The Interaction Mechanism of Intramuscular Gene Delivery Materials with Cell Membranes
Abstract
1. Introduction
2. Computational Details
2.1. The Force Field Selection
2.2. The MD Simulation Software
2.3. Modeling of Material Molecules
2.4. Establishment of Cell Membranes
2.5. Molecular Dynamics Simulation Ideas
3. Results and Discussion
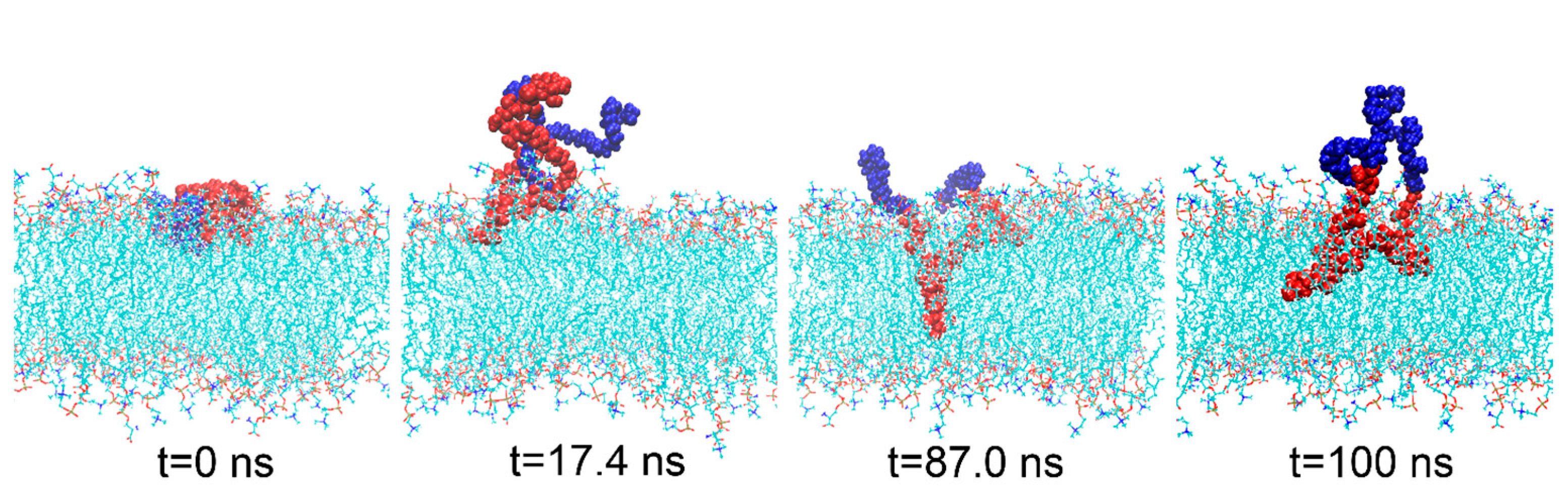
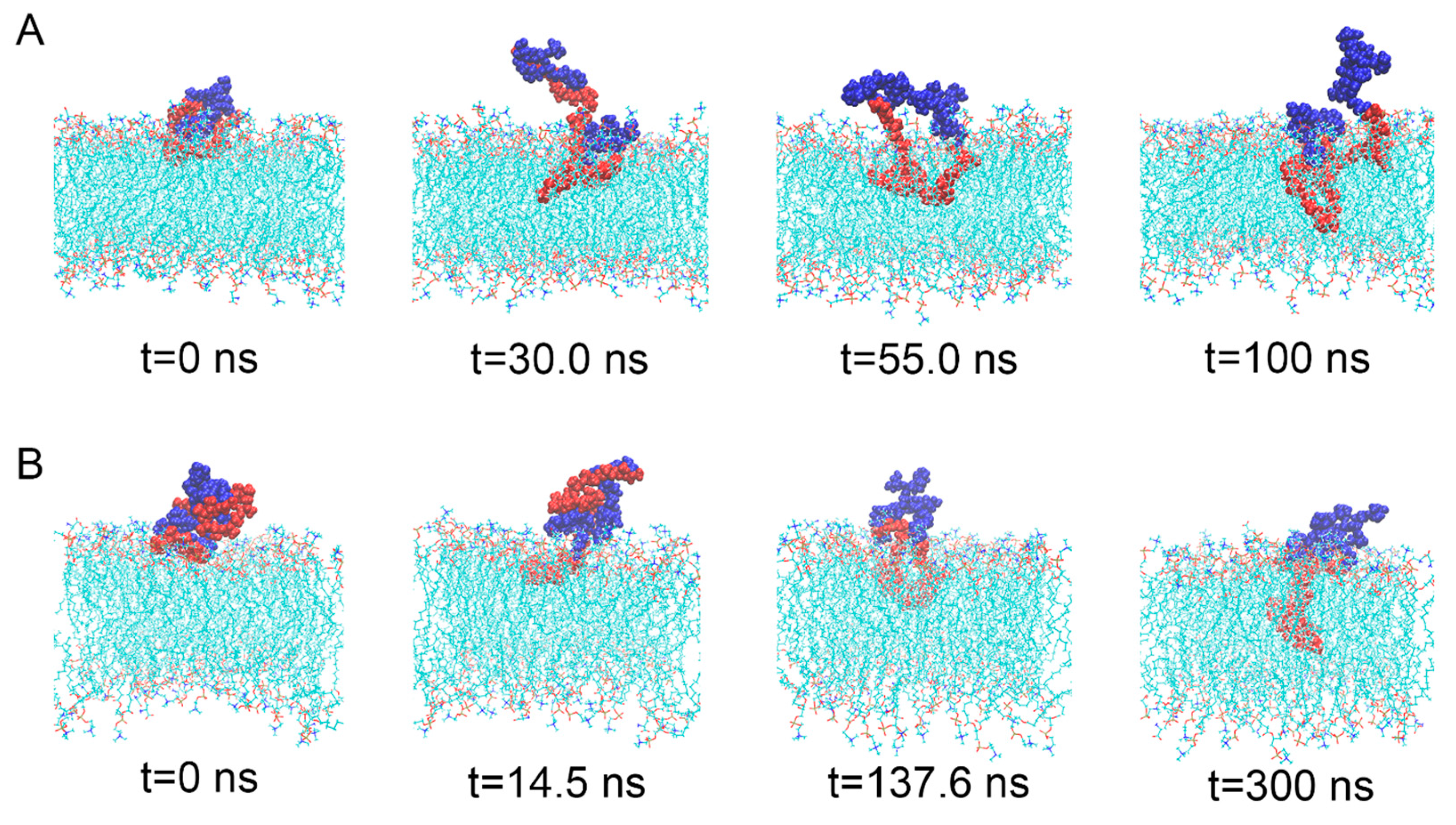
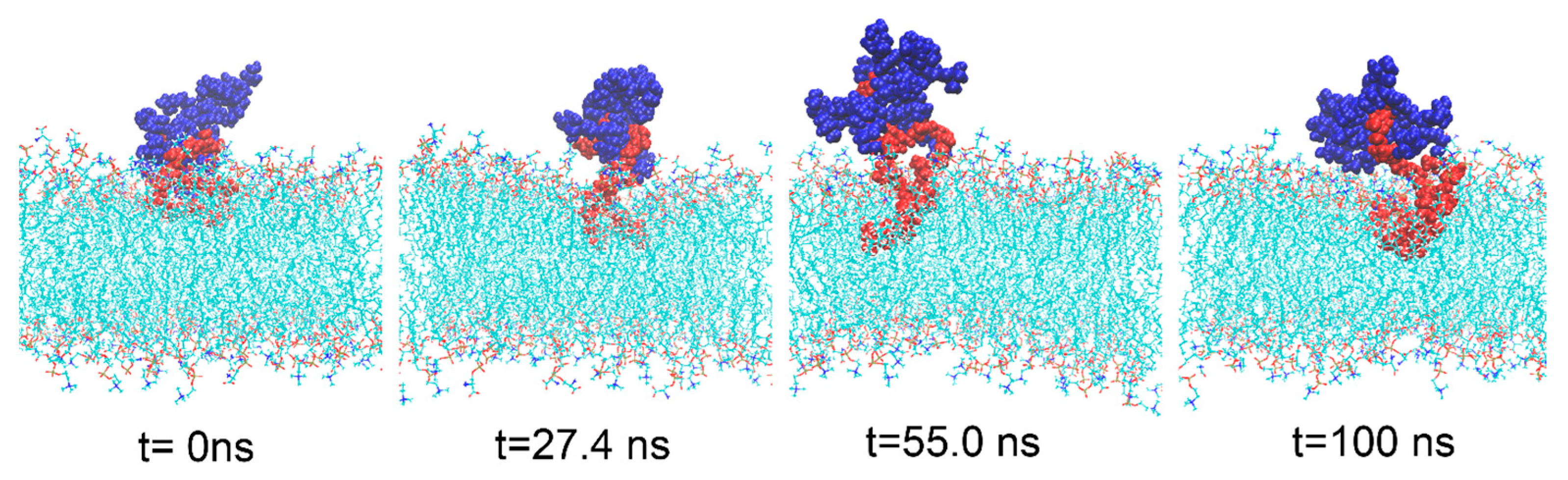
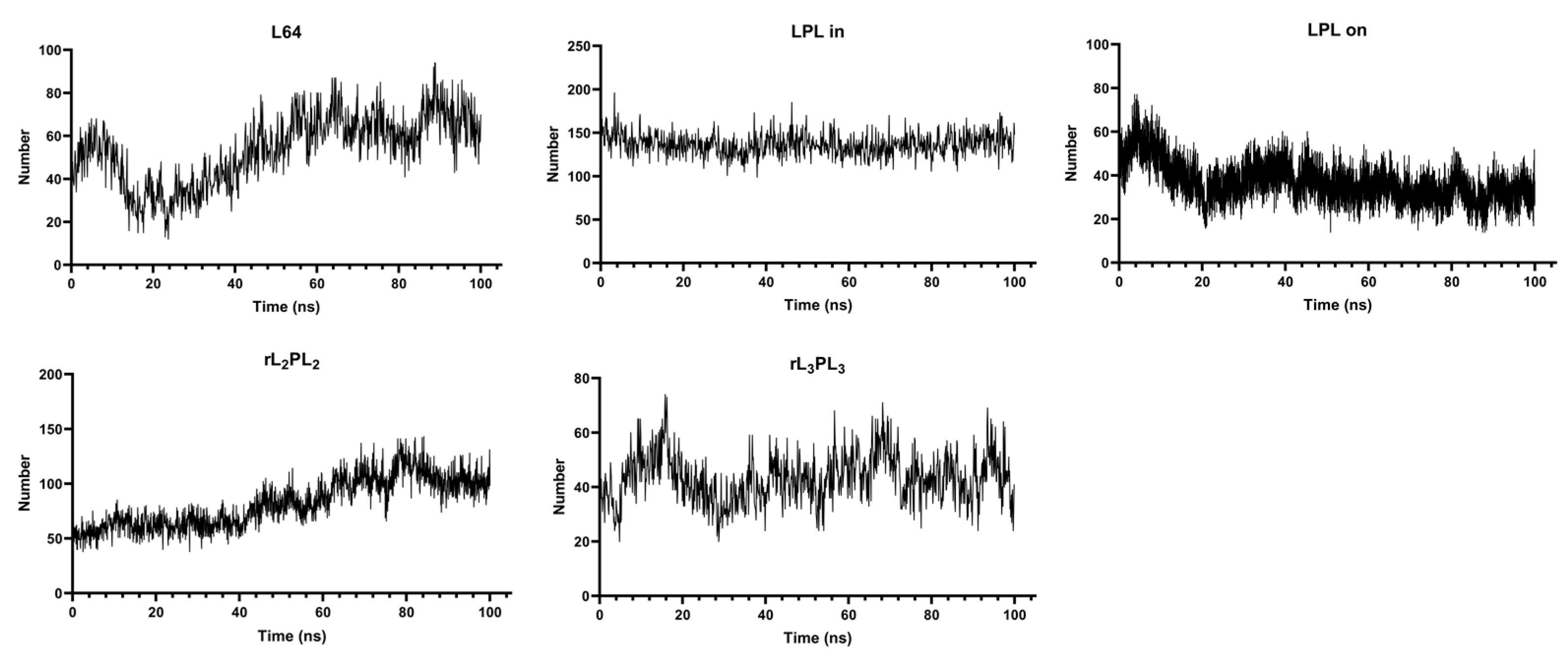
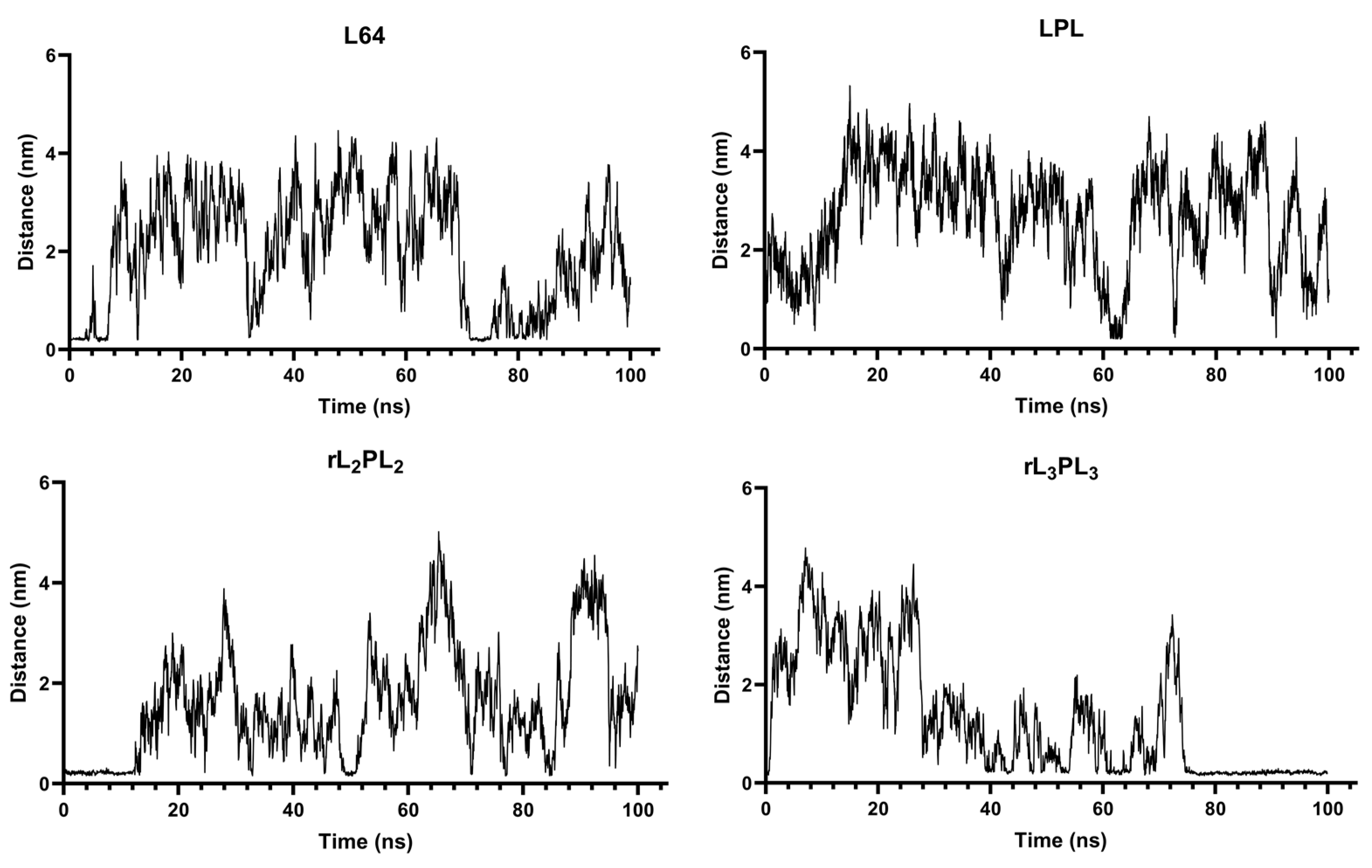
3.1. Interaction Process between Material Molecules and Phospholipid Membranes
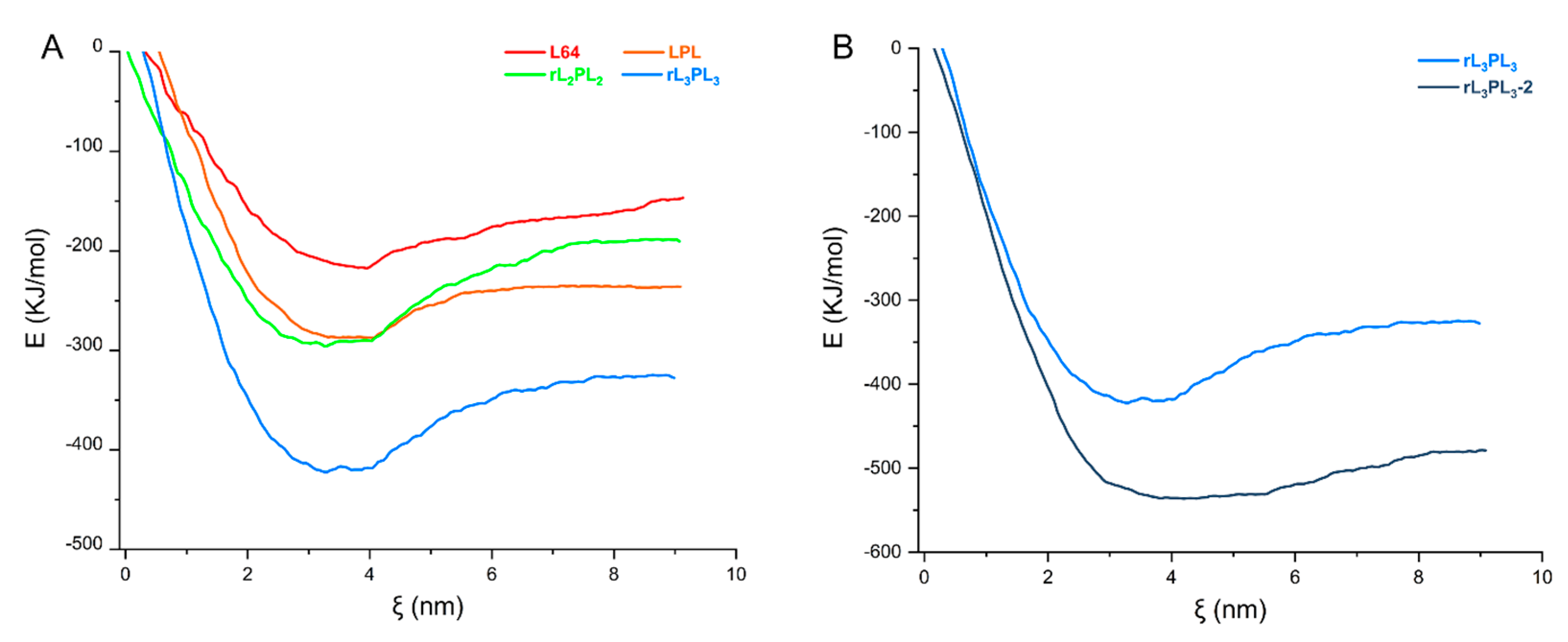
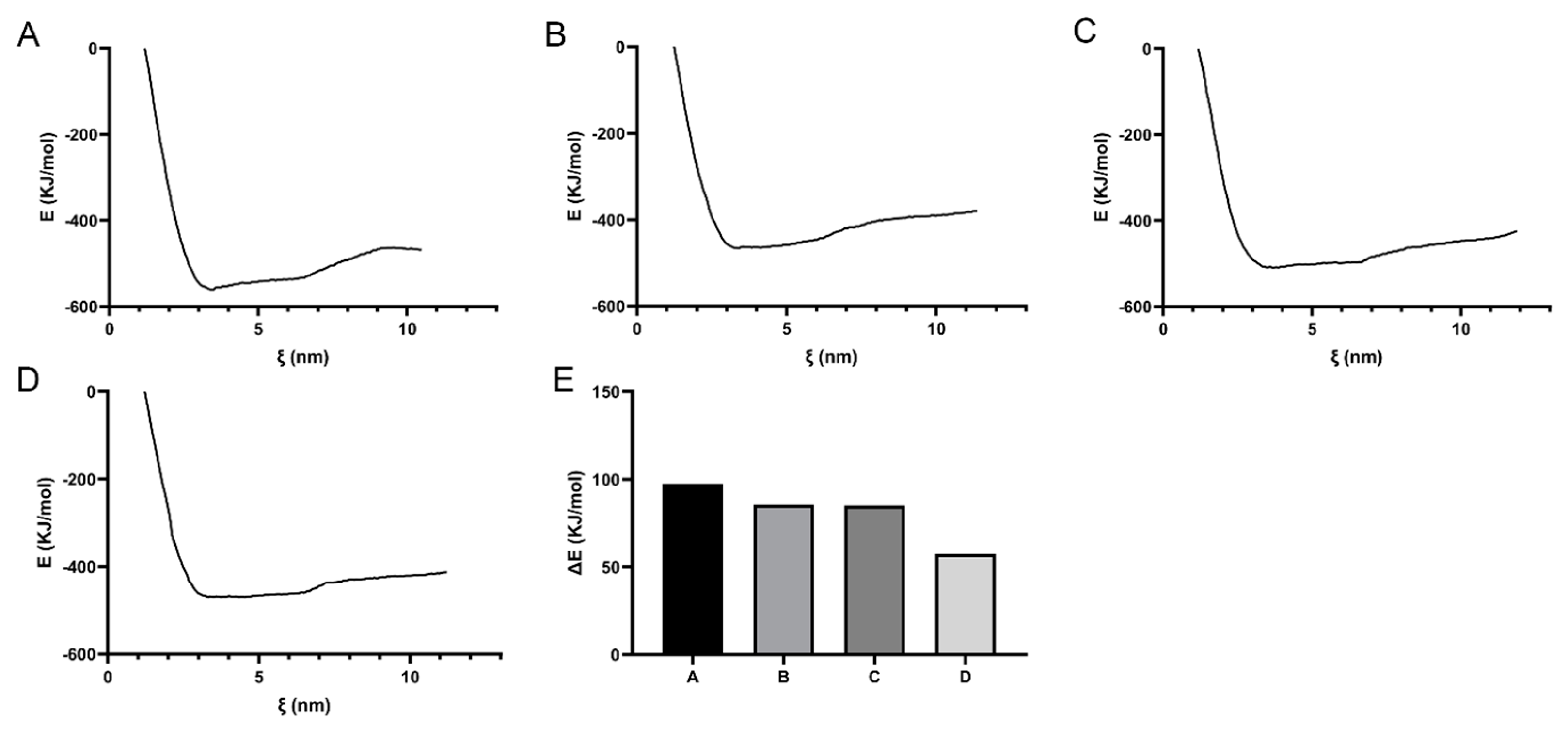
3.2. Transmembrane Free Energy Analysis of the Materials
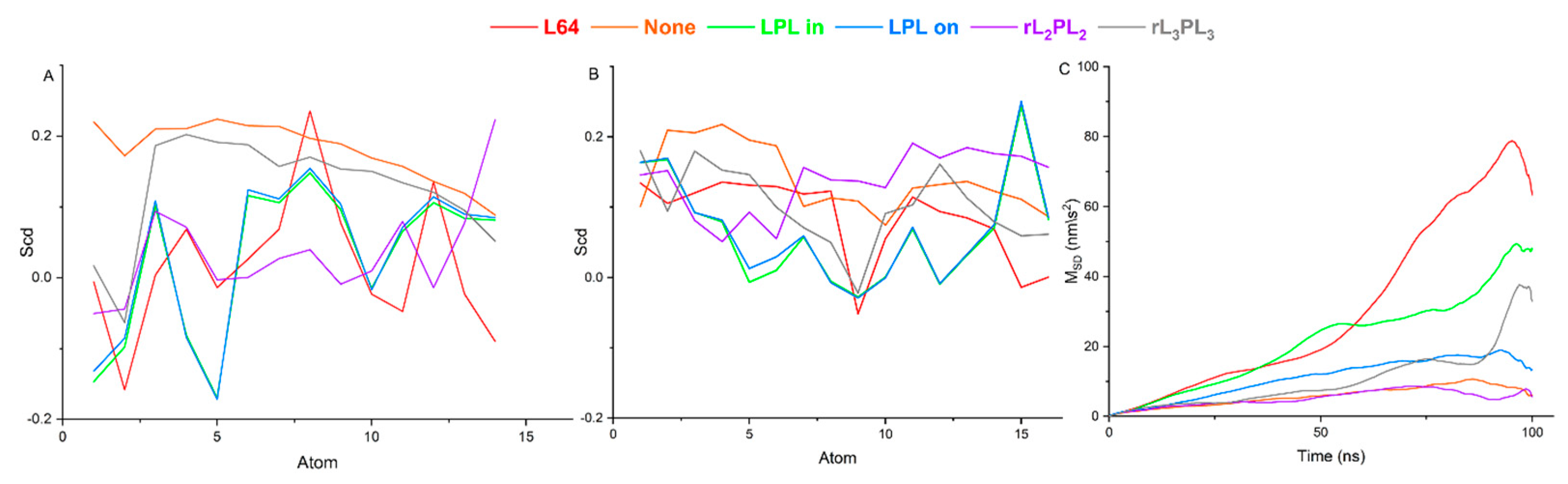
3.3. Parametric Analysis of Phospholipid Membranes
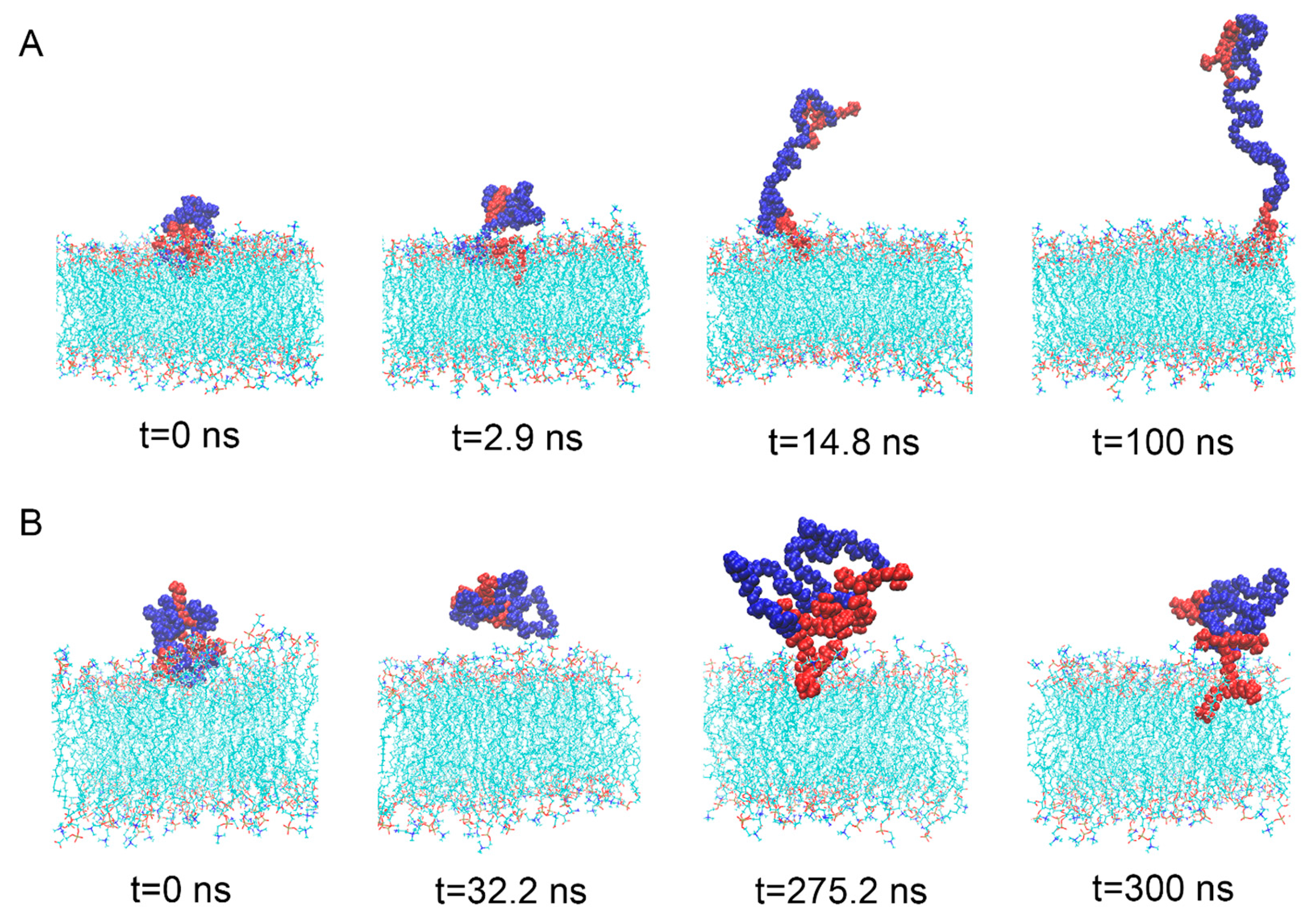
3.4. The Interaction between DNA Molecule and Material Molecule
3.5. Transmembrane Free Energy of DNA Molecule
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle In Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, S.; Li, C.; Geng, Y.; Wang, G.; Gu, Z. Pluronic L64-mediated stable HIF-1α expression in muscle for therapeutic angiogenesis in mouse ischemic limb. Int. J. Nanomed. 2014, 9, 3439–3452. [Google Scholar] [CrossRef]
- Gao, X.; Kim, K.-S.; Liu, D. Nonviral Gene Delivery: What We Know and What Is Next. AAPS J. 2007, 9, E92–E104. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Conwell, C.-C.; Huang, L. Recent advances in non-viral gene delivery. Adv. Genet. 2005, 53, 3–18. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Ma, N.; Steinhoff, G. Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 2013, 14, 46–60. [Google Scholar] [CrossRef]
