Effects of Hyperlipidemia on Osseointegration of Dental Implants and Its Strategies
Abstract
1. Introduction
2. Implants in Hyperlipidemia
2.1. Negative Effects of Titanium on the Osseointegration under Hyperlipidemic Conditions
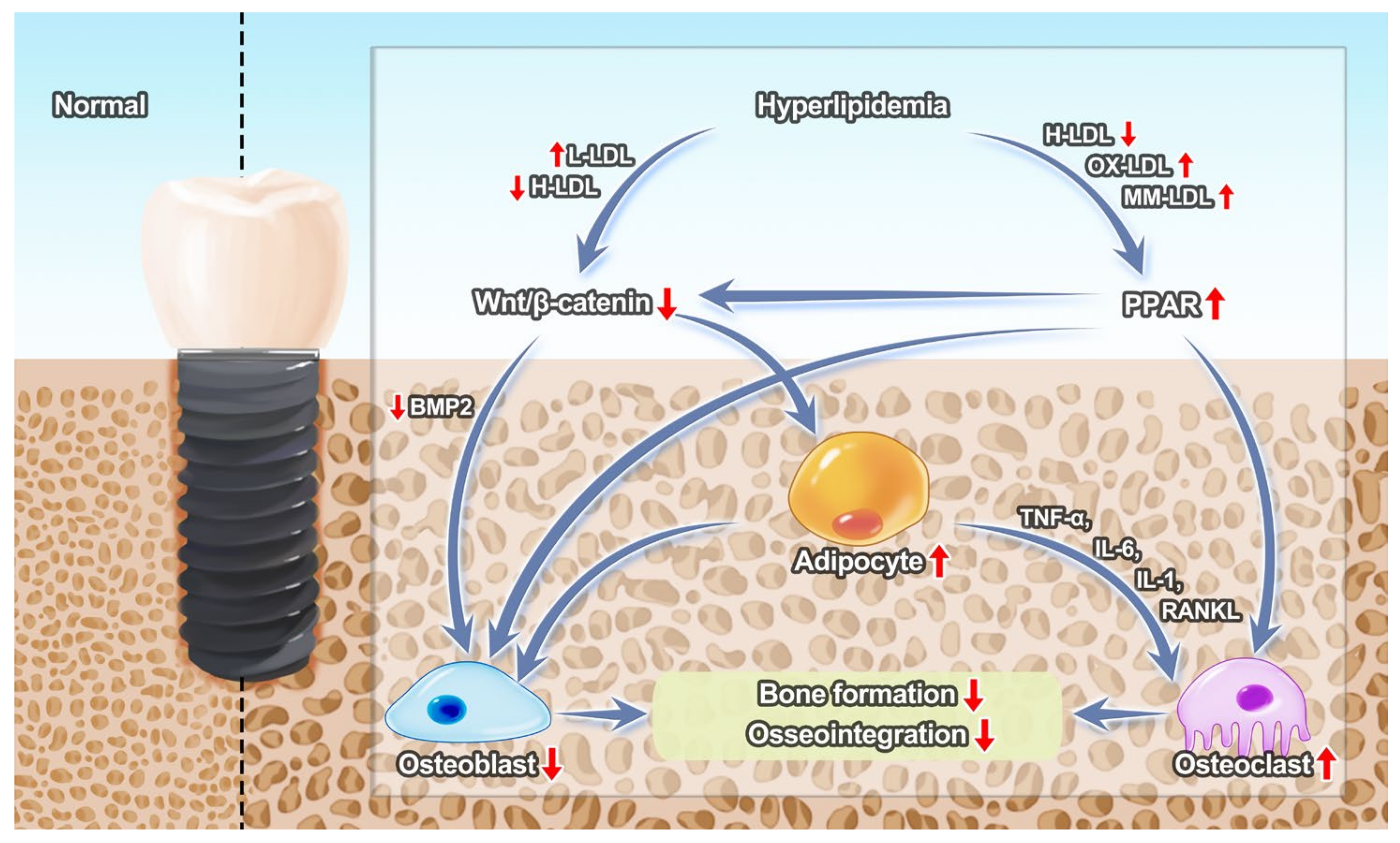
2.2. Effects of Hyperlipidemia on Bone Tissue around Implants
2.3. Effects of Hyperlipidemia on Inflammation about Implants
3. Local Drugs Injection
4. Implant Surface Modification under Hyperlipidemic Conditions
4.1. Statin-Based Implant Surface Modification
4.2. Vitamin D Based Implant Modification
5. Bone Grafting Material Modification
6. Promising Strategies for Promoting Implant Osseointegration
6.1. The Application of Statins
6.2. Cerium Oxide Based Implant Modification
7. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, Itc81–itc96. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Zhao, K.; Gao, L.; Zhao, J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021, 33, 1911–1925. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Tekin, M.B.; Toker, H. The effect of hyperlipidemia on bone graft regeneration of peri-implantal created defects in rabbits. Int. J. Implant Dent. 2019, 5, 18. [Google Scholar] [CrossRef]
- Wang, Y.N.; Jia, T.; Zhang, D. Hyperlipidemia Impairs Implant Osseointegration. Clin. Oral Implant. Res. 2019, 30, 137. [Google Scholar] [CrossRef]
- Keuroghlian, A.; Barroso, A.D.; Kirikian, G.; Bezouglaia, O.; Tintut, Y.; Tetradis, S.; Moy, P.; Pirih, F.; Aghaloo, T. The effects of hyperlipidemia on implant osseointegration in the mouse femur. J. Oral Implantol. 2015, 41, e7–e11. [Google Scholar] [CrossRef]
- Dundar, S.; Bozoglan, A.; Bulmus, O.; Tekin, S.; Yildirim, T.T.; Kirtay, M.; Toy, V.E.; Gul, M.; Bozoglan, M.Y. Effects of restraint stress and high-fat diet on osseointegration of titanium implants: An experimental study. Braz. Oral Res. 2020, 34, e008. [Google Scholar] [CrossRef]
- Dündar, S.; Yaman, F.; Ozupek, M.F.; Saybak, A.; Gul, M.; Asutay, F.; Kirtay, M.; Ozercan, I.H. The effects of high-fat diet on implant osseointegration: An experimental study. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Guo, Q.; Wang, C.; Ma, X.; He, H.; Oh, Y.; Feng, Y.; Wu, Q.; Gu, N. Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice. J. Appl. Toxicol. 2015, 35, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Murphy, R.C. Oxidized lipids formed non-enzymatically by reactive oxygen species. J. Biol. Chem. 2008, 283, 15513–15514. [Google Scholar] [CrossRef]
- Ani, M.; Moshtaghie, A.A.; Ahmadvand, H. Comparative effects of copper, iron, vanadium and titanium on low density lipoprotein oxidation in vitro. Iran. Biomed. J. 2007, 11, 113–118. [Google Scholar]
- Wang, Y.N.; Jia, T.T.; Feng, Y.; Liu, S.Y.; Zhang, W.J.; Zhang, D.J.; Xu, X. Hyperlipidemia Impairs Osseointegration via the ROS/Wnt/β-Catenin Pathway. J. Dent. Res. 2021, 100, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, Z.; Zhang, W. Modulation of Bone and Marrow Niche by Cholesterol. Nutrients 2019, 11, 1394. [Google Scholar] [CrossRef]
- Pelton, K.; Krieder, J.; Joiner, D.; Freeman, M.R.; Goldstein, S.A.; Solomon, K.R. Hypercholesterolemia promotes an osteoporotic phenotype. Am. J. Pathol. 2012, 181, 928–936. [Google Scholar] [CrossRef]
