1. Introduction
Advances in assisted reproductive technologies (ARTs) have redefined the landscape of fertility care, opening new avenues for diagnosis and treatment that were once unimaginable. The convergence of artificial intelligence (AI) with traditional reproductive methodologies is catalyzing a transformative shift in the field. AI-driven Computer-Aided Sperm Analysis (CASA) systems, leveraging advanced machine learning (ML) and deep learning (DL) techniques, are at the forefront of this change [
1]. These systems provide an automated, objective, and high-throughput evaluation of key sperm parameters—motility, morphology, and DNA integrity—thus overcoming many limitations of subjective, manual analysis.
Historically, sperm evaluation has depended on labor-intensive manual examinations that are inherently prone to variability and inconsistency. With the advent of AI, significant improvements in precision and reproducibility have been achieved. Modern CASA systems integrate sophisticated image processing algorithms and pattern recognition techniques to extract nuanced details from sperm samples. This enhanced analytical capability not only refines diagnostic accuracy but also offers clinicians critical insights for tailoring personalized treatment strategies, ultimately improving outcomes in procedures such as in vitro fertilization (IVF) [
2].
The integration of advanced ML techniques, DL architectures, and big data analytics has further propelled the evolution of AI applications in reproductive medicine [
3,
4]. Using extensive open datasets alongside state-of-the-art ML and DL models, researchers can develop robust predictive systems that correlate subtle variations in sperm quality with clinical outcomes. This comprehensive, data-driven approach facilitates the creation of individualized treatment protocols and shifts the paradigm from traditional methodologies to algorithmically enhanced precision medicine.
Despite these promising advancements, several challenges remain. Issues such as data variability, standardization of evaluation protocols, and the ethical management of sensitive reproductive information continue to pose significant hurdles. Moreover, integrating AI into clinical practice necessitates rigorous validation through controlled trials and the establishment of clear regulatory frameworks to ensure patient safety and data privacy. Addressing these challenges is critical to fully harnessing AI’s potential in revolutionizing reproductive healthcare [
5,
6].
This review provides an in-depth exploration of current AI methodologies applied to sperm analysis, tracing the evolution from classical ML techniques to state-of-the-art deep learning models. By examining both established practices and emerging innovations, the paper highlights how AI-driven CASA systems are transforming fertility diagnostics and treatment. Ultimately, this updated perspective invites readers to envision a future where fertility care is not only more accessible and efficient but also uniquely tailored to the individual needs of patients, marking a significant step forward in the field of reproductive medicine.
Following this introduction,
Section 2 reviews the medical background of ARTs and the evolution of CASA systems.
Section 3 examines how machine learning, deep learning, and big data analytics enhance sperm evaluation.
Section 4 discusses the challenges and ethical considerations in integrating these technologies, and
Section 5 concludes with future research directions and final insights.
2. Background
With significant advancements in AI and ML [
1], these cutting-edge technologies’ cross-disciplinary applications have increased exponentially. An area where AI has shown tremendous potential for transformative change is within healthcare, particularly fertility techniques. ARTs increasingly employ AI tools to tackle intricate challenges, from understanding disease mechanisms and predicting treatment outcomes to personalizing patient care.
ML and DL, key subfields of AI, offer viable solutions to managing complex, diverse, and large-scale clinical data. ML is adept at identifying patterns in large datasets, aiding predictive modeling for fertility outcomes and optimizing treatment protocols. DL, on the other hand, has proven indispensable for analyzing medical imaging data related to ARTs, such as semen morphology and ultrasound images. DL algorithms exhibit a remarkable ability to detect critical features in imaging data that signify underlying fertility-related problems.
Applications of AI within ARTs are manifold. Predictive analytics can project the success rate of treatments, including IVF, using datasets encompassing patient age, hormone levels, and medical history. Furthermore, AI enables personalized treatment strategies by scrutinizing individual patient profiles and determining the appropriate treatment regime. In the sphere of clinical diagnostics, DL algorithms allow the automated evaluation of semen and embryos’ quality, leading to improved success rates of IVF. Lastly, AI facilitates the real-time monitoring of patients undergoing treatment, aiding healthcare professionals in spotting any divergences from the expected route and enabling early interventions.
2.1. Medical Background on ARTs
IVF is a process that involves the fertilization of oocytes outside the female reproductive system, typically in a laboratory setting [
7]. The process begins with the monitoring and stimulation of a woman’s ovulatory process, followed by the removal of ova from the ovaries and their fertilization with sperm in a laboratory [
2]. The resulting embryos are then cultured for a period of time before being transferred into the uterus [
8] (see full process in
Figure 1). This technique, initially introduced in the 1970s, has been used to treat female infertility caused by damaged or blocked fallopian tubes [
9]. The success of IVF has been attributed to various factors, including the development of novel laboratory procedures and techniques [
10].
Research has shown that the efficiency of IVF can be improved through various methods. Ref. [
11] demonstrated that simulating the oviductal environment can reduce polyspermy and increase IVF output in pigs. Ref. [
12] suggested that gamete micromanipulation can enhance the efficiency of mammalian fertilization in vitro. Ref. [
13] highlighted the role of assisted fertilization technology in improving IVF success rates. Refs. [
14,
15] discussed the importance of nutrient management and use efficiency in enhancing IVF outcomes. Refs. [
7,
16] proposed the use of foliar fertilization and egg yolk-treated sperm, respectively, as potential complementary techniques to improve IVF efficiency.
IVF has seen significant advancements in recent years, with improved success rates and the development of new technologies and techniques [
10,
13,
17,
18,
19,
20]. Challenges remain, particularly in the management of poor responders and the reduction in multiple gestation risks [
13,
17]. Future directions for improvement include the development of non-invasive embryo selection methods, the use of transcriptomics, proteomics, metabolomics, and time-lapse imaging technologies [
17]. The viability of early-stage embryo cultures is a key area for improvement, with co-culture on tubal epithelial cells showing promise [
21]. The refinement and enhancement of medication delivery systems also present an opportunity for improvement [
18]. Overall, the field of IVF continues to evolve, with a focus on increasing success rates and ensuring the safety of the procedure.
AI applications and research in contemporary reproductive medicine (RM), particularly focusing on the non-surgical aspects of RM [
21], primarily utilize supervised learning methods such as decision trees (DTs), random forests (RFs), support vector machines (SVMs), Naïve Bayes (NB), and DL classifiers. These AI methods are applied to determine the morphokinetic parameters of embryos that are most optimal using a combination of computer vision image processing methods, and DL techniques [
22]. Specifically, an ensemble comprising eight different DL models is used to rank the best embryos or predict pregnancy outcomes [
23]. One of the main limitations in conducting comparative studies between different methods is the lack of sufficiently large and well-known datasets, often necessitating the use of reference datasets like ImageNET [
24]. In this field, we can find proposals for predicting IVF and intracytoplasmic sperm injection (ICSI) outcomes [
25] through a hybrid feature-selection method and the application of classic ML models such as RFs, SVMs, and logistic regression (LR). Unsupervised learning models have not yet been fully integrated into RM. These algorithms excel at class discovery by utilizing unlabeled data to uncover the inherent structure and relationships within the data [
26]. Common types of unsupervised ML employed include principal component analysis and K-means clustering, which are predominantly applied in image processing. These methods can predict pregnancy outcomes based on the quality of oocytes [
27]. Additionally, unsupervised DL techniques are used for longitudinal follicle tracking to enhance the effectiveness of the IVF procedure [
28].
2.2. Automated Sperm Quality Assessment: Introduction to CASA Systems
The evolution of sperm analysis has undergone significant transformations over the decades, particularly with the advent of CASA systems. Initially, the assessment of male fertility potential relied primarily on routine semen analysis, which established baseline values for various semen parameters. These parameters were outlined in successive editions of the World Health Organization (WHO) guidelines published in 1980, 1987, 1992, 1999, and 2010, creating a framework for predicting conception chances based on semen quality [
29].
