Pupillometry as a Window into Young Children’s Sustained Attention
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
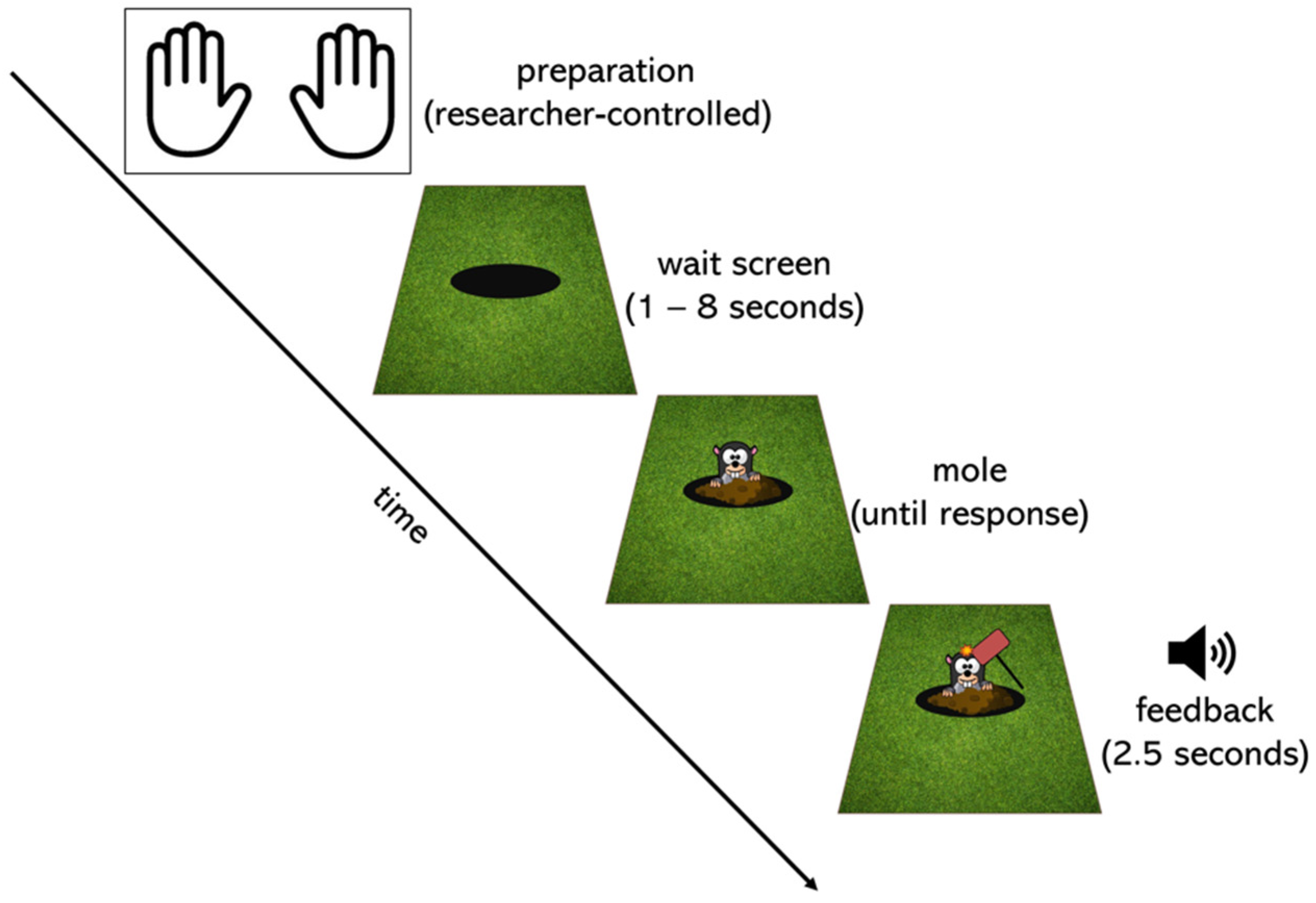
2.2. Task Design and Procedure
2.3. Pupillometry
2.4. Data Analysis
3. Results
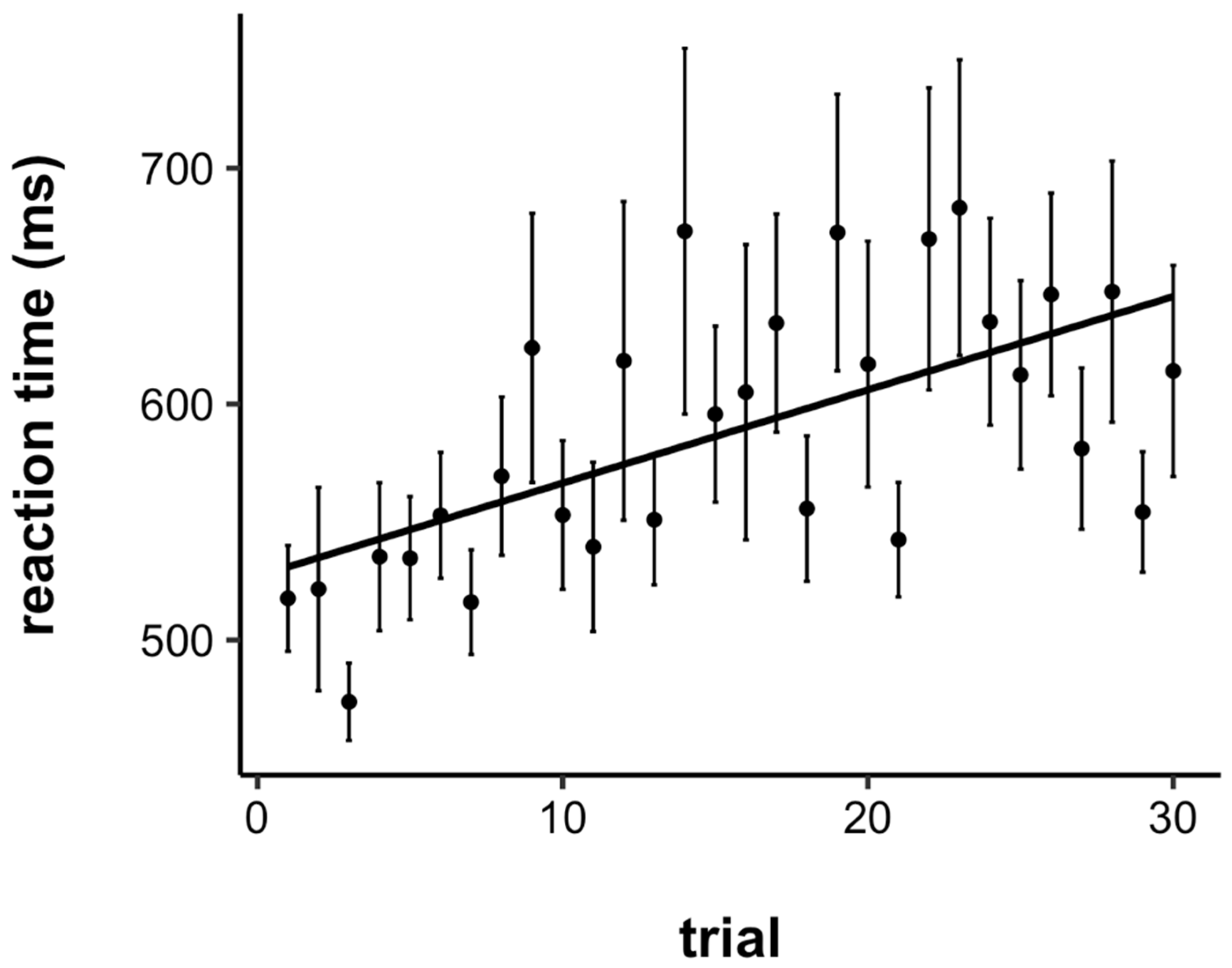
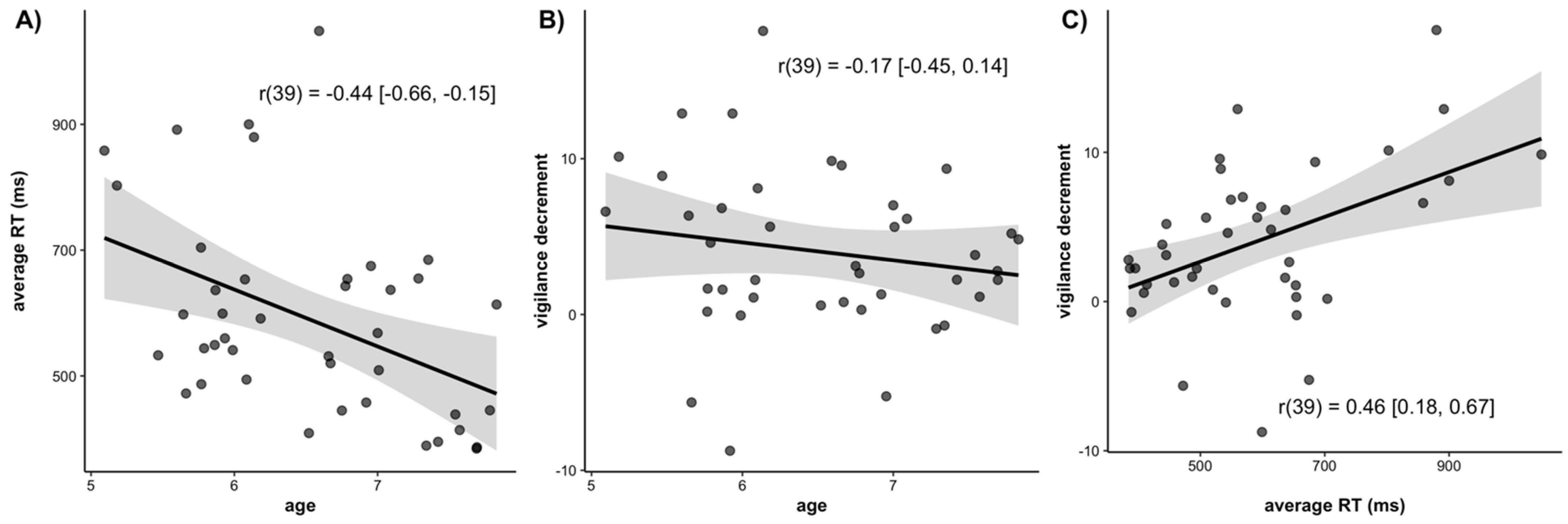
3.1. Reaction Time (RT)
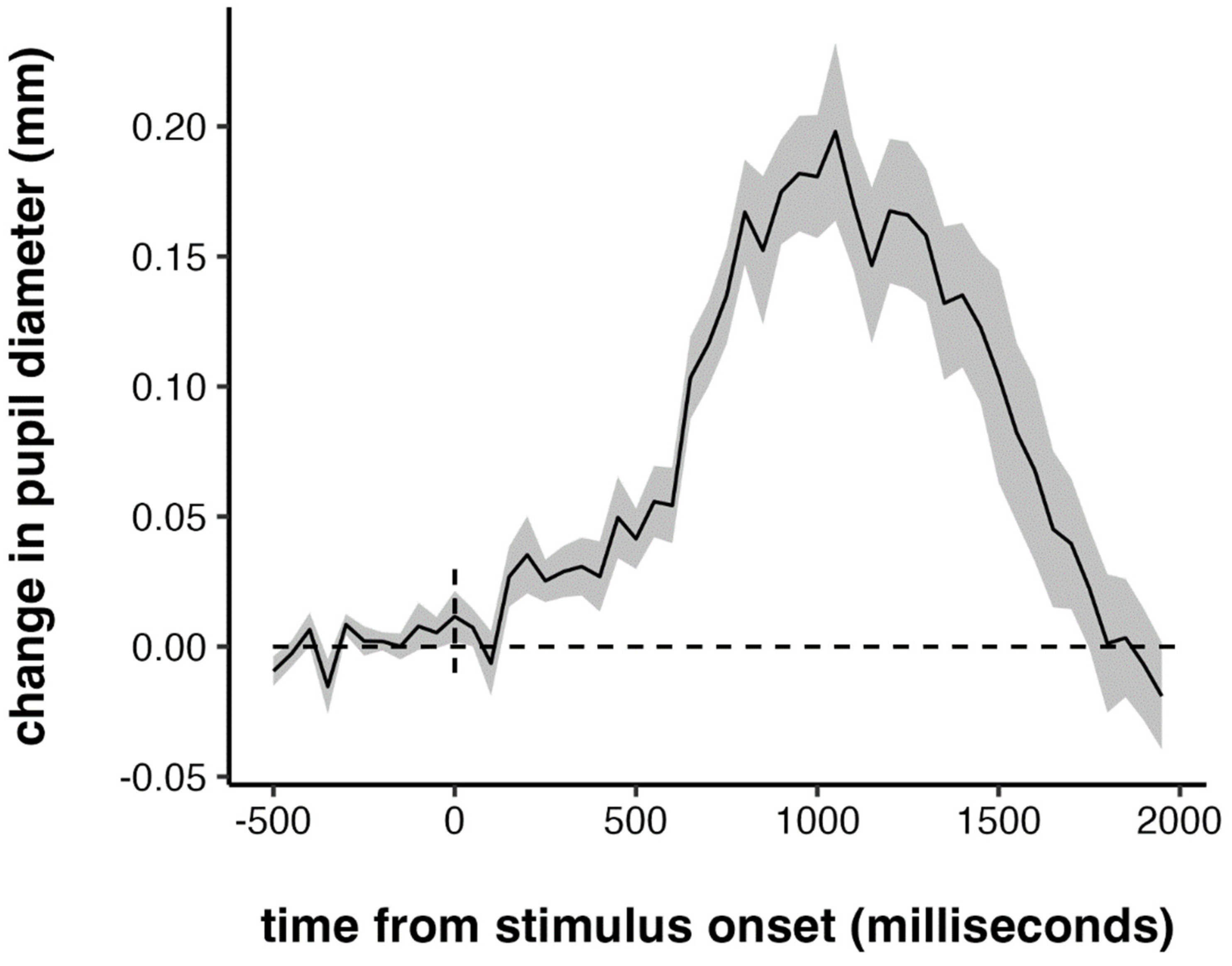
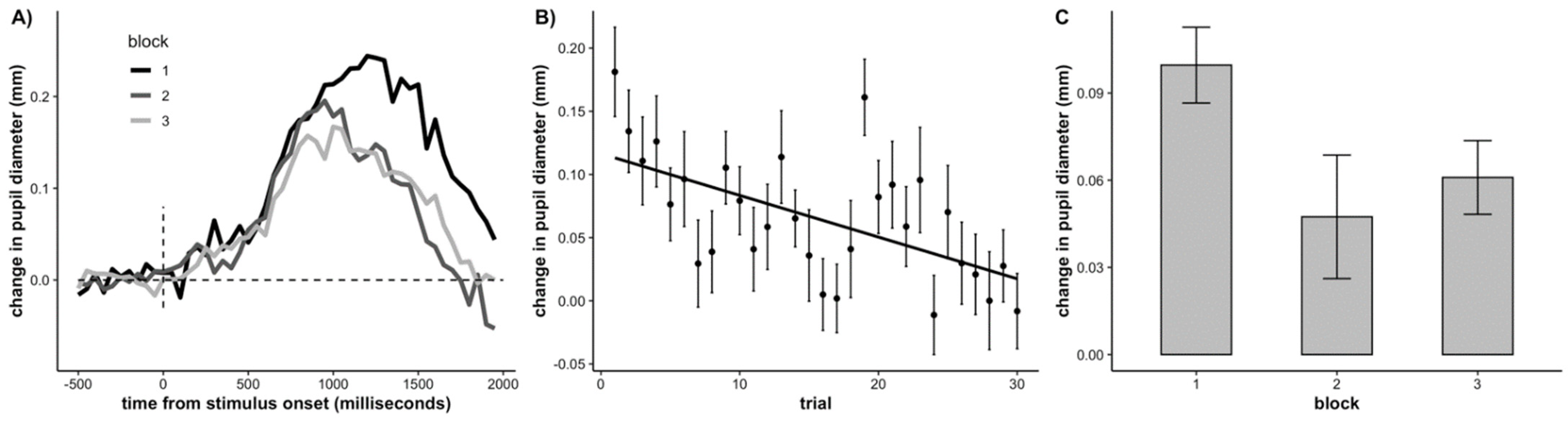
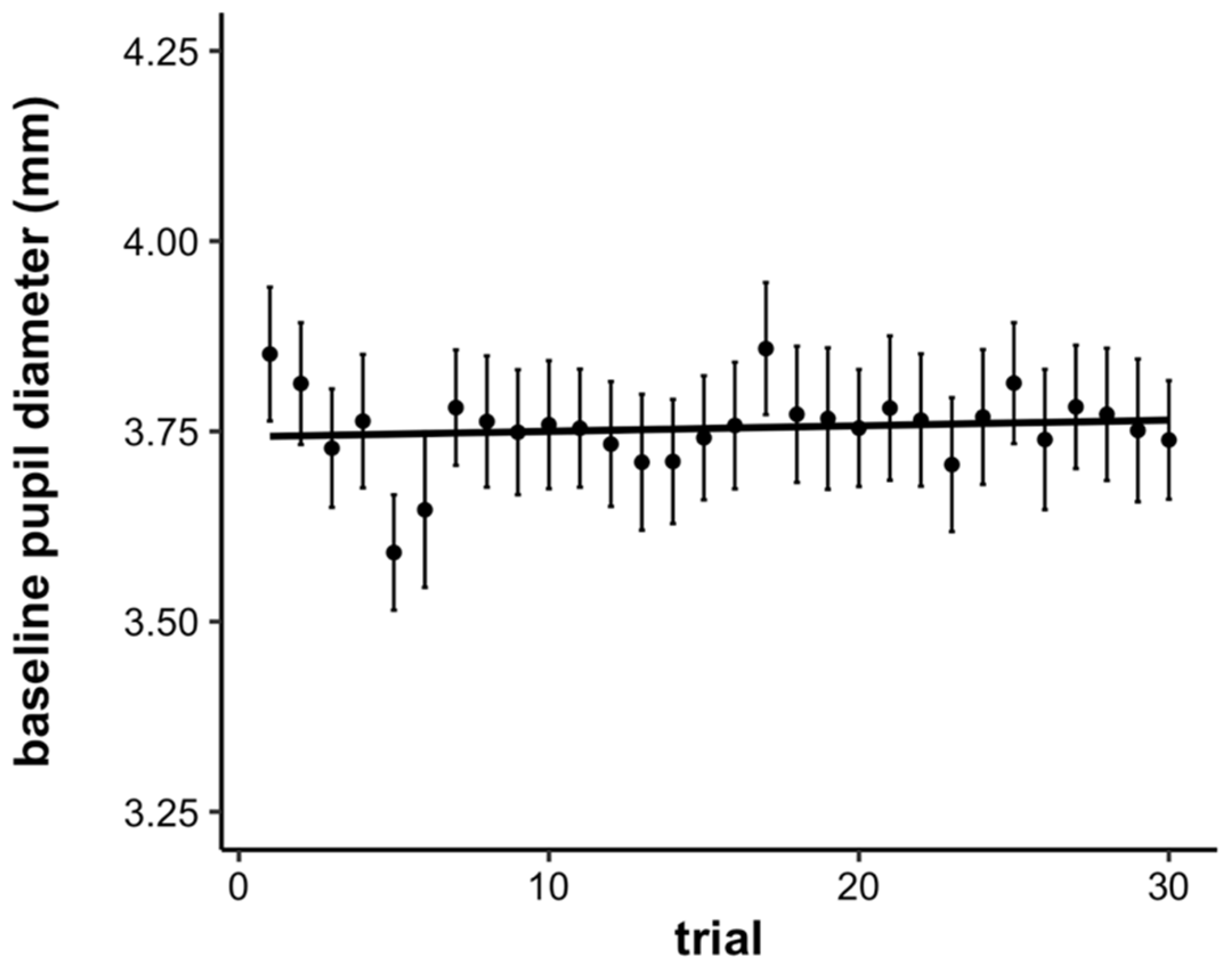
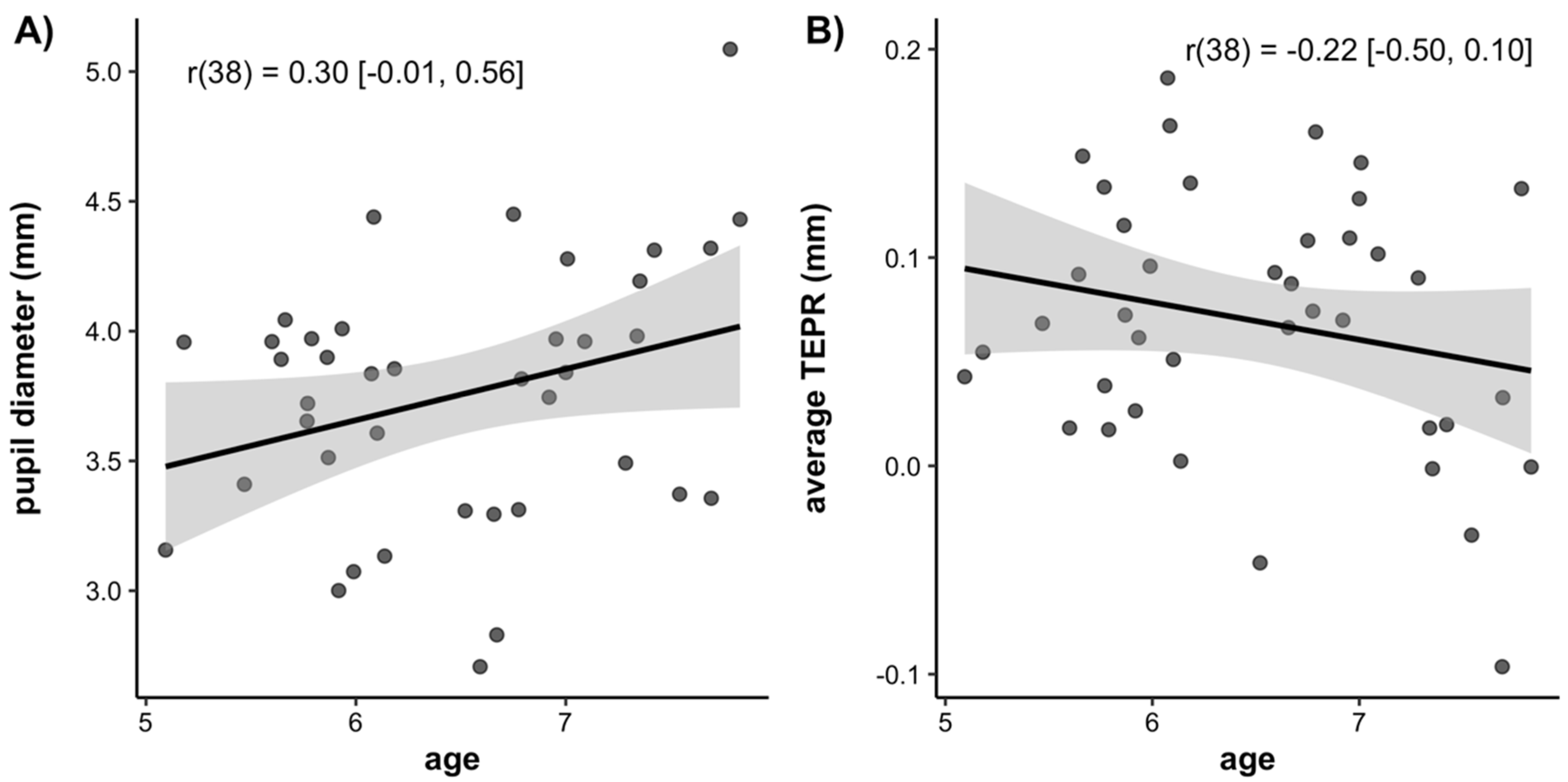
3.2. Pupillometry
3.3. Age Related Differences
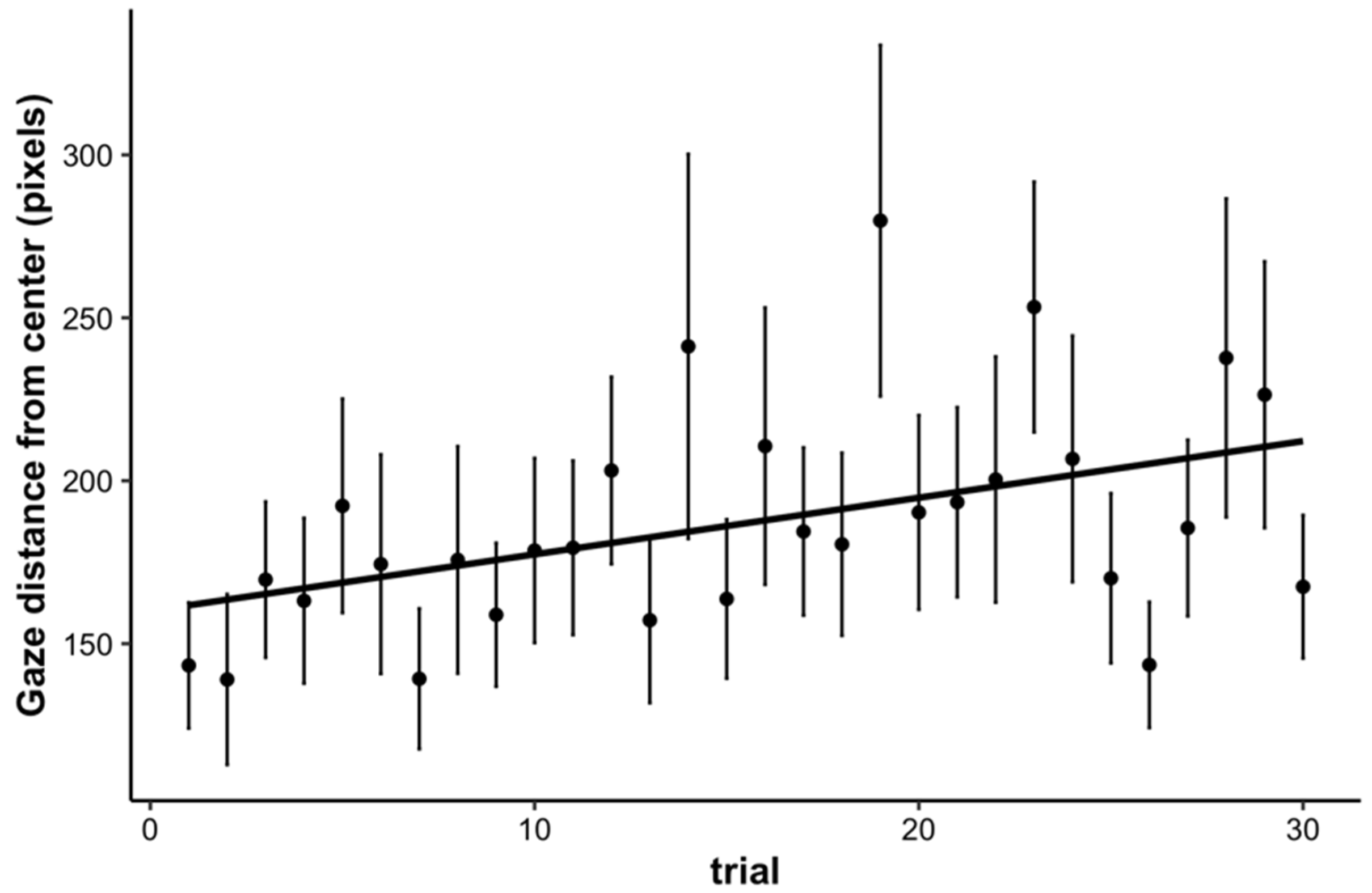
3.4. Fixations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackermann, Lena, Robert Hepach, and Nivedita Mani. 2020. Children Learn Words Easier When They Are Interested in the Category to Which the Word Belongs. Developmental Science 23: e12915. [Google Scholar] [CrossRef] [PubMed]
- Ahern, Sylvia, and Jackson Beatty. 1979. Pupillary Responses During Information Processing Vary with Scholastic Aptitude Test Scores. Science 205: 1289–92. [Google Scholar] [CrossRef] [PubMed]
- Alnæs, Dag, Markus Handal Sneve, Thomas Espeseth, Tor Endestad, Steven Harry Pieter van de Pavert, and Bruno Laeng. 2014. Pupil Size Signals Mental Effort Deployed during Multiple Object Tracking and Predicts Brain Activity in the Dorsal Attention Network and the Locus Coeruleus. Journal of Vision 14: 1. [Google Scholar] [CrossRef]
- Andrade, Brendan F., Darlene A. Brodeur, Daniel A. Waschbusch, Sherry H. Stewart, and Robin McGee. 2009. Selective and Sustained Attention as Predictors of Social Problems in Children With Typical and Disordered Attention Abilities. Journal of Attention Disorders 12: 341–52. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, Gary, and Jonathan D. Cohen. 2005. An integrative theory of locus coeruleus-norepinephrine function: Adaptive Gain and Optimal Performance. Annual Review of Neuroscience 28: 403–50. [Google Scholar] [CrossRef] [PubMed]
- Beatty, Jackson. 1982a. Task-Evoked Pupillary Responses, Processing Load, and the Structure of Processing Resources. Psychological Bulletin 91: 276–92. [Google Scholar] [CrossRef]
