An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications
Abstract
:1. Introduction
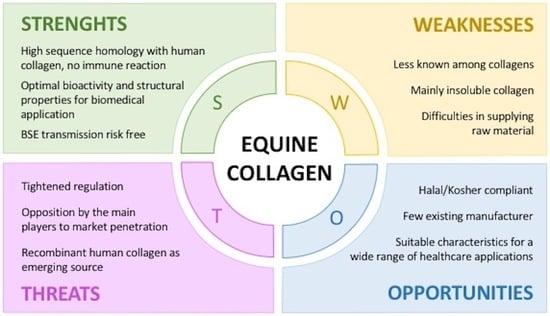
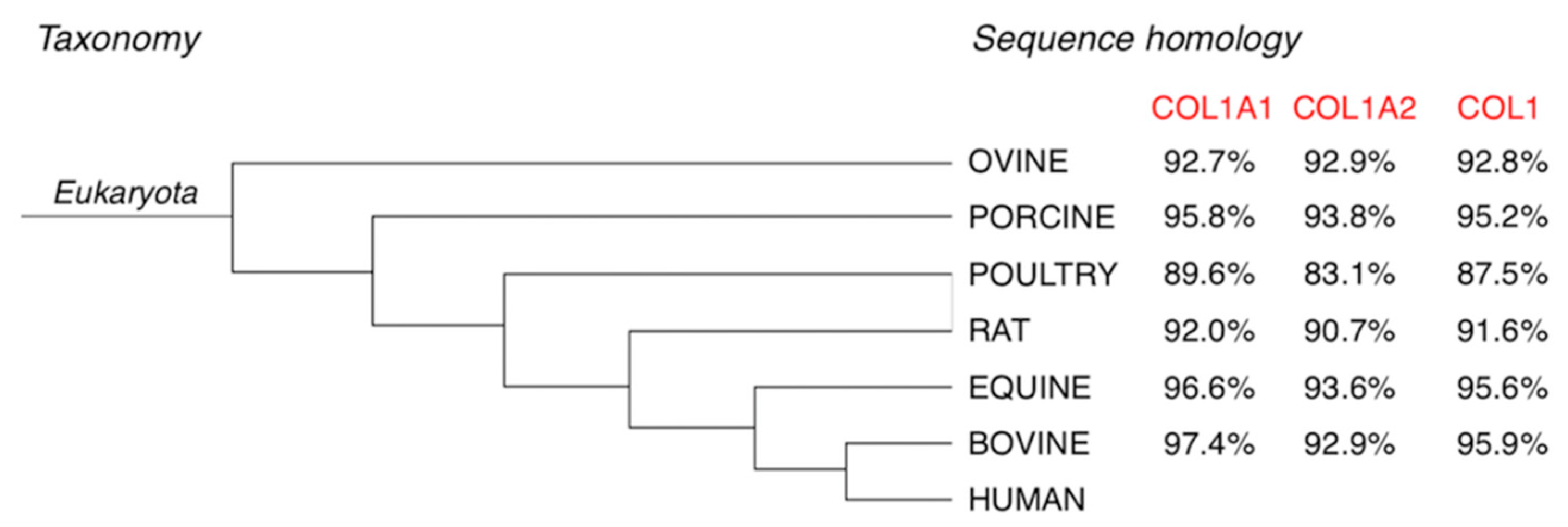
2. Why Equine Collagen
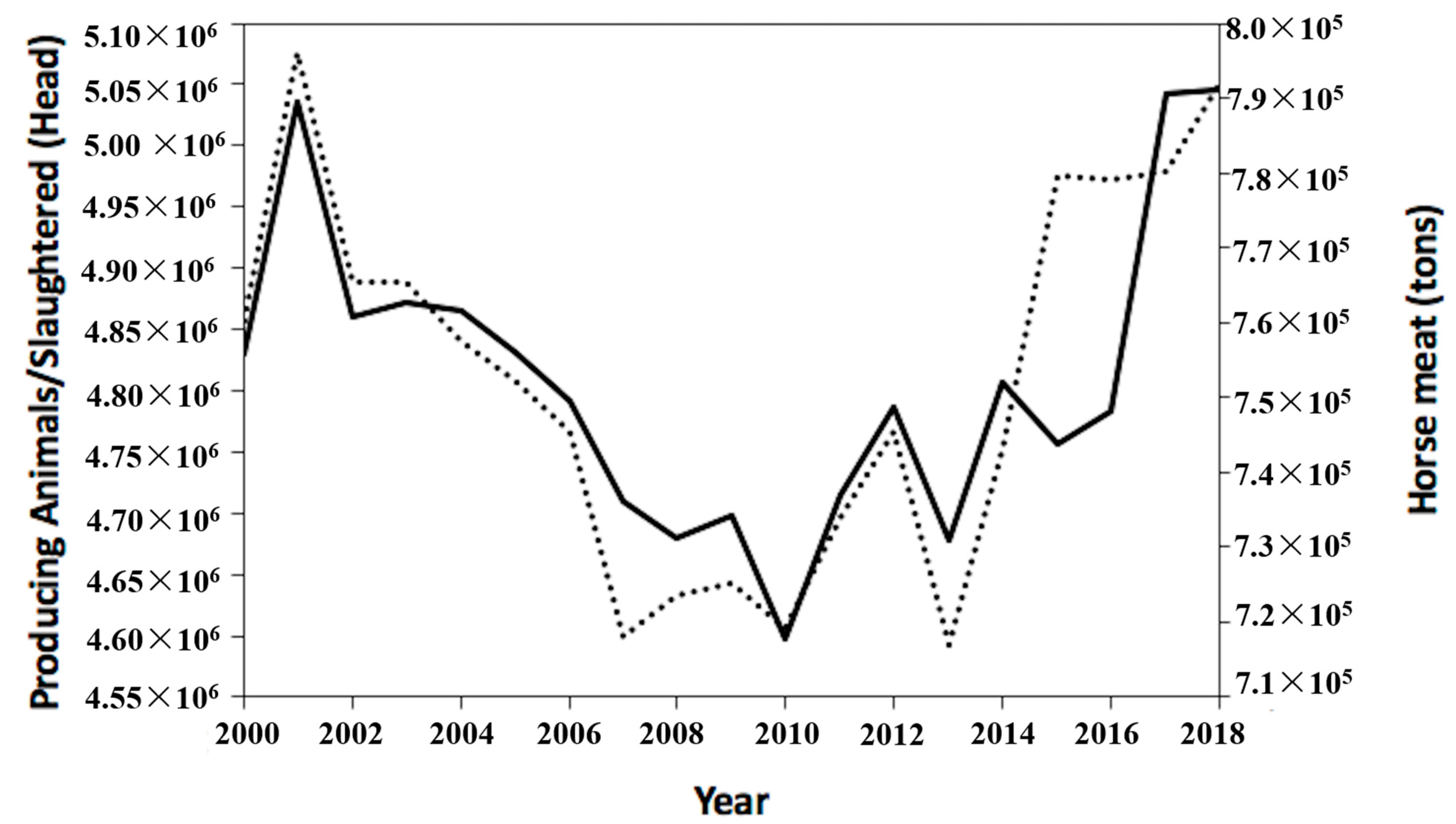
3. Equine By-Products Accessibility
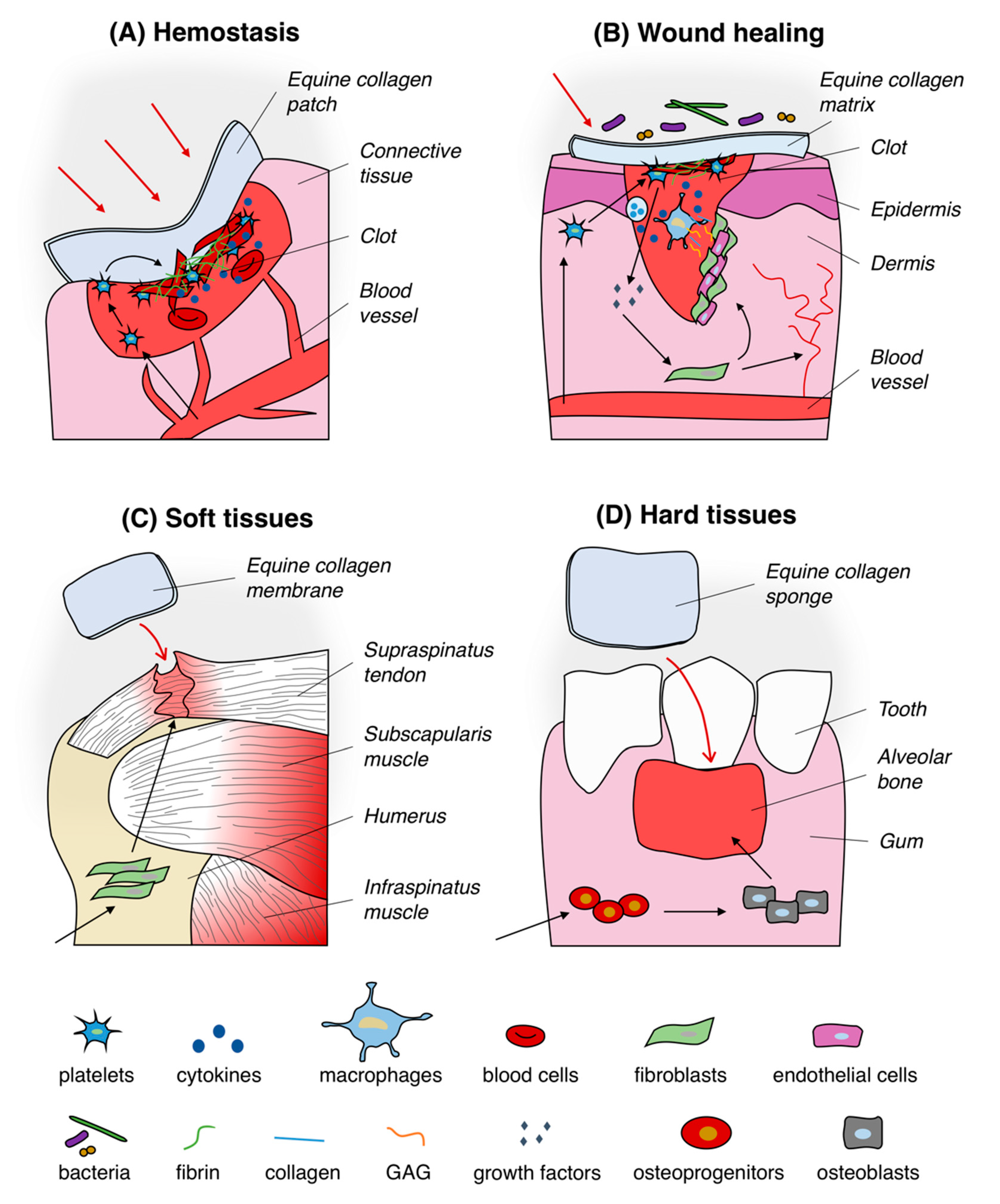
4. Currently Approved Equine Collagen-Based Devices
4.1. Hemostatic System
4.2. Healing of Wounds
4.3. Soft Tissues Regeneration
4.4. Hard Tissues Regeneration
5. Equine Collagen-Based Device Market
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.; Dwivedi, S.; Chandra, S.; Srivastava, A.; Vijay, P. Collagen: A brief analysis. J. Oral Maxillofac. Pathol. 2019, 10, 11–17. [Google Scholar] [CrossRef]
- Nimni, M.E.; Harkness, R.D. Molecular structures and functions of collagen. In Collagen; Nimni, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 1–78. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Ann. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornstein, P.; Sage, H. Structurally distinct collagen types. Ann. Rev. Biochem. 1980, 49, 957–1003. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astbury, W.T. X-ray adventures among the proteins. Trans. Faraday Soc. 1938, 34, 378–388. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F.H.C. The structure of collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Kartha, G. Structure of collagen. Nature 1955, 176, 593–595. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [Green Version]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens—An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Privalov, P.L.; Tiktopulo, E.I.; Tischekno, V.M. Stability and mobility of the collagen structure. J. Mol. Biol. 1979, 127, 203–216. [Google Scholar] [CrossRef]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef] [PubMed]
- Ignat’eva, N.Y.; Danilov, N.A.; Averkiev, S.V.; Obrezkova, M.V.; Lunin, V.V.; Sobol’, E.N. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. Sci. J. Anal. Chem. 2007, 62, 51–57. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. gelatine-based biomaterials and their biocompatibility: Review and perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Makareeva, E.; Leikin, S. Collagen Structure, Folding and Function. In Osteogenesis Imperfecta—A Translational Approach to Brittle Bone Disease; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Exposito, J.-Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The fibrillar collagen family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stover, D.A.; Verrelli, B.C. Comparative vertebrate evolutionary analyses of type i collagen: Potential of COL1a1 gene structure and intron variation for common bone-related diseases. Mol. Biol. Evol. 2011, 28, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [Green Version]
- Davidenko, N.; Hamaia, S.; Bax, D.V.; Malcor, J.D.; Schuster, C.F.; Gullberg, D.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Selecting the correct cellular model for assessing of the biological response of collagen-based biomaterials. Acta Biomater. 2018, 65, 88–101. [Google Scholar] [CrossRef]
- Suesca, E.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C.; Fontanilla, M.R. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 333–341. [Google Scholar] [CrossRef]
- Rhee, S.; Grinnell, F. Fibroblast mechanics in 3D collagen matrices. Adv. Drug. Deliv. Rev. 2007, 59, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Friess, W. Collagen—biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larghezza Masci, V.; Taddei, A.R.; Gambellini, G.; Giorgi, F.; Fausto, A.M. Ultrastructural investigation on fibroblast interaction with collagen scaffold. J. Biomed. Mater. Res. Part A 2016, 104A, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Croce, M.; Silvestri, C.; Guerra, D.; Carnevali, E.; Boraldi, F.; Tiozzo, R. Adhesion and proliferation of human dermal fibroblasts on collagen matrix. J. Biom. Appl. 2004, 18, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Lauer-Fields, J.L.; Juska, D.; Fields, G.B. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002, 66, 19–32. [Google Scholar] [CrossRef]
