean. Prod. 2019, 216, 167–171. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, F.; Zhu, H. Single electrode nylon fibers enhanced polytetrafluoroethylene electret film with hollow cylinder structure for mechanical energy harvesting. Energy Technol. 2018, 6, 1112–1118. [Google Scholar] [CrossRef]
- Pang, H.; Tian, K.; Li, Y.; Su, C.; Duan, F.; Xu, Y. Super-hydrophobic PTFE hollow fiber membrane fabricated by electrospinning of Pullulan/PTFE emulsion for membrane deamination. Sep. Purif. Technol. 2021, 274, 118186. [Google Scholar] [CrossRef]
- Chu, Y.; Zhong, J.; Liu, H.; Ma, Y.; Liu, N.; Song, Y.; Liang, J.; Shao, Z.; Sun, Y.; Dong, Y.; et al. Human pulse diagnosis for medical assessments using a wearable piezoelectret sensing system. Adv. Funct. Mater. 2018, 28, 1803413–1803422. [Google Scholar] [CrossRef]
- Zhong, J.; Ma, Y.; Song, Y.; Zhong, Q.; Chu, Y.; Karakurt, I.; Bogy, D.B.; Lin, L. A flexible piezoelectret actuator/sensor patch for mechanical human-machine interfaces. ACS Nano 2019, 13, 7107–7116. [Google Scholar] [CrossRef] [PubMed]
- Erhard, D.P.; Richter, F.; Bartz, C.B.A.; Schmidt, H.-W. Fluorinated aromatic polyimides as high-performance electret materials. Macromol. Rapid Commun. 2015, 36, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.-T.; Chau, N.M.; Huynh, D.P.; Huynh, N.D.; Choi, D.; Do, H.N. Honeycomb-patterned polyimide-based triboelectric nanogenerator with excellent thermal stability and enhanced electrification performance. ACS Appl. Energy Mater. 2022, 5, 9791–9800. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, R.; Gong, P.; Li, J.; Li, J.; Ao, D.; Wang, P.; Yang, Y.; Man, Y.; Qu, Y. Bioelectric effect of a chitosan bioelectret membrane on bone regeneration in rabbit cranial defects. J. Bioact. Compat. Polym. 2012, 27, 122–132. [Google Scholar] [CrossRef]
- Kumar, R.L.; Narayan, A.K.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohyd. Polym. 2016, 151, 172–188. [Google Scholar]
- Zhong, J.; Zhong, Q.; Zang, X.; Wu, N.; Li, W.; Chu, Y.; Lin, L. Flexible PET/EVA-based piezoelectret generator for energy harvesting in harsh environments. Nano Energy 2017, 37, 268–274. [Google Scholar] [CrossRef]
- Ignatova, M.; Yovcheva, T.; Viraneva, A.; Mekishev, G.; Manolova, N.; Rashkov, I. Study of charge storage in the nanofibrous poly(ethylene terephthalate) electrets prepared by electrospinning or by corona discharge method. Eur. Polym. J. 2008, 44, 1962–1967. [Google Scholar] [CrossRef]
- Antosik, A.K.; Kowalska, U.; Stobińska, M.; Dzięcioł, P.; Pieczykolan, M.; Kozłowska, K.; Bartkowiak, A. Development and characterization of bioactive polypropylene films for food packaging applications. Polymers 2021, 13, 3478. [Google Scholar] [CrossRef]
- Tong, L.; Kwok, D.T.K.; Wang, H.; Wu, L.; Chu, P.K. Surface structures and osteoblast activity on biomedical polytetrafluoroethylene treated by long-pulse, high-frequency oxygen plasma immersion ion implantation. Adv. Eng. Mater. 2010, 12, B163–B169. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohyd. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.; Abdullah, N.H.; Hamidon, M.N.; Matori, K.A.; Yusof, J.M.; Hasan, I.H. Effect of sintering temperature and piezoelectric coefficient (d33) enhancement on rice husk silica ceramics with BaTiO3 addition. Int. J. Nanotechnol. 2020, 16, 706–714. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, C.; Wu, Z.; Hu, L.; Zhang, W.; Zhao, K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration. Sci. Rep. 2017, 7, 43360. [Google Scholar] [CrossRef]