- Aied, A.; Greiser, U.; Pandit, A.; Wang, W. Polymer gene delivery: Overcoming the obstacles. Drug Discov. Today. 2013, 18, 1090–1098. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non-viral vectors in gene therapy-an overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Wirth, T.; Parker, N.; Ylä-Herttuala, S. History of gene therapy. Gene 2013, 525, 162–169. [Google Scholar] [CrossRef]
- Mumper, R.J.; Duguid, J.G.; Anwer, K.; Barron, M.K.; Nitta, H.; Rolland, A.P. Polyvinyl derivatives as novel interactive polymers for controlled gene delivery to muscle. Pharm. Res. 1996, 13, 701–709. [Google Scholar] [CrossRef]
- Osada, K.; Shiotani, T.; Tockary, T.A.; Kobayashi, D.; Oshima, H.; Ikeda, S.; Christie, R.J.; Itaka, K.; Kataoka, K. Enhanced gene expression promoted by the quantized folding of pDNA within polyplex micelles. Biomaterials 2012, 33, 325–332. [Google Scholar] [CrossRef]
- Qi, R.; Gao, Y.; Tang, Y.; He, R.-R.; Liu, T.-L.; He, Y.; Sun, S.; Li, B.-Y.; Li, Y.-B.; Liu, G. PEG-conjugated PAMAM Dendrimers Mediate Efficient Intramuscular Gene Expression. AAPS J. 2009, 11, 395–405. [Google Scholar] [CrossRef]
- Chang, C.-W.; Choi, D.; Kim, W.J.; Yockman, J.W.; Christen, L.V.; Kim, Y.-H.; Kim, S.W. Non-ionic amphiphilic biodegradable PEG–PLGA–PEG copolymer enhances gene delivery efficiency in rat skeletal muscle. J. Control. Release 2007, 118, 245–253. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef]
- Pitard, B.; Pollard, H.; Agbulut, O.; Lambert, O.; Vilquin, J.T.; Cherel, Y.; Abadie, J.; Samuel, J.L.; Rigaud, J.L.; Menoret, S.; et al. A nonionic amphiphile agent promotes gene delivery in vivo to skeletal and cardiac muscles. Hum. Gene Ther. 2002, 13, 1767–1775. [Google Scholar] [CrossRef]
