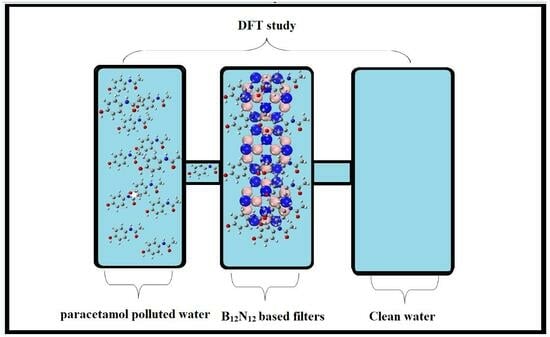
Theoretical Modeling of B12N12 Nanocage for the Effective Removal of Paracetamol from Drinking Water
Abstract
:1. Introduction
2. Computational Details
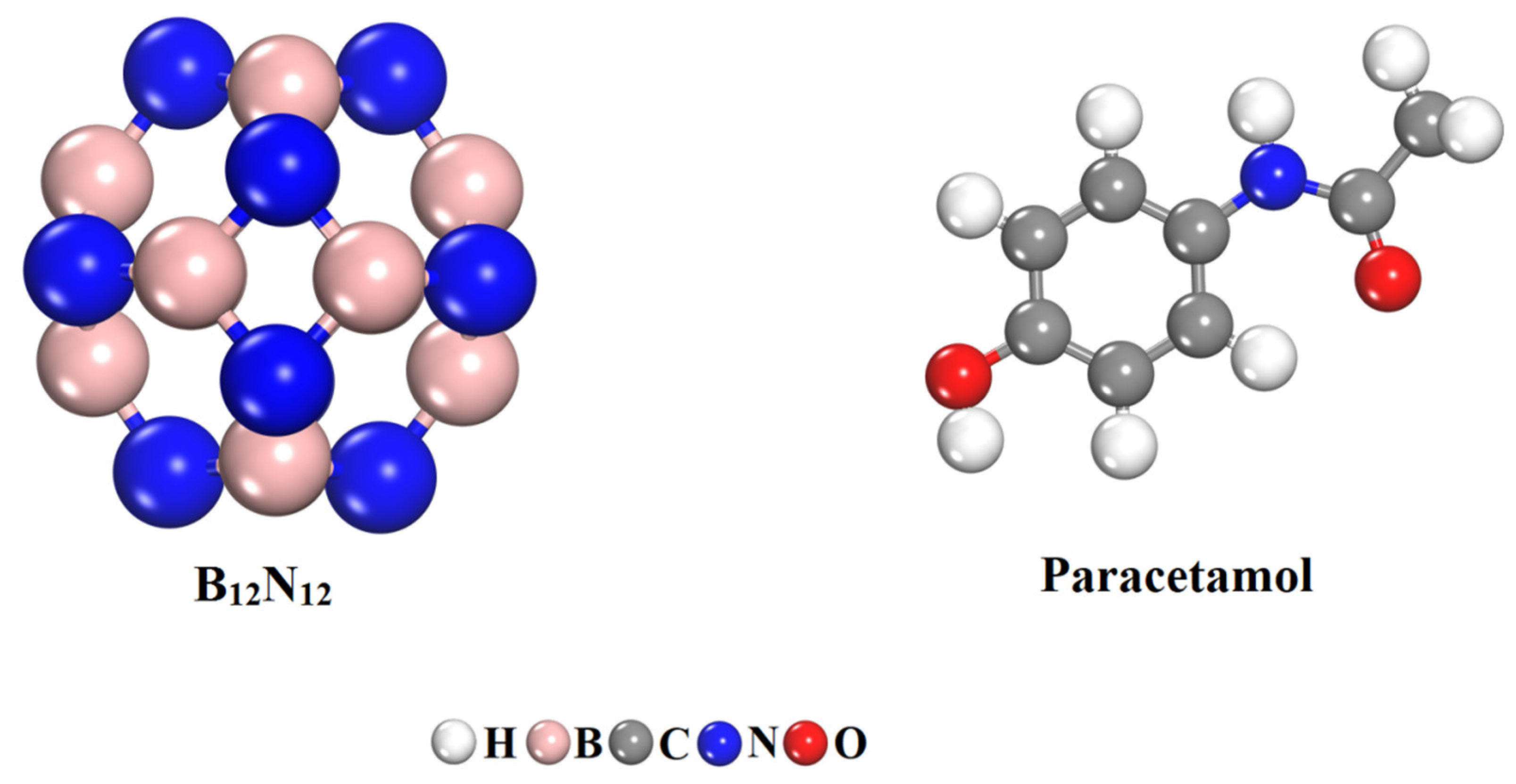
2.1. Optimization of Geometry of B12N12 Nanocage and Paracetamol
2.2. Optimization of B12N12 Complexes and Estimation of Binding Energies
2.3. Reduced Density Gradient (RDG) Analysis and Noncovalent Interaction (NCI)
2.4. Natural Bonding Orbital (NBO) Analysis
2.5. Quantum Theory of Atoms in Molecules (QTAIM) Analysis
2.6. Frontier Molecular Orbital (FMO) Analysis
2.7. Electron Density Difference (EDD) Analysis
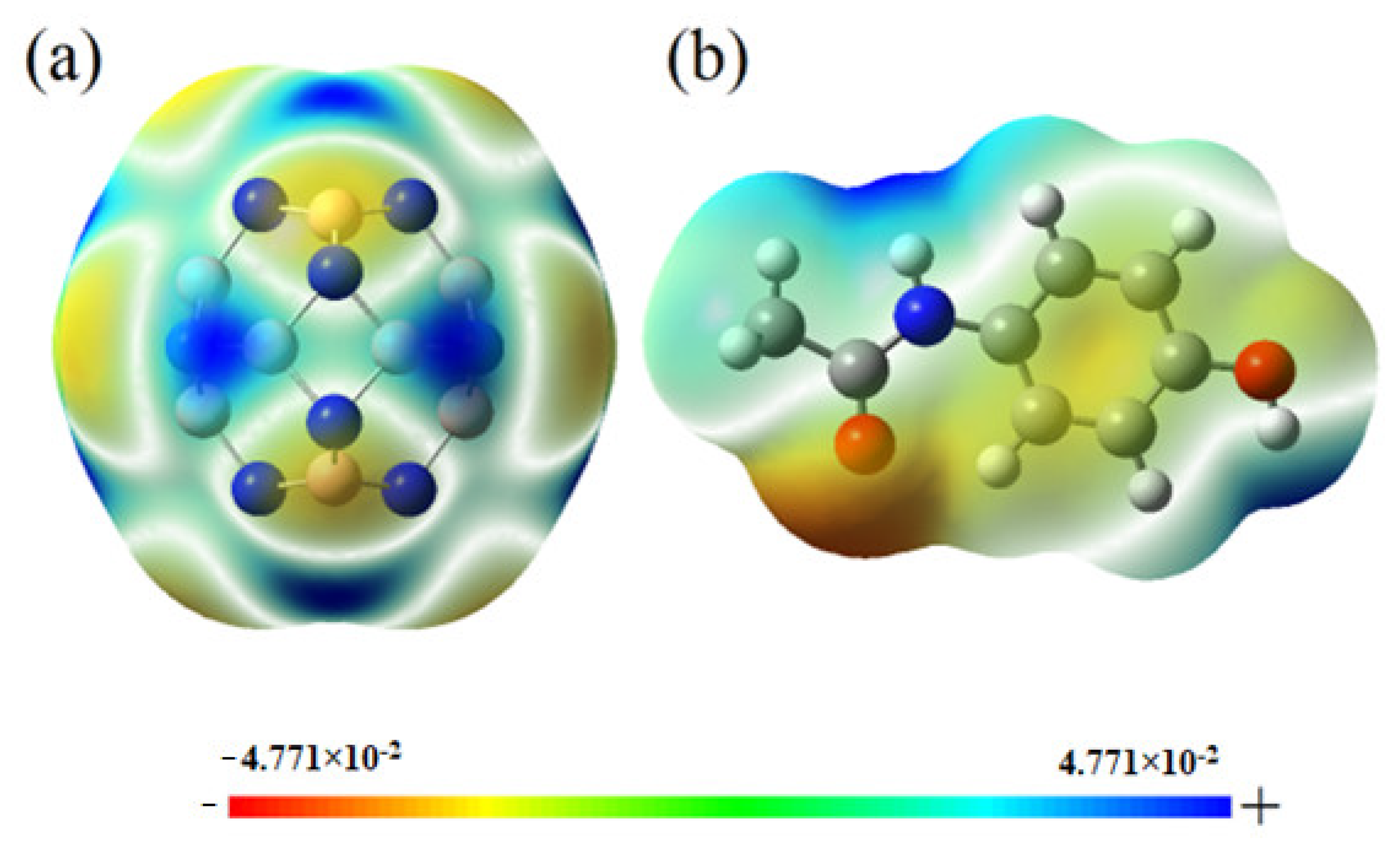
2.8. Effect of Solvent on the Adsorption and Dipole Moment Analysis
3. Thermodynamics Analysis
4. Recovery Time Calculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Guo, W.; Li, P.; Du, Q.; Zhou, Y.; Xu, D.; Zhang, Z. Hydrogeochemical processes regulating the groundwater geochemistry and human health risk of groundwater in the rural areas of the Wei River Basin, China. Expo. Health 2023, 1–16. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; He, S.; Wang, L. Occurrence and distribution of groundwater fluoride and manganese in the Weining Plain (China) and their probabilistic health risk quantification. Expo. Health 2022, 14, 263–279. [Google Scholar] [CrossRef]
- Alam, S.M.K.; Li, P.; Fida, M. Groundwater nitrate pollution due to excessive use of N-fertilizers in Rural Areas of Bangladesh: Pollution status, health risk, source contribution, and future impacts. Expo. Health 2023, 1–24. [Google Scholar] [CrossRef]