- Zafar, M.U.; Bravo-Cordero, J.J.; Torramade-Moix, S.; Escolar, G.; Jerez-Dolz, D.; Lev, E.I.; Badimon, J.J. Effects of electret coating technology on coronary stent thrombogenicity. Platelets 2022, 33, 312–319. [Google Scholar] [CrossRef]
- Yang, L.; Ji, H.; Zhu, K.; Wang, J.; Qiu, J. Dramatically improved piezoelectric properties of poly(vinylidene fluoride) composites by incorporating aligned TiO2@MWCNTs. Compos. Sci. Technol. 2016, 123, 259–267. [Google Scholar] [CrossRef]
- Hamdi, O.; Mighri, F.; Rodrigue, D. Time and thermal stability improvement of polyethylene ferroelectrets. J. Appl. Polym. Sci. 2019, 136, 47646. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Zheng, N.; Yang, J.; Jiang, Z.; Zhang, C.; Yao, Z.; Tang, L.; Cao, K. Controllable preparation of porous polypropylene piezoelectrets using crystallization self-reinforcement method induced by the variable thermal history. Macromol. Mater. Eng. 2022, 307, 2200314. [Google Scholar] [CrossRef]
- Chen, L.; Cao, J.; Li, G.; Fang, P.; Gong, X.; Zhang, X. Property assessment and application exploration for layered polytetrafluoroethylene piezoelectrets. IEEE Sens. J. 2019, 19, 11262–11271. [Google Scholar] [CrossRef]
- Zhang, Y.; Bowen, C.R.; Deville, S. Ice-templated poly(vinylidene fluoride) ferroelectrets. Soft Matter 2019, 15, 825–832. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Zhang, X. Energy harvesting from vibration using flexible floroethylenepropylene piezoelectret films with cross-tunnel structure. IEEE Trans. Dielect Elect. Insul. 2015, 22, 1349–1354. [Google Scholar] [CrossRef]
- Prokhorov, E.; Bárcenas, G.L.; Sánchez, B.L.E.; Franco, B.; Padilla-Vaca, F.; Landaverde, M.A.H.; Limón, J.M.Y.; López, R.A. Chitosan-BaTiO3 nanostructured piezopolymer for tissue engineering. Colloids Surf. B 2020, 196, 111296. [Google Scholar] [CrossRef]
- Okosun, F.; Guerin, S.; Celikin, M.; Pakrashi, V. Flexible amino acid-based energy harvesting for structural health monitoring of water pipes. Cell Rep. Phys. Sci. 2021, 2, 100434. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Si, Y.; Yin, X.; Yu, J.; Ding, B. Electrospun polyvinylidene fluoride/SiO2 nanofibrous membranes with enhanced electret property for efficient air filtration. Compos. Commun. 2019, 13, 57–62. [Google Scholar] [CrossRef]
- Minami, T.; Utsubo, T.; Miyata, T.; Ohbayashi, Y. Inorganic electret using SiO2 thin films prepared by radio-frequency magnetron sputtering. J. Vac. Sci. Technol. A 2003, 21, 1178–1182. [Google Scholar] [CrossRef]
- Horiuchia, N.; Madokorob, K.; Nozakia, K.; Nakamuraa, M.; Katayamab, K.; Nagaia, A.; Yamashita, K. Electrical conductivity of polycrystalline hydroxyapatite and its application to electret formation. Solid State Ion. 2018, 315, 19–25. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakamura, S.; Yamashita, K. Enhanced osteobonding by negative surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 2001, 57, 477–484. [Google Scholar] [CrossRef]
- Wada, N.; Horiuchi, N.; Mukougawa, K.; Nozaki, K.; Nakamura, M.; Nagai, A.; Okura, T.; Yamashita, K. Electrostatic induction power generator using hydroxyapatite ceramic electrets. Mater. Res. Bull. 2016, 74, 50–56. [Google Scholar] [CrossRef]
- Kumar, D.; Gittings, J.P.; Turner, I.G.; Bowen, C.R.; Bastida-Hidalgo, A.; Cartmell, S.H. Polarization of hydroxyapatite: Influence on osteoblast cell proliferation. Acta Biomater. 2010, 6, 1549–1554. [Google Scholar] [CrossRef]
- Nishigaki, T.; Nishikawa, H.; Kusunoki, M.; Hontsu, S. Measurement of piezoelectric properties of pulsed laser deposited hydroxyapatite thin films on platinum or titanium substrate. Bioceram. Dev. Appl. 2013, 3, 008. [Google Scholar] [CrossRef]