- Ohuchi, E.; Imai, K.; Fujii, Y.; Sato, H.; Seiki, M.; Okada, Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997, 272, 2446–2451. [Google Scholar] [CrossRef] [Green Version]
- Bohn, G.; Liden, B.; Schultz, G.; Yang, Q.; Gibson, D.J. Ovine-based collagen matrix dressing: Next-generation collagen dressing for wound care. Adv. Wound Care 2016, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chvapil, M. Collagen sponge: Theory and practice of medical applications. J. Biomed. Mater. Res. 1977, 11, 721–741. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Avila Rodriguez, M.I.; Rodriguez Barroso, L.G.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Bozec, L.; Knowles, J.C.; Syed, O.; Mudera, V.; Day, R.; Hyun, J.K. Collagen—Emerging collagen based therapies hit the patient. Adv. Drug. Deliv. Rev. 2013, 65, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Tampieri, A.; Salvatore, L.; Sannino, A.; Hee, J.; Ghiron, L.; Condorelli, G. Collagen based scaffold for biomedical applications. J. Biotechnol. 2010, 150. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Sparavigna, A.; Tateo, A.; Inselvini, E.; Tocchio, M.; Orlandini, M.C.; Botali, G. Anti-age activity and tolerance evaluation of collagen micro-injection treatment associated to topical application of a cosmetic formulation. J. Clin. Exp. Dermatol. Res. 2017, 8, 1000391. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Naing, M.W. Production of recombinant collagen: State of the art and challenges. J. Biol. Eng. 2017, 1, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Yulianti, D.H.; Rukmana, T.I. Isolation, purification, and characterization of bovine tendon collagen and analysis of glycine, proline, and hydroxyproline by high-performance liquid chromatography-fluorescence. Int. J. Appl. Pharm. 2018, 10, 311–315. [Google Scholar] [CrossRef]
- Gorlov, I.F.; Titov, E.I.; Semenov, G.V.; Slozhenkina, M.I.; Sokolov, A.Y.; Omarov, R.S.; Goncharov, A.I.; Zlobina, E.Y.; Litvinova, E.V.; Karpenko, E.V. Collagen from porcine skin: A method of extraction and structural properties. Int. J. Food Prop. 2018, 21, 1031–1042. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 1. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, E.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An insight on type I collagen from horse tendon for the manufacture of implantable devices. Int. J. Biol. Macromol. 2020, 154, 291–306. [Google Scholar] [CrossRef]
- Rittié, L. Type I collagen purification from rat tail tendons. Methods Mol. Biol. 2017, 1627, 287–308. [Google Scholar] [CrossRef] [PubMed]
- AraÚJo, Í.B.D.S.; Bezerra, T.K.A.; Nascimento, E.S.d.; Gadelha, C.A.d.A.; Santi-Gadelha, T.; Madruga, M.S. Optimal conditions for obtaining collagen from chicken feet and its characterization. Food Sci. Technol. 2018, 38, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef] [PubMed]
- Grønlien, K.G.; Pedersen, M.E.; Sanden, K.W.; Høst, V.; Karlsen, J.; Tønnesen, H.H. Collagen from Turkey (Meleagris gallopavo) tendon: A promising sustainable biomaterial for pharmaceutical use. Sustain. Chem. Pharm. 2019, 100166. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Stephens, C.B.; Bertran, K.; Swayne, D.E.; Spackman, E. The pathogenesis of H7N8 low and highly pathogenic avian influenza viruses from the United States 2016 outbreak in chickens, turkeys and mallards. PLoS ONE 2017, 12, e0177265. [Google Scholar] [CrossRef]
