In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Materials
2.3. Rat Subcutaneous Implantation
2.4. Micro-Raman Spectrometry
2.5. Surface Ultrastructural and Elemental Characterization
2.6. Elemental Mapping Analysis
3. Results
3.1. Micro-Raman Spectrometry
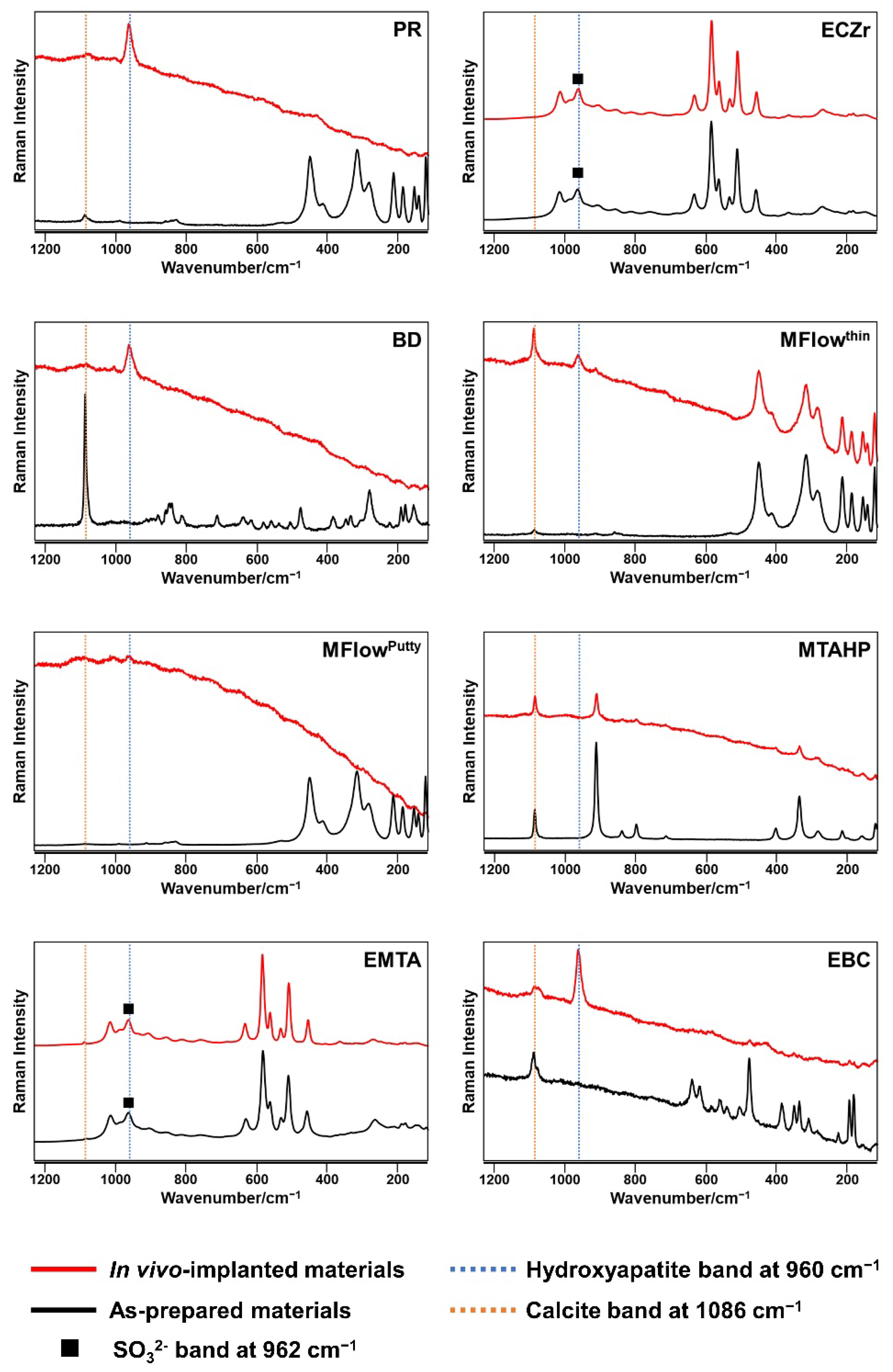
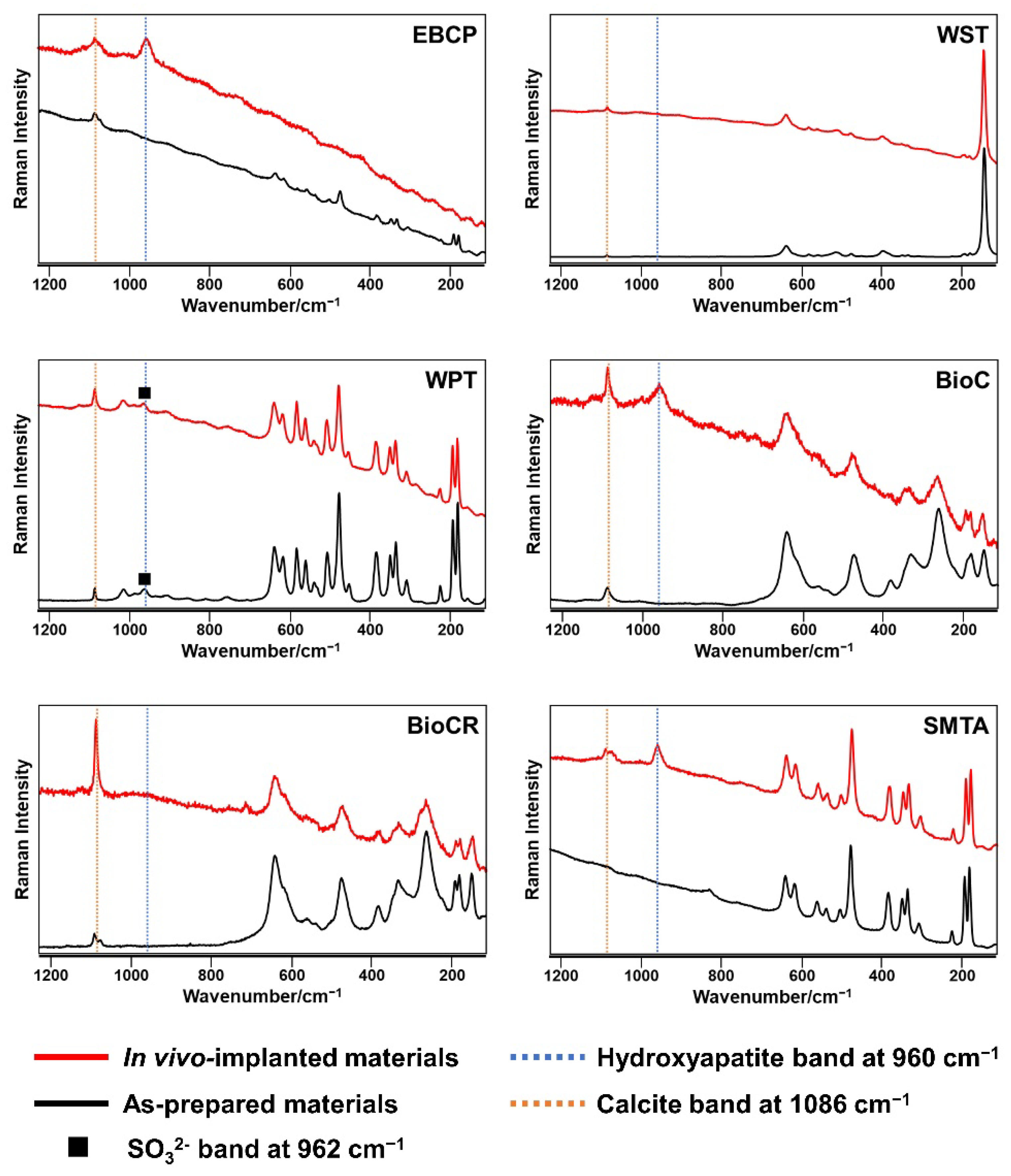
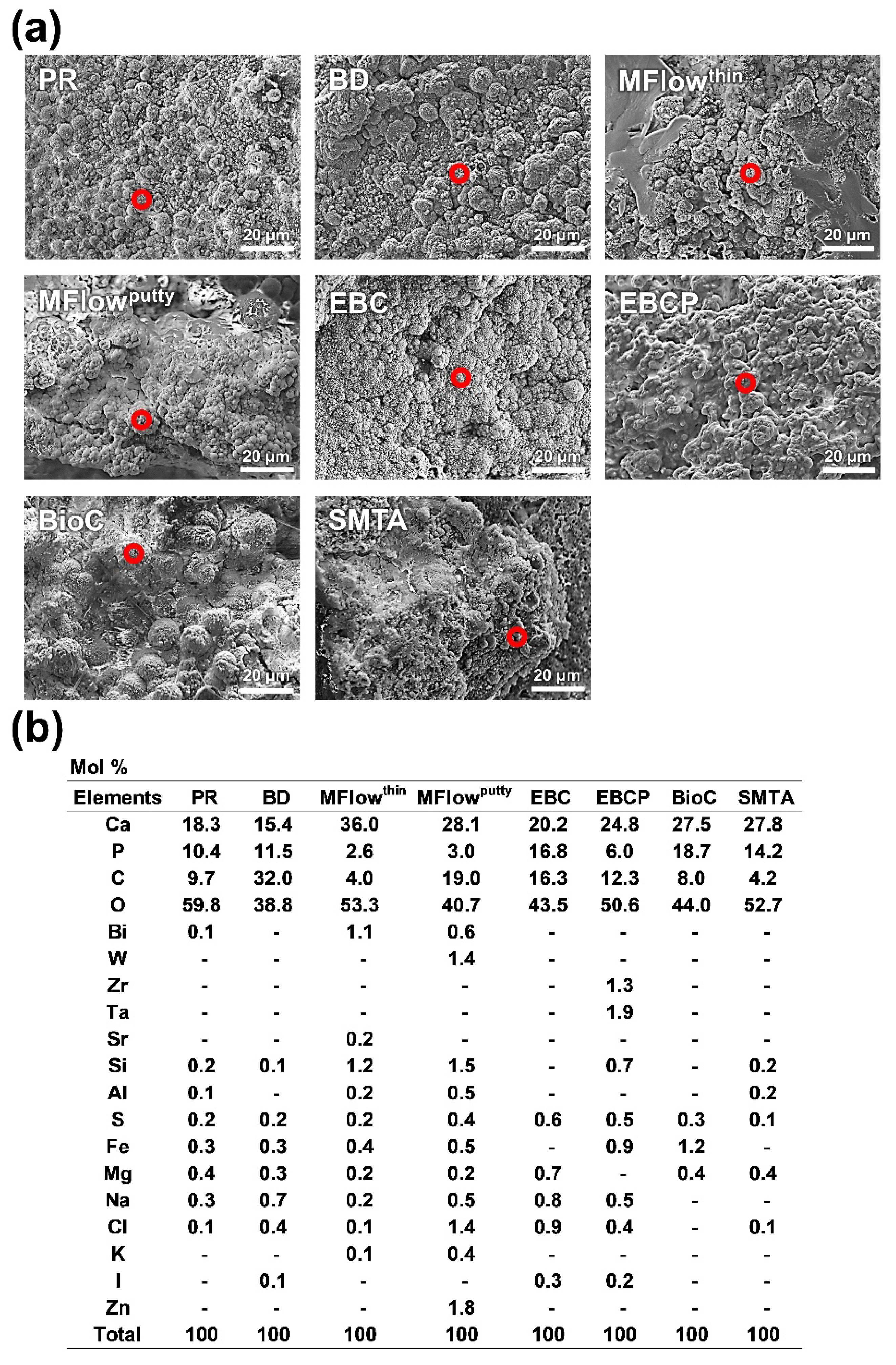
3.2. Surface Ultrastructural and Elemental Characterization
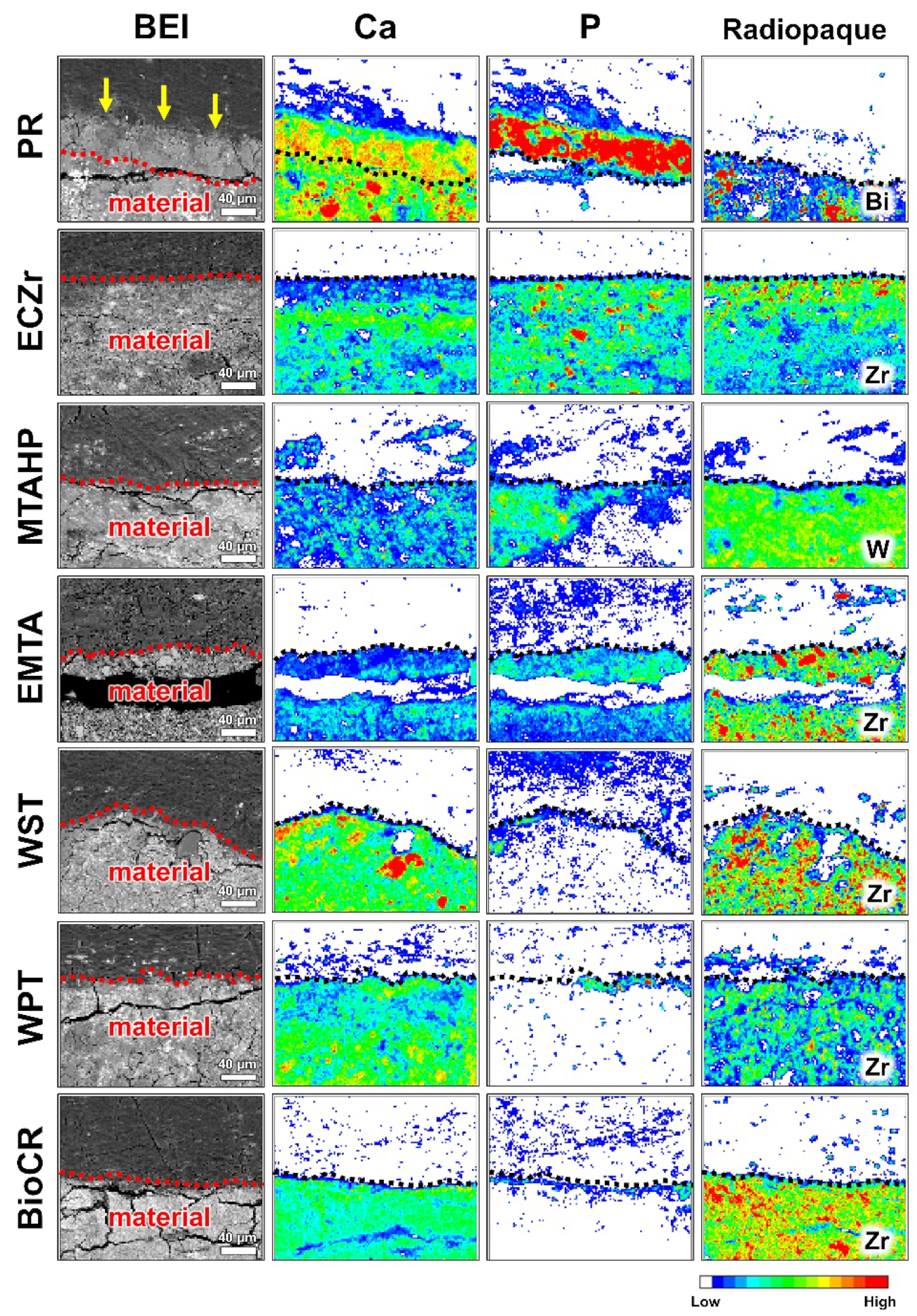
3.3. Elemental Mapping Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pushpalatha, C.; Dhareshwar, V.; Sowmya, S.V.; Augustine, D.; Vinothkumar, T.S.; Renugalakshmi, A.; Shaiban, A.; Kakti, A.; Bhandi, S.H.; Dubey, A.; et al. Modified Mineral Trioxide Aggregate-A Versatile Dental Material: An Insight on Applications and Newer Advancements. Front. Bioeng. Biotechnol. 2022, 10, 941826. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part II: Leakage and Biocompatibility Investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part III: Clinical Applications, Drawbacks, and Mechanism of Action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Primus, C.M.; Tay, F.R.; Niu, L.N. Bioactive Tri/Dicalcium Silicate Cements for Treatment of Pulpal and Periapical Tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Hinata, G.; Yoshiba, K.; Han, L.; Edanami, N.; Yoshiba, N.; Okiji, T. Bioactivity and Biomineralization Ability of Calcium Silicate-Based Pulp-Capping Materials after Subcutaneous Implantation. Int. Endod. J. 2017, 50, e40–e51. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.F.; Santos, A.S.; Figueiredo, C.P.; Baggio, C.H.; Felippe, M.C.S.; Felippe, W.T.; Cordeiro, M.M. Host-Mineral Trioxide Aggregate Inflammatory Molecular Signaling and Biomineralization Ability. J. Endod. 2010, 36, 1347–1353. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Ciapetti, G.; Perut, F.; Taddei, P.; Modena, E.; Rossi, P.L.; Prati, C. Biomimetic Calcium-Silicate Cements Aged in Simulated Body Solutions. Osteoblast Response and Analyses of Apatite Coating. J. Appl. Biomater. Biomech. 2009, 7, 160–170. [Google Scholar]
- Niu, L.N.; Jiao, K.; Wang, T.D.; Zhang, W.; Camilleri, J.; Bergeron, B.E.; Feng, H.L.; Mao, J.; Chen, J.H.; Pashley, D.H.; et al. A Review of the Bioactivity of Hydraulic Calcium Silicate Cements. J. Dent. 2014, 42, 517–533. [Google Scholar] [CrossRef]
- Martin, R.L.; Monticelli, F.; Brackett, W.W.; Loushine, R.J.; Rockman, R.A.; Ferrari, M.; Pashley, D.H.; Tay, F.R. Sealing Properties of Mineral Trioxide Aggregate Orthograde Apical Plugs and Root Fillings in an in Vitro Apexification Model. J. Endod. 2007, 33, 272–275. [Google Scholar] [CrossRef]