- Fu, C.; Savino, K.; Gabrys, P.; Zeng, A.; Guan, B.; Olvera, D.; Wang, C.; Song, B.; Awad, H.; Gao, Y.; et al. Hydroxyapatite thin films with giant electrical polarization. Chem. Mater. 2015, 27, 1164–1171. [Google Scholar] [CrossRef]
- Ren, W.; Yang, G.-D.; Feng, A.-L.; Miao, R.-X.; Xia, J.-B.; Wang, Y.-G. Annealing effects on the optical and electrochemical properties of tantalum pentoxide films. J. Adv. Ceram. 2021, 10, 704–713. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, Z.; Cheng, T.; Fei, T.; Fu, Z.; Liu, X.; Zhang, J.; Yang, J.-Y. Finite temperature ultraviolet-visible dielectric functions of tantalum pentoxide: A combined spectroscopic elipsometry and first-principles study. Photonics 2022, 9, 440. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.-W.; Shih, Y.-H.; Huang, C.-H.; Lee, S.-A.; Chen, Y.-S.; Lin, J.-H. Filtration efficiency of electret air filters reinforced by titanium dioxide. Appl. Sci. 2020, 10, 2686. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Mitra, I.; Bose, S. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar] [CrossRef]
- Paxtona, N.C.; Allenbya, M.C.; Lewisb, P.M.; Woodruff, M.A. Biomedical applications of polyethylene. Eur. Polym. J. 2019, 118, 412–428. [Google Scholar] [CrossRef]
- Balasubramaniyan, K.; Bhoobalan, K.; Jayaraman, D.; Sounderraj, S.; Muthuukumar, K.R.; Santhini, E. Development and assessment of biologically compatible anterior cruciate ligament using braided ultra-high molecular weight polyethylene. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1306–1318. [Google Scholar] [CrossRef]
- Galikhanov, M.F.; Minzagirova, A.M.; Spiridonova, R.R. Modifying the properties of polyethylene electrets through the incorporation of montmorillonite. Surf. Engin. Appl. Electrochem. 2020, 55, 679–683. [Google Scholar] [CrossRef]
- Rostami, M.; Azdast, T.; Hasanzadeh, R.; Moradian, M. A study on fabrication of nanocomposite polyethylene foam through extrusion foaming procedure. Cell. Polym. 2021, 40, 231–243. [Google Scholar] [CrossRef]
- Abolfazl, M.; Denis, R. Energy absorption capacity of ferroelectrets based on porous polypropylene. Polym. Eng. Sci. 2018, 58, 300–309. [Google Scholar]
- Mohebbi, A.; Mighri, F.; Ajji, A.; Rodrigue, D. Polymer ferroelectret based on polypropylene foam: Piezoelectric properties prediction using dynamic mechanical analysis. Polym. Adv. Technol. 2017, 28, 476–483. [Google Scholar] [CrossRef]
- Wang, J.; Rychkov, D.; Gerhard, R. Chemical modification with orthophosphoric acid enhances surface-charge stability on polypropylene electrets. Appl. Phys. Lett. 2017, 110, 192901. [Google Scholar] [CrossRef]
- Mao, H.; Ma, P.; Jiang, G. Filtration efficiency investigation of mesh fabrics by polytetrafluoroethylene filament with surface static electricity. J. Text. Inst. 2018, 110, 451–459. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, Y.; Mo, X.; Chen, S.; Xu, Z.; Zhou, B.; Zhou, H.; Hu, B.; Zhou, J. Electrospun polytetrafluoroethylene nanofibrous membrane for high-performance self-powered sensors. Nanoscale Res. Lett. 2019, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Yin, X.; Yu, J.; Ding, B. Electret polyvinylidene fluoride nanofibers hybridized by polytetrafluoroethylene nanoparticles for high-efficiency air filtration. ACS Appl. Mater. Interfaces 2016, 8, 23985–23994. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A comparative analysis of the basic properties and applications of poly (vinylidene fluoride) (PVDF) and poly (methyl methacrylate) (PMMA). Polym. Bull. 2021, 79, 5635–5665. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Lolla, D.; Lolla, M.; Abutaleb, A.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, polarization of electrospun polyvinylidene fluoride electret fibers and effect on capturing nanoscale solid aerosols. Materials 2016, 9, 671. [Google Scholar] [CrossRef]