- Schofield, J.D.; Freeman, I.L.; Jackson, D.S. The isolation, and amino acid and carbohydrate composition, of polymeric collagens prepared from various human tissues. Biochem. J. 1971, 124, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; de Wet, W.; Bernard, M.; Ding, J.; Morabito, M.; Myers, J.; Williams, C.; Ramirez, F. Human proα1(I) collagen gene structure reveals evolutionary conservation of a pattern of introns and exons. Nature 1984, 310, 337–340. [Google Scholar] [CrossRef]
- Exposito, J.-Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of Collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef]
- Ramshaw, J.A. Biomedical applications of collagens. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Ghodbane, S.A.; Dunn, M.G. Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J. Biomed. Mater. Res. A 2016, 104, 2685–2692. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, P.; Parma, B. Immunological safety evaluation of a horse collagen haemostatic pad. Arzneimittelforschung 2001, 51, 414–419. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Screen, H.R.C. Tendon structure and composition. Adv. Exp. Med. Biol. 2016, 920, 3–10. [Google Scholar] [CrossRef]
- Franchi, M.; Trire, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen structure of tendon relates to function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and supramolecular X-ray characterization of engineered tissues from equine tendon, bovine dermis, and fish skin type-I collagen. Macromol. Biosci. 2020, 2000017. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J. Equine Xenografts for Reconstructive Surgery; The Podiatry Institute: Decatur, GA, USA, 2012; Volume 49. [Google Scholar]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [Green Version]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W. Modelling methods for in vitro biomechanical properties of the skin: A review. Biomed. Eng. Lett. 2015, 5, 241–250. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Campa, L.; Natali, M.L.; Salvatore, L.; Madaghiele, M.; De Caro, L.; et al. Investigations of processing-induced structural changes in horse type-I collagen at sub and supramolecular levels. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Office of the Chief Economist; World Agricultural Outlook Board. U.S. Department of Agriculture USDA Agricultural Projections to 2025. Available online: https://www.ers.usda.gov/webdocs/publications/37809/56729_oce-2016-1.pdf?v=0 (accessed on 12 March 2020).
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Delgado, L.M.; Shologu, N.; Fuller, K.; Zeugolis, D.I. Acetic acid and pepsin result in high yield, high purity and low macrophage response collagen for biomedical applications. Biomed. Mater. 2017, 12, 065009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingsworth, L.R.; De Lustro, F.; Brennan, J.E.; Sawamura, S.; Mc Pherson, J. The human immune response to reconstituted bovine collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a bovine collagen implant—Clinical and immunological study in 705 patients. J. Am. Acad. Dermatol. 1989, 21, 1203–1208. [Google Scholar] [CrossRef]
- Lucey, P.; Goldberg, D.J. Complications of collagen fillers. Facial Plast. Surg. 2014, 30, 615–622. [Google Scholar] [CrossRef]
- Economic Impacts of Feed-Related Regulatory Responses to Bovine Spongiform Encephalopathy. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiBjOvpi_jrAhWC_KQKHcUNBDAQFjABegQIBRAB&url=https%3A%2F%2Fwww.ers.usda.gov%2Fwebdocs%2Foutlooks%2F37404%2F11780_ldpm17001.pdf%3Fv%3D9127.5&usg=AOvVaw0JaunsaXpe44ih6d_sVTCe (accessed on 18 July 2020).
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, S.; Maiti, T.K. Caprine (goat) collagen: A potential biomaterial for skin tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 23, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Collagen Business Case Report. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj-ro_TjPjrAhVDDuwKHe2cA64QFjABegQIAhAB&url=https%3A%2F%2Fwww.mla.com.au%2Fdownload%2Ffinalreports%3FitemId%3D3811&usg=AOvVaw36Mod_yBSWK0Z5EK3jRqHM (accessed on 22 June 2020).