- García, A.J.; Ducheyne, P.; Boettiger, D. Effect of Surface Reaction Stage on Fibronectin-Mediated Adhesion of Osteoblast-like Cells to Bioactive Glass. J. Biomed. Mater. Res. 1998, 40, 48–56. [Google Scholar] [CrossRef]
- Tziafas, D.; Pantelidou, O.; Alvanou, A.; Belibasakis, G.; Papadimitriou, S. The Dentinogenic Effect of Mineral Trioxide Aggregate (MTA) in Short-Term Capping Experiments. Int. Endod. J. 2002, 35, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kohout, G.D.; He, J.; Primus, C.M.; Opperman, L.A.; Woodmansey, K.F. Comparison of Quick-Set and Mineral Trioxide Aggregate Root-End Fillings for the Regeneration of Apical Tissues in Dogs. J. Endod. 2015, 41, 248–252. [Google Scholar] [CrossRef]
- Silva, R.A.B.; Borges, A.T.N.; Hernandéz-Gatón, P.; de Queiroz, A.M.; Arzate, H.; Romualdo, P.C.; Nelson-Filho, P.; Silva, L.A.B. Histopathological, Histoenzymological, Immunohistochemical and Immunofluorescence Analysis of Tissue Response to Sealing Materials after Furcation Perforation. Int. Endod. J. 2019, 52, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral Trioxide Aggregate and Other Bioactive Endodontic Cements: An Updated Overview—Part I: Vital Pulp Therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Characterization and Hydration Kinetics of Tricalcium Silicate Cement for Use as a Dental Biomaterial. Dent. Mater. 2011, 27, 836–844. [Google Scholar] [CrossRef]
- Ber, B.S.; Hatton, J.F.; Stewart, G.P. Chemical Modification of ProRoot MTA to Improve Handling Characteristics and Decrease Setting Time. J. Endod. 2007, 33, 1231–1234. [Google Scholar] [CrossRef]
- Marciano, M.A.; Duarte, M.A.H.; Camilleri, J. Dental Discoloration Caused by Bismuth Oxide in MTA in the Presence of Sodium Hypochlorite. Clin. Oral Investig. 2015, 19, 2201–2209. [Google Scholar] [CrossRef]
- Del Carmen Jiménez-Sánchez, M.; Segura-Egea, J.J.; Díaz-Cuenca, A. MTA HP Repair Stimulates in Vitro an Homogeneous Calcium Phosphate Phase Coating Deposition. J. Clin. Exp. Dent. 2019, 11, e322–e326. [Google Scholar] [CrossRef]
- Han, L.; Kodama, S.; Okiji, T. Evaluation of Calcium-Releasing and Apatite-Forming Abilities of Fast-Setting Calcium Silicate-Based Endodontic Materials. Int. Endod. J. 2015, 48, 124–130. [Google Scholar] [CrossRef]