- Greer, A.I.M.; Vasiev, I.; Della-Rosa, B.; Gadegaard, N. Fluorinated ethylene-propylene: A complementary alternative to PDMS for nanoimprint stamps. Nanotechnology 2016, 27, 155301. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Wang, F.; Wirges, W.; Gerhard, R.; Basso, H.C. Three-layer piezoelectrets from fluorinated ethylene-propylene (FEP) copolymer films. Appl. Phys. A 2010, 103, 455–461. [Google Scholar] [CrossRef]
- Wang, B.; Liu, C.; Xiao, Y.; Zhong, J.; Li, W.; Cheng, Y.; Hu, B.; Huang, L.; Zhou, J. Ultrasensitive cellular fluorocarbon piezoelectret pressure sensor for self-powered human physiological monitoring. Nano Energy 2017, 32, 42–49. [Google Scholar] [CrossRef]
- Wang, P.F.; Wu, S.K.; Shi, X.Y.; Deng, B.M.; Sun, C. The aggregation behaviour of chitosan bioelectret in aqueous solution using a fluorescence probe. J. Mater. Sci. 1998, 33, 1753–1757. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Zhao, J.; Wang, S. Multifunctional hydrogels based on chitosan, hyaluronic acid and other biological macromoleculesfor the treatment of inflammatory bowel disease: A review. Int. J. Biol. Macromol. 2023, 227, 505–523. [Google Scholar] [CrossRef]
- Qu, Y.; Ao, D.; Wang, P.; Wang, Y.; Kong, X.; Man, Y. Chitosan/nano-hydroxyapatite composite electret membranes enhance cell proliferation and osteoblastic expression in vitro. J. Bioact. Compat. Polym. 2014, 29, 3–14. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Wang, Q.; Yang, J.; Gong, P.; Man, Y.; Zhang, J. In vitro and in vivo evaluation of porous chitosan electret membrane for bone regeneration. J. Bioact. Compat. Polym. 2018, 33, 426–438. [Google Scholar] [CrossRef]
- Tonndorf, R.; Aibibu, D.; Cherif, C. Collagen multifilament spinning. Mater. Sci. Eng. C 2020, 106, 110105. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.T.; Oecal, S.; Mörgelin, M.; Schmid, P.W.N.; Buchner, J.; Baumann, U.; Gebauer, J.M. Collagen’s primary structure determines collagen: HSP47 complex stoichiometry. J. Biol. Chem. 2021, 297, 101169. [Google Scholar] [CrossRef]
- Rezaei, N.; Lyons, A.; Forde, N.R. Nano-Mechanical studies of collagen: The influence of ionic strength, pH and collagen sources on molecular flexibility. Biophys. J. 2017, 112, 488a. [Google Scholar] [CrossRef]
- Yang, X.L.; Gu, J.W.; Zhu, H.S. Preparation of bioelectret collagen and its influence on cell culture in vitro. J. Mater. Sci. Mater. Med. 2006, 17, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Bongioanni, A.; Bueno, M.S.; Mezzano, B.E.A.; Longhi, M.R.; Garnero, C. Amino acids and its pharmaceutical applications: A mini review. Int. J. Pharm. 2022, 613, 121375. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Pearce, E.L. Amino assets: How amino acids support immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- O’Donnell, J.; Sarkar, S.M.; Guerin, S.; Borda, G.G.; Silien, C.; Soulimane, T.; Thompson, D.; O’Reilly, E.; Tofail, S.A.M. Piezoelectricity in the proteinogenic amino acid L-leucine: A novel piezoactive bioelectret. IEEE Trans. Dielect Elect. Insul. 2020, 27, 1465–1468. [Google Scholar] [CrossRef]