- Adelmann-Grill, B.C.; Otto, K. Immunological safety evaluation of a haemostatic agent and wound dressing made of horse collagen fibrils. Arzneimittelforschung 1987, 37, 802–805. [Google Scholar] [PubMed]
- Abdal-Hameed, I.A. Clinical and radiographical assessment of topical application of collagen fibrils on tooth socket healing. Al–Rafidain Dent. J. 2014, 14, 244–251. [Google Scholar] [CrossRef]
- Steel, K.E.; Twenhafel, N.A. Review paper: Pathology of animal models of alphavirus encephalitis. Vet. Pathol. 2010, 47, 790–805. [Google Scholar] [CrossRef]
- Wong, K.T. Alphaviral Equine Encephalomyelitis (Eastern, Western, and Venezuelan). In Infections of the Central Nervous System, 1st ed.; Chrétien, F., Wong, K.T., Sharer, L.R., Keohane, C., Gray, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Brown, E.M.; Farrell, H.M.; Wildermuth, R.J. Influence of neutral salts on the hydrothermal stability of acid-soluble collagen. J. Protein Chem. 2000, 19, 85–92. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S.; Foresti, E.; Parma, B.; Rubini, K.; Sidoti, M.C.; Roveri, N. Films of self-assembled purely helical type I collagen molecules. J. Mater. Chem. 2004, 14. [Google Scholar] [CrossRef]
- Böhm, S.; Strauß, C.; Stoiber, S.; Kasper, C.; Charwat, V. Impact of source and manufacturing of collagen matrices on fibroblast cell growth and platelet aggregation. Materials 2017, 10, 1086. [Google Scholar] [CrossRef] [Green Version]
- Belaunzaran, X.; Bessa, R.J.B.; Lavín, P.; Mantecón, A.R.; Kramer, J.K.G.; Aldai, N. Horse-meat for human consumption—Current research and future opportunities. Meat Sci. 2015, 108, 74–81. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Bastianello Campagnol, P.C.; Zhu, Z.; Alpas, H.; Barba, F.J.; Tomasevic, I. Technological aspects of horse meat products—A review. Food Res. Int. 2017, 102, 176–183. [Google Scholar] [CrossRef]
- Balji, Y.; Knicky, M.; Zamaratskaia, G. Perspectives and safety of horsemeat consumption. Int. J. Food Sci. Technol. 2020, 55, 942–952. [Google Scholar] [CrossRef]
- Carmichael, D.J.; Lawrie, R.A. Bovine collagen. I. Changes in collagen solubility with animal age. Int. J. Food Sci. Technol. 1967, 2, 299–311. [Google Scholar] [CrossRef]
- Gibson, T. Evolution of catgut ligatures: The endeavours and success of Joseph Lister and William Macewen. Br. J. Surg. 1990, 77, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Pullinger, B.; Pirie, A. Chronic inflammation due to implanted collagen. J. Pathol. Bacter. 1942, 54, 341–344. [Google Scholar] [CrossRef]
- Knapp, T.R.; Kaplan, E.N.; Daniels, J.R. Injectable collagen for soft tissue augmentation. Plast. Reconstr. Surg. 1977, 60, 398–405. [Google Scholar]
- Sezer, A.D.; Cevher, E. Biopolymers as wound healing materials: Challenges and new strategies. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: London, UK, 2011; pp. 383–414. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef]
- George, B. Overview of current regulations governing medical devices. Int. J. Drug Regul. Aff. 2019, 7, 62–66. [Google Scholar] [CrossRef]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2009, 20, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.D.O.M.D.; Lopes, A.M.; Mazzola, P.G.; Yagui, C.D.O.R.; Penna, T.C.V.; Pessoa, J.A. Methods of endotoxin removal from biological preparations: A review. J. Pharm. Pharm. Sci. 2007, 10, 388–404. [Google Scholar]
- Salvatore, L.; Madaghiele, M.; Parisi, C.; Gatti, F.; Sannino, A. Crosslinking of micropatterned collagen-based nerve guides to modulate the expected half-life. J. Biomed. Mater. Res. A 2014, 102, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Calo, E.; Salvatore, L.; Bonfrate, V.; Pedone, D.; Frigione, M.; Sannino, A. Assessment of collagen crosslinking and denaturation for the design of regenerative scaffolds. J. Biomed. Mater. Res. A 2016, 104, 186–194. [Google Scholar] [CrossRef]
- Sandor, M.; Xu, H.; Connor, J.; Lombardi, J.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng. Part A 2008, 14, 2021–2031. [Google Scholar] [CrossRef]
- Maternini, M.; Guttadauro, A.; Mascagni, D.; Milito, G.; Stuto, A.; Renzi, A.; Ripamonti, L.; Bottini, C.; Nudo, R.; Del Re, L.; et al. Non cross-linked equine collagen (Salvecoll-E gel) for treatment of complex ano-rectal fistula. Asian J. Surg. 2019, 43, 401–404. [Google Scholar] [CrossRef]
- Monaco, G.; Cholas, R.; Salvatore, L.; Madaghiele, M.; Sannino, A. Sterilization of collagen scaffolds designed for peripheral nerve regeneration: Effect on microstructure, degradation and cellular colonization. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Stupin, V.A.; Gabitov, R.B.; Sinelnikova, T.G.; Silina, E.V. Biological mechanisms of chronic wound and diabetic foot healing: The role of collagen. Ser. J. Exp. Clin. Res. 2018, 19, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Collagen T-Barrier Membrane. Available online: https://www.bebdental.it/pro/en/bone-regeneration/ (accessed on 24 October 2020).