- Yamamoto, S.; Han, L.; Noiri, Y.; Okiji, T. Evaluation of the Ca Ion Release, pH and Surface Apatite Formation of a Prototype Tricalcium Silicate Cement. Int. Endod. J. 2017, 50, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.J.; Patel, K.D.; Knowles, J.C.; Lee, J.H.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Siboni, F.; Taddei, P.; Prati, C.; Gandolfi, M.G. Properties of NeoMTA Plus and MTA Plus Cements for Endodontics. Int. Endod. J. 2017, 50, e83–e94. [Google Scholar] [CrossRef] [PubMed]
- Zamparini, F.; Siboni, F.; Prati, C.; Taddei, P.; Gandolfi, M.G. Properties of Calcium Silicate-Monobasic Calcium Phosphate Materials for Endodontics Containing Tantalum Pentoxide and Zirconium Oxide. Clin. Oral Investig. 2019, 23, 445–457. [Google Scholar] [CrossRef]
- Zamparini, F.; Prati, C.; Taddei, P.; Spinelli, A.; Di Foggia, M.; Gandolfi, M.G. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef]
- Moinzadeh, A.T.; Aznar Portoles, C.; Schembri Wismayer, P.; Camilleri, J. Bioactivity Potential of EndoSequence BC RRM Putty. J. Endod. 2016, 42, 615–621. [Google Scholar] [CrossRef]
- Meschi, N.; Li, X.; Van Gorp, G.; Camilleri, J.; Van Meerbeek, B.; Lambrechts, P. Bioactivity Potential of Portland Cement in Regenerative Endodontic Procedures: From Clinic to Lab. Dent. Mater. 2019, 35, 1342–1350. [Google Scholar] [CrossRef]
- Yang, X.; Tian, J.; Li, M.; Chen, W.; Liu, H.; Wang, Z.; Haapasalo, M.; Shen, Y.; Wei, X. Biocompatibility of a New Calcium Silicate-Based Root Canal Sealer Mediated via the Modulation of Macrophage Polarization in a Rat Model. Materials 2022, 15, 1962. [Google Scholar] [CrossRef]
- Belal, R.S.I.; Edanami, N.; Yoshiba, K.; Yoshiba, N.; Ohkura, N.; Takenaka, S.; Noiri, Y. Comparison of Calcium and Hydroxyl Ion Release Ability and in Vivo Apatite-Forming Ability of Three Bioceramic-Containing Root Canal Sealers. Clin. Oral Investig. 2022, 26, 1443–1451. [Google Scholar] [CrossRef]
- Xin, R.; Leng, Y.; Chen, J.; Zhang, Q. A Comparative Study of Calcium Phosphate Formation on Bioceramics in Vitro and in Vivo. Biomaterials 2005, 26, 6477–6486. [Google Scholar] [CrossRef]
- Kazanci, M.; Fratzl, P.; Klaushofer, K.; Paschalis, E.P. Complementary Information on In Vitro Conversion of Amorphous (Precursor) Calcium Phosphate to Hydroxyapatite from Raman Microspectroscopy and Wide-Angle X-ray Scattering. Calcif. Tissue Int. 2006, 79, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Maguregui, M.; Castro, K.; Morillas, H.; Trebolazabala, J.; Knuutinen, U.; Wiesinger, R.; Schreiner, M.; Madariaga, J.M. Multianalytical Approach to Explain the Darkening Process of Hematite Pigment in Paintings from Ancient Pompeii after Accelerated Weathering Experiments. Anal. Methods 2013, 6, 372–378. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nesic, S.; Young, D. Effect of Impurities on the Corrosion Behavior of CO2 Transmission Pipeline Steel in Supercritical CO2-Water Environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 Nano- and Micro-Particles by Dry Ice Carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; De Dorigo, E.S.; Rossi, P.L.; Prati, C. Kinetics of Apatite Formation on a Calcium-Silicate Cement for Root-End Filling during Ageing in Physiological-like Phosphate Solutions. Clin. Oral Investig. 2010, 14, 659–668. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; Prati, C. Apatite-Forming Ability (Bioactivity) of ProRoot MTA. Int. Endod. J. 2010, 43, 917–929. [Google Scholar] [CrossRef]
- Taddei, P.; Tinti, A.; Gandolfi, M.G.; Rossi, P.L.; Prati, C. Vibrational Study on the Bioactivity of Portland Cement-Based Materials for Endodontic Use. J. Mol. Struct. 2009, 924, 548–554. [Google Scholar] [CrossRef]
- Maltezos, C.; Glickman, G.N.; Ezzo, P.; He, J. Comparison of the Sealing of Resilon, Pro Root MTA, and Super-EBA as Root-End Filling Materials: A Bacterial Leakage Study. J. Endod. 2006, 32, 324–327. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Iezzi, G.; Piattelli, A.; Prati, C.; Scarano, A. Osteoinductive Potential and Bone-Bonding Ability of ProRoot MTA, MTA Plus and Biodentine in Rabbit Intramedullary Model: Microchemical Characterization and Histological Analysis. Dent. Mater. 2017, 33, e221–e238. [Google Scholar] [CrossRef]
- Ha, W.N.; Bentz, D.P.; Kahler, B.; Walsh, L.J. D90: The Strongest Contributor to Setting Time in Mineral Trioxide Aggregate and Portland Cement. J. Endod. 2015, 41, 1146–1150. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Saavedra, F.; Bombarda, G.F.; Gomes, B.P.F.D.A.; De-Jesus-soares, A.; Zaia, A.A.; Duarte, M.A.H.; Tanomaru-Filho, M.; Marciano, M.A. Dental Discoloration Caused by Grey-MTAFlow Cement: Analysis of Its Physicochemical, Biological and Antimicrobial Properties. J. Appl. Oral Sci. 2020, 28, 1–15. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. A Microstructure Insight of MTA Repair HP of Rapid Setting Capacity and Bioactive Response. Materials 2020, 13, 1641. [Google Scholar] [CrossRef] [PubMed]
- Inami, C.; Endoh, C.; Ichinohe, H.; Itsuno, S. Effect of Direct Pulp Capping with a Novel Chemically Curable Mineral Trioxide Aggregate Material using Tri-Butylborane as a Polymerization Initiator. J. Hard Tissue Biol. 2019, 28, 383–390. [Google Scholar] [CrossRef]
- Inami, C.; Nishitani, Y.; Haraguchi, N.; Itsuno, S. Evaluation of the Solubility, Calcium-Release Ability, and Apatite-Forming Ability of a Novel Chemically Curable Mineral Trioxide Aggregate Material. J. Hard Tissue Biol. 2019, 28, 273–280. [Google Scholar] [CrossRef]