- Mindt, S.; Aida, S.; Merx, K.; Müller, A.; Gutting, T.; Hedtke, M.; Neumaier, M.; Hofheinz, R.-D. Therapeutic drug monitoring (TDM) of 5-fluorouracil (5-FU): New preanalytic aspects. Clin. Chem. Lab. Med. 2019, 57, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuch, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S.; et al. 5-FU metabolism in cancer and orally-administrable 5-FU drugs. Cancers 2010, 2, 1717–1730. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Zhou, J. Effective sustained release of 5-FU-loaded PLGA implant for improving therapeutic index of 5-FU in colon tumor. Int. J. Pharm. 2018, 550, 380–387. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Zhang, X.; Ye, C.; Wang, S.; An, X. Development of PVA-based microsphere as a potential embolization agent. Mater. Sci. Eng. C 2022, 135, 112677. [Google Scholar] [CrossRef]
- Yuan, W.; Xu, L.; Huang, P.; An, X.; Cui, L.; Jiang, J. Inhibition effects of a negative electret 5-FU patch on the growth of a hypertrophic scar. Plasm. Sci. Technol. 2018, 20, 054011. [Google Scholar] [CrossRef]
- Yuan, W.; Liang, H.; Huang, P.; An, X.; Jiang, J.; Cui, L. Evaluations of dielectric property and drug release profile of 5-FU patches based on plasma charged electrets. Plasm. Sci. Tech. 2018, 20, 054015. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, J.; Long, Y.; Xie, Y.; Nie, J.; Chen, L. Magnetic materials in promoting bone regeneration. Front. Mater. 2019, 6, 268. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C. Nanomaterial-based bone regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xiong, Y. Applications of bone regeneration hydrogels in the treatment of bone defects: A review. J. Mater. Sci. 2022, 57, 887–913. [Google Scholar] [CrossRef]
- Aleksandrova, S.A.; Aleksandrova, O.I.; Khomutov, V.P.; Morgunov, M.S.; Blinova, M.I. The influence of an electret-generated electric field based on a tantalum oxide anode on differentiation properties of bone marrow stromal cells from patients with osteoarthritis. Cell Tiss. Biol. 2019, 13, 144–151. [Google Scholar] [CrossRef]
- Yu, B.; Qiao, Z.; Cui, J.; Lian, M.; Han, Y.; Zhang, X.; Wang, W.; Yu, X.; Yu, H.; Wang, X.; et al. A host-coupling bio-nanogenerator for electrically stimulated osteogenesis. Biomaterials 2021, 276, 120997. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B 2023, 222, 113096. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable wound dressing based on carboxymethyl chitosan triple-network hydrogel for effective wound antibacterial and hemostasis. Int. J. Biol. Macromol. 2022, 185, 1235–1245. [Google Scholar] [CrossRef]
- Cai, J.; Guo, J.; Wang, S. Application of polymer hydrogels in the prevention of postoperative adhesion: A review. Gels 2023, 9, 98. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, Y.; Zheng, X.; Zhang, Y.; Zhao, J.; Wang, S.; Gu, Y. Rapidly degrading and mussel-inspired multifunctional carboxymethyl chitosan/montmorillonite hydrogel for wound hemostasis. Int. J. Biol. Macromol. 2023, 242, 124960. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, Y.; Chen, Z.; Yu, Y.; Wang, H.; Lu, X.; Zhao, J.; Wang, S. Chitosan-based multifunctional hydrogel for sequential wound inflammation elimination, infection inhibition, and wound healing. Int. J. Biol. Macromol. 2023, 235, 123847. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, H.; Wang, S.; Zhao, J.; Hu, L. Preparation and characterization of biocompatible iron/zirconium/polydopamine /carboxymethyl chitosan hydrogel with fenton catalytic properties and photothermal efficacy. Gels 2023, 9, 452. [Google Scholar] [CrossRef]
- Fan, P.; Zeng, Y.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Chitosan-based hemostatic hydrogels: The concept, mechanism, application, and prospects. Molecules 2023, 28, 1473. [Google Scholar] [CrossRef]