With technological advancements, CASA systems emerged as a revolutionary tool in andrology labs. Over approximately 40 years, CASA technology has evolved through enhancements in imaging devices, computational power, and software algorithms. While the foundational concepts of identifying sperm and analyzing their motility have remained largely consistent, the capabilities of CASA systems have expanded considerably. Many major spermatology laboratories now utilize CASA, although the extent of their application varies significantly [
4,
30]. The reliance on CASA systems reflects a broader trend in fertility treatments, where traditional methods like density gradient centrifugation (DGC) and swim-up techniques have been refined but often rely on sedimentation principles to isolate motile sperm based on physical characteristics. In contrast, CASA and other advanced methodologies emphasize a more nuanced analysis of sperm functionality, such as DNA integrity and gene expression, thus paving the way for a deeper understanding of male fertility potential [
31].
Additionally, the integration of AI into sperm analysis represents the latest frontier in this field. AI methods have shown promise in predicting seminal quality by analyzing environmental factors and lifestyle influences on sperm parameters, thereby enhancing the predictive power of sperm analysis [
21]. As these technologies continue to advance, they hold the potential to significantly elevate the standards of ARTs, marking a pivotal shift in the approach to male fertility assessment and treatment. A summary of the sperm analysis systems discussed in this section, outlining their key features, advantages, limitations, and ethical considerations, is presented in
Table 1.
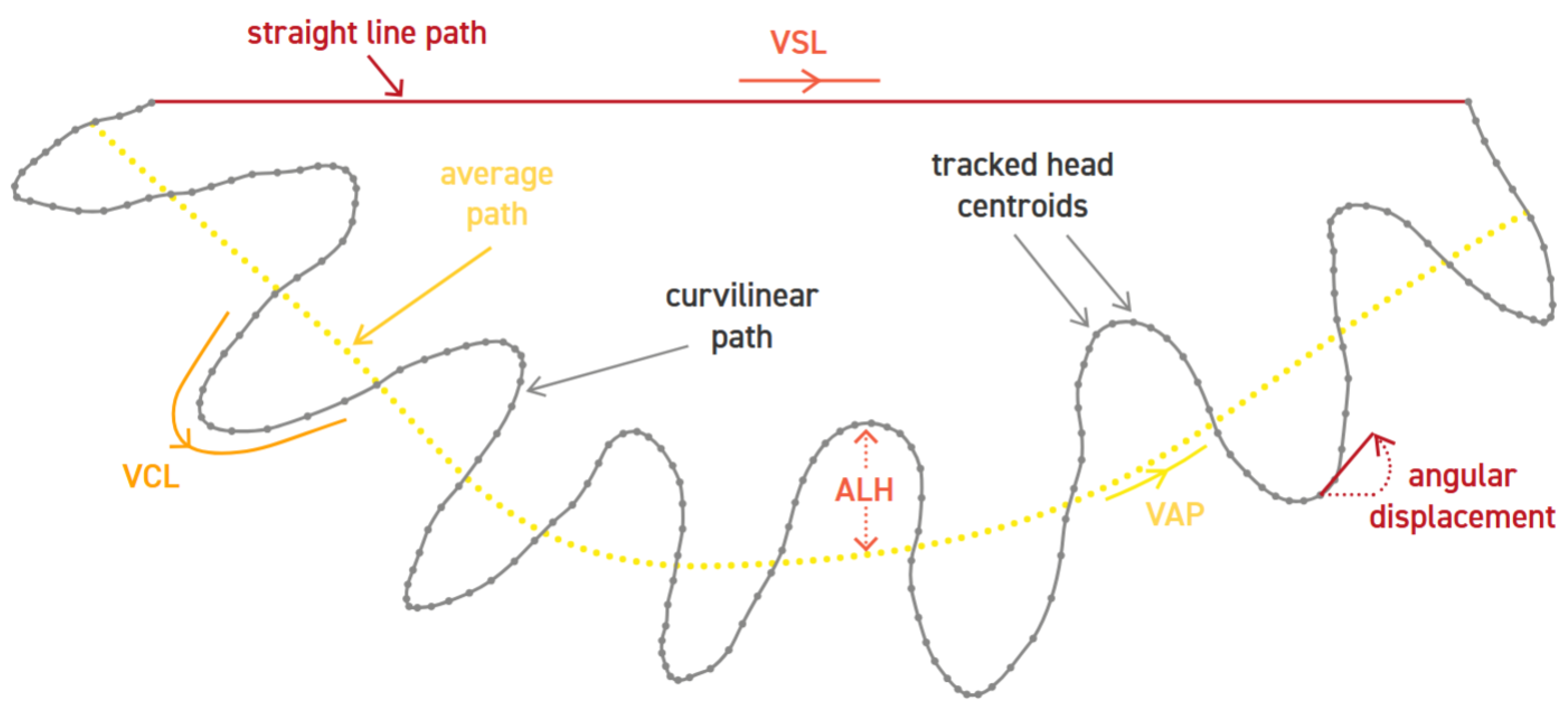
Among the core parameters evaluated by AI-based CASA systems, sperm motility plays a central role in determining male fertility potential. Motility analysis involves tracking the movement patterns of spermatozoa across sequential frames captured in video microscopy. Through algorithms capable of multi-object tracking and trajectory classification, CASA systems quantify kinematic features such as curvilinear velocity (VCL), straight-line velocity (VSL), and linearity (LIN), as seen in
Figure 2. These quantitative measures offer a standardized and reproducible assessment of sperm motion, which is crucial for predicting the ability of sperm to reach and fertilize the oocyte.
In parallel, morphology and DNA integrity represent complementary aspects of sperm quality that influence fertilization success and embryo development. Morphological assessment involves the classification of sperm based on structural attributes, including head shape, midpiece alignment, and tail length. An example of head morphology classification can be found in
Figure 3. AI algorithms trained on labeled datasets can detect subtle morphological deviations that might be overlooked in manual inspections. On the other hand, DNA integrity is typically evaluated using fluorescent staining and specialized imaging techniques that reveal fragmentation or chromatin condensation. While more challenging to automate, recent deep learning approaches have shown promise in identifying DNA damage from image-based data, thus broadening the scope of CASA systems beyond conventional motility and morphology analysis.
3. Artificial Intelligence and Machine Learning in Sperm Analysis
In recent years, the integration of AI and ML into Computer-Assisted Sperm Analysis (CASA) systems has significantly advanced reproductive health diagnostics. Traditional manual sperm analysis, often prone to human error and variability, has benefited from the precision and automation offered by CASA systems, which now incorporate sophisticated ML algorithms to enhance the assessment of key parameters such as motility, morphology, and DNA integrity. By analyzing motility, or the sperm’s ability to move effectively, ML models can precisely categorize movement patterns, providing a robust metric for fertility assessment. Morphology, which examines the size and shape of sperm, is evaluated using pattern recognition techniques, allowing for a standardized, high-accuracy determination of normal and abnormal forms. Furthermore, DNA integrity analysis, essential for understanding genetic viability, is improved through ML models trained to detect subtle genetic anomalies. Together, these AI-enhanced CASA systems provide a comprehensive, reliable, and repeatable approach to sperm analysis, transforming the field with faster, more accurate insights that aid in fertility diagnostics and treatments.
3.1. Machine Learning in CASA Systems
AI and ML are increasingly applied to CASA and ARTs. These technologies offer improved objectivity, efficiency, and accuracy in semen analysis and sperm selection [
3,
4]. CASA systems have evolved over 40 years, providing rapid analyses of sperm motility and morphology [
33]. AI algorithms can process large datasets, potentially enhancing sperm evaluation and selection for fertility treatments [
32,
35]. ML techniques have shown promise in embryo assessment and predicting treatment outcomes [
36]. However, challenges remain in standardizing AI applications for clinical use [
35]. The need for external validation of ML models and the use of big data analytics to enhance prediction accuracy are emphasized by [
37]. The recent development of a public benchmark for DL-powered morphology classification of human sperm by [
5] underscores the growing significance of these technologies in the field of CASA.
3.1.1. Classic Machine Learning
Classic ML methods have played a significant role in enhancing the field of CASA, particularly in the areas of sperm motility and morphology analysis. These traditional methods, including SVMs, RFs, and k-Nearest Neighbors (kNN), have been effectively utilized to analyze and predict outcomes in CASA systems [
38].