- Guiglia, R.; Di Fede, O.; Lo Russo, L.; Sprini, D.; Rini, G.B.; Campisi, G. Osteoporosis, jawbones and periodontal disease. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e93–e99. [Google Scholar] [CrossRef]
- Mayta-Tovalino, F.; Mendoza-Martiarena, Y.; Romero-Tapia, P.; Álvarez-Paucar, M.; Gálvez-Calla, L.; Calderón-Sánchez, J.; Bolaños-Cardenas, R.; Diaz-Sarabia, A. An 11-Year Retrospective Research Study of the Predictive Factors of Peri-Implantitis and Implant Failure: Analytic-Multicentric Study of 1279 Implants in Peru. Int. J. Dent. 2019, 2019, 3527872. [Google Scholar] [CrossRef]
- During, A.; Penel, G.; Hardouin, P. Understanding the local actions of lipids in bone physiology. Prog. Lipid Res. 2015, 59, 126–146. [Google Scholar] [CrossRef]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Brunetti, G.; Faienza, M.F.; Colucci, S.; Grano, M. Osteoporosis and obesity: Role of Wnt pathway in human and murine models. World J. Orthop. 2014, 5, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.J.; Kim, J.M.; Lee, S.Y.; Bae, M.A.; Ahn, J.H.; Han, D.C.; Kwon, B.M. PPARγ agonists induce adipocyte differentiation by modulating the expression of Lipin-1, which acts as a PPARγ phosphatase. Int. J. Biochem. Cell Biol. 2016, 81, 57–66. [Google Scholar] [CrossRef]
- Takada, I.; Kouzmenko, A.P.; Kato, S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Salmi, A.; Quacquarelli, F.; Chauveau, C.; Clabaut, A.; Broux, O. An integrative bioinformatics approach to decipher adipocyte-induced transdifferentiation of osteoblast. Genomics 2022, 114, 110422. [Google Scholar] [CrossRef]
- Hardouin, P.; Pansini, V.; Cortet, B. Bone marrow fat. Joint Bone Spine 2014, 81, 313–319. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089. [Google Scholar] [CrossRef]
- Muruganandan, S.; Ionescu, A.M.; Sinal, C.J. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. Int. J. Mol. Sci. 2020, 21, 2277. [Google Scholar] [CrossRef]
- Yang, D.; Wan, Y. Molecular determinants for the polarization of macrophage and osteoclast. Semin. Immunopathol. 2019, 41, 551–563. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Maglakelidze, N.; Galogre, A.; Tsagareli, Z. Functional-morphologic aspects of changes of mucosal gingiva microcirculatory bed vessels in experimental gingivitis against the background of hypercholesterolemia. Georgian Med. News 2005, 121, 71–74. [Google Scholar]
- Cutler, C.W.; Shinedling, E.A.; Nunn, M.; Jotwani, R.; Kim, B.O.; Nares, S.; Iacopino, A.M. Association between periodontitis and hyperlipidemia: Cause or effect? J. Periodontol. 1999, 70, 1429–1434. [Google Scholar] [CrossRef]
- Norata, G.D.; Grigore, L.; Raselli, S.; Redaelli, L.; Hamsten, A.; Maggi, F.; Eriksson, P.; Catapano, A.L. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: Molecular mechanisms and gene expression studies. Atherosclerosis 2007, 193, 321–327. [Google Scholar] [CrossRef]
- Chen, S.; Lin, G.; Lei, L.; You, X.; Wu, C.; Xu, W.; Huang, M.; Luo, L.; Wang, Z.; Li, Y.; et al. Hyperlipidemia modifies innate immune responses to lipopolysaccharide via the TLR-NF-κB signaling pathway. Inflammation 2013, 36, 968–976. [Google Scholar] [CrossRef]