- Cheah, Y.J.; Buyong, M.R.; Yunus, M.H.M. Wound healing with electrical stimulation technologies: A review. Polymers 2021, 13, 3790. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S. Polysaccharides-based multifunctional hydrogel bio-adhesives for wound healing: A review. Gels 2023, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Kou, T.; Zhu, H. A study on bioelectret collagen. J. Appl. Polym. Sci. 1997, 64, 267–271. [Google Scholar] [CrossRef]
- Nagai, A.; Yamashita, K.; Imamura, M.; Azuma, H. Hydroxyapatite electret accelerates reendothelialization and attenuates intimal hyperplasia occurring after endothelial removal of the rabbit carotid artery. Life Sci. 2008, 82, 1162–1168. [Google Scholar] [CrossRef]
- Okabayashi, R.; Nakamura, M.; Okabayashi, T.; Tanaka, Y.; Nagai, A.; Yamashita, K. Efficacy of polarized hydroxyapatite and silk fibroin composite dressing gel on epidermal recovery from full-thickness skin wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 641–646. [Google Scholar] [CrossRef]
- Kerkmeijer, L.S.; Tenekecioglu, E.; Wykrzykowska, J.J. Stent thrombosis in patients with drug-eluting stents and bioresorbable vascular scaffolds the feared complication. Pol. Arch. Intern. Med. 2018, 128, 943–950. [Google Scholar] [CrossRef]
- Ishihara, T.; Okada, K.; Kida, H.; Tsujimura, T.; Iida, O.; Okuno, S.; Hata, Y.; Toyoshima, T.; Higashino, N.; Kikuchi, A.; et al. Long-term outcomes and clinical predictors of mortality following occurrence of stent thrombosis. J. Am. Heart Assoc. 2022, 11, 023276. [Google Scholar] [CrossRef]
- Ogino, M.; Naemura, K.; Sasaki, S.; Minami, J.; Kano, T.; Ito, N.; Kasai, R.; Kamijyo, F.; Kusumoto, N.; Akimoto, K.; et al. Triboelectric charging of polytetrafluoroethylene antithrombotic catheters. J. Artif. Organs 2019, 22, 300–306. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wang, C.; Li, F.; Qiao, Z.; Zeng, L.; Wang, Z.; Liu, H.; Ding, J.; Yang, H. Conductive composite fiber with optimized alignment guides neural regeneration under electrical stimulation. Adv. Healthc. Mater. 2021, 10, e2000604. [Google Scholar] [CrossRef]
- Yao, X.; Qian, Y.; Fan, C. Electroactive nanomaterials in the peripheral nerve regeneration. J. Mater. Chem. B 2021, 9, 6958–6972. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, J.; Chen, Y.; Zheng, Y.; Li, J.; Zhao, J.; Zhang, J.; Liu, Y.; Liu, X.; Wang, S. MoS2-ALG-Fe/GOx hydrogel with Fenton catalytic activity for combined cancer photothermal, starvation, and chemodynamic therapy. Colloids Surf. B 2020, 195, 111243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, J.; Chen, Z.; Wu, H.; Wang, S. A molybdenum-based nanoplatform with multienzyme mimicking capacities for oxidative stress-induced acute liver injury treatment. Inorg. Chem. Front. 2023, 10, 1305–1314. [Google Scholar] [CrossRef]
- Sengera, J.-L.; Chanb, K.M.; Macandilia, H.; Chana, A.W.M.; Vergec, V.M.K.; Jonesd, K.E.; Webber, C.A. Conditioning electrical stimulation promotes functional nerve regeneration. Exp. Neurol. 2019, 315, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Valentini, R.F.; Sabatini, A.M.; Dario, P.; Aebischer, P. Polymer electret guidance channels enhance peripheral nerve regeneration in mice. Brain Res. 1989, 480, 300–304. [Google Scholar] [CrossRef]
- Makohliso, S.A.; Valentini, R.F.; Aebischer, P. Magnitude and polarity of a fluoroethylene propylene electret substrate charge influences neurite outgrowth in vitro. J. Biomed. Mater. Res. 1993, 27, 1075–1085. [Google Scholar] [CrossRef]
- Mao, R.; Yu, B.; Cui, J.; Wang, Z.; Huang, X.; Yu, H.; Lin, K.; Shen, S.G.F. Piezoelectric stimulation from electrospun composite nanofibers for rapid peripheral nerve regeneration. Nano Energy 2022, 98, 107322. [Google Scholar] [CrossRef]