SVMs are particularly noted for their ability to handle high-dimensional data, making them suitable for the complex datasets typically found in CASA studies. SVMs work by finding the hyperplane that best separates the classes in the training data, which can be used for classification tasks such as predicting the motility of sperm [
39].
RF, Adaptive Boosting, Gradient Boosting, kNN, and Naïve Bayes are other classic ML algorithms that have been explored for sperm motility classification. In [
38], the authors utilized these classifiers to build a grading model for sperm motility, finding that the SVM performed best in classifying sperm with high motility.
In the domain of sperm morphology, classic ML techniques have also been applied. In [
40], authors developed an automatic framework to classify sperm heads as normal or abnormal using a supervised learning model trained on a novel set of morphological features.
These classic ML methods are generally less complex computationally than DL models, which can make them more accessible for use in clinical settings where resources and technical expertise might be limited. Moreover, the interpretability of these methods is a significant advantage, as it allows healthcare providers to make informed decisions based on the model’s findings.
However, while these traditional ML methods have proven useful, they also have limitations. Classic ML models often require extensive feature engineering and parameter tuning to achieve optimal performance. This process can be time-consuming and requires domain expertise, which may not always be readily available. In [
41], the authors pointed out that none of the standard descriptors or classification approaches is best suited for tackling the problem of sperm head classification, indicating the need for more specialized descriptors and classification methods.
3.1.2. Deep Learning
DL surpasses classic ML in CASA because it can automatically learn intricate patterns from complex datasets like images and videos. Its ability to handle high-dimensional data without extensive feature engineering makes it particularly effective for CASA tasks.
The application of DL in CASA has significantly advanced the field of male infertility diagnostics. Traditional manual methods for sperm morphology analysis are labor-intensive, subjective, and prone to variability, which has driven the development of automated systems using DL techniques [
42,
43].
DL frameworks have been employed to automate the classification of sperm morphology, focusing on various parts of the sperm cell, including the head, midpiece, and tail. These frameworks utilize convolutional neural networks (CNNs) and other advanced architectures to analyze and classify sperm images, providing a more standardized and efficient approach compared to manual examination [
5,
44].
The use of pre-trained models and transfer learning has been explored to enhance the performance of these systems. For example, models like VGG16 have been adapted for sperm morphology classification, leveraging their ability to learn complex features from large datasets [
6,
43]. Additionally, novel DL algorithms have been developed to address specific challenges in sperm morphology analysis, such as detecting deformities in the acrosome and vacuole regions [
45].
Overall, the integration of DL into CASA systems offers a promising solution to the limitations of traditional methods, providing higher precision, consistency, and efficiency in sperm morphology analysis. This technological advancement holds the potential to improve the accuracy of infertility diagnostics and optimize treatment outcomes [
46,
47]. However, ongoing research is necessary to validate these models across diverse datasets and clinical settings to ensure their robustness and generalizability [
5,
6].
3.1.3. Big Data
The role of big data in CASA is becoming increasingly significant as the volume of data generated by clinics continues to grow. CASA systems generate a wealth of data, capturing detailed parameters such as mean path velocity (VAP), curvilinear velocity (VCL), straight-line velocity (VSL), and the amplitude of lateral head displacement (ALH) [
48]. This extensive data collection has opened new avenues for big data analytics in reproductive science. The ability to effectively analyze this vast quantity of data can lead to more informed clinical decisions, personalized treatment plans, and improved patient outcomes [
37].
One of the primary advantages of big data in CASA is its potential to enhance predictive modeling. By analyzing large datasets, ML algorithms can identify patterns and correlations that may not be visible in smaller datasets. These predictions help clinicians tailor treatments to individual patients, potentially increasing the likelihood of successful outcomes.
Despite the potential, the current state of big data for CASA is still evolving. One of the primary challenges is the need for large, annotated datasets to train ML models for improved accuracy and reliability in sperm motility and kinematics assessment [
49]. The VISEM-Tracking dataset represents a significant step forward in this regard, enabling the training of complex models.
However, the use of big data in CASA also presents several challenges. Data privacy and security are major concerns, as the sensitive nature of reproductive health data requires stringent measures to protect patient confidentiality. Ensuring the accuracy and consistency of big data is another challenge, as data collected from different sources may vary in quality and format, making it difficult to integrate and analyze effectively.
In conclusion, big data holds tremendous potential to transform CASA by enabling more precise and personalized treatments, enhancing predictive modeling, and improving the safety and quality of care. However, realizing this potential requires overcoming significant challenges related to data privacy, integration, and analysis. As technology advances and the field of RM continues to evolve, the strategic use of big data will likely play an increasingly central role in shaping the future of CASA.
3.2. Sperm Quality Assessment with Artificial Intelligence
A range of AI tools have been developed and applied to assisted reproduction, particularly in IVF procedures. These tools have been shown to improve the efficiency of various steps in the ART process, including patient risk identification, gamete production assessment, and clinical management [
50]. They also have the potential to predict IVF outcomes, decrease variability, and enhance the selection of sperm and embryos [
51,
52]. Despite these advancements, the routine use of AI in assisted reproduction clinics requires further validation and ethical considerations [
53].
Clinical decision-making in semen analysis is often impacted by the subjective nature of evaluating microscopic sperm parameters such as concentration, motility, and morphology, in addition to human error and intra-operator variability. These factors can compromise the accuracy of the results.
3.2.1. Sperm Motility
Over the past 25 years, the introduction of Computer-Aided Semen Analysis (CASA) [
54] has supplemented traditional semen analysis to offer more consistent and less subjective outcomes. CASA systems, which utilize cameras and software to analyze microscopic details and provide sperm parameter data, excel in evaluating sperm kinematic parameters and hyperactivation. These automated systems deliver more objective and reproducible results than manual assessments. Nevertheless, certain uncertainties have hindered the widespread adoption of CASA. Evaluating sperm morphology remains problematic for CASA due to the wide range of abnormalities and different classification systems. Future advancements in artificial intelligence optical microscopic (AIOM) technologies may address these issues by enabling CASA systems to classify sperm morphology accurately. Despite progress in DL networks, further research is needed to confirm their efficacy.
Accurately monitoring changes in sperm motility is crucial for understanding various genetic and biochemical factors affecting fertilization. CASAnova [
55] is an automated method for classifying all patterns of human sperm motility during in vitro capacitation. Using support vector machine-based DT and data from 2817 sperm tracks, CASAnova assigns one of five classifications (progressive, intermediate, hyperactivated, slow, or weakly motile) to each sperm based on kinematic parameters. The program achieves an overall accuracy of 89.9% and effectively detects shifts in motility patterns during capacitation, showcasing its sensitivity and utility for high-throughput monitoring of sperm motility alterations.
We can find an interesting proposal that combines sperm mobility measures with sperm intracellular pH (pHi) and develops a gradient-boosted machine learning model to forecast successful conventional IVF outcomes among normospermic patients [
56]. Utilizing sperm samples from 76 IVF patients, pHi and hyperactivated motility were measured. A gradient-boosted machine learning model was then trained using clinical data, sperm pHi, and membrane potential to predict successful conventional IVF. Validation on an independent dataset of 18 patients demonstrated the model’s ability to predict successful IVF with a mean accuracy of 0.72, mean sensitivity of 0.65, and mean specificity of 0.80. These findings suggest a correlation between sperm pHi and conventional IVF outcomes and highlight the predictive capability of the machine learning model in assessing successful fertilization in normospermic patients undergoing IVF.
To aid in the early detection of seminal disorders and assist in identifying suitable donor candidates, the application of artificial neural networks (ANNs) [
57], specifically multilayer perceptron (MLP) networks, has proven highly effective in predicting sperm concentration and motility based on lifestyle and environmental data gathered from questionnaires [
58]. Since lifestyle and environmental factors must be considered to evaluate semen quality by processing fertility-related data, other AI techniques have also been applied, such as SVMs, particle swarm optimization [
59], and fuzzy radial basis function neural networks [
60].