- Beatty, Jackson. 1982b. Phasic Not Tonic Pupillary Responses Vary with Auditory Vigilance Performance. Psychophysiology 19: 167–72. [Google Scholar] [CrossRef]
- Benitez, Viridiana L., Catarina Vales, Rima Hanania, and Linda B. Smith. 2017. Sustained Selective Attention Predicts Flexible Switching in Preschoolers. Journal of Experimental Child Psychology 156: 29–42. [Google Scholar] [CrossRef]
- Benitez, Viridiana L., Martin Zettersten, and Erica Wojcik. 2020. The Temporal Structure of Naming Events Differentially Affects Children’s and Adults’ Cross-Situational Word Learning. Journal of Experimental Child Psychology 200: 104961. [Google Scholar] [CrossRef]
- Boersma, Frederic, Keri Wilton, Richard Barham, and Walter Muir. 1970. Effects of Arithmetic Problem Difficulty on Pupillary Dilation in Normals and Educable Retardates. Journal of Experimental Child Psychology 9: 142–55. [Google Scholar] [CrossRef]
- Bonmassar, Carolina, Andreas Widmann, and Nicole Wetzel. 2020. The Impact of Novelty and Emotion on Attention-Related Neuronal and Pupil Responses in Children. Developmental Cognitive Neuroscience 42: 100766. [Google Scholar] [CrossRef] [PubMed]
- Brandes-Aitken, Annie, Stephen Braren, Margaret Swingler, Kristin Voegtline, and Clancy Blair. 2019. Sustained Attention in Infancy: A Foundation for the Development of Multiple Aspects of Self-Regulation for Children in Poverty. Journal of Experimental Child Psychology 184: 192–209. [Google Scholar] [CrossRef] [PubMed]
- Brisson, Julie, Marc Mainville, Dominique Mailloux, Christelle Beaulieu, Josette Serres, and Sylvain Sirois. 2013. Pupil Diameter Measurement Errors as a Function of Gaze Direction in Corneal Reflection Eyetrackers. Behavior Research Methods 45: 1322–31. [Google Scholar] [CrossRef] [PubMed]
- Burton, Birgitte Klee, Signe Vangkilde, Anders Petersen, Lene Theil Skovgaard, Jens Richardt Jepsen, Nicoline Hemager, Camilla Jerlang Christiani, Katrine Soeborg Spang, Ditte Ellersgaard, Aja Greve, and et al. 2018. Sustained Attention and Interference Control Among 7-Year-Old Children With a Familial High Risk of Schizophrenia or Bipolar Disorder—A Nationwide Observational Cohort Study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3: 704–12. [Google Scholar] [CrossRef]