- ISO 23317:2014(E). International Organization for Standardization (2014) International Standard: Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. ISO: Geneva, Switzerland, 2014. Available online: https://www.iso.org/obp/ui/#iso:std:iso:23317:ed-3:v1:en (accessed on 17 March 2023).
- Lanfranco, A.M.; Schofield, P.F.; Murphy, P.J.; Hodson, M.E.; Mosselmans, J.F.W.; Valsami-Jones, E. Characterization and Identification of Mixed-Metal Phosphates in Soils: The Application of Raman Spectroscopy. Mineral. Mag. 2003, 67, 1299–1316. [Google Scholar] [CrossRef]
- Bósio, C.C.; Felippe, G.S.; Bortoluzzi, E.A.; Felippe, M.C.S.; Felippe, W.T.; Rivero, E.R.C. Subcutaneous Connective Tissue Reactions to IRoot SP, Mineral Trioxide Aggregate (MTA) Fillapex, DiaRoot BioAggregate and MTA. Int. Endod. J. 2014, 47, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Alves Silva, E.C.; Tanomaru-Filho, M.; da Silva, G.F.; Delfino, M.M.; Cerri, P.S.; Guerreiro-Tanomaru, J.M. Biocompatibility and Bioactive Potential of New Calcium Silicate-Based Endodontic Sealers: Bio-C Sealer and Sealer Plus BC. J. Endod. 2020, 46, 1470–1477. [Google Scholar] [CrossRef]
- Bueno, C.R.E.; Vasques, A.M.V.; Cury, M.T.S.; Sivieri-Araújo, G.; Jacinto, R.C.; Gomes-Filho, J.E.; Cintra, L.T.A.; Dezan-Júnior, E. Biocompatibility and Biomineralization Assessment of Mineral Trioxide Aggregate Flow. Clin. Oral Investig. 2019, 23, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Leng, Y. Theoretical Analysis of Calcium Phosphate Precipitation in Simulated Body Fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Nekoofar, M.H.; Namazikhah, M.S.; Sheykhrezae, M.S.; Mohammadi, M.M.; Kazemi, A.; Aseeley, Z.; Dummer, P.M.H. pH of Pus Collected from Periapical Abscesses. Int. Endod. J. 2009, 42, 534–538. [Google Scholar] [CrossRef]
- Wang, K.; Leng, Y.; Lu, X.; Ren, F.; Ge, X.; Ding, Y. Theoretical Analysis of Protein Effects on Calcium Phosphate Precipitation in Simulated Body Fluid. CrystEngComm 2012, 14, 5870–5878. [Google Scholar] [CrossRef]
- Edanami, N.; Belal, R.S.I.; Takenaka, S.; Yoshiba, K.; Yoshiba, N.; Ohkura, N.; Takahara, S.; Noiri, Y. Apatite-Forming Ability of Flowable vs. Putty Formulations of Newly Developed Bioactive Glass-Containing Endodontic Cement. Appl. Sci. 2021, 11, 8969. [Google Scholar] [CrossRef]