In addition to proposals focused on final decision-making, there are approaches that combine different ML techniques to improve recorded data, for example, the integration of SPARCOM with model-based DL through algorithm unfolding to develop a compact neural network that leverages domain knowledge [
61]. Their findings show that the learned SPARCOM (LSPARCOM) network can produce super-resolution images from a limited number of high-emitter-density frames without needing optical system details. The authors believe LSPARCOM has the potential to enable efficient and interpretable live-cell imaging and could be widely applied in single-molecule localization microscopy of biological structures. Additionally, we can find multiple automated techniques for non-invasive measurement of single-sperm motility and morphology, offering precise quantitative data for sperm selection in ICSI, focused on improving the tracking of the movement of the spermatozoa [
62]. Using an adapted probabilistic data association filter for multi-sperm tracking and integrating total variation norm into image processing, the methods address challenges like intersecting sperm and uneven image intensity. Accurate coordinate transformation predicts sperm position during magnification switches. The experimental results demonstrate 95.6% accuracy in motility measurement and less than 10% error in morphology measurement.
Table 2 summarizes the main artificial intelligence techniques analyzed in this subsection, highlighting their respective advantages and limitations.
3.2.2. Sperm Morphology
Sperm morphology is a valuable indicator of sperm dysfunction in both basic and clinical research [
63]. Numerous studies [
4] have focused on this topic, with the main objective being the comparison between manual methods and automated systems, such as the X1 PRO semen analyzer [
64]. This device is capable of providing reliable results when the percentage of normal sperm forms is ≥4%. The LensHookeVR X12 semen analysis system uses a DL algorithm designed to automatically analyze sperm morphology, concentrating on features like the sperm head, midpiece, tail, and excess residual cytoplasm, which can provide reproducible and reliable male infertility diagnosis [
65]. These studies highlight the importance and the need for reliable automated procedures in an increasingly demanding environment.
Due to the complexity of the field and the growing number of AI-based approaches, it is crucial to establish standardized protocols to ensure optimal outcomes and minimize inter-expert variability [
66]. To address this, the development of a gold standard for sperm morphology analysis (SCIAN-MorphoSpermGS) has been proposed [
41]. Additionally, a benchmark set of experiments has been introduced, using this gold standard to systematically evaluate and compare classification performance across several supervised learning algorithms, including one-Nearest Neighbor, NB, DTs, and SVMs.
Lastly, CNNs have demonstrated excellent performance in classifying sperm based on their morphology [
67]. The primary advantage of these proposed models is that they require no manual intervention for proper functioning, enabling fully automated morphological classification of sperm images. A comprehensive summary of the AI-based methods applied to sperm morphology assessment, along with their respective advantages and limitations, is presented in
Table 3.
3.2.3. Sperm DNA Integrity
The sperm’s DNA fragmentation is another parameter to consider. The Comet assay [
34] offers us the method that lets us select the sperm with the highest DNA integrity through different AI techniques. In this method, the intact DNA forms the comet’s head, while the fragmented strands create the comet’s tail. By comparing the relative fluorescence of the tail to that of the head, the level of DNA damage can be quantified. We can find methods that offer a groundbreaking approach to assessing individual sperm DNA quality prior to clinical selection, filling a critical gap in current RM [
47]. This method employs deep CNNs to analyze sperm images, enabling the prediction of DNA quality without invasive tests. By training the network on images of approximately 1000 sperm cells, a moderate correlation between the images and DNA quality is achieved, facilitating the identification of cells with higher genetic integrity. Compatible with current sperm selection practices, this technology provides clinicians with the ability to swiftly predict DNA quality and select high-quality sperm, potentially enhancing outcomes in assisted reproductive techniques.
As discussed earlier, the different parameters used to assess male fertility are interrelated. Research has identified a correlation between DNA fragmentation and progressive sperm motility [
68], and there is also evidence linking and predicting sperm DNA integrity from morphological parameters at the single-cell level using LR [
69]. Given these interconnections, employing a combination of AI tools to analyze various characteristics and synthesize them into a comprehensive final analysis could enhance the overall assessment by integrating diverse sources of information.
Finally, ML has significantly expanded in the field of male fertility prediction. This includes specific optimizations of fertility prediction algorithms in men [
70], the development of tools to evaluate male infertility or potential sperm donors by predicting semen profiles and sperm concentration based on questionnaires [
71], and the prediction of biochemical markers in seminal plasma [
72], such as total protein, zinc content, and glucosidase activity, among others. These are some of the latest AI applications in the study of male fertility.
Table 4 summarizes the AI-driven approaches to sperm DNA integrity evaluation, outlining their advantages and limitations.
4. Sperm Open Data
Open datasets in male infertility research hold immense potential for propelling advancements in RM and enhancing outcomes for individuals undergoing ARTs, including IVF. The transparent availability of fertility data fosters seamless collaboration between researchers and clinicians, facilitating efficient scientific progress and enriching the standard of patient care. The analyzed papers showcase a wide spectrum of sperm datasets, differing in their focus, scale, and utility, underscoring the varied methodologies employed in male infertility research for sperm analysis. A summary table of all the analyzed datasets can be seen in
Table 5.
In SVIA, ref. [
76] introduces the dataset, which includes microscopic videos and images of sperm, designed to evaluate computer vision techniques in sperm analysis. This extensive dataset contains 101 videos, 130,042 images, and 127,600 object information, making it a comprehensive resource for testing object detection, tracking, and image classification technologies in the context of sperm analysis.
The VISEM-tracking dataset [
49] focuses on tracking human spermatozoa through video recordings. This dataset includes 20 video recordings, each 30 s long, providing 29,196 frames (see an example in
Figure 4). It is designed to improve the accuracy and reliability of CASA systems by providing data that can be used to train supervised machine learning models to assess sperm motility and kinematics.
The VISEM dataset [
77] presents a multimodal dataset that includes videos, biological analysis data, and participant data, offering a rich source for studying human spermatozoa under various conditions. This dataset is notable for its comprehensive approach, combining video data with detailed biological and participant information, which can be used to explore new research questions in human reproduction.
McCallum et al. [
47] discuss a DL-based approach for selecting human sperm with high DNA integrity using a dataset of 1000 sperm cells known as HSMA-DS. That dataset is particularly focused on the DNA quality of sperm and is used to train a CNN to predict DNA quality from brightfield images alone.