- Chatham, Christopher H., Michael J. Frank, and Yuko Munakata. 2009. Pupillometric and Behavioral Markers of a Developmental Shift in the Temporal Dynamics of Cognitive Control. Proceedings of the National Academy of Sciences 106: 5529–33. [Google Scholar] [CrossRef]
- Cheng, Chen, Zsuzsa Kaldy, and Erik Blaser. 2019. Focused Attention Predicts Visual Working Memory Performance in 13-Month-Old Infants: A Pupillometric Study. Developmental Cognitive Neuroscience 36: 100616. [Google Scholar] [CrossRef]
- Choudhury, Naseem, and Kathleen S. Gorman. 2000. The Relationship between Sustained Attention and Cognitive Performance in 17–24-Month Old Toddlers. Infant and Child Development 9: 127–46. [Google Scholar] [CrossRef]
- Colombo, John, and Carol L. Cheatham. 2006. The Emergence and Basis of Endogenous Attention in Infancy and Early Childhood. Advances in Child Development and Behavior 34: 283–322. [Google Scholar] [CrossRef]
- Coors, Annabell, Monique M. B. Breteler, and Ulrich Ettinger. 2022. Processing Speed, but Not Working Memory or Global Cognition, Is Associated with Pupil Diameter during Fixation. Psychophysiology 6: e14089. [Google Scholar] [CrossRef]
- Davies, Patrick T., Meredith J. Woitach, Marcia A. Winter, and E. Mark Cummings. 2008. Children’s Insecure Representations of the Interparental Relationship and Their School Adjustment: The Mediating Role of Attention Difficulties. Child Development 79: 1570–82. [Google Scholar] [CrossRef]
- Dinges, David F, and John W Powell. 1985. Microcomputer Analyses of Performance on a Portable, Simple Visual RT Task during Sustained Operations. Behavior Research Methods, Instruments, & Computers 17: 652–55. [Google Scholar]
- Dowle, Matt, and Arun Srinivasan. 2018. Data.Table: Extension of data.frame. Available online: https://CRAN.R-project.org/package=data.table (accessed on 2 October 2022).
