Dynamics of Blood Flows in the Cardiocirculatory System
Abstract
1. Introduction
1.1. Adopted Model: Features, Limitations and Scientific Context
1.2. Contribution and Structure of the Paper
2. Mathematical Model
2.1. Newtonian Blood Flow
2.2. Basic Equations
2.3. Interfaces
2.3.1. Riemann Problem at the Interface
2.3.2. Inflow, Interface, and Terminal Boundary Conditions
- Case 1 (pure resistance). We assume that the power exchanges between the different parts of the human body involved in the network are mostly due to dissipative effects, described by the only terminal resistance, . Such an assumption reads asNotice that corresponds to a free terminal site, namely , and we obtainFinally, means there are no reflected characteristics at the terminal site; indicates a complete reflection, i.e., blockage condition, .
- Case 2 (RC cell). This case deals with a typical RC cell, i.e., a simple circuit with a resistor and a capacitor. In analogy with other situations that involve more electric components, this situation obeys the need to provide suitable modeling for both the dissipative and conservative effects within the human body. A similar situation is not unusual considering that, especially in the cardiovascular system, the heart is used to suffering the effects of other tissues (dissipative phenomena) and radiating energy (conservative effect) to the individual organs of the body by pumping blood. From a mathematical point of view, without affecting the generality of the discussion, we consider the lumped parameter model that consists of only a resistance that, under suitable assumptions, refers to either dissipative or conservative effects. In this case, assuming that and are, respectively, the pressures at the inlet and at the end of the arterial domain, we have the following nonlinear equation for :The last equation is easily solved (numerically) by using Newton’s method and assuming as a starting point. Finally, we haveand the terminal conditions reduce to