- Wei, H.; Tarling, E.J.; McMillen, T.S.; Tang, C.; LeBoeuf, R.C. ABCG1 regulates mouse adipose tissue macrophage cholesterol levels and ratio of M1 to M2 cells in obesity and caloric restriction. J. Lipid Res. 2015, 56, 2337–2347. [Google Scholar] [CrossRef]
- Levy, R.I.; Morganroth, J.; Rifkind, B.M. Treatment of hyperlipidemia. N. Engl. J. Med. 1974, 290, 1295–1301. [Google Scholar] [CrossRef]
- Kane, J.P.; Malloy, M.J. Treatment of hyperlipidemia. Annu. Rev. Med. 1990, 41, 471–482. [Google Scholar] [CrossRef]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar]
- Ruan, F.; Zheng, Q.; Wang, J. Mechanisms of bone anabolism regulated by statins. Biosci. Rep. 2012, 32, 511–519. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A. Potential mechanisms and applications of statins on osteogenesis: Current modalities, conflicts and future directions. J. Control. Release 2015, 215, 12–24. [Google Scholar] [CrossRef]
- Meisel, P.; Kroemer, H.K.; Nauck, M.; Holtfreter, B.; Kocher, T. Tooth loss, periodontitis, and statins in a population-based follow-up study. J. Periodontol. 2014, 85, e160–e168. [Google Scholar] [CrossRef] [PubMed]
- Blais, J.E.; Ye, X.; Wan, E.Y.F.; Wong, W.C.W.; Wong, I.C.K.; Tomlinson, B.; Chan, E.W. Effectiveness of Simvastatin Versus Gemfibrozil for Primary Prevention of Cardiovascular Events: A Retrospective Cohort Study of 223,699 Primary Care Patients. Clin. Drug Investig. 2022, 42, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Curtis, A.J.; Ernst, M.E.; Ryan, J.; Zoungas, S.; Wolfe, R.; McNeil, J.J.; Murray, A.M.; Reid, C.M.; Chowdhury, E.K.; et al. Comparison of statins for primary prevention of cardiovascular disease and persistent physical disability in older adults. Eur. J. Clin. Pharmacol. 2022, 78, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Kim, S.T.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Yu, J.I.; Park, H.C.; Choi, D.H.; et al. A Phase II Study of Preoperative Chemoradiotherapy with Capecitabine Plus Simvastatin in Patients with Locally Advanced Rectal Cancer. Cancer Res. Treat. 2023, 55, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.K.; Royea, J.; Hamel, E. Simvastatin rescues memory and granule cell maturation through the Wnt/β-catenin signaling pathway in a mouse model of Alzheimer’s disease. Cell Death Dis. 2022, 13, 325. [Google Scholar] [CrossRef]
- Lv, J.; Xiu, P.; Tan, J.; Jia, Z.; Cai, H.; Liu, Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: Implantation of electron beam melting-fabricated porous Ti6Al4V scaffolds incorporating growth factor-doped fibrin glue. Biomed. Mater. 2015, 10, 035013. [Google Scholar] [CrossRef]
- Maeda, T.; Kawane, T.; Horiuchi, N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology 2003, 144, 681–692. [Google Scholar] [CrossRef]
- Liu, S.; Bertl, K.; Sun, H.; Liu, Z.H.; Andrukhov, O.; Rausch-Fan, X. Effect of simvastatin on the osteogenetic behavior of alveolar osteoblasts and periodontal ligament cells. Hum. Cell 2012, 25, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Okamura, A.; Koyano, K. Simvastatin promotes osteogenesis around titanium implants. Clin. Oral Implants Res. 2004, 15, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Ogino, Y.; Moriyama, Y.; Atsuta, I.; Jinno, Y.; Kihara, M.; Tsukiyama, Y.; Koyano, K. Simvastatin enhances bone formation around titanium implants in rat tibiae. J. Oral Rehabil. 2010, 37, 123–130. [Google Scholar] [CrossRef]