- Buonomo, O.C.; Piccirillo, R.; Caramanica, A.; Andrich, R.; Tirelli, C.; La Pinta, M.; Scardamaglia, F. The use of an equine collagen fleece (Gentafleece®) in T1 breast cancer surgery: Preliminary results. Cancer Res. 2009, 69, 4147–4150. [Google Scholar] [CrossRef]
- Amado, S.; Rodrigues, J.M.; Luís, A.L.; Armada-da-Silva, P.A.S.; Vieira, M.; Gartner, A.; Simões, M.J.; Veloso, A.P.; Fornaro, M.; Raimondo, S.; et al. Effects of collagen membranes enriched with in vitro-differentiated N1E-115 cells on rat sciatic nerve regeneration after end-to-end repair. J. Neuroneg. Rehab. 2010, 7. [Google Scholar] [CrossRef] [Green Version]
- Sahin, M. The role of topical Genta Fleece HD and gentamicin spray in prevention of sternum wound infections after open heart surgery: A comparative study. Arch. Med. Sci. Atheroscler. Dis. 2018, 3, e29–e34. [Google Scholar] [CrossRef]
- Schonleber, F.; Reck, T.; Tannapfel, A.; Hohenberger, W.; Schneider, I. Collagen foil (TissuFoil E) reduces the formation of adhesions when using polypropylene mesh for the repair of experimental abdominal wall defects. Int. J. Colorectal Dis. 2006, 21, 840–846. [Google Scholar] [CrossRef]
- Glaum, R.; Wiedmann-Al-Ahmad, M.; Huebner, U.; Schmelzeisen, R. Tissue engineering of composite grafts: Cocultivation of human oral keratinocytes and human osteoblast-like cells on laminin-coated polycarbonate membranes and equine collagen membranes under different culture conditions. J. Biomed. Mater. Res. A 2010, 93, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, J.; de Buys Roessingh, A.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for paediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- Knopp, U.; Christmann, F.; Reusche, E.; Sepehrnia, A. A new collagen biomatrix of equine origin versus a cadaveric dura graft for the repair of dural defects—A comparative animal experimental study. Acta Neurochir. 2005, 147, 877–887. [Google Scholar] [CrossRef]
- Cappabianca, P.; Esposito, F.; Cavallo, L.M.; Messina, A.; Solari, D.; Di Somma, L.G.M.; De Vitiis, E. Use of equine collagen foil as dura mater substitute in endoscopic endonasal transsphenoidal surgery. Surg. Neurol. 2006, 65, 144–149. [Google Scholar] [CrossRef]
- Biroli, F.; Esposito, F.; Fusco, M.; Bani, G.G.; Signorelli, A.; De Vitiis, O.; Cappabianca, P.; Cavallo, L.M. Novel equine collagen-only dural substitute. Neurosurgery 2008, 62, ONSE273–ONSE274. [Google Scholar] [CrossRef] [PubMed]
- Gazzeri, R.; Neroni, M.; Alfieri, A.; Galarza, M.; Faiola, A.; Esposito, S.; Giordano, M. Transparent equine collagen biomatrix as dural repair. A prospective clinical study. Acta Neurochir. 2009, 151, 537–543. [Google Scholar] [CrossRef]
- Parlato, C.; Di Nuzzo, G.; Luongo, M.; Parlato, S.R.; Accardo, M.; Cuccurullo, L.; Moraci, A. Use of a collagen biomatrix (TissuDura®) for dura repair: A long-term neuroradiological and neuropathological evaluation. Acta Neurochir. 2011, 153, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Petter-Puchner, A.H.; Froetscher, W.; Krametter-Froetscher, R.; Dragan Lorinson, D.; Redl, H.; Van Griensven, M. The long-term neurocompatibility of human fibrin sealant and equine collagen as biomatrices in experimental spinal cord injury. Exp. Toxicol. Pathol. 2007, 58, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Renard, J.; Billia, M.; Morra, I.; Terrone, C.; Scarpa, R.M. Biological glues and collagen fleece for hemostasis during laparoscopic partial nephrectomy: Technique and results of prospective study. J. Endourol. 2007, 21, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl, S.M.; Grognuz, A.; Michetti, M.; Raffoul, W.; Applegate, L.A. Enhancement of human adipose-derived stem cell expansion and stability for clinical use. Int. J. Stem Cell Res. Ther. 2015, 2, 007. [Google Scholar] [CrossRef]
- Holzer, B.; Grußner, U.; Bruckner, B.; Houf, M.; Kiffner, E.; Schildberg, F.W.; Vogel, P.; Rosen, H.R. Efficacy and tolerance of a new gentamicin collagen fleece (Septocoll) after surgical treatment of a pilonidal sinus. Colorect. Dis. 2003, 5, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Andreasi Bassi, M.; Ludovichetti, M.; Pagnutti, S. Maxillary sinus lift with a collagenic equine heterologous bone substitute. Histomorphometric analysis. Ital. Oral Surg. 2012, 11, 56–62. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Andreasi Bassi, M.; Cinci, L.; Pieri, L.; Ammirabile, G.; Pagnutti, S. Treatment of a bone defect consequent to the removal of a periapical cyst with equine bone and equine membranes: Clinical and histological outcome. Minerva Stomatol. 2012, 61, 1–14. [Google Scholar]
- Tizzoni, R.; Tizzoni, M. How do GTR and GBR Differ? A periodontitis case treated using an equine-derived, enzyme-deantigenic, collagen-preserving bone graft, and collagen membranes. J. Contemp. Dent. Pract. 2019, 20, 639–644. [Google Scholar] [CrossRef]
- Jacomacci, W.P.; Pavan, A.J.; Zanoni, J.N.; Camarini, E.T. Bovine and equine biomaterials in mandibular alveolar dog model: Split-mouth study. Braz. Dent. Sci. 2016, 19, 75–81. [Google Scholar] [CrossRef]
- Gigante, A.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Enea, D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc. Tech. 2012, 1, e175–e180. [Google Scholar] [CrossRef]
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Gigante, A. One-step cartilage repair in the knee: Collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee 2015, 22, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonida, A.; Todeschini, G.; Lomartire, G.; Cinci, L.; Pieri, L. Socket preservation using enzyme-treated equine bone granules and an equine collagen matrix: A case report with histological and histomorphometrical assessment. J. Contemp. Dent. Pract. 2016, 17, 890–896. [Google Scholar] [CrossRef]
- Tarquini, G. Coronally advanced flap technique to treat class i and ii gingival recession in combination with connective tissue graft or equine collagen matrix: A retrospective study. Int. J. Periodontics Restor. Dent. 2017, 37, e217–e223. [Google Scholar] [CrossRef]
- Karr, J.C.; Taddei, A.R.; Picchietti, S.; Gambellini, G.; Fausto, A.M.; Giorgi, F. A morphological and biochemical analysis comparative study of the collagen products Biopad, Promogram, Puracol, and Colactive. Adv. Skin Wound Care 2011, 24, 208–216. [Google Scholar] [CrossRef]
- Cecen, B.; Kozaci, D.; Yuksel, M.; Erdemli, D.; Bagriyanik, A.; Havitcioglu, H. Biocompatibility of MG-63 cells on collagen, poly-L-lactic acid, hydroxyapatite scaffolds with different parameters. J. Appl. Biomater. Funct. Mater. 2015, 13, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Knaepler, H. Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection in orthopaedic surgery. Int. J. Surg. 2012, 10, S15–S20. [Google Scholar] [CrossRef] [Green Version]
- Chia, C.L.K.; Shelat, V.G.; Low, W.; George, S.; Rao, J. The use of collatamp G, local gentamicin-collagen sponge, in reducing wound infection. Int. Surg. 2014, 99, 565–570. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J. Orthop. Res. 2010, 28, 116–124. [Google Scholar] [CrossRef]