- Guo, Y.; Li, P.; He, X.; Wang, L. Groundwater quality in and around a landfill in northwest China: Characteristic pollutant identification, health risk assessment, and controlling factor analysis. Expo. Health 2022, 14, 885–901. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Li, Y.; Xiang, G. Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: A case study of Tongchuan, Northwest China. Expo. Health 2019, 11, 95–107. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Guo, W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 11–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Xu, B. Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ. Earth Sci. 2018, 77, 273. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Hinchey, E.K.; Boehme, S.E.; Daughton, C.G.; Ruhoy, I.S.; Conerly, O.; Daniels, R.L.; Lauer, L.; McCarthy, M.; Nettesheim, T.G.; et al. Disposal practices for unwanted residential medications in the United States. Environ. Int. 2009, 35, 566–572. [Google Scholar] [CrossRef]
- Valcárcel, Y.; Alonso, S.G.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 2011, 84, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Caragea, G.; Avram, O.; Pauna, A.; Costea, A.C.; Tudosie, M. Acetaminophen, a therapeutic or an extremely toxic remedy—A review. J. Mind Med. Sci. 2022, 9, 102–110. [Google Scholar] [CrossRef]
- Kim, J.W.; Yoon, S.M.; Lee, S.J.; Narumiya, M.; Nakada, N.; Han, I.S.; Tanaka, H. Occurrence and fate of PPCPs wastewater treatment plants in Korea. Int. Proc. Chem. Biol. Environ. Eng. 2012, 35, 57–61. [Google Scholar]
- Badar, Z.; Shanableh, A.; El-Keblawy, A.; Mosa, K.A.; Semerjian, L.; Al Mutery, A.; Hussain, M.I.; Bhattacharjee, S.; Tsombou, F.M.; Ayyaril, S.S.; et al. Assessment of uptake, accumulation and degradation of paracetamol in spinach (Spinacia oleracea L.) under controlled laboratory conditions. Plants 2022, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Juárez, J.I.; Torres-Palma, R.A.; Botero-Coy, A.M.; Hernández, F. Pharmaceuticals and environmental risk assessment in municipal wastewater treatment plants and rivers from Peru. Environ. Int. 2021, 155, 106674. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Beelen, E.S.E. Municipal Waste Water Treatment Plant (WWTP) Effluents: A Concise Overview of the Occurrence of Organic Substances; Association of River Waterworks-RIWA: Nieuwegein, The Netherlands, 2007; ISBN 978-90-6683-124-7. [Google Scholar]