- Zhao, W.; Lemaître, J.; Bowen, P. A Comparative Study of Simulated Body Fluids in the Presence of Proteins. Acta Biomater. 2017, 53, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kang, C.M.; Song, J.S.; Shin, Y.S.; Kim, S.Y.; Kim, S.O.; Choi, H.J. Biological Efficacy of Two Mineral Trioxide Aggregate (MTA)-Based Materials in a Canine Model of Pulpotomy. Dent. Mater. J. 2017, 36, 41–47. [Google Scholar] [CrossRef]
- Alnassar, I.; Altinawi, M.K.; Rekab, M.S.; Alzoubi, H.; Katbeh, I. Evaluation of Bioceramic Putty in Pulpotomy of Immature Permanent Molars with Symptoms of Irreversible Pulpitis. Cureus 2022, 14, e31806. [Google Scholar] [CrossRef]
- Liu, W.N.; Chang, J.; Zhu, Y.Q.; Zhang, M. Effect of Tricalcium Aluminate on the Properties of Tricalcium Silicate-Tricalcium Aluminate Mixtures: Setting Time, Mechanical Strength and Biocompatibility. Int. Endod. J. 2011, 44, 41–50. [Google Scholar] [CrossRef]
- Chang, K.C.; Chang, C.C.; Huang, Y.C.; Chen, M.H.; Lin, F.H.; Lin, C.P. Effect of Tricalcium Aluminate on the Physicochemical Properties, Bioactivity, and Biocompatibility of Partially Stabilized Cements. PLoS ONE 2014, 9, e106754. [Google Scholar] [CrossRef]
- López-GarcíA, S.; Lozano, A.; Garcíabernal, D.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Moraleda, J.M.; Murcia, L.; Rodríguez-Lozano, F.J. Biological Effects of New Hydraulic Materials on Human Periodontal Ligament Stem Cells. J. Clin. Med. 2019, 8, 1216. [Google Scholar] [CrossRef]
- Seux, D.; Couble, M.L.; Hartmann, D.J.; Gauthier, J.P.; Magloire, H. Odontoblast-like Cytodifferentiation of Human Dental Pulp Cells in Vitro in the Presence of a Calcium Hydroxide-Containing Cement. Arch. Oral Biol. 1991, 36, 117–128. [Google Scholar] [CrossRef]
- Monchau, F.; Hivart, P.; Genestie, B.; Chai, F.; Descamps, M.; Hildebrand, H.F. Calcite as a Bone Substitute. Comparison with Hydroxyapatite and Tricalcium Phosphate with Regard to the Osteoblastic Activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 490–498. [Google Scholar] [CrossRef]
- Edanami, N.; Yoshiba, N.; Ohkura, N.; Takeuchi, R.; Tohma, A.; Noiri, Y.; Yoshiba, K. Characterization of Dental Pulp Myofibroblasts in Rat Molars after Pulpotomy. J. Endod. 2017, 43, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Edanami, N.; Ibn Belal, R.S.; Yoshiba, K.; Yoshiba, N.; Ohkura, N.; Takenaka, S.; Noiri, Y. Effect of a Resin-Modified Calcium Silicate Cement on Inflammatory Cell Infiltration and Reparative Dentin Formation after Pulpotomy in Rat Molars. Aust. Endod. J. 2022, 48, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, N.; Noiri, Y.; Matsui, S.; Kuremoto, K.; Maezono, H.; Ishimoto, T.; Nakano, T.; Ebisu, S.; Hayashi, M. Development of a Root Canal Treatment Model in the Rat. Sci. Rep. 2017, 7, 3315. [Google Scholar] [CrossRef] [PubMed]