- Case 3 (compliance). As the previous case can generate oscillations for pressure and flow, a more complicated model that deals with compliance can be considered. Compliance is a capacitor, C, that models the stabilization of the pressure. In this case, it is necessary to compute by using a recursive equation of the following form:where F is the suitable continuous function described in [18]. In this case, the terminal conditions are written as
3. Numerical Schemes
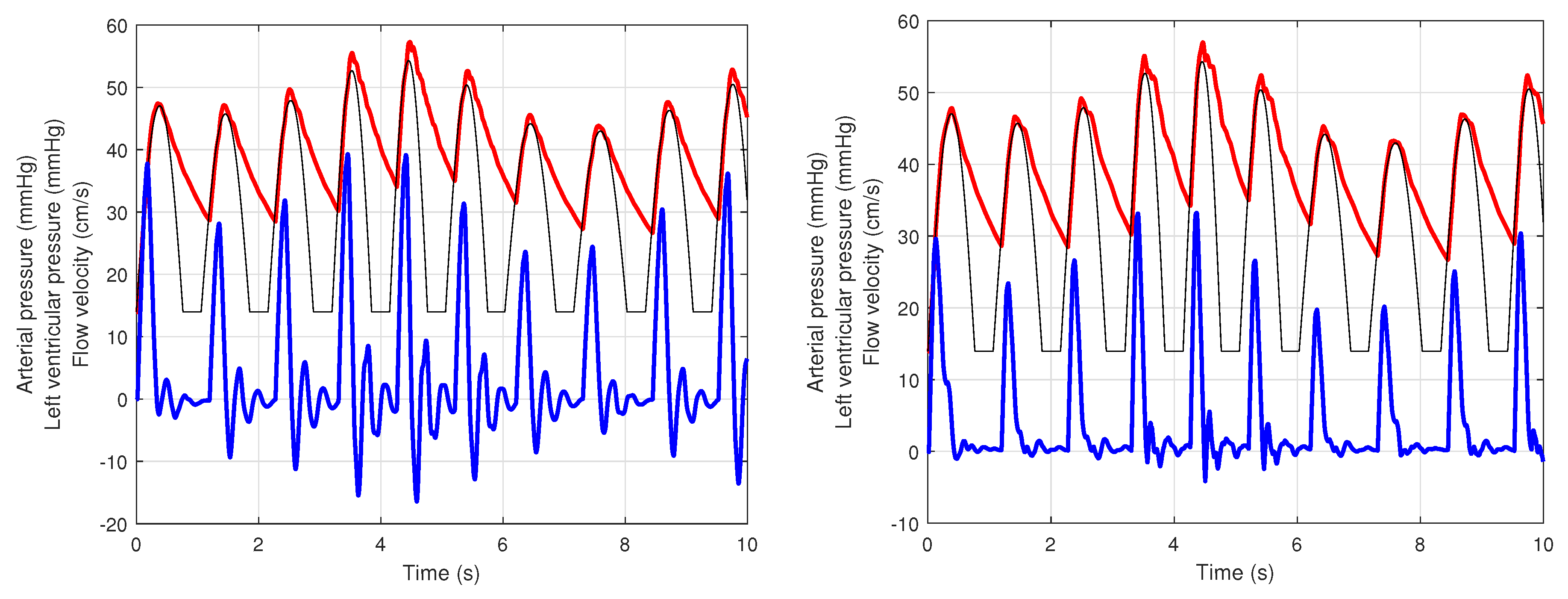
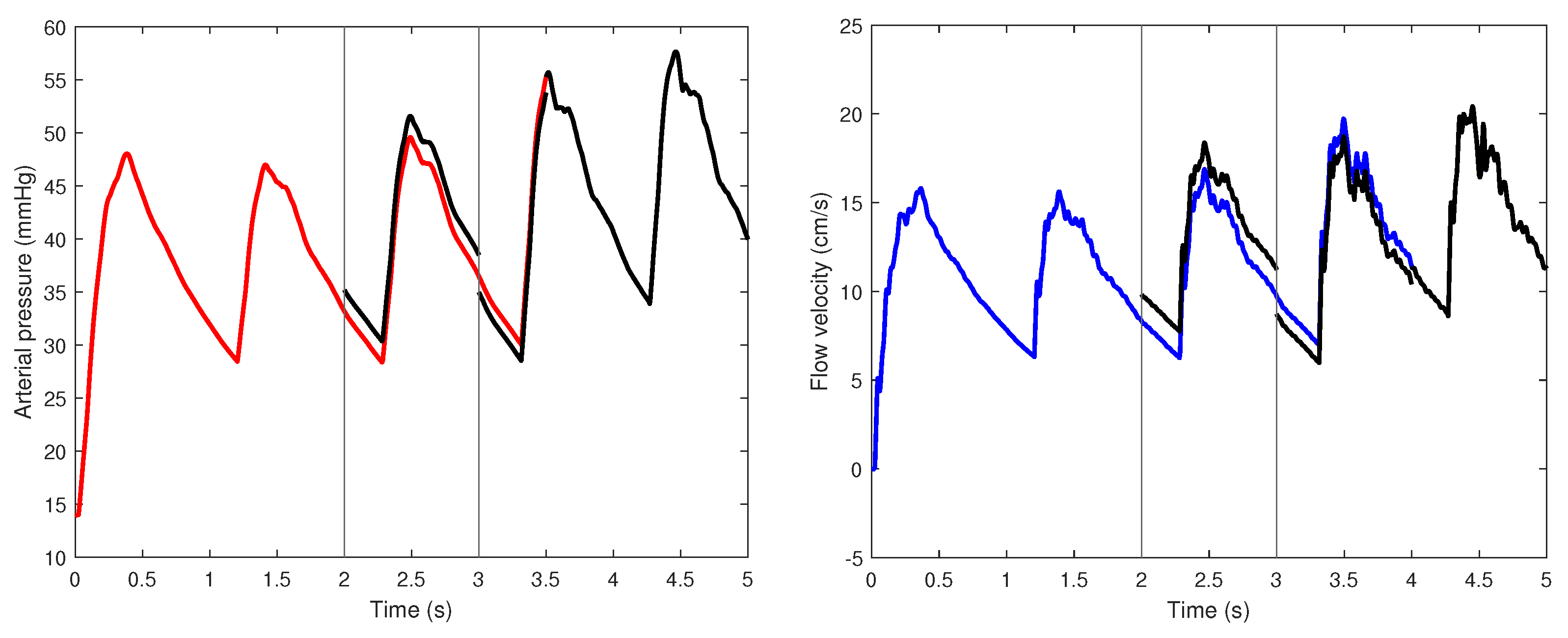
4. Simulation Results
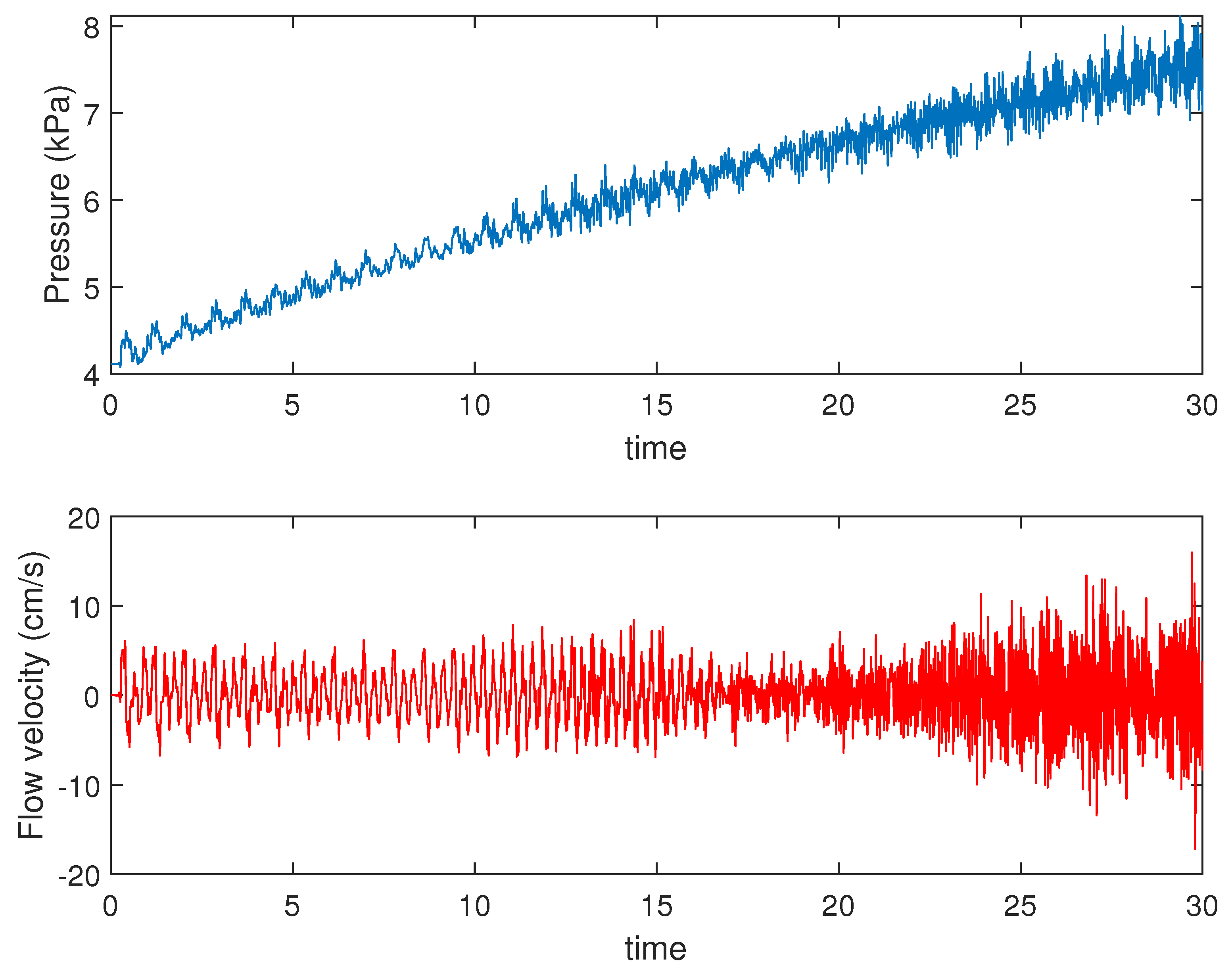
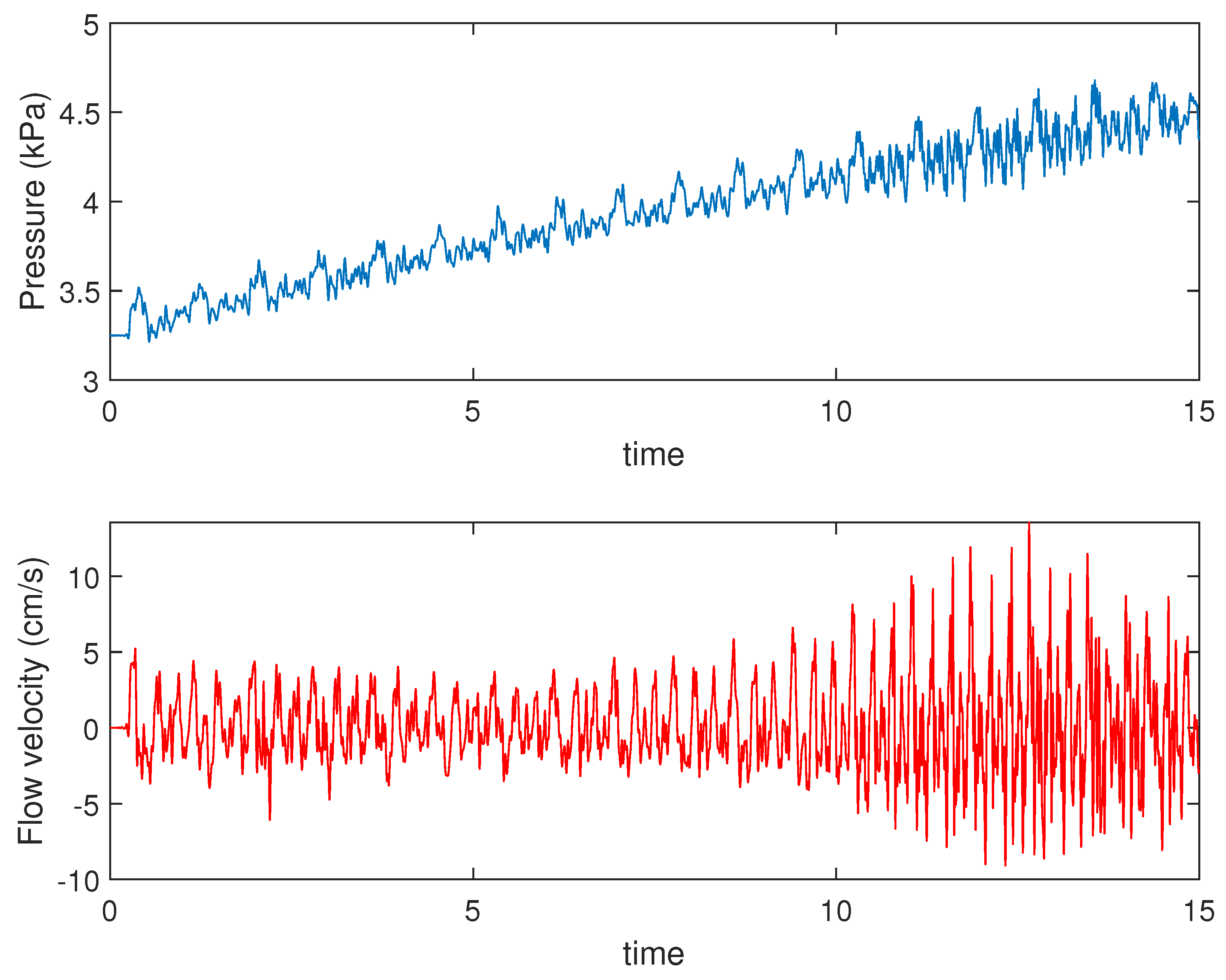
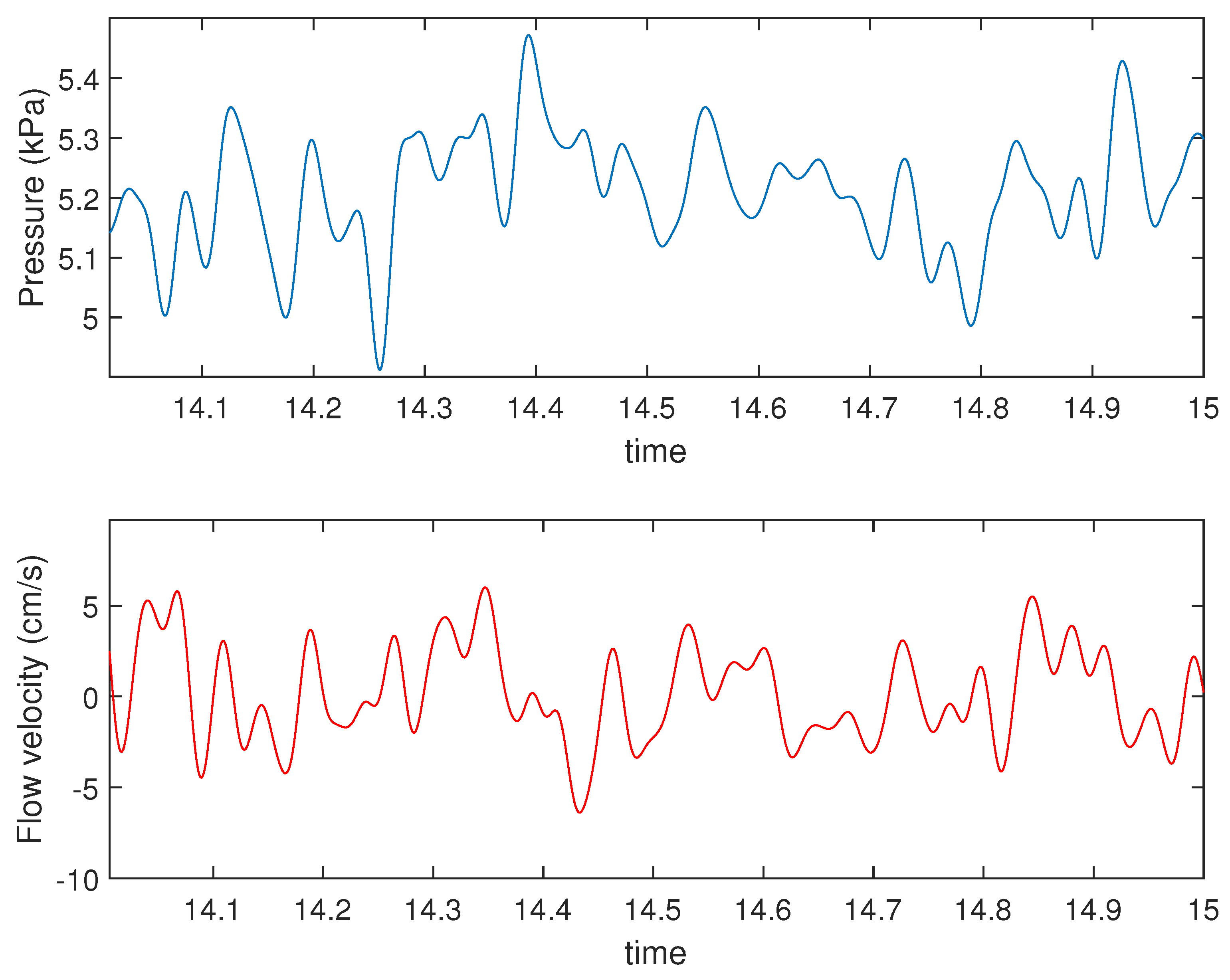
4.1. Presence of Mayer Waves
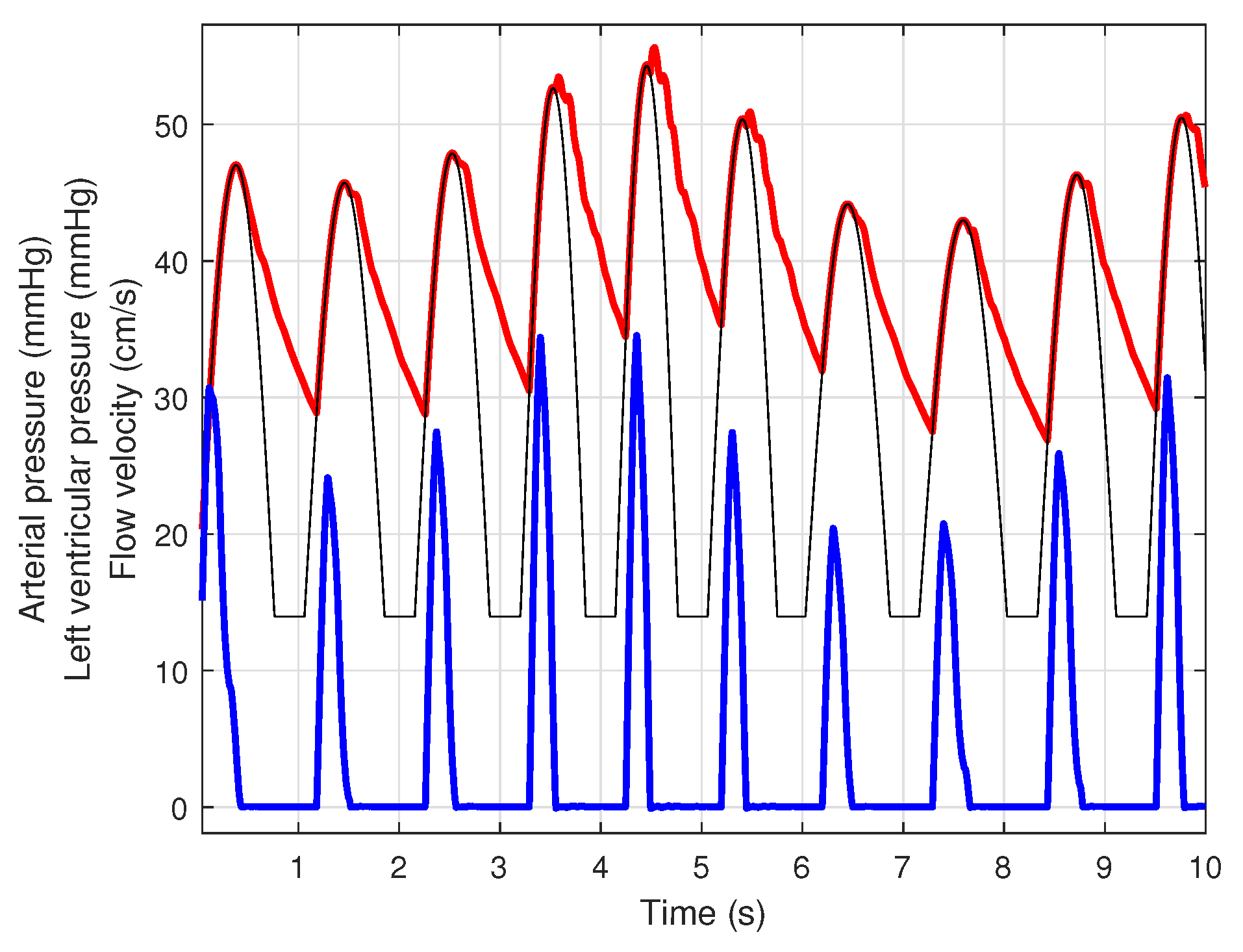
4.2. Principal Arteries
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Acronym | Meaning |
|---|---|
| PDE | Partial differential equation |
| ODE | Ordinary differential equation |
| 3D | Three-dimensional |
| 1D | One-dimensional |
| 0D | Zero-dimensional |
| FFT | Fast Fourier transform |
| RS | Riemann solver |
| RP | Riemann problem |
| HR | Heart rate for the heart model |
| Notation | Meaning |
|---|---|
| set of edges | |
| set of interfaces | |
| k-th edge | |
| j-th interface | |
| cross section area for the k-th edge | |
| blood pressure for the k-th edge | |
| average flow velocity for the k-th edge | |
| friction force (for unit length) for the k-th edge | |
| blood density for the k-th edge | |
| fluid viscosity for the k-th edge | |
| Young modulus for the k-th edge | |
| wall density for the k-th edge | |
| wall thickness for the k-th edge | |
| unstressed radius for the k-th edge | |
| wall displacement for the k-th edge | |
| external pressure for the k-th edge | |
| Poisson ratio for the k-th edge | |
| coefficient of the arterial wall for the k-th edge | |