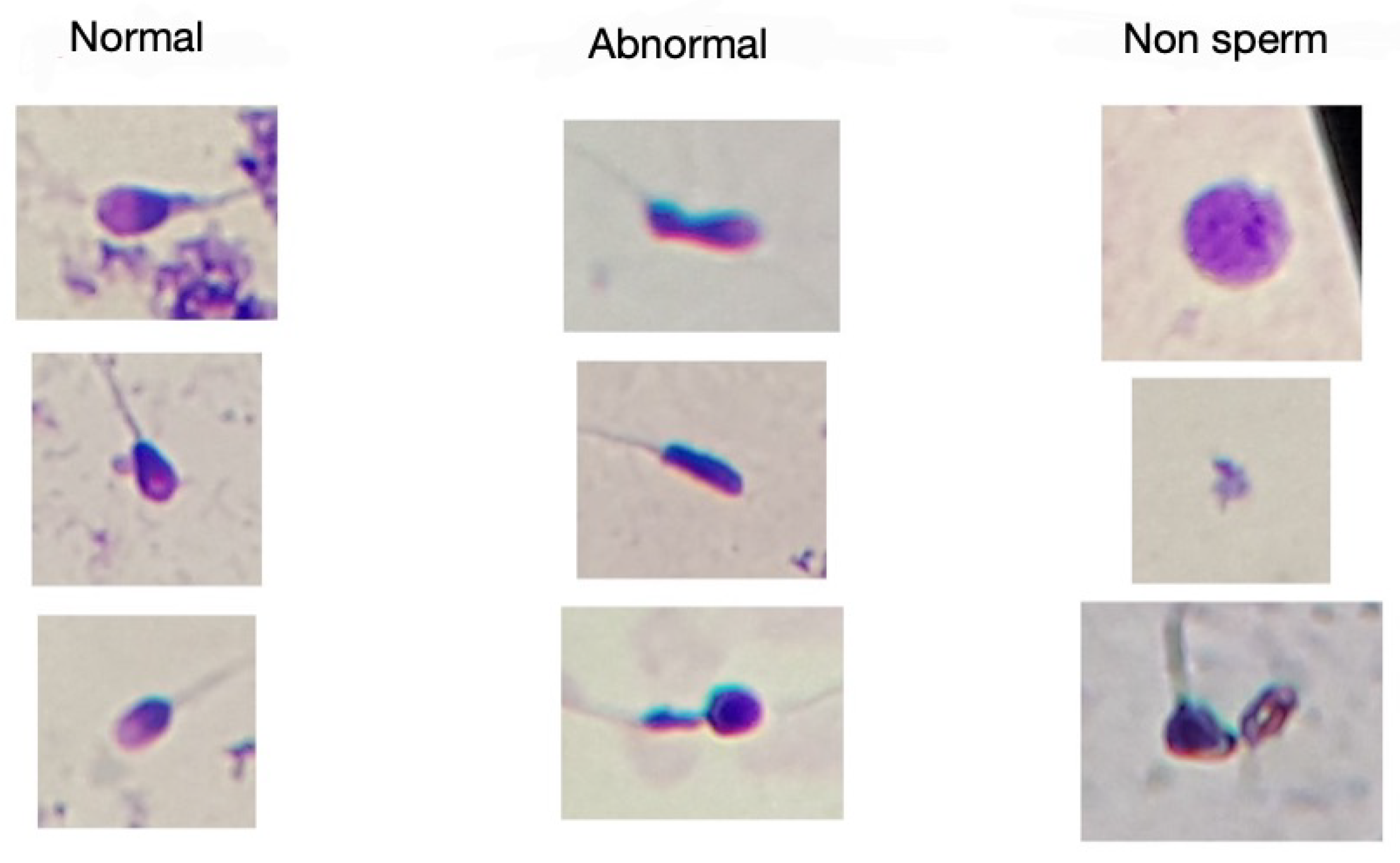
A hybrid human sperm detection and classification system was tested on a novel publicly available morphological sperm image dataset [
75]. That dataset included images labeled by experts as non-sperm, normal, and abnormal sperm, making it a valuable resource for developing and testing new machine learning methods for sperm morphology analysis.
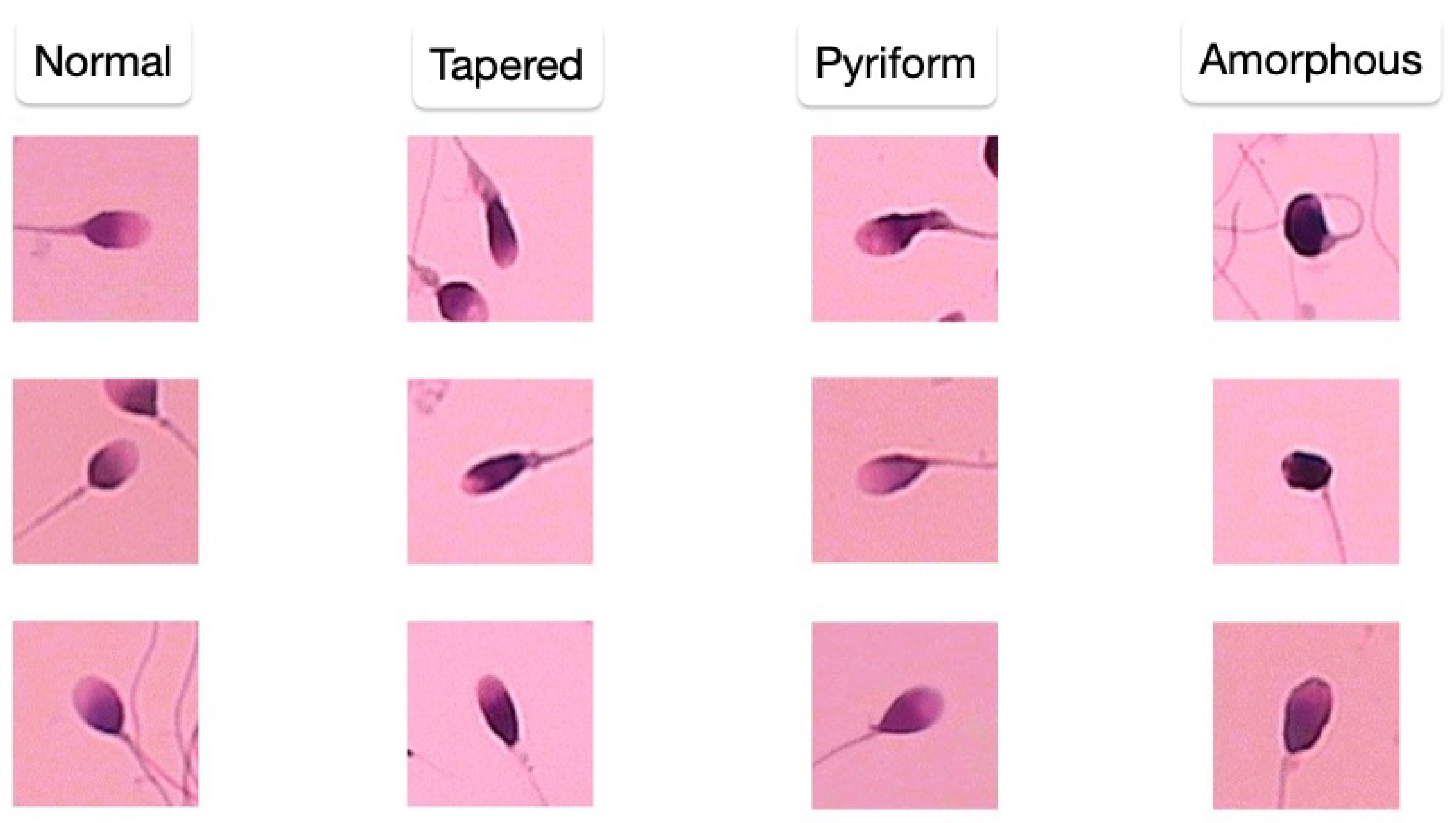
The SCIAN-MorphoSpermGS dataset [
41] provides a gold-standard dataset for morphological sperm analysis (
Figure 5). The dataset includes sperm head images classified into normal, tapered, and amorphous categories, serving as a benchmark for evaluating sperm classification techniques.
The HuSHeM dataset [
74] includes images of human sperm heads classified into four categories (see
Figure 6). The dataset supports the development of dictionary-learning techniques for classifying sperm head shapes. The classification is performed through expert annotation, ensuring reliable identification of each morphological type.
To provide a detailed comparison of how morphology is evaluated in SCIAN-MorphoSpermGS and HuSHeM datasets and the reported performance levels of these specific techniques relevant to the classifications shown in
Figure 5 and
Figure 6,
Table 6 summarizes the key aspects of the approaches from [
41,
74]. This table highlights the datasets used, the methods for feature quantification or representation, the classification algorithms employed, and key performance metrics achieved by these studies, offering insight into their respective strengths and limitations in classifying these complex morphological types.
Javadi’s paper [
45] introduces the MHSMA dataset, which consists of 1540 sperm images used to train a deep learning model for detecting morphological deformities in sperm parts such as the acrosome and head.
Ghasemian discusses the HSMA-DS dataset, comprising 1457 sperm images used to develop an automatic sperm morphology analysis system [
73]. That dataset is significant for its focus on normal and abnormal sperm, providing a balanced view for training classification systems.
In conclusion, these datasets collectively enhance research capabilities in sperm analysis by providing diverse, well-annotated resources that address various aspects of sperm morphology and function. They enable the development of advanced computational models that can lead to more accurate and less subjective analyses of male fertility. The availability of these datasets encourages collaborative and comparative research, potentially accelerating advancements in the diagnosis and treatment of male infertility. Integrating these datasets with ML models represents a promising direction for future research, potentially leading to breakthroughs in RM.