- Ebert, Kerry Danahy, and Kathryn Kohnert. 2011. Sustained Attention in Children With Primary Language Impairment: A Meta-Analysis. Journal of Speech, Language & Hearing Research 54: 1372–84. [Google Scholar] [CrossRef]
- Eckstein, Maria K., Belén Guerra-Carrillo, Alison T. Miller Singley, and Silvia A. Bunge. 2017. Beyond Eye Gaze: What Else Can Eyetracking Reveal about Cognition and Cognitive Development? Developmental Cognitive Neuroscience 25: 69–91. [Google Scholar] [CrossRef] [PubMed]
- Esterman, Michael, and David Rothlein. 2019. Models of Sustained Attention. Current Opinion in Psychology 29: 174–80. [Google Scholar] [CrossRef]
- Fisher, Anna V. 2019. Selective Sustained Attention: A Developmental Foundation for Cognition. Current Opinion in Psychology 29: 248–53. [Google Scholar] [CrossRef]
- Fortenbaugh, Francesca C., Joseph DeGutis, Laura Germine, Jeremy B. Wilmer, Mallory Grosso, Kathryn Russo, and Michael Esterman. 2015. Sustained Attention Across the Life Span in a Sample of 10,000: Dissociating Ability and Strategy. Psychological Science 26: 1497–510. [Google Scholar] [CrossRef]
- Frye, Douglas, Philip David Zelazo, and Tibor Palfai. 1995. Theory of Mind and Rule-Based Reasoning. Cognitive Development 10: 483–527. [Google Scholar] [CrossRef]
- Gagl, Benjamin, Stefan Hawelka, and Florian Hutzler. 2011. Systematic Influence of Gaze Position on Pupil Size Measurement: Analysis and Correction. Behavior Research Methods 43: 1171–81. [Google Scholar] [CrossRef]
- Gallardo-Moreno, Geisa B., Andrés A. González-Garrido, Teresita Villaseñor-Cabrera, Francisco J. Alvarado-Rodríguez, Vanessa D. Ruiz-Stovel, Miriam E. Jiménez-Maldonado, Nayeli Contreras-Piña, and Fabiola R. Gómez-Velázquez. 2020. Sustained Attention in Schoolchildren with Type-1 Diabetes. A Quantitative EEG Study. Clinical Neurophysiology 131: 2469–78. [Google Scholar] [CrossRef]
- Gardner-Neblett, Nicole, Jamie DeCoster, and Bridget K. Hamre. 2014. Linking Preschool Language and Sustained Attention with Adolescent Achievement through Classroom Self-Reliance. Journal of Applied Developmental Psychology 35: 457–67. [Google Scholar] [CrossRef]