| unstressed cross section area for the k-th edge | |
| forward characteristic information | |
| backward characteristic information | |
| characteristic velocity for the k-th edge | |
| characteristic velocity, dependent on only , for the k-th edge | |
| inflow time-dependent area | |
| inflow time-dependent velocity | |
| inflow time-dependent flow rate | |
| resistance for boundary condition “pure resistance” | |
| resistance for boundary condition “RC cell” or “compliance” | |
| C | capacitor for boundary condition “compliance” |
| Edge | Length (mm) | Area (mm2) | () | |
|---|---|---|---|---|
| 1 | 40 | 598 | 0.388 | NN |
| 2 | 20 | 515 | 0.348 | NN |
| 3 | 34 | 122 | 0.932 | NN |
| 4 | 34 | 056 | 0.170 | NN |
| 5 | 177 | 43 | 0.206 | NN |
| 6 | 148 | 12 | 10.360 | 0.91 |
| 7 | 422 | 51 | 1.864 | NN |
| 8 | 235 | 11 | 1.150 | 0.82 |
| 9 | 67 | 15 | 8.984 | NN |
| 10 | 79 | 3 | 51.576 | 0.96 |
| 11 | 171 | 13 | 9.784 | 0.89 |
| 12 | 176 | 12 | 10.576 | 0.78 |
| 13 | 177 | 12 | 9.868 | 0.79 |
| 14 | 39 | 314 | 0.496 | NN |
| 15 | 208 | 43 | 2.076 | NN |
| 16 | 176 | 12 | 10.576 | 0.78 |
| 17 | 177 | 12 | 9.868 | 0.79 |
| 18 | 52 | 314 | 0.496 | NN |
| 19 | 34 | 56 | 1.664 | NN |
| 20 | 148 | 12 | 10.360 | 0.90 |
| 21 | 422 | 51 | 1.864 | NN |
| 22 | 235 | 11 | 11.464 | 0.82 |
| 23 | 67 | 15 | 8.984 | NN |
| 24 | 79 | 3 | 51.576 | 0.96 |
| 25 | 171 | 13 | 9.784 | 0.89 |
| 26 | 80 | 20 | 0.035 | 0.63 |
| 27 | 104 | 302 | 0.468 | NN |
| 28 | 53 | 191 | 0.668 | NN |
| 29 | 20 | 48 | 1.900 | NN |
| 30 | 10 | 13 | 7.220 | NN |
| 31 | 66 | 15 | 4.568 | 0.93 |
| 32 | 71 | 10 | 6.268 | 0.92 |
| 33 | 63 | 24 | 3.324 | 0.93 |
| 34 | 59 | 43 | 2.276 | 0.93 |
| 35 | 10 | 125 | 0.908 | NN |
| 36 | 32 | 33 | 2.264 | 0.86 |
| 37 | 10 | 102 | 1.112 | NN |
| 38 | 32 | 16 | 4.724 | 0.86 |
| 39 | 106 | 70 | 1.524 | NN |
| 40 | 50 | 8 | 7.580 | 0.92 |
| 41 | 10 | 58 | 1.596 | NN |
| 42 | 59 | 33 | 2.596 | NN |