- Lavigne, M.D.; Pohlschmidt, M.; Novo, J.F.; Higgins, B.; Alakhov, V.; Lochmuller, H.; Sakuraba, H.; Goldspink, G.; MacDermot, K.; Górecki, D.C. Promoter dependence of plasmid-pluronics targeted alpha galactosidase A expression in skeletal muscle of Fabry mice. Mol. Ther. 2005, 12, 985–990. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, J.; Sriadibhatla, S.; Gebhart, C.; Alakhov, V.; Kabanov, A. Promoter- and strain-selective enhancement of gene expression in a mouse skeletal muscle by a polymer excipient Pluronic P85. J. Control. Release 2005, 108, 496–512. [Google Scholar] [CrossRef]
- Lui, S.; Ma, L.; Tan, R.; Lu, Q.; Geng, Y.; Wang, G.; Gu, Z. Safe and efficient local gene delivery into skeletal muscle via a combination of Pluronic L64 and modified electrotransfer. Gene Ther. 2014, 21, 558–565. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Sun, Z.; Han, F.; Tang, J.Z.; Gao, R.; Wang, G. The proper strategy to compress and protect plasmid DNA in the Pluronic L64-electropulse system for enhanced intramuscular gene delivery. Regen. Biomater. 2019, 6, 289–298. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Liu, S.; Bi, F.; Liu, M.; Wang, G. Intramuscular expression of plasmid-encoded FVII-Fc immunoconjugate for tumor immunotherapy by targeting tumoral blood vessels and cells. Front. Oncol. 2021, 11, 638591. [Google Scholar] [CrossRef]
- Deng, L.; Yang, P.; Li, C.; Xie, L.; Lu, W.; Zhang, Y.; Liu, M.; Wang, G. Prolonged control of insulin-dependent diabetes via intramuscular expression of plasmid-encoded single-strand insulin analogue. Genes Dis. 2022; in press. [Google Scholar] [CrossRef]