- Edanami, N.; Yoshiba, K.; Shirakashi, M.; Ibn Belal, R.S.; Yoshiba, N.; Ohkura, N.; Tohma, A.; Takeuchi, R.; Okiji, T.; Noiri, Y. Impact of Remnant Healthy Pulp and Apical Tissue on Outcomes after Simulated Regenerative Endodontic Procedure in Rat Molars. Sci. Rep. 2020, 10, 20967. [Google Scholar] [CrossRef] [PubMed]
| Material (Abbreviation) | Composition (wt%) |
|---|---|
| White ProRoot MTA (PR) | Powder: Portland cement (tricalcium silicate, dicalcium silicate, tricalcium aluminate, and other trace compounds) (60–90%), bismuth oxide (10–40%), and gypsum |
| Liquid: distilled water | |
| Endocem Zr (ECZr) | Powder: natural pure cement (calcium oxide, silicon dioxide, aluminum oxide, and other mineral oxides) (50%) and zirconium oxide (50%) |
| Liquid: distilled water | |
| Biodentine (BD) | Powder: tricalcium silicate, dicalcium silicate, calcium carbonate (10–25%), zirconium oxide, calcium oxide, and iron oxide |
| Liquid: calcium chloride, hydrosoluble polymer, and distilled water | |
| MTA Flow (MFlow) | Powder: tricalcium silicate (<50%), bismuth trioxide (<30%), dicalcium silicate (<20%), calcium sulfate (<3%), and silica (<0.1%) |
| Liquid: water-soluble silicone-based gel | |
| MTA REPAIR HP (MTAHP) | Powder: tricalcium silicate (45–55%), calcium tungstate (20–30%), dicalcium silicate (10–15%), tricalcium aluminate (5–12%), and calcium oxide (1–5%) |
| Liquid: distilled water and organic plasticizer | |
| Endoseal MTA (EMTA) | Ready-to-use paste: radiopacifiers (zirconium oxide and bismuth oxide) (47.28%), natural pure cement (calcium oxide, silicon dioxide, aluminum oxide, and other mineral oxides) (27.81%), and thickening agents (24.91%) |
| EndoSequence BC Sealer (EBC) | Ready-to-use paste: zirconium oxide (35–45%), tricalcium silicate (20–35%), dicalcium silicate (7–15%), calcium hydroxide (1–4%), calcium phosphate monobasic, filler, and thickening agents |
| EndoSequence BC RRM Putty (EBCP) | Ready-to-use putty: tricalcium silicate (30–36%), zirconium oxide (15–18%), tantalum pentoxide (12–15%), dicalcium silicate (9–13%), calcium sulfate (3–8%), calcium phosphate monobasic, filler, and thickening agents |
| Well-Root ST (WST) | Ready-to-use paste: calcium aluminosilicate compound, zirconium oxide, filler, and thickening agents |
| Well-Root PT (WPT) | Ready-to-use putty: calcium aluminosilicate compound, zirconium oxide, filler, and thickening agents |
| BioC Sealer (BioC) | Ready-to-use paste: tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, zirconium oxide, silicon oxide, polyethylene glycol, and iron oxide |