| 43 | 58 | 33 | 2.596 | NN |
| 44 | 144 | 25 | 5.972 | NN |
| 45 | 50 | 18 | 12.536 | 0.93 |
| 46 | 443 | 14 | 10.236 | NN |
| 47 | 126 | 13 | 10.608 | 0.89 |
| 48 | 321 | 11 | 23.232 | 0.72 |
| 49 | 343 | 6 | 36.972 | 0.72 |
| 50 | 145 | 25 | 5.972 | NN |
| 51 | 51 | 18 | 12.536 | 0.93 |
| 52 | 444 | 14 | 10.236 | NN |
| 53 | 127 | 13 | 10.608 | 0.89 |
| 54 | 323 | 11 | 23.232 | 0.72 |
| 55 | 344 | 6 | 36.972 | 0.72 |
References
- Pozrikidis, C. Numerical simulation of blood flow through microvascular capillary networks. Bull. Math. Biol. 2009, 71, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Zamir, M. Hemo-Dynamics; Biological and Medical Physics, Biomedical Engineering; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Chen, Q.; Jiang, L.; Li, C.; Hu, D.; Bu, J.W.; Cai, D.; Du, J.L. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol. 2012, 10, e1001374. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Microcirculation in Fractal Branching Networks; Springer: Tokyo, Japan, 2014. [Google Scholar]
- Muller, L.O.; Toro, E.F. A global multi-scale model for the human circulation with emphasis on the venous system. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 681–725. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.U.; Vaughan, G.D.A.; Sainsbury, C.; Johnson, M.; Peskin, C.S.; Olufsen, M.S.; Hill, N.A. Numerical simulation of blood flow and pressure drop in the pulmonary arterial and venous circulation. Biomech. Model. Mechanobiol. 2014, 13, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Formaggia, L.; Lamponi, D.; Quarteroni, A. One-dimensional models for blood flow in arteries. J. Eng. Math. 2003, 47, 251–276. [Google Scholar] [CrossRef]
- Formaggia, L.; Lamponi, D.; Tuveri, M.; Veneziani, A. Numerical modeling of 1D arterial networks coupled with a lumped parameters description of the heart. Comp. Meth. Biomech. Biomed. Eng. 2006, 9, 273–288. [Google Scholar] [CrossRef]