- Pu, L.; Geng, Y.; Liu, S.; Chen, J.; Luo, K.; Wang, G.; Gu, Z. Electroneutralized Amphiphilic Triblock Copolymer with a Peptide Dendron for Efficient Muscular Gene Delivery. ACS Appl. Mater. Interfaces 2014, 6, 15344–15351. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Wang, J.; Li, N.; Chai, Q.; Irache, J.M.; Wang, G.; Tang, J.Z.; Gu, Z. Synthesis of Electroneutralized Amphiphilic Copolymers with Peptide Dendrons for Intramuscular Gene Delivery. ACS Appl. Mater. Interfaces 2016, 8, 13724–13734. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Jiao, Y.; Pu, L.; Tang, J.Z.; Wang, G. The Progress of Non-Viral Materials and Methods for Gene Delivery to Skeletal Muscle. Pharmaceutics 2022, 14, 2428. [Google Scholar] [CrossRef]
- Chen, J.; Luo, J.; Zhao, Y.; Pu, L.; Lu, X.; Gao, R.; Wang, G.; Gu, Z. Increase in Transgene Expression by Pluronic L64-Mediated Endosomal/Lysosomal Escape through Its Membrane-Disturbing Action. ACS Appl. Mater. Interfaces 2015, 7, 7282–7293. [Google Scholar] [CrossRef]
- Salsbury, F.R., Jr. Molecular dynamics simulations of protein dynamics and their relevance to drug discovery. Curr. Opin. Pharmacol. 2010, 10, 738–744. [Google Scholar] [CrossRef]
- Dallavalle, M.; Calvaresi, M.; Bottoni, A.; Melle-Franco, M.; Zerbetto, F. Graphene can wreak havoc with cell membranes. ACS Appl. Mater. Interfaces 2015, 7, 4406–4414. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Zhao, L.; Gu, Z.; Duan, G.; Zhou, B.; Yang, Z.; Zhou, R. Superior Compatibility of C2N with Human Red Blood Cell Membranes and the Underlying Mechanism. Small 2018, 14, e180539. [Google Scholar] [CrossRef]
- Gao, X.; Hong, S.; Liu, Z.; Yue, T.; Dobnikar, J.; Zhang, X. Membrane potential drives direct translocation of cell-penetrating peptides. Nanoscale 2019, 11, 1949–1958. [Google Scholar] [CrossRef]
- Tian, L.; Wu, G. Microsecond molecular dynamics simulation of the adsorption and penetration of oil droplets on cellular membrane. J. Hazard. Mater. 2020, 397, 122683. [Google Scholar] [CrossRef]