5. Future Challenges
Integrating AI into assisted reproductive technologies continues to advance, yet several challenges remain that must be addressed to fully harness its potential. A primary issue is the development of robust, clinically generalizable models. Many current AI systems depend on high-quality, standardized datasets, and the variability across clinical settings can impede the accuracy and reliability of these models. Expanding and harmonizing datasets, while ensuring rigorous validation through controlled studies, is essential.
Another challenge lies in the evolving regulatory and ethical landscape. As AI applications in fertility care progress toward clinical use, establishing clear guidelines and regulatory frameworks becomes critical. These must address not only patient safety, data privacy, and security but also complex ethical issues, such as consent and the long-term implications of AI-driven reproductive decisions.
Interoperability with existing clinical infrastructure presents an additional hurdle. AI tools must seamlessly integrate with electronic health records and ART management systems, necessitating standardized data formats and communication protocols to ensure smooth and effective deployment.
An emerging challenge is the need for explainable AI (XAI). As deep learning models become more complex, ensuring transparency and interpretability is crucial for building trust among clinicians and patients. XAI techniques can provide insights into how decisions are made, facilitating accountability and informed clinical decision-making, which are essential in sensitive areas like reproductive healthcare.
Finally, the successful adoption of AI in this field depends on comprehensive training and education for healthcare providers. Clinicians need to understand how to interpret and effectively incorporate AI insights into patient care, which calls for multidisciplinary collaboration between medical professionals, data scientists, and regulatory experts.
By addressing these challenges, the field can move toward a future where AI not only improves diagnostic accuracy and treatment outcomes but also supports ethical, efficient, and patient-centered fertility care.
6. Conclusions
The integration of AI and ML has brought significant improvements in diagnostic accuracy, efficiency, and objectivity within ARTs, especially in the evaluation of male fertility. Notably, CASA systems have enhanced the assessment of critical sperm parameters such as motility, morphology, and DNA integrity, providing more consistent and reproducible results compared to manual methods. Furthermore, DL techniques have enabled advanced image classification, supporting fully automated fertility diagnostics with demonstrated effectiveness. AI-driven sperm selection also contributes by reducing human error and accelerating analysis, which in turn helps improve IVF outcomes. However, several challenges remain before these technologies can be fully integrated into routine clinical workflows. Key limitations include the following:
The lack of standardized, open-access datasets, which limits model generalizability and validation.
Unresolved ethical, regulatory, and data security issues that could hinder widespread adoption.
The need for further clinical validation to confirm real-world efficacy and long-term benefits.
Practical barriers such as integrating AI systems with existing workflows and training healthcare professionals.
Looking ahead, advancing AI in ARTs will require sustained interdisciplinary collaboration and the development of robust, standardized datasets. Additionally, clear regulatory frameworks and guidelines must be established to ensure safe and ethical implementation. With these measures, AI holds strong potential to personalize fertility treatments, enhance success rates, and increase access to reproductive healthcare globally.
Author Contributions
Conceptualization, F.J.B., D.G.-G. and C.F.-B.; Investigation, F.J.B., D.G.-G. and C.F.-B.; Writing—Original Draft, F.J.B., D.G.-G. and C.F.-B.; Writing—Review and Editing, F.J.B., D.G.-G. and C.F.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCIU/AEI/10.13039/501100011033 and by ERDF/EU (FederaMed project) grant number PID2021-123960OB-I00. This work was also partially supported by the Knowledge Generation Project PID2023-150070NB-I00, funded by the Ministry of Science, Innovation, and Universities of Spain. Finally, the research reported in this paper was also funded by the European Union (BAG-INTEL) project, grant agreement no. 101121309).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors would also like to thank Clínica Sanabria, Unidad de Reproducción Vithas, and Unidad de Reproducción del Hospital Materno Infantil for their collaboration and support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| AIOM | Artificial Intelligence Optical Microscopy |
| ALH | Amplitude of Lateral Head Displacement |
| ANN | Artificial Neural Network |
| ARTs | Assisted Reproductive Technologies |
| CASA | Computer-Aided Sperm Analysis |
| CNN | Convolutional Neural Network |
| DGC | Density Gradient Centrifugation |
| DL | Deep Learning |
| DT | Decision Tree |
| HSMA-DS | Human Sperm Morphology Analysis DataSet |
| HuSHeM | Human Sperm Head Morphology |
| ICSI | Intracytoplasmic Sperm Injection |
| IVF | In Vitro Fertilization |
| kNN | k-Nearest Neighbors |
| LR | Logistic Regression |
| LSPARCOM | Learned SPARCOM |
| ML | Machine Learning |
| MLP | Multilayer Perceptron |
| MHSMA | Modified Human Sperm Morphology Analysis |
| NB | Naïve Bayes |
| pHi | Intracellular pH |
| RF | Random Forest |
| RM | Reproductive Medicine |
| SCIAN-MorphoSpermGS | Gold Standard for Sperm Morphology Analysis |
| SMIDS | Sperm Morphology Image Data Set |
| SVIA | Sperm Videos and Images Analysis |
| SVM | Support Vector Machine |
| VAP | Mean Path Velocity |
| VCL | Curvilinear Velocity |
| VISEM | Video Dataset of Human Semen |
| VISEM-Tracking | VISEM Tracking Dataset |
| VSL | Straight-Line Velocity |
| XAI | Explainable Artificial Intelligence |
References
- Baker, S.; Xiang, W. Artificial intelligence of things for smarter healthcare: A survey of advancements, challenges, and opportunities. IEEE Commun. Surv. Tutor. 2023, 25, 1261–1293. [Google Scholar] [CrossRef]
- Tal, R.; Talarczyk-Desole, J.; Bentov, Y.; Lin, Y.J.; Fujiwara, T.; Tiboni, G.M. Advances in In Vitro Fertilization; Scientific Research Publishing: Wuhan, China, 2017; ISBN 978-1-61896-332-1. [Google Scholar]
- Panner Selvam, M.K.; Moharana, A.K.; Baskaran, S.; Finelli, R.; Hudnall, M.C.; Sikka, S.C. Current Updates on Involvement of Artificial Intelligence and Machine Learning in Semen Analysis. Medicina 2024, 60, 279. [Google Scholar] [CrossRef] [PubMed]
- Cherouveim, P.; Velmahos, C.; Bormann, C.L. Artificial intelligence for sperm selection—A systematic review. Fertil. Steril. 2023, 120, 24–31. [Google Scholar] [CrossRef]
- Shahali, S.; Murshed, M.; Spencer, L.; Tunc, O.; Pisarevski, L.; Conceicao, J.; McLachlan, R.; O’Bryan, M.K.; Ackermann, K.; Zander-Fox, D.; et al. Morphology Classification of Live Unstained Human Sperm Using Ensemble Deep Learning. Adv. Intell. Syst. 2024, 6, 2400141. [Google Scholar] [CrossRef]
- Yamashita, H.; Yasui, N.; Uchida, N.; Sukenobe, Y.; Ibayashi, M.; Saito, M.; Hiraoka, K.; Kawai, K. Investigation of deep learning based detection of sperm morphological defects. Fertil. Steril. 2019, 112, e281. [Google Scholar] [CrossRef]