- Moreno-González, R.; Rodríguez-Mozaz, S.; Gros, M.; Barceló, D.; León, V.M. Seasonal distribution of pharmaceuticals in marine water and sediment from a mediterranean coastal lagoon (SE Spain). Environ. Res. 2015, 138, 326–344. [Google Scholar] [CrossRef]
- Kummerová, M.; Zezulka, Š.; Babula, P.; Tříska, J. Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Z.; Liu, Y.; Cao, T.; Zhang, H.; Hao, M.; Chen, R.; Dzakpasu, M.; Wang, X.C. Phytoremediation mechanisms and plant eco-physiological response to microorganic contaminants in integrated vertical-flow constructed wetlands. J. Hazard. Mater. 2022, 424, 127611. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Polak, D.; Zielińska, I.; Szwast, M.; Kogut, I.; Małolepszy, A. Modification of ceramic membranes with carbon compounds for pharmaceutical substances removal from water in a filtration—Adsorption system. Membranes 2021, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: A review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Deepa, J.R.; Nair, A.S. Fabrication of chemically modified graphene oxide/nano hydroxyapatite composite for adsorption and subsequent photocatalytic degradation of aureomycine hydrochloride. J. Ind. Eng. Chem. 2017, 47, 415–430. [Google Scholar] [CrossRef]
- El-Sayed, M.E.A. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.-V.N.; Prakash, D.G.; Joseph, A.A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Ma, J. Enhanced adsorption and removal of ciprofloxacin on regenerable long TiO2 nanotube/graphene oxide hydrogel adsorbents. J. Nanomater. 2015, 2015, 675862. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Liu, S.; Shu, D.; Zeng, G.; Hu, X.; Tan, X.; Jiang, L.; Yan, Z.; Cai, X. Cu (II)-influenced adsorption of ciprofloxacin from aqueous solutions by magnetic graphene oxide/nitrilotriacetic acid nanocomposite: Competition and enhancement mechanisms. Chem. Eng. J. 2017, 319, 219–228. [Google Scholar] [CrossRef]
- Shi, S.; Fan, Y.; Huang, Y. Facile low temperature hydrothermal synthesis of magnetic mesoporous carbon nanocomposite for adsorption removal of ciprofloxacin antibiotics. Ind. Eng. Chem. Res. 2013, 52, 2604–2612. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Leo, B.F.; Murphy, F. The toxic truth about carbon nanotubes in water purification: A perspective view. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, M.; Shahab, S.; Khaleghian, M.; Kumar, R. Interaction between new anti-cancer drug syndros and CNT (6, 6-6) nanotube for medical applications: Geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO-LUMO investigation. Appl. Surf. Sci. 2018, 434, 504–513. [Google Scholar] [CrossRef]
- Sheikhi, M.; Shahab, S.; Khaleghian, M.; Hajikolaee, F.H.; Balakhanava, I.; Alnajjar, R. Adsorption properties of the molecule resveratrol on CNT (8, 0-10) nanotube: Geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO-LUMO investigations. J. Mol. Struct. 2018, 1160, 479–487. [Google Scholar] [CrossRef]
- Sheikhi, M.; Shahab, S.; Alnajjar, R.; Ahmadianarog, M. Adsorption properties of the new anti-cancer drug alectinib on CNT (6, 6-6) nanotube: Geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO--LUMO investigations. J. Clust. Sci. 2019, 30, 83–96. [Google Scholar] [CrossRef]
- Kim, J.H.; Pham, T.V.; Hwang, J.H.; Kim, C.S.; Kim, M.J. Boron nitride nanotubes: Synthesis and applications. Nano Converg. 2018, 5, 17. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Pang, H.; Zhang, R.; Song, W.; Fu, D.; Hayat, T.; Wang, X. Boron nitride-based materials for the removal of pollutants from aqueous solutions: A review. Chem. Eng. J. 2018, 333, 343–360. [Google Scholar] [CrossRef]