- Kon, E.; Muttini, A.; Arcangeli, E.; Delcogliano, M.; Filardo, G.; Aldini, N.N.; Pressato, D.; Quarto, R.; Zaffagnini, S.; Marcacci, M. Novel nanostructured scaffold for osteochondral regeneration: Pilot study in horses. J. Tissue Eng. Regen. Med. 2010, 4, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Condello, V.; Filardo, G.; Madonna, V.; Andriolo, L.; Screpis, D.; Bonomo, M.; Zappia, M.; Dei Giudici, L.; Zorzi, C. Use of a biomimetic scaffold for the treatment of osteochondral lesions in early osteoarthritis. BioMed Res. Int. 2018, 2018, 7937089. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosi, R.; Valli, F.; De Luca, P.; Ursino, N.; Usuelli, F.G. MaioRegen osteochondral substitute for the treatment of knee defects: A systematic review of the literature. J. Clin. Med. 2019, 8, 783. [Google Scholar] [CrossRef] [Green Version]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Carmela, C.; Ferrari, F. Electrospinning technologies in wound dressing applications. In Therapeutic Dressing and Wound Healing Applications; Boateng, J., Ed.; Wiley: Hoboken, NJ, USA, 2020; p. 333. [Google Scholar]
- Snyder, D.L.; Sullivan, N.; Margolis, D.J.; Schoelles, K.M. Skin Substitutes for Treating Chronic Wounds; Technology Assessment Program Project ID No. WNDT0818; Prepared by the ECRI Institute-Penn Medicine Evidence-based Practice Center under Contract No. HHSA 290-2015-00005-I: Rockville, MD, USA, 2020. [Google Scholar]
- Spanel-Borowski, K. The chick chorioallantoic membrane as test system for biocompatible materials. Res. Exp. Med. 1989, 189, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Schillinger, U.; Putz, U.; Stemberger, A.; Plank, C. Non viral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J. Gene Med. 2002, 4, 634–643. [Google Scholar] [CrossRef]
- Czerny, M.; Verrel, F.; Weber, H.; Müller, N.; Kircheis, L.; Lang, W.; Steckmeier, B.; Trubel, W. Collagen patch coated with fibrin glue components. Treatment of suture hole bleedings in vascular reconstruction. Cardiovasc. Surg. 2000, 41, 553–557. [Google Scholar]
- Joseph, T.; Adeosun, A.; Paes, T.; Bahal, V. Randomised controlled trial to evaluate the efficacy of TachoComb H patches in controlling PTFE suture-hole bleeding. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 549–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malicev, E.; Radosavljevic, D.; Velikonja, N.K. Fibrin gel improved the spatial uniformity and phenotype of human chondrocytes seeded on collagen scaffolds. Biotechnol. Bioeng. 2006, 96, 364–370. [Google Scholar] [CrossRef]
- Ikeda, T.; Miyata, Y.; Tsutani, Y.; Misumi, K.; Arihiro, K.; Okada, M. Fibrinogen/thrombin-based collagen fleece (TachoComb®) promotes regeneration in pulmonary arterial injury. Eur. J. Cardiothorac. Surg. 2012, 41, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, S.; Origasa, H.; Tetens, V.; Kobayashi, M. Comparison of TachoSil and TachoComb in patients undergoing liver resection—A randomized, double-blind, non-inferiority trial. Langenbecks Arch. Surg. 2017, 402, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Siemer, S.; Lahme, S.; Altziebler, S.; Machtens, S.; Strohmaier, W.; Wechsel, H.-W.; Goebell, P.; Schmeller, N.; Oberneder, R.; Stolzenburg, J.-U.; et al. Efficacy and safety of TachoSil W as haemostatic treatment versus standard suturing in kidney tumour resection: A randomised prospective study. Eur. Urolog. 2007, 52, 1156–1163. [Google Scholar] [CrossRef]
- Maisano, F.; Kjærgård, H.K.; Bauernschmitt, R.; Pavie, A.; Rábago, G.; Laskar, M.; Marstein, J.P.; Falk, V. TachoSil surgical patch versus conventional haemostatic fleece material for control of bleeding in cardiovascular surgery: A randomised controlled trial. Eur. J. Cardiothorac. Surg. 2009, 36, 708–714. [Google Scholar] [CrossRef]
- Briceno, J.; Naranjo, A.; Ciria, R.; Diaz-Nieto, R.; Sanchez-Hidalgo, J.-M.; Luque, A.; Rufian, S.; Lopez-Cillero, P. A prospective study of the efficacy of clinical application of a new carrier-bound fibrin sealant after liver resection. Arch. Surg. 2010, 145, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, L.; Seiler, C.M.; Broelsch, C.E.; de Hemptinne, B.; Klempnauer, J.; Mischinger, H.-J.; Gassel, H.-J.; Rokkjaer, M.; Schauer, R.; Larsen, P.N.; et al. Hemostatic efficacy of TachoSil in liver resection compared with argon beam coagulator treatment: An open, randomized, prospective, multicenter, parallel-group trial. Surgery 2011, 149, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, I.; Vasdev, N.; Soomro, N. A review of current hemostatic agents and tissue sealants used in laparoscopic partial nephrectomy. Rev. Urol. 2011, 13, 131–138. [Google Scholar] [CrossRef]
- Tinelli, A. Post-cesarean section hemorrage treated by a collagen patch coated with the human coagulation factors. J. Clin. Case Rep. 2011, 1, 1000e103. [Google Scholar] [CrossRef]
- Vida, V.L.; Padalino, M.A.; Barzon, E.; Stellin, G. Efficacy of fibrinogen/thrombin-coated equine collagen patch in controlling lymphatic leaks. J. Cardiovasc. Surg. 2012, 27, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Vida, V.L.; De Francesci, M.; Barzon, E.; Padalino, M.A.; Scattolin, F.; Stellin, G. The use of fibrinogen/thrombin-coated equine collagen patch in children requiring reoperations for congenital hearth disease. A single center clinical experience. J. Cardiovasc. Surg. 2014, 55, 401–406. [Google Scholar]
- Odermatt, E.K.; Steuer, H.; Lembert, N. Efficacy of a collagen hemostat versus a carrier-bound fibrin sealant. J. Thrombo. Cir. 2017, 3, 1000120. [Google Scholar] [CrossRef]
- Deponti, D.; Di Giancamillo, A.; Gervaso, F.; Scandone, C.; Addis, A.; Agnoletto, M.; Domenicucci, M.; Sannino, A.; Domeneghini, C.; Peretti, G.M. Alternative cell sources for tendon/ligament engineering. J. Orthop. 2013, 5, 93–99. [Google Scholar]
- Bang, K.; Sampram, E.; Funding, M.; Moller Christensen, T.; Baandrup, U.; Hjortdal, V.E. Gentacoll® hampers epithelialisation and neovascularisation in excisional wounds in hairless mice. Scand. J. Plast. Reconstr. Hand Surg. 1998, 32, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.G. Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection following cardiac surgery. Int. J. Surg. 2012, 10, S10–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.-H.; Park, J.; Park, J.; Kim, M.; Kim, D.; Song, M.-Y.; Kang, G.-I.; Hwang, W.-K.; Ku, S.-K.; Jang, K.-H.; et al. Evaluation for biocompatibility of gentamicin-collagen sponge on the experimental animal wound model. J. Vet. Clin. 2015, 32, 404. [Google Scholar] [CrossRef]
- Bargholz, C. Perforation repair with mineral trioxide aggregate: A modified matrix concept. Int. Endod. J. 2005, 38, 59–69. [Google Scholar] [CrossRef]
- Kleinheinz, J.; Stratmann, U.; Joos, U.; Wiesmann, H.-P. VEGF-activated angiogenesis during bone regeneration. J. Oral Maxillofac. Surg. 2005, 63, 1310–1316. [Google Scholar] [CrossRef]
- Weinzierl, K.; Hemprich, A.; Frerich, B. Bone engineering with adipose tissue derived stromal cells. J. Craniomaxillofac. Surg. 2006, 34, 466–471. [Google Scholar] [CrossRef]
- Kleinheinz, J.; Jung, S.; Wermker, K.; Fischer, C.; Joos, U. Release kinetics of VEGF165 from a collagen matrix and structural matrix changes in a circulation model. Head Face Med. 2010, 6. [Google Scholar] [CrossRef] [Green Version]