- Mynard, J.P.; Smolich, J.J. One-dimensional haemodynamic modeling and wave dynamics in the entire adult circulation. Ann. Biomed. Eng. 2015, 43, 1443–1460. [Google Scholar] [CrossRef]
- Olufsen, M.S.; Ottesen, J.T.; Tran, H.; Ellwein, L.; Lipsitz, L.A.; Novak, V. Blood pressure and blood flow variation during postural change from sitting to standing: Model development and validation. J. Appl. Physiol. 2005, 99, 1523–1537. [Google Scholar] [CrossRef]
- Ottesen, J.T.; Olufsen, M.S.; Larsen, J.K. Applied Mathematical Models in Human Physiology; SIAM: Philadelphia, PA, USA, 2004. [Google Scholar]
- Shi, Y.; Lawford, P.; Hose, R. Review of zero-D and 1-D models of blood flow in the cardiovascular system. Biomed. Eng. Online 2011, 10, 33. [Google Scholar] [CrossRef]
- van de Vosse, F.N.; Stergiopulos, N. Pulse wave propagation in the arterial tree. Annu. Rev. Fluid Mech. 2011, 43, 467–499. [Google Scholar] [CrossRef]
- Quarteroni, A.; Manzoni, A.; Negri, F. Reduced Basis Methods for Partial Differential Equations, An Introduction; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ottesen, J.T. Modelling of the baroreflex-feedback mechanism with time-delay. J. Math. Biol. 1997, 36, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Batzel, J.J.; Kappel, F.; Schneditz, D.; Tran, H.T. Cardiovascular and Respiratory Systems: Modeling, Analysis, and Control; SIAM: Philadelphia, PA, USA, 2007. [Google Scholar]
- Cascaval, R.C.; D’Apice, C.; D’Arienzo, M.P.; Manzo, R. Boundary control for an arterial system. J. Fluid Flow Heat Mass Transf. 2016, 3, 25–33. [Google Scholar] [CrossRef]
- Alastruey, J. Numerical Modelling of Pulse Wave Propagation in the Cardiovascular System: Development, Validation and Clinical Applications. Ph.D. Thesis, Imperial College London, London, UK, 2007. [Google Scholar]
- Cheng, Y.; Shu, C.W. A discontinuous Galerkin finite element method for time dependent partial differential equations with higher oder derivatives. Math. Comput. 2008, 77, 699–730. [Google Scholar] [CrossRef]