- Du, Z.; Chen, J.; Yan, F.; Xiao, Y. Effects of Simvastatin on bone healing around titanium implants in osteoporotic rats. Clin. Oral Implants Res. 2009, 20, 145–150. [Google Scholar] [CrossRef]
- Sun, T.; Xing, H.L.; Chen, Z.Z.; Tao, Z.S.; Li, J. Simvastatin reverses the harmful effects of high fat diet on titanium rod osseointegration in ovariectomized rats. J. Bone Miner. Metab. 2021, 39, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yang, N.; Fu, X.; Cui, Y.; Guo, Q.; Ma, T.; Yin, X.; Leng, H.; Song, C. Single-dose local simvastatin injection improves implant fixation via increased angiogenesis and bone formation in an ovariectomized rat model. Med. Sci. Monit. 2015, 21, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Ayukawa, Y.; Ogino, Y.; Atsuta, I.; Koyano, K. Topical application of statin affects bone healing around implants. Clin. Oral Implants Res. 2008, 19, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Ayukawa, Y.; Ogino, Y.; Atsuta, I.; Todo, M.; Takao, Y.; Koyano, K. Local application of fluvastatin improves peri-implant bone quantity and mechanical properties: A rodent study. Acta Biomater. 2010, 6, 1610–1618. [Google Scholar] [CrossRef]
- Masuzaki, T.; Ayukawa, Y.; Moriyama, Y.; Jinno, Y.; Atsuta, I.; Ogino, Y.; Koyano, K. The effect of a single remote injection of statin-impregnated poly (lactic-co-glycolic acid) microspheres on osteogenesis around titanium implants in rat tibia. Biomaterials 2010, 31, 3327–3334. [Google Scholar] [CrossRef]
- Ren, H.; Huo, F.; Wang, Z.; Liu, F.; Dong, X.; Wang, F.; Fan, X.; Yuan, M.; Jiang, X.; Lan, J. Sdccag3 Promotes Implant Osseointegration during Experimental Hyperlipidemia. J. Dent. Res. 2020, 99, 938–948. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Liu, F.; Xu, J.; Liu, Q.; Yin, K.; Lan, J. MicroRNA-29a-3p enhances dental implant osseointegration of hyperlipidemic rats via suppressing dishevelled 2 and frizzled 4. Cell. Biosci. 2018, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontology 2000 2019, 79, 178–189. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Nyan, M.; Hao, J.; Miyahara, T.; Noritake, K.; Rodriguez, R.; Kasugai, S. Accelerated and enhanced bone formation on novel simvastatin-loaded porous titanium oxide surfaces. Clin. Implant Dent. Relat. Res. 2014, 16, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.S.; Frank, M.J.; Rubert, M.; Monjo, M.; Lyngstadaas, S.P.; Haugen, H.J. Simvastatin-activated implant surface promotes osteoblast differentiation in vitro. J. Biomater. Appl. 2014, 28, 897–908. [Google Scholar] [CrossRef]
- Yang, G.; Song, L.; Guo, C.; Zhao, S.; Liu, L.; He, F. Bone responses to simvastatin-loaded porous implant surfaces in an ovariectomized model. Int. J. Oral Maxillofac. Implants 2012, 27, 369–374. [Google Scholar]
- Lopez-Alvarez, M.; Lopez-Puente, V.; Rodriguez-Valencia, C.; Angelome, P.C.; Liz-Marzan, L.M.; Serra, J.; Pastoriza-Santos, I.; Gonzalez, P. Osteogenic effects of simvastatin-loaded mesoporous titania thin films. Biomed. Mater. 2018, 13, 025017. [Google Scholar] [CrossRef]
- Zhao, S.; Wen, F.; He, F.; Liu, L.; Yang, G. In vitro and in vivo evaluation of the osteogenic ability of implant surfaces with a local delivery of simvastatin. Int. J. Oral Maxillofac. Implants 2014, 29, 211–220. [Google Scholar] [CrossRef]