- Gilzenrat, Mark S., Sander Nieuwenhuis, Marieke Jepma, and Jonathan D. Cohen. 2010. Pupil Diameter Tracks Changes in Control State Predicted by the Adaptive Gain Theory of Locus Coeruleus Function. Cognitive, Affective, & Behavioral Neuroscience 10: 252–69. [Google Scholar] [CrossRef]
- Hayes, Taylor R., and Alexander A. Petrov. 2016. Mapping and Correcting the Influence of Gaze Position on Pupil Size Measurements. Behavior Research Methods 48: 510–27. [Google Scholar] [CrossRef]
- Hepach, Robert, and Gert Westermann. 2016. Pupillometry in Infancy Research. Journal of Cognition and Development 17: 359–77. [Google Scholar] [CrossRef]
- Huang-Pollock, Cynthia, Roger Ratcliff, Gail McKoon, Alexandra Roule, Tyler Warner, Jason Feldman, and Shane Wise. 2020. A Diffusion Model Analysis of Sustained Attention in Children with Attention Deficit Hyperactivity Disorder. Neuropsychology 34: 641–53. [Google Scholar] [CrossRef] [PubMed]
- Isbell, Elif, Susan D. Calkins, Margaret M. Swingler, and Esther M. Leerkes. 2018. Attentional Fluctuations in Preschoolers: Direct and Indirect Relations with Task Accuracy, Academic Readiness, and School Performance. Journal of Experimental Child Psychology 167: 388–403. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Elizabeth, Alison Miller Singley, Andrew Peckham, Sheri Johnson, and Silvia Bunge. 2014. Task-Evoked Pupillometry Provides a Window into the Development of Short-Term Memory Capacity. Frontiers in Psychology 5: 218. [Google Scholar] [CrossRef] [PubMed]
- Jongman, Suzanne R., Ardi Roelofs, and Antje S. Meyer. 2015. Sustained Attention in Language Production: An Individual Differences Investigation. Quarterly Journal of Experimental Psychology 68: 710–30. [Google Scholar] [CrossRef]
- Joshi, Siddhartha, Yin Li, Rishi M. Kalwani, and Joshua I. Gold. 2016. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 89: 221–34. [Google Scholar] [CrossRef]
- Just, Marcel Adam, Patricia A. Carpenter, and Akira Miyake. 2003. Neuroindices of Cognitive Workload: Neuroimaging, Pupillometric and Event-Related Potential Studies of Brain Work. Theoretical Issues in Ergonomics Science 4: 56–88. [Google Scholar] [CrossRef]