- Tomasiello, S.; Macías-Díaz, J.E.; Alba-Pérez, J. An alternative formulation of the differential quadrature method with a neural network perspective. Int. J. Comput. Math. 2023, 100, 1248–1263. [Google Scholar] [CrossRef]
- Hasan, M.; Patel, B.P.; Pradyumna, S. A benchmark study on the axial velocity profile of wave propagation in deformable blood vessels. Phys. Fluids 2021, 33, 041905. [Google Scholar] [CrossRef]
- Alastruey, J.; Khir, A.W.; Matthys, K.S.; Segers, P.; Sherwin, S.J.; Verdonck, P.R.; Parker, K.H.; Peir, J. Pulse wave propagation in a model human arterial network: Assessment of 1-D visco-elastic simulations against in vivo measurements. J. Biomech. 2011, 44, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Alastruey, J.; Parker, K.H.; Peiro, J.; Sherwin, S.J. Analysing the pattern of pulse waves in arterial networks: A time-domain study. J. Eng. Math. 2009, 64, 331–351. [Google Scholar] [CrossRef]
- Reymond, P.; Merenda, F.; Perren, F.; Rüfenacht, D.; Stergiopulos, N. Validation of a one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H208–H222. [Google Scholar] [CrossRef]
- Sherwin, S.J.; Formaggia, L.; Peiro, J.; Franke, V. Computational modeling of 1D blood flow with variable mechanical properties and its application to the simulation of wave propagation in the human arterial system. Int. J. Numer. Methods Fluids 2003, 43, 673–700. [Google Scholar] [CrossRef]
- Steele, B.N.; Valdez-Jasso, D.; Haider, M.A.; Olufsen, M.S. Predicting arterial flow and pressure dynamics using a 1D fluid dynamics model with a viscoelastic wall. SIAM J. Appl. Math. 2011, 71, 1123–1143. [Google Scholar] [CrossRef]