- Pullisaar, H.; Tiainen, H.; Landin, M.A.; Lyngstadaas, S.P.; Haugen, H.J.; Reseland, J.E.; Østrup, E. Enhanced in vitro osteoblast differentiation on TiO2 scaffold coated with alginate hydrogel containing simvastatin. J. Tissue Eng. 2013, 4, 2041731413515670. [Google Scholar] [CrossRef]
- Fang, W.; Zhao, S.; He, F.; Liu, L.; Yang, G. Influence of simvastatin-loaded implants on osseointegration in an ovariectomized animal model. Biomed. Res. Int. 2015, 2015, 831504. [Google Scholar] [CrossRef]
- Salomo-Coll, O.; Mate-Sanchez de Val, J.E.; Ramirez-Fernandez, M.P.; Hernandez-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Topical applications of vitamin D on implant surface for bone-to-implant contact enhance: A pilot study in dogs part II. Clin. Oral Implants Res. 2016, 27, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Lin, A.; Wang, C.J.; Park, S.; Nishimura, I. Vitamin D and bone physiology: Demonstration of vitamin D deficiency in an implant osseointegration rat model. J. Prosthodont. 2009, 18, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ochiai-Shino, H.; Onodera, S.; Saito, A.; Shibahara, T.; Azuma, T. Promoting effect of 1,25(OH)2 vitamin D3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte-like cells. Open Biol. 2015, 5, 140201. [Google Scholar] [CrossRef]
- Sakai, S.; Takaishi, H.; Matsuzaki, K.; Kaneko, H.; Furukawa, M.; Miyauchi, Y.; Shiraishi, A.; Saito, K.; Tanaka, A.; Taniguchi, T.; et al. 1-Alpha, 25-dihydroxy vitamin D3 inhibits osteoclastogenesis through IFN-beta-dependent NFATc1 suppression. J. Bone Miner. Metab. 2009, 27, 643–652. [Google Scholar] [CrossRef]
- Shiraishi, A.; Takeda, S.; Masaki, T.; Higuchi, Y.; Uchiyama, Y.; Kubodera, N.; Sato, K.; Ikeda, K.; Nakamura, T.; Matsumoto, T.; et al. Alfacalcidol inhibits bone resorption and stimulates formation in an ovariectomized rat model of osteoporosis: Distinct actions from estrogen. J. Bone Miner. Res. 2000, 15, 770–779. [Google Scholar] [CrossRef]
- Tsurukami, H.; Nakamura, T.; Suzuki, K.; Sato, K.; Higuchi, Y.; Nishii, Y. A novel synthetic vitamin D analogue, 2 beta-(3-hydroxypropoxy)1 alpha, 25-dihydroxyvitamin D3 (ED-71), increases bone mass by stimulating the bone formation in normal and ovariectomized rats. Calcif. Tissue Int. 1994, 54, 142–149. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Luu, W.; Li, D.; Sharpe, L.J.; Brown, A.J. DHCR7: A vital enzyme switch between cholesterol and vitamin D production. Prog. Lipid Res. 2016, 64, 138–151. [Google Scholar] [CrossRef]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Mansour, G.; Al Ashwah, A.; Koura, A. Evaluation of simvastatin grafting around immediate dental implants in dogs. Implant Dent. 2014, 23, 195–199. [Google Scholar] [CrossRef]
- Wang, C.Z.; Wang, Y.H.; Lin, C.W.; Lee, T.C.; Fu, Y.C.; Ho, M.L.; Wang, C.K. Combination of a Bioceramic Scaffold and Simvastatin Nanoparticles as a Synthetic Alternative to Autologous Bone Grafting. Int. J. Mol. Sci 2018, 19, 4099. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.Y.; Zhang, S.Y.; Feng, L.; Ren, J. Effects of simvastatin gel on bone regeneration in alveolar defects in miniature pigs. Chin. Med. J. 2011, 124, 3953–3958. [Google Scholar] [PubMed]