- Karatekin, Canan, David J. Marcus, and Jane W. Couperus. 2007. Regulation of Cognitive Resources during Sustained Attention and Working Memory in 10-Year-Olds and Adults. Psychophysiology 44: 128–44. [Google Scholar] [CrossRef]
- Karatekin, Canan. 2004. Development of Attentional Allocation in the Dual Task Paradigm. International Journal of Psychophysiology 52: 7–21. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, Sean D., John A. Stern, Timothy B. Brown, and John W. Rohrbaugh. 2009. Detecting Phasic Lapses in Alertness Using Pupillometric Measures. Applied Ergonomics 40: 978–86. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, Alexandra, Per B. Brockhoff, and Rune H. B. Christensen. 2017. LmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software 82: 1–26. [Google Scholar] [CrossRef]
- Laeng, Bruno, Sylvain Sirois, and Gustaf Gredebäck. 2012. Pupillometry: A Window to the Preconscious? Perspectives on Psychological Science 7: 18–27. [Google Scholar] [CrossRef] [PubMed]
- Martin, Joel T., Annalise H. Whittaker, and Stephen J. Johnston. 2022. Pupillometry and the Vigilance Decrement: Task-evoked but Not Baseline Pupil Measures Reflect Declining Performance in Visual Vigilance Tasks. European Journal of Neuroscience 55: 778–99. [Google Scholar] [CrossRef]
- Massar, Stijn A. A., Julian Lim, Karen Sasmita, and Michael W. L. Chee. 2016. Rewards Boost Sustained Attention through Higher Effort: A Value-Based Decision Making Approach. Biological Psychology 120: 21–27. [Google Scholar] [CrossRef]
- Massar, Stijn A. A., Julian Lim, Karen Sasmita, and Michael W. L. Chee. 2019. Sleep Deprivation Increases the Costs of Attentional Effort: Performance, Preference and Pupil Size. Neuropsychologia 123: 169–77. [Google Scholar] [CrossRef]
- Mathôt, Sebastiaan, Jasper Fabius, Elle Van Heusden, and Stefan Van der Stigchel. 2018. Safe and Sensible Preprocessing and Baseline Correction of Pupil-Size Data. Behavior Research Methods 50: 94–106. [Google Scholar] [CrossRef]