- Canic, S.; Hartley, C.J.; Rosenstrauch, D.; Tambaca, J.; Guidoboni, G.; Mikelic, A. Blood flow in compliant arteries: An effective viscoelastic reduced model, numerics and experimental validation. Ann. Biomed. Eng. 2006, 34, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Cascaval, R.C. A Boussinesq model for pressure and flow velocity waves in arterial segments. Math. Comp. Simul. 2012, 82, 1047–1055. [Google Scholar] [CrossRef]
- Tonini, A.; Vergara, C.; Regazzoni, F.; Dede’, L.; Scrofani, R.; Cogliati, C.; Quarteroni, A. A mathematical model to assess the effects of COVID-19 on the cardiocirculatory system. Sci. Rep. 2024, 14, 8304. [Google Scholar] [CrossRef] [PubMed]
- Zingaro, A.; Fumagalli, I.; Dede’, L.; Fedele, M.; Africa, P.C.; Corno, A.F.; Quarteroni, A. A geometric multiscale model for the numerical simulation of blood flow in the human left heart. Discret. Contin. Dyn. Syst.-Ser. S 2022, 15, 2391–2427. [Google Scholar] [CrossRef]
- Zingaro, A.; Bucelli, M.; Piersanti, R.; Regazzoni, F.; Dede’, L.; Quarteroni, A. An electromechanics-driven fluid dynamics model for the simulation of the whole human heart. J. Comput. Phys. 2024, 504, 112885. [Google Scholar] [CrossRef]
- Sazonov, I.; Nithiarasu, P. A novel, FFT-based one-dimensional blood flow solution method for arterial network. Biomech. Model. Mechanobiol. 2019, 18, 1311–1334. [Google Scholar] [CrossRef]
- Baranovskii, E.S. Feedback Optimal Control Problem for a Network Model of Viscous Fluid Flows. Math. Notes 2022, 112, 26–39. [Google Scholar] [CrossRef]
- Syed, F.; Khan, S.; Toma, M. Modeling Dynamics of the Cardiovascular System Using Fluid-Structure Interaction Methods. Biology 2023, 12, 1026. [Google Scholar] [CrossRef]
- D’Apice, C.; Manzo, R.; Piccoli, B. A fluid dynamic model for telecommunication networks with sources and destinations. SIAM J. Appl. Math. 2008, 68, 981–1003. [Google Scholar] [CrossRef][Green Version]
- Rarità, L.; Stamova, I.; Tomasiello, S. Numerical schemes and genetic algorithms for the optimal control of a continuous model of supply chains. Appl. Math. Comput. 2021, 388, 125464. [Google Scholar] [CrossRef]