- Harvey, M.J.; Fabritiis, G.D. High-throughput molecular dynamics the powerful new tool for drug discovery. Drug Discov. Today 2012, 17, 1059–1062. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Hildebrand, P.W.; Rose, A.S.; Tiemann, J.K.S. Bringing Molecular Dynamics Simulation Data into View. Trends Biochem. Sci. 2019, 44, 902–913. [Google Scholar] [CrossRef]
- He, X.; Lin, M.; Lu, T.; Qu, Z.; Xu, F. Molecular analysis of interactions between a PAMAM dendrimer-paclitaxel conjugate and a biomembrane. Phys. Chem. Chem. Phys. 2015, 17, 29507–29517. [Google Scholar] [CrossRef]
- Voulgarakis, N.-K.; Rasmussen, K.-Ø.; Welch, P.-M. Dendrimers as synthetic gene vectors: Cell membrane attachment. J. Chem. Phys. 2009, 130, 155101. [Google Scholar] [CrossRef]
- Drenscko, M.; Loverde, S.-M. Molecular dynamics simulations of the interaction of phospholipid bilayers with polycaprolactone. Mol. Simul. 2019, 45, 859–867. [Google Scholar] [CrossRef]
- Sun, C.; Tang, T. Study on the role of polyethylenimine as gene delivery carrier using molecular dynamics simulations. J. Adhes. Sci. Technol. 2012, 28, 399–416. [Google Scholar] [CrossRef]
- Arvayo-Zatarain, J.A.; Favela-Rosales, F.; Contreras-Aburto, C.; Urrutia-Bañuelos, E.; Maldonado, A. Molecular dynamics simulation study of the effect of halothane on mixed DPPC/DPPE phospholipid membranes. J. Mol. Model. 2019, 25, 4. [Google Scholar] [CrossRef]
- Sasaki, E.; Hayashi, Y.; Kimura, Y.; Sashida, S.; Hamano, N.; Nirasawa, K.; Hamada, K.; Katagiri, F.; Kikkawa, Y.; Sakai, T.; et al. Alpha-dystroglycan binding peptide A2G80-modified stealth liposomes as a muscle-targeting carrier for Duchenne muscular dystrophy. J. Control. Release 2021, 329, 1037–1045. [Google Scholar] [CrossRef]