- Lu, X.; Sun, C.; Chen, L.; Feng, Z.; Gao, H.; Hu, S.; Dong, M.; Wang, J.; Zhou, W.; Ren, N.; et al. Stemness maintenance and massproduction of neural stem cells on poly L-Lactic Acid nanofibrous membrane based on piezoelectriceffect. Small 2022, 18, 2107236. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Li, T.; Wei, Z.; Xiong, R.; Qian, L.; Ma, J.; Yuan, T.; Wu, Q.; Lai, C.; Ma, X.; et al. Biofeedback electrostimulation for bionic and long-lasting neural modulation. Nat. Commun. 2022, 13, 5302. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fan, P.; Li, J.; Wang, S. Preparation of Biocompatible Manganese Selenium-Based Nanoparticles with Antioxidant and Catalytic Functions. Molecules 2023, 28, 4498. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, X.; Zhao, J.; Tang, J.; Hu, L.; Wang, S. Glucose oxidase-loaded colloidal stable WS2 nanobowls for combined starvation/photothermal therapy of colorectal tumors. Int. J. Pharm. 2023, 636, 112848. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, L.; Lu, X.; Yang, Y.; Peng, H.; Sun, Z.; Xu, J.; Chu, H. Real-time drug release monitoring from pH-responsive CuS-encapsulated metal-organic frameworks. RSC Adv. 2022, 12, 11119–11127. [Google Scholar] [CrossRef] [PubMed]
- Musaie, K.; Abbaszadeh, S.; Nosrati-Siahmazgi, V.; Qahremani, M.; Wang, S.; Eskandari, M.R.; Niknezhad, S.V.; Haghi, F.; Li, Y.; Xiao, B.; et al. Metal-coordination synthesis of a natural injectable photoactive hydrogel with antibacterial and blood-aggregating functions for cancer thermotherapy and mild-heating wound repair. Biomater. Sci. 2023, 11, 2486–2503. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Mao, Y.; Li, Z. Self-powerbility in electrical stimulation drug delivery system. Adv. Mater. Technol. 2021, 7, 2100055. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; Valle, L.J.d.; Alemán, C. Cell responses to electrical pulse stimulation for anticancer drug release. Materials 2019, 12, 2633. [Google Scholar] [CrossRef]
- Cui, L.; Jiang, J.; Zhang, L.; Song, C.; Zhao, W.; Lin, J. Enhancing effect of electret on transdermal drug delivery. J. Electrost. 2001, 51–52, 153–158. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, P.; Liang, Y.Y.; Guo, X.; Jiang, J.; Cui, L.L. Optimizing the formulation of cyclosporine A electret patch and the controlled release of drug. J. Phys. Conf. Ser. 2013, 418, 012147. [Google Scholar] [CrossRef]
- Murthy, N.S.; Boguda, V.A.; Payasada, K. Electret enhances transdermal drug permeation. Biol. Pharm. Bull. 2008, 31, 99–102. [Google Scholar]
- Tu, Y.; Wang, X.; Lu, Y.; Zhang, H.; Yu, Y.; Chen, Y.; Liu, J.; Sun, Z.; Cui, L.; Gao, J.; et al. Promotion of the transdermal delivery of protein drugs by N-trimethyl chitosan nanoparticles combined with polypropylene electret. Int. J. Nanomed. 2016, 11, 5549–5561. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Zou, X.; Li, Z.; Liu, W.; Liu, G.; Ma, M.; Zheng, Y. Ultrasonic/electrical dual stimulation response nanocomposite bioelectret for controlled precision drug release. Mater. Today Bio. 2023, 20, 100665. [Google Scholar] [CrossRef]
- Lee, H.-B.; Meeseepong, M.; Trung, T.Q.; Kim, B.-Y.; Lee, N.-E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 2020, 156, 112133. [Google Scholar] [CrossRef]
- Johansson, D.; Malmgren, K.; Murphy, M.A. Wearable sensors for clinical applications in epilepsy, parkinson’s disease, and stroke: A mixed-methods systematic review. J. Neurol. 2018, 265, 1740–1752. [Google Scholar] [CrossRef]
- Kumar, S.; Victoria-Castro, A.M.; Melchinger, H.; O’Connor, K.D.; Psotka, M.; Desai, N.R.; Ahmad, T.; Wilson, F.P. Wearables in cardiovascular disease. In Journal of Cardiovascular Translational Research; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–12. [Google Scholar]