- de Falco, M.; Gaeta, M.; Loia, V.; Rarità, L.; Tomasiello, S. Differential quadrature-based numerical solutions of a fluid dynamic model for supply chains. Commun. Math. Sci. 2016, 14, 1467–1476. [Google Scholar] [CrossRef]
- Manzo, R.; Piccoli, B.; Rarità, L. Optimal distribution of traffic flows at junctions in emergency cases. Eur. J. Appl. Math. 2012, 23, 515–535. [Google Scholar] [CrossRef]
- Kupenko, O.P.; Manzo, R. Approximation of an optimal control problem in coefficient for variational inequality with anisotropic p-Laplacian. Nonlinear Differ. Equ. Appl. 2016, 23, 35. [Google Scholar] [CrossRef]
- Sarkar, N.; Sharma, S.D.; Chakraborty, S.; Roy, S. A comparative study of Newtonian and non-Newtonian blood flow through Bi-Leaflet Mechanical Heart Valve. Comput. Fluids 2024, 279, 106337. [Google Scholar] [CrossRef]
- Smith, N.P.; Pullan, A.J.; Hunter, P.J. An anatomically based model of transient coronary blood flow in the heart. SIAM J. Appl. Math. 2001, 62, 990–1018. [Google Scholar] [CrossRef]
- Sherwin, S.J.; Franke, V.E.; Peiró, J.; Parker, K.H. One-dimensional modelling of a vascular network in space-time variables. J. Eng. Math. 2003, 47, 217–250. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Arienzo, M.P.; Rarità, L. Dynamics of Blood Flows in the Cardiocirculatory System. Computation 2024, 12, 194. https://doi.org/10.3390/computation12100194
D’Arienzo MP, Rarità L. Dynamics of Blood Flows in the Cardiocirculatory System. Computation. 2024; 12(10):194. https://doi.org/10.3390/computation12100194
Chicago/Turabian StyleD’Arienzo, Maria Pia, and Luigi Rarità. 2024. "Dynamics of Blood Flows in the Cardiocirculatory System" Computation 12, no. 10: 194. https://doi.org/10.3390/computation12100194
APA StyleD’Arienzo, M. P., & Rarità, L. (2024). Dynamics of Blood Flows in the Cardiocirculatory System. Computation, 12(10), 194. https://doi.org/10.3390/computation12100194