| BioC Repair (BioCR) | Ready-to-use putty: tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, zirconium oxide, silicon oxide, polyethylene glycol, and iron oxide |
| Super MTA Paste (SMTA) | Paste: Portland cement (tricalcium silicate, dicalcium silicate, tricalcium aluminate, and other trace compounds) (30–40%), zirconium oxide (30–40%), and hydroxypropyl methacrylate (20–30%) |
| Catalyst: partially oxidized tri-n-butylborane, n-hexane, and ethanol |
| Material | Abbreviation | HA Detected via Micro-Raman? (Figure 1) | Calcite Detected via Micro-Raman? (Figure 1) | HA-Like Spherulites Detected via SEM-WDX? (Figure 2) | Ca-P-Rich Layer Detected in Elemental Mapping? (Figure 3) |
|---|---|---|---|---|---|
| White ProRoot MTA | PR | yes | yes | yes | yes |
| Endocem Zr | ECZr | no | no | no | no |
| Biodentine | BD | yes | yes | yes | - |
| MTA Flow with a thin consistency | MFlowthin | yes | yes | yes | - |
| MTA Flow with a putty consistency | MFlowputty | yes | yes | yes | - |
| MTA REPAIR HP | MTAHP | no | yes | no | no |
| Endoseal MTA | EMTA | no | yes | no | no |
| EndoSequence BC Sealer | EBC | yes | yes | yes | - |
| EndoSequence BC RRM Putty | EBCP | yes | yes | yes | - |
| Well-Root ST | WST | no | yes | no | no |
| Well-Root PT | WPT | no | yes | no | no |
| BioC Sealer | BioC | yes | yes | yes | - |
| BioC Repair | BioCR | no | yes | no | no |
| Super MTA Paste | SMTA | yes | yes | yes | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edanami, N.; Takenaka, S.; Ibn Belal, R.S.; Yoshiba, K.; Takahara, S.; Yoshiba, N.; Ohkura, N.; Noiri, Y. In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model. J. Funct. Biomater. 2023, 14, 213. https://doi.org/10.3390/jfb14040213
Edanami N, Takenaka S, Ibn Belal RS, Yoshiba K, Takahara S, Yoshiba N, Ohkura N, Noiri Y. In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model. Journal of Functional Biomaterials. 2023; 14(4):213. https://doi.org/10.3390/jfb14040213
Chicago/Turabian StyleEdanami, Naoki, Shoji Takenaka, Razi Saifullah Ibn Belal, Kunihiko Yoshiba, Shintaro Takahara, Nagako Yoshiba, Naoto Ohkura, and Yuichiro Noiri. 2023. "In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model" Journal of Functional Biomaterials 14, no. 4: 213. https://doi.org/10.3390/jfb14040213
APA StyleEdanami, N., Takenaka, S., Ibn Belal, R. S., Yoshiba, K., Takahara, S., Yoshiba, N., Ohkura, N., & Noiri, Y. (2023). In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model. Journal of Functional Biomaterials, 14(4), 213. https://doi.org/10.3390/jfb14040213