- Nirasawa, K.; Hamada, K.; Naraki, Y.; Kikkawa, Y.; Sasaki, E.; Endo-Takahashi, Y.; Hamano, N.; Katagiri, F.; Nomizu, M.; Negishi, Y. Development of A2G80 peptide-gene complex for targeted delivery to muscle cells. J. Control. Release 2021, 329, 988–996. [Google Scholar] [CrossRef]
- Pomel, C.; Leborgne, C.; Cheradame, H.; Scherman, D.; Kichler, A.; Guegan, P. Synthesis and evaluation of amphiphilic poly(tetrahydrofuran-b-ethylene oxide) copolymers for DNA delivery into skeletal muscle. Pharm. Res. 2008, 25, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Rasolonjatovo, B.; Illy, N.; Bennevault, V.; Mathé, J.; Midoux, P.; Le Gall, T.; Haudebourg, T.; Montier, T.; Lehn, P.; Pitard, B.; et al. Temperature-Sensitive Amphiphilic Non-Ionic Triblock Copolymers for Enhanced In Vivo Skeletal Muscle Transfection. Macromol. Biosci. 2020, 20, e1900276. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Spoel, D.V.D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Kutzner, C.; Páll, S.; Fechner, M.; Esztermann, A.; Groot, B.L.D.; Grubmüller, H. More Bang for Your Buck: Improved use of GPU Nodes for GROMACS 2018. J. Comput. Chem. 2019, 40, 2418–2431. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- MacKerell, A.D., Jr.; Vanommeslaeghe, K. Automation of the CHARMM General Force Field (CGenFF) I: Bond Perception and Atom Typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of Bonded Parameters and Partial Atomic Charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef]