- Yogi, R.; Jaiswal, N.K. Adsorption of CO gas molecules on zigzag BN/AlN nanoribbons for nano sensor applications. Phys. Lett. A 2019, 383, 532–538. [Google Scholar] [CrossRef]
- Ali, Q.; Khan, A.A.; Yar, M.; Khan, M.; Ahmad, R.; Ahmad, I. Theoretical insight of ciprofloxacin removal from water using boron nitride (B12N12) nanocage. Surf. Interfaces 2022, 31, 101982. [Google Scholar] [CrossRef]
- Tazikeh-Lemeski, E.; Soltani, A.; Baei, M.T.; Javan, M.B.; Rad, S.M. Theoretical study on pure and doped B12 N12 fullerenes as thiophene sensor. Adsorption 2018, 24, 585–593. [Google Scholar] [CrossRef]
- Rad, A.S. Study on the surface interaction of Furan with X12Y12 (X = B, Al, and Y = N, P) semiconductors: DFT calculations. Heteroat. Chem. 2016, 27, 316–322. [Google Scholar]
- Beheshtian, J.; Kamfiroozi, M.; Bagheri, Z.; Peyghan, A.A. B12N12 nano-cage as potential sensor for NO2 detection. Chin. J. Chem. Phys. 2012, 25, 60. [Google Scholar] [CrossRef]
- Vessally, E.; Esrafili, M.D.; Nurazar, R.; Nematollahi, P.; Bekhradnia, A. A DFT study on electronic and optical properties of aspirin-functionalized B 12 N 12 fullerene-like nanocluster. Struct. Chem. 2017, 28, 735–748. [Google Scholar] [CrossRef]
- Farrokhpour, H.; Jouypazadeh, H.; Sohroforouzani, S.V. Interaction of different types of nanocages (Al12N12, Al12P12, B12N12, Be12O12, Mg12O12, Si12C12 and C24) with HCN and ClCN: DFT, TD-DFT, QTAIM, and NBO calculations. Mol. Phys. 2020, 118, 1626506. [Google Scholar] [CrossRef]
- Badran, H.M.; Eid, K.M.; Ammar, H.Y. A DFT study on the effect of the external electric field on ammonia interaction with boron nitride nano-cage. J. Phys. Chem. Solids 2020, 141, 109399. [Google Scholar] [CrossRef]