- Berryhill, S.; Morton, C.J.; Dean, A.; Berryhill, A.; Provencio-Dean, N.; Patel, S.I.; Estep, L.; Combs, D.; Mashaqi, S.; Gerald, L.B.; et al. Effect of wearables on sleep in healthy individuals: A randomized crossover trial and validation study. J. Clin. Sleep Med. 2020, 16, 775–783. [Google Scholar] [CrossRef]
- Honda, S.; Hara, H.; Arie, T.; Akita, S.; Takei, K. A wearable, flexible sensor for real-time, home monitoring of sleep apnea. Iscience 2022, 25, 104163. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Dong, R.; Tao, K. Wearable device oriented flexible and stretchable energy harvester based on embedded liquid-metal electrodes and FEP electret film. Sensors 2020, 20, 458. [Google Scholar] [CrossRef]
- Wang, H.L.; Guo, Z.H.; Zhu, G.; Pu, X.; Wang, Z.L. Boosting the power and powering the impedance of triboelectric nanogenerators through manipulating the permittivity for wearable energy harvesting. ACS Nano 2021, 15, 7513–7521. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, J.; Zeng, H.; Chen, Z.; Tao, K.; Wu, J.; Li, Y. An electret/hydrogel-based tactile sensor boosted by micro-patterned and electrostatic promoting methods with flexibility and wide-temperature tolerance. Micromachines 2021, 12, 1462. [Google Scholar] [CrossRef]
- Wu, N.; Cheng, X.; Zhong, Q.; Zhong, J.; Li, W.; Wang, B.; Hu, B.; Zhou, J. Cellular polypropylene piezoelectret for human body energy harvesting and health monitoring. Adv. Funct. Mater. 2015, 25, 4788–4794. [Google Scholar] [CrossRef]
- Yan, C.; Deng, W.; Jin, L.; Yang, T.; Wang, Z.; Chu, X.; Su, H.; Chen, J.; Yang, W. Epidermis-inspired ultrathin 3D cellular sensor array for self-powered biomedical monitoring. ACS Appl. Mater. Interfaces 2018, 10, 41070–41075. [Google Scholar] [CrossRef]


| Classification | Material Name | Fabrication Method | Piezoelectric Coefficient d33/d31 (pC/N) | Ref. |
|---|---|---|---|---|
| Inorganic | Silicon dioxide (SiO2) | High-temperature sintering | 4 | [53] |
| Hydroxyapatite (HA) | Slip casting | 6.8 | [54] | |
| Tantalum pentoxide (Ta2O5) | Vacuum plasma spray technique | - | [55] | |
| Titanium dioxide (TiO2) | Solution cast and mechanical rolling | 41 | [56] | |
| Macromolecules | Polyethylene (PE) | Extrusion film blowing | 935 | [57] |
| Polypropylene (PP) | Crystallization self-reinforcement foaming | 700 | [58] | |
| Polytetrafluoroethylene (PTFE) | Secret commercial technology | 1300 | [59] | |
| Polyvinylidene fluoride (PVDF) | Freeze casting | 264 | [60] | |
| Fluorinated ethylene propylene (FEP) | Template | 1000 | [61] | |
| Chitosan (CS) | Solvent casting | 11.29 | [62] | |
| Amino acid | Evaporative crystallization | 0.9 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhao, J.; Xie, P.; Wang, S. Biomedical Applications of Electrets: Recent Advance and Future Perspectives. J. Funct. Biomater. 2023, 14, 320. https://doi.org/10.3390/jfb14060320
Zhang X, Zhao J, Xie P, Wang S. Biomedical Applications of Electrets: Recent Advance and Future Perspectives. Journal of Functional Biomaterials. 2023; 14(6):320. https://doi.org/10.3390/jfb14060320
Chicago/Turabian StyleZhang, Xinyuan, Jiulong Zhao, Pei Xie, and Shige Wang. 2023. "Biomedical Applications of Electrets: Recent Advance and Future Perspectives" Journal of Functional Biomaterials 14, no. 6: 320. https://doi.org/10.3390/jfb14060320
APA StyleZhang, X., Zhao, J., Xie, P., & Wang, S. (2023). Biomedical Applications of Electrets: Recent Advance and Future Perspectives. Journal of Functional Biomaterials, 14(6), 320. https://doi.org/10.3390/jfb14060320