- Barak, Y.; Amit, A.; Lessing, J.B.; Paz, G.; Homonnai, Z.T.; Yogev, L. Improved fertilization rate in an in vitro fertilization program by egg yolk-treated sperm. Fertil. Steril. 1992, 58, 197–198. [Google Scholar] [CrossRef]
- Moon, S.Y. In Vitro Fertilization Program. J. Korean Med Assoc. 2007, 50, 431. [Google Scholar] [CrossRef]
- Zaninovic, N.; Rosenwaks, Z. Artificial intelligence in human in vitro fertilization and embryology. Fertil. Steril. 2020, 114, 914–920. [Google Scholar] [CrossRef]
- Bing, Y.; Ouellette, R.J. Fertilization in vitro. In Human Embryogenesis: Methods and Protocols; Vaillancourt, C., Lafond, J., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 251–266. [Google Scholar]
- Soriano-Úbeda, C.; García-Vázquez, F.A.; Romero-Aguirregomezcorta, J.; Matás, C. Improving porcine in vitro fertilization output by simulating the oviductal environment. Sci. Rep. 2017, 7, 43616. [Google Scholar] [CrossRef]
- Gordon, J.W. Use of Micromanipulation for Increasing the Efficiency of Mammalian Fertilization In Vitro. Ann. N. Y. Acad. Sci. 1988, 541, 601–613. [Google Scholar] [CrossRef]
- Leung, C. Recent advances in clinical aspects of in vitro fertilisation. Hong Kong Med J. 2000, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.G.; Rosen, C.J.; Shiffler, A.K.; Taysom, T.W. Enhanced Efficiency Fertilizers for Improved Nutrient Management: Potato (Solanum Tuberosum). Crop Manag. 2008, 7, 1–16. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Improving Nutrient Use Efficiency in Crops. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Alexander, A.; Schroeder, M. Fertilizer use efficiency. J. Plant Nutr. 1987, 10, 1391–1399. [Google Scholar] [CrossRef]
- Huang, J.Y.J.; Rosenwaks, Z. In vitro fertilisation treatment and factors affecting success. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 777–788. [Google Scholar] [CrossRef]
- Janát-Amsbury, M.M.; Gupta, K.M.; Kablitz, C.D.; Peterson, C.M. Drug delivery for in vitro fertilization: Rationale, current strategies and challenges. Adv. Drug Deliv. Rev. 2009, 61, 871–882. [Google Scholar] [CrossRef]
- Fechner, A.J. The state of the art of in vitro fertilization. Front. Biosci. 2011, E3, 264–278. [Google Scholar] [CrossRef]
- Letur-Könirsch, H.; Olivennes, F.; Raoul-Duval, A.; Frydman, R. Techniques, interrogations and results of medically assisted procreation. Pediatrie 1993, 48, 872–882. [Google Scholar]
- Medenica, S.; Zivanovic, D.; Batkoska, L.; Marinelli, S.; Basile, G.; Perino, A.; Cucinella, G.; Gullo, G.; Zaami, S. The Future Is Coming: Artificial Intelligence in the Treatment of Infertility Could Improve Assisted Reproduction Outcomes—The Value of Regulatory Frameworks. Diagnostics 2022, 12, 2979. [Google Scholar] [CrossRef]
- VerMilyea, M.; Hall, J.; Diakiw, S.; Johnston, A.; Nguyen, T.; Perugini, D.; Miller, A.; Picou, A.; Murphy, A.; Perugini, M. Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF. Hum. Reprod. 2020, 35, 770–784. [Google Scholar] [CrossRef]
- Kragh, M.F.; Karstoft, H. Embryo selection with artificial intelligence: How to evaluate and compare methods? J. Assist. Reprod. Genet. 2021, 38, 1675–1689. [Google Scholar] [CrossRef]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. Imagenet large scale visual recognition challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Mai, Q. Multi-Omics Analysis and Machine Learning Prediction Model for Pregnancy Outcomes After Intracytoplasmic Sperm Injection–in vitro Fertilization. Front. Public Health 2022, 10, 924539. [Google Scholar] [CrossRef]
- Hahne, F.; Huber, W.; Gentleman, R.; Falcon, S.; Gentleman, R.; Carey, V. Unsupervised machine learning. In Bioconductor Case Studies; Springer: New York, NY, USA, 2008; pp. 137–157. [Google Scholar]
- Milewska, A.J.; Jankowska, D.; Citko, D.; Wiesak, T.; Acacio, B.; Milewski, R. The use of principal component analysis and logistic regression in prediction of infertility treatment outcome. Stud. Log. GRAMMAR Rhetor. 2014, 39, 7–23. [Google Scholar] [CrossRef]
- Srivastava, D.; Gupta, S.; Kudavelly, S.; Suryanarayana K, V.; Ga, R. Unsupervised Deep Learning based Longitudinal Follicular Growth Tracking during IVF Cycle using 3D Transvaginal Ultrasound in Assisted Reproduction. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual Conference, 1–5 November 2021; pp. 3209–3212. [Google Scholar]
- Milachich, T.; Dyulgerova-Nikolova, D. The Sperm: Parameters and Evaluation. In Innovations in Assisted Reproduction Technology; Sharma, N., Chakrabarti, S., Barak, Y., Ellenbogen, A., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 1. [Google Scholar]
- Si, K.; Huang, B.; Jin, L. Application of artificial intelligence in gametes and embryos selection. Hum. Fertil. 2023, 26, 757–777. [Google Scholar] [CrossRef] [PubMed]
- You, J.B.; McCallum, C.; Wang, Y.; Riordon, J.; Nosrati, R.; Sinton, D. Machine learning for sperm selection. Nat. Rev. Urol. 2021, 18, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial intelligence in reproductive medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef]
- Amann, R.P.; Waberski, D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17.e3. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Fernandez, E.I.; Ferreira, A.S.; Cecílio, M.H.M.; Chéles, D.S.; de Souza, R.C.M.; Nogueira, M.F.G.; Rocha, J.C. Artificial intelligence in the IVF laboratory: Overview through the application of different types of algorithms for the classification of reproductive data. J. Assist. Reprod. Genet. 2020, 37, 2359–2376. [Google Scholar] [CrossRef]
- Zaninovic, N.; Elemento, O.; Rosenwaks, Z. Artificial intelligence: Its applications in reproductive medicine and the assisted reproductive technologies. Fertil. Steril. 2019, 112, 28–30. [Google Scholar] [CrossRef]
- Ranjini, K.; Suruliandi, A.; Raja, S.P. Machine Learning Techniques for Assisted Reproductive Technology: A Review. J. Circ. Syst. Comput. 2020, 29, 2030010. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, F. P-052 Machine learning-based sperm motility grading model. Hum. Reprod. 2024, 39, deae108.429. [Google Scholar] [CrossRef]
- Hidayatullah, P.; Mengko, T.L.E.R.; Munir, R.; Barlian, A. Bull Sperm Tracking and Machine Learning-Based Motility Classification. IEEE Access 2021, 9, 61159–61170. [Google Scholar] [CrossRef]
- Revollo, N.V.; Sarmiento, G.N.R.; Delrieux, C.; Herrera, M.; González-José, R. Supervised machine learning classification of human sperm head based on morphological features. In Trends and Advancements of Image Processing and Its Applications; Springer: Cham, Switzerland, 2021; pp. 177–191. [Google Scholar]
- Chang, V.; Garcia, A.; Hitschfeld, N.; Härtel, S. Gold-standard for computer-assisted morphological sperm analysis. Comput. Biol. Med. 2017, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Mustafa, G.; Ma, J. Deep Learning-Based Morphological Classification of Human Sperm Heads. Diagnostics 2020, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Riordon, J.; McCallum, C.; Sinton, D. Deep learning for the classification of human sperm. Comput. Biol. Med. 2019, 111, 103342. [Google Scholar] [CrossRef]
- Chandra, S.; Gourisaria, M.K.; Gm, H.; Konar, D.; Gao, X.; Wang, T.; Xu, M. Prolificacy Assessment of Spermatozoan via State-of-the-Art Deep Learning Frameworks. IEEE Access 2022, 10, 13715–13727. [Google Scholar] [CrossRef]