- Jin, H.; Ji, Y.; Cui, Y.; Xu, L.; Liu, H.; Wang, J. Simvastatin-Incorporated Drug Delivery Systems for Bone Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 2177–2191. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Bae, M.S.; Qiao, L.; Heo, D.N.; Lee, J.B.; Lee, W.J.; Park, J.H.; Lee, D.-W.; Hwang, Y.-S.; Kwon, I.K. In vitro evaluation of simvastatin acid (SVA) coated beta-tricalcium phosphate (β-TCP) particle on bone tissue regeneration. Macromol. Res. 2012, 20, 754–761. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, L.-Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.-W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. Part A 2015, 103, 3580–3589. [Google Scholar] [CrossRef]
- Delan, W.K.; Zakaria, M.; Elsaadany, B.; ElMeshad, A.N.; Mamdouh, W.; Fares, A.R. Formulation of simvastatin chitosan nanoparticles for controlled delivery in bone regeneration: Optimization using Box-Behnken design, stability and in vivo study. Int. J. Pharm. 2020, 577, 119038. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, H.Z.; Zhang, Z.Y. 3D printed poly(ε-caprolactone) scaffolds function with simvastatin-loaded poly(lactic-co-glycolic acid) microspheres to repair load-bearing segmental bone defects. Exp. Ther. Med. 2019, 17, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, J.E.; Balikov, D.A.; Bae, M.S.; Heo, D.N.; Lee, D.; Rim, H.J.; Lee, D.-W.; Sung, H.-J.; Kwon, I.K. Poly(l-Lactic Acid)/Gelatin Fibrous Scaffold Loaded with Simvastatin/Beta-Cyclodextrin-Modified Hydroxyapatite Inclusion Complex for Bone Tissue Regeneration. Macromol. Biosci. 2016, 16, 1027–1038. [Google Scholar] [CrossRef]
- Park, Y.S.; David, A.E.; Park, K.M.; Lin, C.-Y.; Than, K.D.; Lee, K.; Park, J.B.; Jo, I.; Park, K.D.; Yang, V.C. Controlled Release of Simvastatin from In situ Forming Hydrogel Triggers Bone Formation in MC3T3-E1 Cells. AAPS J. 2013, 15, 367–376. [Google Scholar] [CrossRef]
- Tanigo, T.; Takaoka, R.; Tabata, Y. Sustained release of water-insoluble simvastatin from biodegradable hydrogel augments bone regeneration. J. Control. Release 2010, 143, 201–206. [Google Scholar] [CrossRef]
- Sukul, M.; Min, Y.-K.; Lee, S.-Y.; Lee, B.-T. Osteogenic potential of simvastatin loaded gelatin-nanofibrillar cellulose-β tricalcium phosphate hydrogel scaffold in critical-sized rat calvarial defect. Eur. Polym. J. 2015, 73, 308–323. [Google Scholar] [CrossRef]
- Bae, M.S.; Yang, D.H.; Lee, J.B.; Heo, D.N.; Kwon, Y.-D.; Youn, I.C.; Choi, K.; Hong, J.H.; Kim, G.T.; Choi, Y.S.; et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials 2011, 32, 8161–8171. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Chen, C.; Aghaloo, T.; Lee, M. Co-delivery of simvastatin and demineralized bone matrix hierarchically from nanosheet-based supramolecular hydrogels for osteogenesis. J. Mater. Chem. B 2021, 9, 7741–7750. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, Y.; Kuang, Y.; Xu, S.; Lu, Q.; Diao, J.; Zhao, N. Hierarchically porous calcium-silicon nanosphere-enabled co-delivery of microRNA-210 and simvastatin for bone regeneration. J. Mater. Chem. B 2021, 9, 3573–3583. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, K.; Hu, T.; Wang, S.; Xu, H.; Zhang, S.; Liu, S.; Xie, Y.; Zheng, X. Titania nanotube array supported nanoceria with redox cycling stability ameliorates oxidative stress-inhibited osteogenesis. Chem. Eng. J. 2021, 415, 128913. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Li, Y.; Wang, S.; Yang, M. The effects of Ce on the proliferation, osteogenic differentiation and mineralization function of MC3T3-E1 cells in vitro. Biol. Trace Elem. Res. 2012, 149, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Zhang, J.C.; Zhang, Q.; Wang, S.X.; Yang, M.S. TGF-β/BMP signaling pathway is involved in cerium-promoted osteogenic differentiation of mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 1105–1114. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K.W.K.; Chu, P.K. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv. Sci. 2018, 5, 1700678. [Google Scholar] [CrossRef]
- Parra-Robert, M.; Zeng, M.; Shu, Y.; Fernández-Varo, G.; Perramón, M.; Desai, D.; Chen, J.; Guo, D.; Zhang, X.; Morales-Ruiz, M.; et al. Mesoporous silica coated CeO(2) nanozymes with combined lipid-lowering and antioxidant activity induce long-term improvement of the metabolic profile in obese Zucker rats. Nanoscale 2021, 13, 8452–8466. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zippl, A.L.; Seeber, B.; Wildt, L. Obesity and infertility: Are hyperlipidemia and hyperinsulinemia the bad guys? Fertil. Steril. 2021, 116, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Defesche, J.C.; Gidding, S.S.; Harada-Shiba, M.; Hegele, R.A.; Santos, R.D.; Wierzbicki, A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Prim. 2017, 3, 17093. [Google Scholar] [CrossRef] [PubMed]
- Poothullil, J.M. Obesity, hyperlipidemia and non-insulin-dependent diabetes: A unified theory. Neurosci. Biobehav. Rev. 1993, 17, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Monteiro, J.; Pellizzer, E.P.; Araújo Lemos, C.A.; de Moraes, S.L.D.; do Egito Vasconcelos, B.C. Is there an association between overweight/obesity and dental implant complications? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 1241–1249. [Google Scholar] [CrossRef]
- Tirone, F.; Salzano, S.; D’Orsi, L.; Paola, P.; Rodi, D. Is a high level of total cholesterol a risk factor for dental implants or bone grafting failure? A retrospective cohort study on 227 patients. Eur. J. Oral Implantol. 2016, 9, 77–84. [Google Scholar]
- Zang, H.; Yang, W.; Tian, X. Simvastatin in the Treatment of Colorectal Cancer: A Review. Evid. Based Complement. Altern. Med. 2022, 2022, 3827933. [Google Scholar] [CrossRef]
- Schulze-Späte, U.; Dietrich, T.; Wu, C.; Wang, K.; Hasturk, H.; Dibart, S. Systemic vitamin D supplementation and local bone formation after maxillary sinus augmentation—A randomized, double-blind, placebo-controlled clinical investigation. Clin. Oral Implants Res. 2016, 27, 701–706. [Google Scholar] [CrossRef]
| No. | Citation | Authors | Carriers | Bioactive Molecules or Drugs | In Vitro | In Vivo | Under Hyperlipidemia Condition |
|---|---|---|---|---|---|---|---|
| 1 | [56] | Tan, Jie et al. | No | simvastatin | No | Yes | No |
| 2 | [57] | Moriyama, Yasuko et al. | PGA gel | fluvastatin | No | Yes | No |
| 3 | [58] | Moriyama, Yasuko et al. | PGA gel | fluvastatin | No | Yes | No |
| 4 | [59] | Masuzaki, Tomohiro et al. | PLGA microspheres | fluvastatin | No | Yes | No |