- Mathôt, Sebastiaan. 2018. Pupillometry: Psychology, Physiology, and Function. Journal of Cognition 1: 16. [Google Scholar] [CrossRef]
- McGarrigle, Ronan, Piers Dawes, Andrew J. Stewart, Stefanie E. Kuchinsky, and Kevin J. Munro. 2017. Measuring Listening-Related Effort and Fatigue in School-Aged Children Using Pupillometry. Journal of Experimental Child Psychology 161: 95–112. [Google Scholar] [CrossRef]
- McQuillan, Maureen E., John E. Bates, Angela D. Staples, Caroline P. Hoyniak, Kathleen M. Rudasill, and Victoria J. Molfese. 2021. Sustained Attention across Toddlerhood: The Roles of Language and Sleep. Developmental Psychology 57: 1042–57. [Google Scholar] [CrossRef] [PubMed]
- Murphy, Peter R., Ian H. Robertson, Joshua H. Balsters, and Redmond G. O’connell. 2011. Pupillometry and P3 Index the Locus Coeruleus–Noradrenergic Arousal Function in Humans. Psychophysiology 48: 1532–43. [Google Scholar] [CrossRef] [PubMed]
- Murphy, Peter R., Redmond G. O’Connell, Michael O’Sullivan, Ian H. Robertson, and Joshua H. Balsters. 2014. Pupil Diameter Covaries with BOLD Activity in Human Locus Coeruleus. Human Brain Mapping 35: 4140–54. [Google Scholar] [CrossRef] [PubMed]
- Rajkowski, Janusz, P. Kubiak, and Gary Aston-Jones. 1993. Correlations between Locus Coeruleus (LC) Neural Activity, Pupil Diameter and Behavior in Monkey Support a Role of LC in Attention. Abstract 19: 974. [Google Scholar]
- Rhoades, Brittany L., Heather K. Warren, Celene E. Domitrovich, and Mark T. Greenberg. 2011. Examining the Link between Preschool Social–Emotional Competence and First Grade Academic Achievement: The Role of Attention Skills. Early Childhood Research Quarterly 26: 182–91. [Google Scholar] [CrossRef]
- Robison, Matthew K., and Nash Unsworth. 2019. Pupillometry Tracks Fluctuations in Working Memory Performance. Attention, Perception, & Psychophysics 81: 407–19. [Google Scholar] [CrossRef]
- Robison, Matthew K. 2018. Regulating Mind-Wandering and Sustained Attention with Goal-Setting, Feedback, and Incentives. Doctoral dissertation, University of Oregon, Eugene, OR, USA. Available online: http://hdl.handle.net/1794/23712 (accessed on 2 October 2022).