- Yu, W.; He, X.; Vanommeslaeghe, K.; MacKerell, A.D., Jr. Extension of the CHARMM General Force Field to Sulfonyl-Containing Compounds and Its Utility in Biomolecular Simulations. J. Comput. Chem. 2012, 33, 2451–2468. [Google Scholar] [CrossRef]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool, Version 1.2.0; Available online: http://avogadro.cc/ (accessed on 13 August 2012).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Tieleman, D.P.; Forrest, L.R.; Sansom, S.P.; Berendsen, H.J.C. Lipid Properties and the Orientation of Aromatic Residues in OmpF, Influenza M2, and Alamethicin Systems: Molecular Dynamics Simulations. Biochemistry 1998, 37, 17554–17561. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Monticelli, L.; Tieleman, D.P. Molecular Dynamics Simulation of a Palmitoyl-Oleoyl Phosphatidylserine Bilayer with Na+ Counterions and NaCl. Biophys. J. 2004, 86, 1601–1609. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for Mixed Bilayers and Its Application to Yeast Membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder Toward Realistic Biological Membrane Simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Roux, B. The calculation of the potential of mean force using computer simulations. Comput. Phys. Comm. 1995, 91, 275–282. [Google Scholar] [CrossRef]
- Kumar, S.; Rosenerg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. Multidimensional Free-Energy Calculations Using the Weighted Histogram Analysis Method. J. Comput. Chem. 1995, 16, 1339–1350. [Google Scholar] [CrossRef]
- Kästner, J.; Thiel, W. Bridging the gap between thermodynamic integration and umbrella sampling provides a novel analysis method: “Umbrella integration”. J. Chem. Phys. 2005, 123, 144104. [Google Scholar] [CrossRef]
- Hub, J.S.; Groot, B.L.; Spoel, D. g_whams-A Free Weighted Histogram Analysis Implementation Including Robust Error and Autocorrelation Estimates. J. Chem. Theory Comput. 2010, 6, 3713–3720. [Google Scholar] [CrossRef]