| 5 | [60] | Ren, H et al. | No | Sdccag3-enhancer | Yes | Yes | Yes |
| 6 | [60] | Ren, H et al. | No | lncRNA-MSTRG.97162.4- enhancer | Yes | Yes | Yes |
| 7 | [60] | Ren, H et al. | No | miR-193a-3p-inhibitor | Yes | Yes | Yes |
| 8 | [61] | Liu, Fei et al. | No | miR-29a-3p- enhancer | Yes | Yes | Yes |
| No. | Citation | Authors | Carriers | Bioactive Molecules or Drugs | In Vitro | In Vivo | Applied to Dental Implant |
|---|---|---|---|---|---|---|---|
| 1 | [64] | Nyan, Myat et al. | porous titanium oxide | simvastatin | No | Yes | Yes |
| 2 | [65] | Walter, Martin Sebastian et al. | / | simvastatin | No | Yes | Yes |
| 3 | [66] | Yang, Guoli et al. | / | simvastatin | No | Yes | Yes |
| 4 | [67] | López-Álvarez, Miriam et al. | mesoporous titanium oxide | simvastatin | Yes | No | Yes |
| 5 | [68] | Zhao, Shifang et al. | calcium phosphate | simvastatin | Yes | Yes | Yes |
| 6 | [69] | Pullisaar, Helen et al. | porous titanium oxide+alginate hydrogel | simvastatin | Yes | No | Yes |
| 7 | [70] | Fang, Wen et al. | nanohydroxyapatite | simvastatin | No | Yes | Yes |
| 8 | [71] | Salomó-Coll, Oscar et al. | / | vitamin D | No | Yes | Yes |
| No. | Citation | Authors | Carriers | Bioactive Molecules or Drugs | In Vitro | In Vivo |
|---|---|---|---|---|---|---|
| 1 | [83] | Yang, Dae Hyeok, et al. | β-tricalcium phosphate | simvastatin acid | Yes | No |
| 2 | [84] | Yan, Qi et al. | PLGA-PEG-PLGA hydrogel | simvastatin | Yes | Yes |
| 3 | [85] | Delan, Wisam Khalaf et al. | chitosan+tripolyphosphate nanoparticles | simvastatin | No | Yes |
| 4 | [86] | Zhang, Zhan-Zhao et al. | PLGA+collagen | simvastatin | Yes | Yes |
| 5 | [87] | Lee, Jung Bok et al. | PLA+gelatin+β-cyclodextrin+hydroxyapatite | simvastatin | Yes | Yes |
| 6 | [88] | Park, Yoon Shin et al. | gelatin-PEG-tyramine hydrogel | simvastatin | Yes | No |
| 7 | [89] | Tanigo, Tomomi et al. | gelatin+L-lactic acid oligomer | simvastatin | Yes | Yes |
| 8 | [90] | Sukul, Mousumi, et al. | gelatin+β-tricalcium phosphate hydrogel | simvastatin | Yes | Yes |
| 9 | [91] | Bae, Min Soo et al. | hyaluronic acid hydrogel | simvastatin | Yes | Yes |
| 10 | [92] | Zhang, Xiao et al. | LAPONITE® hydrogel | simvastatin | Yes | Yes |
| 11 | [93] | Liu, Junjie et al. | calcium–silicon nanospheres | microRNA-210+simvastatin | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Meng, S.; Chen, J.; Wan, Q. Effects of Hyperlipidemia on Osseointegration of Dental Implants and Its Strategies. J. Funct. Biomater. 2023, 14, 194. https://doi.org/10.3390/jfb14040194
Sun H, Meng S, Chen J, Wan Q. Effects of Hyperlipidemia on Osseointegration of Dental Implants and Its Strategies. Journal of Functional Biomaterials. 2023; 14(4):194. https://doi.org/10.3390/jfb14040194
Chicago/Turabian StyleSun, Haiyang, Shuhuai Meng, Junyu Chen, and Qianbing Wan. 2023. "Effects of Hyperlipidemia on Osseointegration of Dental Implants and Its Strategies" Journal of Functional Biomaterials 14, no. 4: 194. https://doi.org/10.3390/jfb14040194
APA StyleSun, H., Meng, S., Chen, J., & Wan, Q. (2023). Effects of Hyperlipidemia on Osseointegration of Dental Implants and Its Strategies. Journal of Functional Biomaterials, 14(4), 194. https://doi.org/10.3390/jfb14040194