- Frerich, B.; Winter, K.; Scheller, K.; Braumann, U.-D. Comparison of different fabrication techniques for human adipose tissue engineering in severe combined immunodeficient mice. Artif. Organs 2011, 36, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Garrier, J.; Bezdetnaya, L.; Barlier, C.; Gräfe, S.; Guillemin, F.; D’Hallewin, M.A. Foslip®-based photodynamic therapy as a means to improve wound healing. Photodiagnosis Photodyn. Ther. 2011, 8, 321–327. [Google Scholar] [CrossRef]
- Krupp, C.; Bargholz, C.; Brüsehaber, M.; Hülsmann, M. Treatment outcome after repair of root perforations with mineral trioxide aggregate: A retrospective evaluation of 90 teeth. J. Endod. 2013, 39, 1364–1368. [Google Scholar] [CrossRef]
- Stimmelmayr, M.; Güth, J.F.; Schlee, M.; Beuer, F. Vertical ridge augmentation using the modified shell technique: A case report. J. Oral Maxillofac. Surg. 2014, 72, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, Z.S.; Berezhanskyy, T.; Utzinger, M.; Aneja, M.K.; Emrich, D.; Erben, R.; Schüler, C.; Altpeter, P.; Ferizi, M.; Hasenpusch, G.; et al. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J. Control. Release 2016, 239, 137–148. [Google Scholar] [CrossRef]
- Brüsehaber, M.; Bargholz, C.; Hülsmann, M. Mineral trioxide aggregate for root-end closure of non-vital immature permanent teeth: A retrospective study. ENDO (Lond. Engl.) 2016, 10, 176–181. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Gredes, T.; Heinemann, F.; Dominiak, M.; Botzenhart, U.; Gedrange, T. Socket augmentation using a commercial collagen-based product—An animal study in pigs. Mater. Sci. Eng. C 2015, 46, 177–183. [Google Scholar] [CrossRef]
- Schnutenhaus, S.; Doering, I.; Dreyhaupt, J.; Rudolph, H.; Luthardt, R.G. Alveolar ridge preservation with a collagen material: A randomized controlled trial. J. Periodontal Implant Sci. 2018, 48, 236–250. [Google Scholar] [CrossRef]
- Gorskiy, V.A.; Sivkov, A.S.; Agapov, M.A.; Titkov, B.E.; Schadskij, S.O. The first experience of using a single-layer intra-abdominal collagen plate. Khirurgiia (Mosk.) 2015, 5, 59–61. [Google Scholar] [CrossRef]
- Marinucci, L.; Lilli, C.; Guerra, M.; Belcastro, S.; Becchetti, E.; Stabellini, G.; Calvi, E.M.; Locci, P. Biocompatibility of collagen membranes crosslinked with glutaraldehyde or diphenylphosphoryl azide: An in vitro study. J. Biomed. Mater. Res. A 2003, 67, 504–509. [Google Scholar] [CrossRef]
- Fontes, C.E.R.; Mardegam, M.J.; Prado-Filho, O.R.; Ferreira, M.C. Comparative analysis of surgical hemostatic sponge in liver injury: Study in rats. Arq. Bras. Cir. Dig. 2018, 31, e1342. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.-C. Comparison of the binding capacity of collagen from different origin for IL-1β and TNF-α. Acta Vulnologica 2009, 7, 71–73. [Google Scholar]
- Fitzgerald, R.H.; Steinberg, J.S. Collagen in wound healing: Are we onto something new or just repeating the past? Foot Ankle J. 2009, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Pallaske, F.; Pallaske, A.; Herklotz, K.; Boese-Landgraf, J. The significance of collagen dressings in wound management: A review. J. Wound Care 2018, 27, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.-C. Influence of the collagen origin on the binding affinity for neutrophil elastase. In Proceedings of the 18th Conference of the European Wound Management Association (EWMA), Lisbon, Portugal, 14–16 May 2008. [Google Scholar]
- Karr, J.C. Healing diabetic foot and heel ulcers with Biopad®, an equine type heterologous lyophilized collagen primary wound dressing. In Proceedings of the Symposium on Advanced Wound Care (SAWC), Atlanta, GA, USA, 23–26 May 2004. [Google Scholar]
- Gigante, A.; Cesari, E.; Busilacchi, A.; Manzotti, S.; Greco, F. Type-I collagen membrane as a scaffold for tendon repair: An in vitro and in vivo study. J. Orthopaed. Traumatol. 2008, 9, 1–94. [Google Scholar]
- Gigante, A.; Busilacchi, A.; Lonzi, B.; Cecconi, S.; Manzotti, S.; Renghini, C.; Giuliani, A.; Mattioli-Belmonte, M. Purified collagen I oriented membrane for tendon repair: An ex vivo morphological study. J. Orthop. Res. 2012, 31, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Filardo, G.; Kon, E.; Panseri, S.; Montesi, M.; Iafisco, M.; Savini, E.; Sprio, S.; Cunha, C.; Giavaresi, G.; et al. Fabrication and pilot in vivo study of a collagen-BDDGE-elastin core-shell scaffold for tendon regeneration. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Negri, S.; Farinato, S.; Fila, C.; Pagliaro, P.; Bellomi, A. Tissue engineering: Chondrocyte cultures on type I collagen support. Cytohistological and immunohistochemical study. J. Orthopaed. Traumatol. 2007, 8, 57–63. [Google Scholar] [CrossRef]
- Grigolo, B.; Desando, G.; Cavallo, C.; Zini, N.; Ghisu, S.; Facchini, A. Evaluation of chondrocyte behavior in a new equine collagen scaffold useful for cartilage repair. J. Biol. Regul. Homeost. Agents 2011, 25, S53–S62. [Google Scholar]
- Bistolfi, A.; Ferracini, R.; Galletta, C.; Tosto, F.; Sgarminato, V.; Digo, E.; Vernè, E.; Massè, A. Regeneration of articular cartilage: Scaffold used inorthopedic surgery. A short handbook of available products for regenerative joints surgery. Clin. Sci. Res. Rep. 2017, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, G.; Forte, S.; Gulino, R.; Cefalì, F.; Figallo, E.; Salvatorelli, L.; Maniscalchi, E.T.; Angelico, G.; Parenti, R.; Gulisano, M.; et al. Combination of collagen-based scaffold and bioactive factors induces adipose-derived mesenchymal stem cells chondrogenic differentiation in vitro. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Sanjurjo-Rodríguez, C.; Castro-Viñuelas, R.; Hermida-Gómez, T.; Fuentes-Boquete, I.M.; De Toro, F.J.; Blanco, F.J.; Díaz-Prado, S.M. Human cartilage engineering in an in vitro repair model using collagen scaffolds and mesenchymal stromal cells. Int. J. Med. Sci. 2017, 14, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiele, M. Hyaluronic acid for advanced therapies: Promises and challenges. Eur. Polym. J. 2019, 117, 134–147. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Gostynska, N.; Campodoni, E.; Dapporto, M.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Ribose mediated crosslinking of collagen-hydroxyapatite hybrid scaffolds for bone tissue regeneration using biomimetic strategies. Mater. Sci. Eng. C 2017, 77, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Gómez–Morales, J.; Fernández Penas, R.; Verdugo-Escamilla, C.; Degli Esposti, L.; Oltolina, F.; Prat, M.; Iafisco, M.; Fernández Sánchez, J.F. Bioinspired mineralization of type I collagen fibrils with apatite in presence of citrate and europium ions. Crystals 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regeneration: A literature review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collagen Market Size, Share & Trends Analysis Report by Source (Bovine, Porcine), by Product (Gelatin, Native, Hydrolyzed), by Application (Food & Beverages, Healthcare), and Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/native-collagen-market (accessed on 13 March 2020).