- Javadi, S.; Mirroshandel, S.A. A novel deep learning method for automatic assessment of human sperm images. Comput. Biol. Med. 2019, 109, 182–194. [Google Scholar] [CrossRef]
- Suleman, M.; Ilyas, M.; Lali, M.I.U.; Rauf, H.T.; Kadry, S. A review of different deep learning techniques for sperm fertility prediction. AIMS Math. 2023, 8, 16360–16416. [Google Scholar] [CrossRef]
- McCallum, C.; Riordon, J.; Wang, Y.; Kong, T.; You, J.B.; Sanner, S.; Lagunov, A.; Hannam, T.G.; Jarvi, K.; Sinton, D. Deep learning-based selection of human sperm with high DNA integrity. Commun. Biol. 2019, 2, 250. [Google Scholar] [CrossRef]
- Agustinus; Pakpahan, C. Computer Assisted Sperm Analysis: A Review. Indones. Androl. Biomed. J. 2020, 1, 60–66. [Google Scholar] [CrossRef]
- Thambawita, V.; Hicks, S.A.; Storås, A.M.; Nguyen, T.; Andersen, J.M.; Witczak, O.; Haugen, T.B.; Hammer, H.L.; Halvorsen, P.; Riegler, M.A. VISEM-Tracking, a human spermatozoa tracking dataset. Sci. Data 2023, 10, 260. [Google Scholar] [CrossRef]
- Bulletti, F.; Berrettini, M.; Sciorio, R.; Bulletti, C. Artificial intelligence algorithms for optimizing assisted reproductive technology programs: A systematic review. Glob. Transl. Med. 2023, 2, 0308. [Google Scholar] [CrossRef]
- Raimundo, J.; Cabrita, P. Artificial intelligence at assisted reproductive technology. Procedia Comput. Sci. 2021, 181, 442–447. [Google Scholar] [CrossRef]
- Naser, M.; Mohamed, M.; Shehata, L.H. Artificial Intelligence in Assisted Reproductive Technology Review. Int. J. Prog. Sci. Technol. 2021, 25, 507–511. [Google Scholar] [CrossRef]
- Pavlovic, Z.J.; Jiang, V.S.; Hariton, E. Current applications of artificial intelligence in assisted reproductive technologies through the perspective of a patient’s journey. Curr. Opin. Obstet. Gynecol. 2024, 36, 211–217. [Google Scholar] [CrossRef]
- Tomlinson, M.J.; Naeem, A. CASA in the medical laboratory: CASA in diagnostic andrology and assisted conception. Reprod. Fertil. Dev. 2018, 30, 850–859. [Google Scholar] [CrossRef]
- Goodson, S.G.; White, S.; Stevans, A.M.; Bhat, S.; Kao, C.Y.; Jaworski, S.; Marlowe, T.R.; Kohlmeier, M.; McMillan, L.; Zeisel, S.H.; et al. CASAnova: A multiclass support vector machine model for the classification of human sperm motility patterns. Biol. Reprod. 2017, 97, 698–708. [Google Scholar] [CrossRef]
- Gunderson, S.J.; Molina, L.C.P.; Spies, N.; Balestrini, P.A.; Buffone, M.G.; Jungheim, E.S.; Riley, J.; Santi, C.M. Machine-learning algorithm incorporating capacitated sperm intracellular pH predicts conventional in vitro fertilization success in normospermic patients. Fertil. Steril. 2021, 115, 930–939. [Google Scholar] [CrossRef]
- Collobert, R.; Bengio, S. Links between perceptrons, MLPs and SVMs. In Proceedings of the 21st International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 23. [Google Scholar]
- Girela, J.L.; Gil, D.; Johnsson, M.; Gomez-Torres, M.J.; De Juan, J. Semen parameters can be predicted from environmental factors and lifestyle using artificial intelligence methods. Biol. Reprod. 2013, 88, 99. [Google Scholar] [CrossRef]
- Sahoo, A.J.; Kumar, Y. Seminal quality prediction using data mining methods. Technol. Health Care 2014, 22, 531–545. [Google Scholar] [CrossRef]
- Candemir, C. Estimating the semen quality from life style using fuzzy radial basis functions. Int. J. Mach. Learn. Comput 2018, 8, 44–48. [Google Scholar] [CrossRef]
- Dardikman-Yoffe, G.; Eldar, Y.C. Learned SPARCOM: Unfolded deep super-resolution microscopy. Opt. Express 2020, 28, 27736–27763. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, Z.; Huang, J.; Wang, X.; Ru, C.; Pu, H.; Xie, S.; Zhang, J.; Moskovtsev, S.; Librach, C.; et al. Automated non-invasive measurement of single sperm’s motility and morphology. IEEE Trans. Med. Imaging 2018, 37, 2257–2265. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Noto, D.; Corsaro, R.; Collodel, G. The relevance of sperm morphology in male infertility. Front. Reprod. Health 2022, 4, 945351. [Google Scholar] [CrossRef]
- Agarwal, A.; Selvam, M.K.P. P–011 Automated sperm morphology assessment using artificial intelligence technology. Hum. Reprod. 2021, 36, deab130.010. [Google Scholar] [CrossRef]
- Chang, A.; Yang, Y.C.; Henthorn, D.; Cheng, Y.S. P-034 Revolutionizing sperm morphology assessment: A novel artificial intelligence optic microscope (AIOM)-powered platform for consistent and reliable analysis in male infertility diagnosis. Hum. Reprod. 2024, 39, deae108.411. [Google Scholar] [CrossRef]
- Fleiss, J.L. Measuring nominal scale agreement among many raters. Psychol. Bull. 1971, 76, 378. [Google Scholar] [CrossRef]
- Yüzkat, M.; Ilhan, H.O.; Aydin, N. Multi-model CNN fusion for sperm morphology analysis. Comput. Biol. Med. 2021, 137, 104790. [Google Scholar] [CrossRef]
- Elbashir, S.; Magdi, Y.; Rashed, A.; Ibrahim, M.A.; Edris, Y.; Abdelaziz, A.M. Relationship between sperm progressive motility and DNA integrity in fertile and infertile men. Middle East Fertil. Soc. J. 2018, 23, 195–198. [Google Scholar] [CrossRef]
- Wang, Y.; Riordon, J.; Kong, T.; Xu, Y.; Nguyen, B.; Zhong, J.; You, J.B.; Lagunov, A.; Hannam, T.G.; Jarvi, K.; et al. Prediction of DNA integrity from morphological parameters using a single-sperm DNA fragmentation index assay. Adv. Sci. 2019, 6, 1900712. [Google Scholar] [CrossRef] [PubMed]
- Engy, E.; Ali, E.; Sally, E.G. An optimized artificial neural network approach based on sperm whale optimization algorithm for predicting fertility quality. Stud. Inform. Control 2018, 27, 349–358. [Google Scholar]
- Badura, A.; Marzec-Wróblewska, U.; Kamiński, P.; Łakota, P.; Ludwikowski, G.; Szymański, M.; Wasilow, K.; Lorenc, A.; Buciński, A. Prediction of semen quality using artificial neural network. J. Appl. Biomed. 2019, 17, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vickram, A.; Kamini, A.R.; Das, R.; Pathy, M.R.; Parameswari, R.; Archana, K.; Sridharan, T. Validation of artificial neural network models for predicting biochemical markers associated with male infertility. Syst. Biol. Reprod. Med. 2016, 62, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, F.; Mirroshandel, S.A.; Monji-Azad, S.; Azarnia, M.; Zahiri, Z. An efficient method for automatic morphological abnormality detection from human sperm images. Comput. Methods Programs Biomed. 2015, 122, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Shaker, F.; Monadjemi, S.A.; Alirezaie, J.; Naghsh-Nilchi, A.R. A dictionary learning approach for human sperm heads classification. Comput. Biol. Med. 2017, 91, 181–190. [Google Scholar] [CrossRef]
- Ilhan, H.O.; Sigirci, I.O.; Serbes, G.; Aydin, N. A fully automated hybrid human sperm detection and classification system based on mobile-net and the performance comparison with conventional methods. Med. Biol. Eng. Comput. 2020, 58, 1047–1068. [Google Scholar] [CrossRef]
- Chen, A.; Li, C.; Zou, S.; Rahaman, M.M.; Yao, Y.; Chen, H.; Yang, H.; Zhao, P.; Hu, W.; Liu, W.; et al. SVIA dataset: A new dataset of microscopic videos and images for computer-aided sperm analysis. Biocybern. Biomed. Eng. 2022, 42, 204–214. [Google Scholar] [CrossRef]
- Haugen, T.B.; Hicks, S.A.; Andersen, J.M.; Witczak, O.; Hammer, H.L.; Borgli, R.; Halvorsen, P.; Riegler, M. Visem: A multimodal video dataset of human spermatozoa. In Proceedings of the 10th ACM Multimedia Systems Conference, Amherst, MA, USA, 18–21 June 2019; pp. 261–266. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).