- Robison, Matthew K., and Gene A. Brewer. 2022. Individual Differences in Working Memory Capacity, Attention Control, Fluid Intelligence, and Pupillary Measures of Arousal. Journal of Experimental Psychology: Learning, Memory, and Cognition 48: 1296–310. [Google Scholar] [CrossRef]
- Robison, Matthew K., Joseph T. Coyne, Ciara Sibley, Noelle L. Brown, Brittany Neilson, and Cyrus Foroughi. 2022. An Examination of Relations Between Baseline Pupil Measures and Cognitive Abilities. Psychophysiology 59: e14124. [Google Scholar] [CrossRef]
- Rose, Susan A., Sam Wass, Jeffery J. Jankowski, Judith F. Feldman, and Aleksandra Djukic. 2017. Sustained Attention in the Face of Distractors: A Study of Children with Rett Syndrome. Neuropsychology 31: 403–10. [Google Scholar] [CrossRef]
- Selezneva, Elena, and Nicole Wetzel. 2022. The Impact of Probabilistic Cues on Sound-Related Pupil Dilation and ERP Responses in 7–9-Year-Old Children. Auditory Perception & Cognition 5: 1–21. [Google Scholar] [CrossRef]
- Sirois, Sylvain, and Iain R. Jackson. 2012. Pupil Dilation and Object Permanence in Infants. Infancy 17: 61–78. [Google Scholar] [CrossRef] [PubMed]
- Sirois, Sylvain, and Julie Brisson. 2014. Pupillometry. WIREs Cognitive Science 5: 679–92. [Google Scholar] [CrossRef]
- Strauch, Christoph, Chin-An Wang, Wolfgang Einhäuser, Stefan Van der Stigchel, and Marnix Naber. 2022. Pupillometry as an Integrated Readout of Distinct Attentional Networks. Trends in Neurosciences 45: 635–47. [Google Scholar] [CrossRef]
- Swaab-Barneveld, Hanna, Leo de Sonneville, Peggy Cohen-Kettenis, Anneke Gielen, Jan Buitelaar, and Herman van engeland. 2000. Visual Sustained Attention in a Child Psychiatric Population. Journal of the American Academy of Child & Adolescent Psychiatry 39: 651–59. [Google Scholar] [CrossRef]
- Tsukahara, Jason S., Tyler L. Harrison, and Randall W. Engle. 2016. The Relationship between Baseline Pupil Size and Intelligence. Cognitive Psychology 91: 109–23. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, Jason S., and Randall W. Engle. 2021. Is baseline pupil size related to cognitive ability? Yes (under proper lighting conditions). Cognition 211: 104643. [Google Scholar] [CrossRef]
- Tucha, Lara, Oliver Tucha, Susanne Walitza, Thomas A. Sontag, Rainer Laufkötter, Martin Linder, and Klaus W. Lange. 2009. Vigilance and Sustained Attention in Children and Adults With ADHD. Journal of Attention Disorders 12: 410–21. [Google Scholar] [CrossRef]
- Unsworth, Nash, and Matthew K. Robison. 2016. Pupillary Correlates of Lapses of Sustained Attention. Cognitive, Affective, & Behavioral Neuroscience 16: 601–15. [Google Scholar] [CrossRef]
- Unsworth, Nash, and Matthew K. Robison. 2017. The Importance of Arousal for Variation in Working Memory Capacity and Attention Control: A Latent Variable Pupillometry Study. Journal of Experimental Psychology: Learning, Memory, and Cognition 43: 1962–87. [Google Scholar] [CrossRef]
- Unsworth, Nash, and Matthew K. Robison. 2018. Tracking Arousal State and Mind Wandering with Pupillometry. Cognitive, Affective, & Behavioral Neuroscience 18: 638–64. [Google Scholar]
- van den Brink, Ruud L., Peter R. Murphy, and Sander Nieuwenhuis. 2016. Pupil Diameter Tracks Lapses of Attention. PLoS ONE 11: e0165274. [Google Scholar] [CrossRef] [PubMed]
- Varazzani, Chiara, Aurore San-Galli, Sophie Gilardeau, and Sebastien Bouret. 2015. Noradrenaline and Dopamine Neurons in the Reward/Effort Trade-off: A Direct Electrophysiological Comparison in Behaving Monkeys. Journal of Neuroscience 35: 7866–77. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, Giacomo, Peter A. J. Fanning, Darren R. Hocking, Stephanie Sievers, and Cheryl Dissanayake. 2017. Social Attention, Joint Attention and Sustained Attention in Autism Spectrum Disorder and Williams Syndrome: Convergences and Divergences. Journal of Autism and Developmental Disorders 47: 1866–77. [Google Scholar] [CrossRef] [PubMed]
- West, Gillian, David R. Shanks, and Charles Hulme. 2021. Sustained Attention, Not Procedural Learning, Is a Predictor of Reading, Language and Arithmetic Skills in Children. Scientific Studies of Reading 25: 47–63. [Google Scholar] [CrossRef]
- Wickham, Hadley. 2016. Ggplot2: Elegant Graphics for Data Analysis. New York: Springer. Available online: http://ggplot2.org (accessed on 2 October 2022).
- Wickham, Hadley. 2017. Tidyverse: Easily Install and Load the Tidyverse. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 2 October 2022).
- Wilke, Claus O. 2020. Cowplot: Streamlined Plot Theme and Plot Annotations for Ggplot2. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 2 October 2022).
- Yu, Chen, Sumarga H. Suanda, and Linda B. Smith. 2019. Infant Sustained Attention but Not Joint Attention to Objects at 9 Months Predicts Vocabulary at 12 and 15 Months. Developmental Science 22: e12735. [Google Scholar] [CrossRef]
- Zelazo, Philip David, Jacob E. Anderson, Jennifer Richler, Kathleen Wallner-Allen, Jennifer L. Beaumont, and Sandra Weintraub. 2013. Ii. Nih Toolbox Cognition Battery (Cb): Measuring Executive Function and Attention. Monographs of the Society for Research in Child Development 78: 16–33. [Google Scholar] [CrossRef]
- Zhang, Felicia, and Lauren L. Emberson. 2020. Using Pupillometry to Investigate Predictive Processes in Infancy. Infancy 25: 758–80. [Google Scholar] [CrossRef]
- Zhang, Felicia, Sagi Jaffe-Dax, Robert C. Wilson, and Lauren L. Emberson. 2018. Prediction in Infants and Adults: A Pupillometry Study. Developmental Science 22: e12780. [Google Scholar] [CrossRef]
- Zhao, Sijia, Gabriela Bury, Alice Milne, and Maria Chait. 2019. Pupillometry as an Objective Measure of Sustained Attention in Young and Older Listeners. Trends in Hearing 23: 2331216519887815. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benitez, V.L.; Robison, M.K. Pupillometry as a Window into Young Children’s Sustained Attention. J. Intell. 2022, 10, 107. https://doi.org/10.3390/jintelligence10040107
Benitez VL, Robison MK. Pupillometry as a Window into Young Children’s Sustained Attention. Journal of Intelligence. 2022; 10(4):107. https://doi.org/10.3390/jintelligence10040107
Chicago/Turabian StyleBenitez, Viridiana L., and Matthew K. Robison. 2022. "Pupillometry as a Window into Young Children’s Sustained Attention" Journal of Intelligence 10, no. 4: 107. https://doi.org/10.3390/jintelligence10040107
APA StyleBenitez, V. L., & Robison, M. K. (2022). Pupillometry as a Window into Young Children’s Sustained Attention. Journal of Intelligence, 10(4), 107. https://doi.org/10.3390/jintelligence10040107