- Sirsi, S.R.; Schray, R.C.; Guan, X.; Lykens, N.M.; Williams, J.H.; Erney, M.L.; Lutz, G.J. Functionalized PEG–PEI Copolymers Complexed to Exon-Skipping Oligonucleotides Improve Dystrophin Expression in mdx Mice. Hum. Gene Ther. 2008, 19, 795–806. [Google Scholar] [CrossRef]
- Lee, J.-L.; Lo, C.-W.; Ka, S.-M.; Chen, A.; Chen, W.-S. Prolonging the expression duration of ultrasound-mediated gene transfection using PEI nanoparticles. J. Control. Release 2012, 160, 64–71. [Google Scholar] [CrossRef]
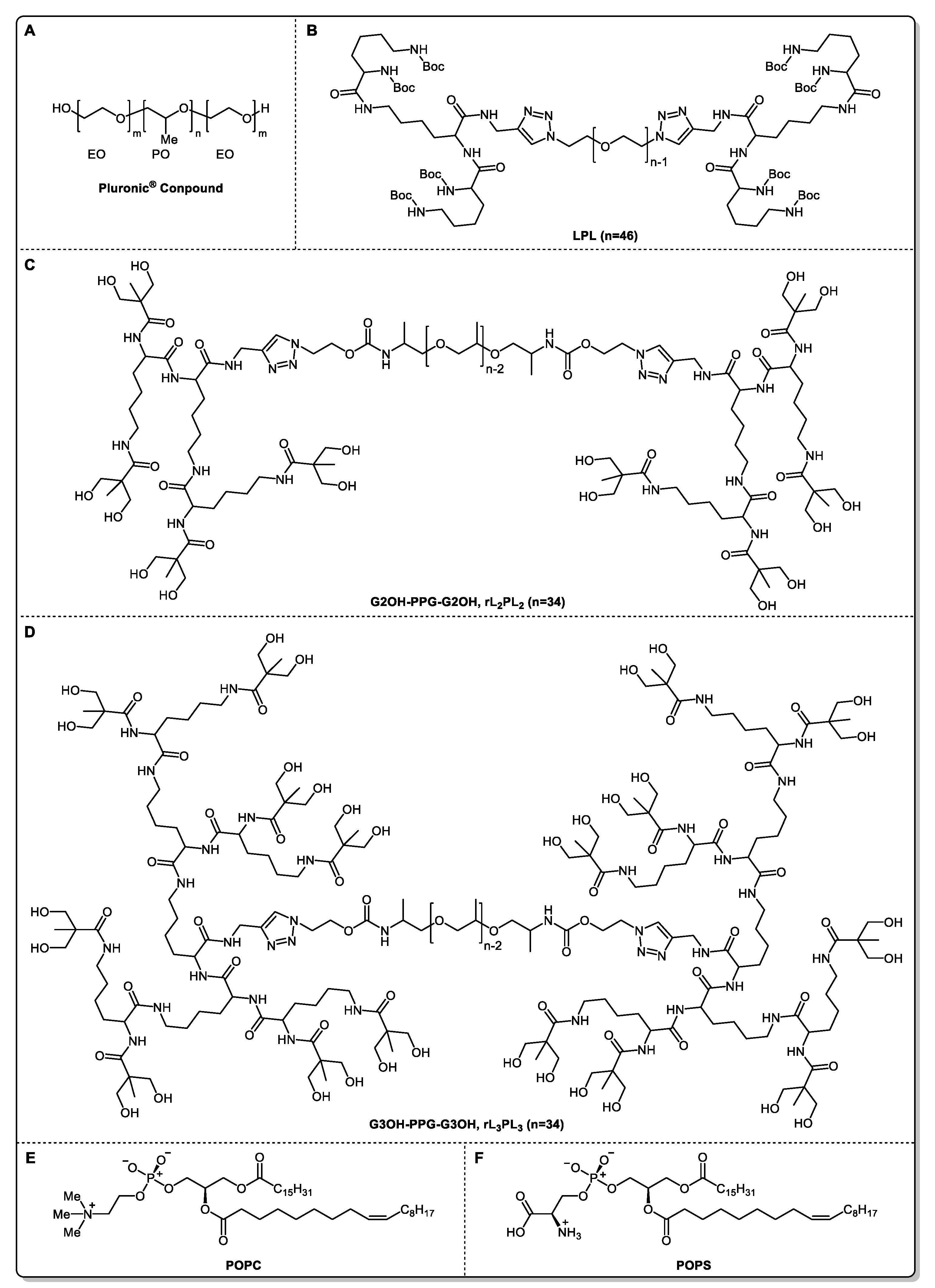

| Molar Mass | Critical Micelle Concentration (mg/mL) | Work Concentration (wt%) | |
|---|---|---|---|
| L64 | 2900 | 0.43 | 0.1 |
| LPL | 3775 | 0.089 | 0.0075 |
| rL2PL2 | 4025 | 0.056 | 0.005 |
| rL3PL3 | 5980 | 0.251 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Jiao, Y.; Pu, L.; Chen, J.; Liu, M.; Tang, J.Z.; Wang, G. The Interaction Mechanism of Intramuscular Gene Delivery Materials with Cell Membranes. J. Funct. Biomater. 2023, 14, 219. https://doi.org/10.3390/jfb14040219
Cui Z, Jiao Y, Pu L, Chen J, Liu M, Tang JZ, Wang G. The Interaction Mechanism of Intramuscular Gene Delivery Materials with Cell Membranes. Journal of Functional Biomaterials. 2023; 14(4):219. https://doi.org/10.3390/jfb14040219
Chicago/Turabian StyleCui, Zhanpeng, Yang Jiao, Linyu Pu, Jianlin Chen, Ming Liu, James Zhenggui Tang, and Gang Wang. 2023. "The Interaction Mechanism of Intramuscular Gene Delivery Materials with Cell Membranes" Journal of Functional Biomaterials 14, no. 4: 219. https://doi.org/10.3390/jfb14040219
APA StyleCui, Z., Jiao, Y., Pu, L., Chen, J., Liu, M., Tang, J. Z., & Wang, G. (2023). The Interaction Mechanism of Intramuscular Gene Delivery Materials with Cell Membranes. Journal of Functional Biomaterials, 14(4), 219. https://doi.org/10.3390/jfb14040219