- Collagen Solutions plc (COS.L) Initiation. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwingsGNqPjrAhWtMewKHcd1BmsQFjAAegQIBhAB&url=https%3A%2F%2Fir.collagensolutions.com%2Fdocs%2Flibrariesprovider18%2Fpdf%2Fresearch%2Fhardman_research_24sept2014.pdf&usg=AOvVaw39AFScS0UOGcFIrPDK0jwQ (accessed on 26 February 2020).
- Tissue Engineered Collagen Biomaterials Market. Available online: https://www.transparencymarketresearch.com/tissue-engineered-collagen-biomaterials-market.html (accessed on 31 October 2020).
- Espicom Business Intelligence. All Change in the Advanced Wound Care Market 2009; Espicom Business Intelligence: Chichester, UK, 2009. [Google Scholar]
- Collagen Extraction. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=2ahUKEwih8Kna4MfoAhVIiFwKHT_CRAQFjABegQIAxAB&url=https%3A%2F%2Fwww.mla.com.au%2Fdownload%2Ffinalreports%3FitemId%3D3749&usg=AOvVaw0lM7SFl1rgXY8rdj6YqMxX (accessed on 25 August 2020).
- Gorham, S.D. Novel materials from biological sources. In Biomaterials; Byrom, D., Ed.; Macmillan Publishers Ltd. and ICI Biological Products Business: New York, NY, USA, 1991; p. 55. [Google Scholar]
- Pappas, A.M.; Hyatt, G.W. The evaluation of collagen film applied to skin defects in mice. Surg. Forum 1960, 10, 844–846. [Google Scholar]
| Amino Acids | Equine [45] | Bovine [45] | Ovine [74] | Rat [80] | Human [52] |
|---|---|---|---|---|---|
| Alanine | 120 | 124 | 113 | 105 | 114 |
| Arginine | 56 | 61 | 63 | 55 | 53 |
| Aspartate | 50 | 53 | 48 | 35 | 45 |
| Cysteine | 0 | 0 | 0 | 1 | 0 |
| Glycine | 219 | 222 | 317 | 332 | 334 |
| Glutamate | 97 | 97 | 76 | 68 | 78 |
| Histidine | 11 | 7 | 0 | 5 | 6 |
| Hydroxy lysine | 13 | 10 | 0 | 12 | 9 |
| Hydroxy proline | 104 | 103 | 94 | 91 | 86 |
| Isoleucine | 13 | 15 | 11 | 12 | 10 |
| Leucine | 32 | 30 | 28 | 27 | 25 |
| Lysine | 26 | 21 | 30 | 27 | 24 |
| Methionine | 4 | 2 | 10 | 7 | 6 |
| Phenylalanine | 19 | 18 | 14 | 11 | 14 |
| Proline | 142 | 147 | 117 | 121 | 120 |
| Serine | 39 | 35 | 35 | 39 | 34 |
| Threonine | 22 | 20 | 18 | 21 | 17 |
| Tryptophan | 0 | 0 | 0 | 0 | 0 |
| Tyrosine | 5 | 2 | 4 | 3 | 4 |
| Valine | 28 | 27 | 23 | 26 | 26 |
| Imino acid | 246 | 250 | 211 | 212 | 205 |
| TOT. | 1000 | 1000 | 1000 | 1000 | 1000 |
| Company | Product | Additives | Form | Application | Ref. |
|---|---|---|---|---|---|
| B. & B. Dental (Bologna, Italy) | T-Barrier | - | Sheet | Hemostasis, Hard tissue | [103] |
| Baxter (Rome, Italy) | Gentafleece | Gentamicin sulphate | Sponge | Hemostasis, Wound healing | [104,105,106] |
| TissuFoil E | - | Sheet | Wound healing | [107,108] | |
| TissuDura | - | Sheet | Wound healing | [109,110,111,112,113,114] | |
| TissueFleece | - | Sponge | Hemostasis | [115,116,117] | |
| Zimmer Biomet (Warsaw, USA) | Septocoll | Gentamicin sulphate | Sponge | Hemostasis | [118] |
| Bioteck (Vicenza, Italy) | Biocollagen | - | Membrane | Hard tissues | [119,120,121] |
| Bio-gen | Spongy bone | Powder | Hard tissues | [122] | |
| MeRG | Glycosaminoglycans | Membrane | Soft tissues | [123,124] | |
| Xenomatrix | - | Sheet | Soft tissues | [125,126] | |
| Euroresearch (Milano, Italy) | Biopad | - | Sponge | Wound healing, Hard tissues | [26,127,128] |
| Bioart | - | Powder | Hard tissues | - | |
| Nithya | - | Gel | Soft tissues, Anti-aging | [39] | |
| Revamil | Honey | Sponge | Wound healing | - | |
| Versuspray | Silver | Powder | Wound healing | - | |
| EUSA Pharma (Langhorne, USA) | Collatamp | Gentamicin sulphate | Sponge | Wound healing | [129,130] |
| Finceramica (Faenza, Italy) | MaioRegen | Hydroxyapatite | Membrane | Soft tissue | [131,132,133,134] |
| Fidia Farmaceutici (Bologna, Italy) | Bionect pad | Hyaluronic acid | Sponge | Wound healing | [135] |
| Innocoll (Athlone, Ireland) | Collexa | Bovine collagen | Sponge | Wound healing | [136] |
| MLM Biologics (Gainesville, USA) | Bio-conneKt | - | Membrane | Wound healing | [136] |
| Nycomed (Munich, Germany) | TachoTop | - | Sponge | Hemostasis, Wound healing | [137,138] |
| TachoComb | Human fibrinogen and bovine thrombin | Sponge | Hemostasis, Wound healing | [139,140,141,142,143] | |
| TachoSil | Human fibrinogen and human thrombin | Sponge | Hemostasis, Wound healing | [143,144,145,146,147,148,149,150,151,152] | |
| Opocrin (Modena, Italy) | Antema | - | Sheet | Hemostasis, Wound healing | [57,153] |
| Resorba Medical GmbH (Nürnberg, Germany) | Genta-coll | Gentamicin sulphate | Sponge | Hemostasis, Hard tissues | [154,155,156] |
| Kollagen | - | Sponge | Hemostasis, Hard tissues | [82,157,158,159,160,161,162,163,164,165,166] | |
| Parasorb | - | Membrane | Hemostasis, Hard tissues | [77,167,168] | |
| Salvecoll (Como, Italy) | Salvecoll-E | - | Gel | Wound healing | [100] |
| Takeda (Tokyo, Japan) | CollGARA | - | Sponge | Hemostasis, Wound healing | [169] |
| GABA Vebas (Roma, Italy) | Paroguide | Chondroitin sulphate | Membrane | Wound healing | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. https://doi.org/10.3390/jfb11040079
Gallo N, Natali ML, Sannino A, Salvatore L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. Journal of Functional Biomaterials. 2020; 11(4):79. https://doi.org/10.3390/jfb11040079
Chicago/Turabian StyleGallo, Nunzia, Maria Lucia Natali, Alessandro Sannino, and Luca Salvatore. 2020. "An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications" Journal of Functional Biomaterials 11, no. 4: 79. https://doi.org/10.3390/jfb11040079
APA StyleGallo, N., Natali, M. L., Sannino, A., & Salvatore, L. (2020). An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. Journal of Functional Biomaterials, 11(4), 79. https://doi.org/10.3390/jfb11040079