- Rad, A.S.; Zareyee, D.; Foukolaei, V.P.; Moghadas, B.K.; Peyravi, M. Study on the electronic structure of Al12N12 and Al12P12 fullerene-like nano-clusters upon adsorption of CH3F and CH3Cl. Mol. Phys. 2016, 114, 3143–3149. [Google Scholar]
- Oku, T.; Nishiwaki, A.; Narita, I. Formation and atomic structure of B12N12 nanocage clusters studied by mass spectrometry and cluster calculation. Sci. Technol. Adv. Mater. 2004, 5, 635. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Wilkinson, K.A.; Sherwood, P.; Guest, M.F.; Naidoo, K.J. Acceleration of the GAMESS-UK electronic structure package on graphical processing units. J. Comput. Chem. 2011, 32, 2313–2318. [Google Scholar] [CrossRef]
- Moellmann, J.; Grimme, S. DFT-D3 study of some molecular crystals. J. Phys. Chem. C 2014, 118, 7615–7621. [Google Scholar] [CrossRef]
- Jumabaev, A.; Holikulov, U.; Hushvaktov, H.; Issaoui, N.; Absanov, A. Intermolecular interactions in ethanol solution of OABA: Raman, FTIR, DFT, M062X, MEP, NBO, FMO, AIM, NCI, RDG analysis. J. Mol. Liq. 2023, 377, 121552. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 16; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Benda, R.; Vezin, T.; Lebental, B. Prediction of the interaction strength of an urea-based probe toward ions in water by means of Density Functional Theory/Polarizable Continuum Model calculations. Int. J. Quantum Chem. 2022, 122, e26901. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Noormohammadbeigi, M.; Kamalinahad, S.; Izadi, F.; Adimi, M.; Ghasemkhani, A. Theoretical investigation of thioguanine isomers anticancer drug adsorption treatment on B12N12 nanocage. Mater. Res. Express 2020, 6, 1250g2. [Google Scholar] [CrossRef]




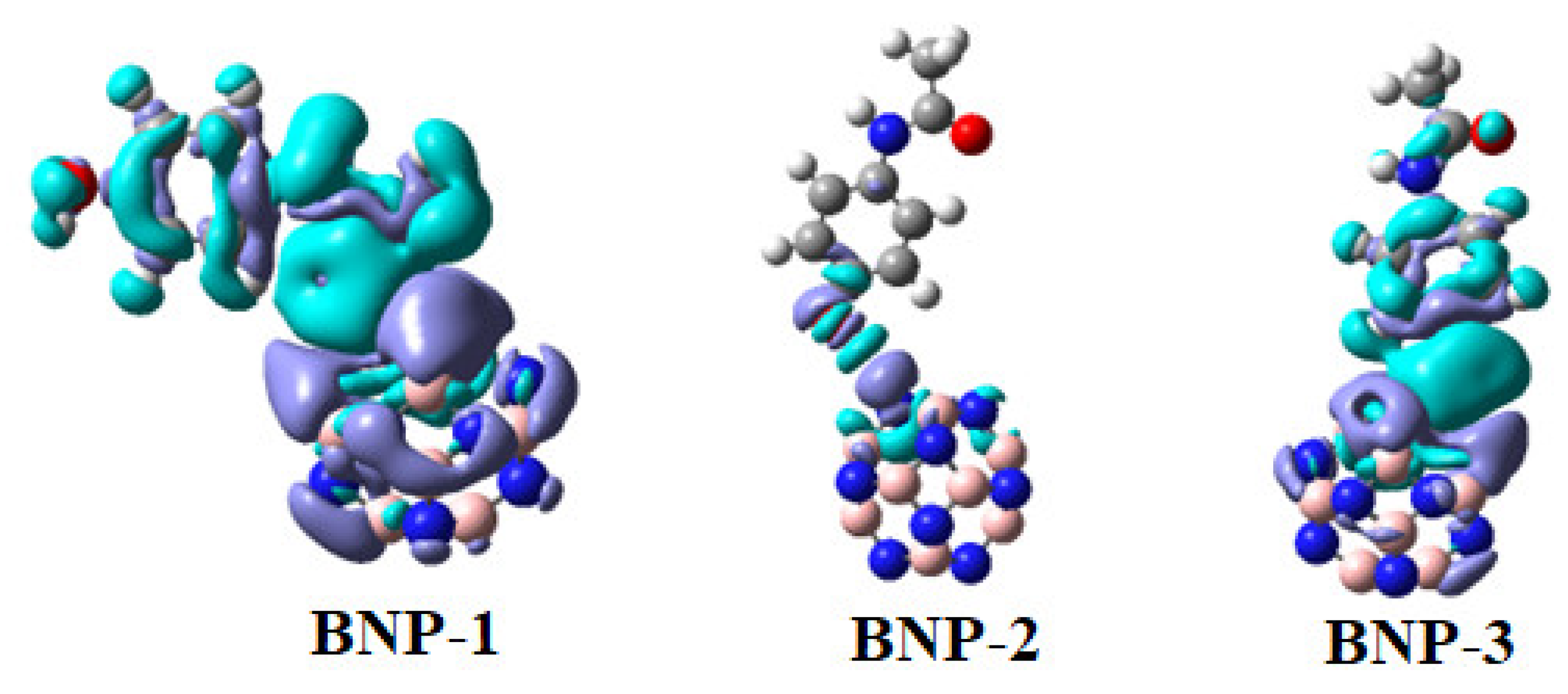

| System | Eads | ELUMO | EHOMO | Eg | Q |
|---|---|---|---|---|---|
| B12N12 | - | −0.84 | −7.70 eV | 6.86 eV | - |
| Paracetamol | - | −0.08 | −5.48 | 5.40 | - |
| BNP-1 | −27.94 | −1.72 | −6.52 | 4.80 | −0.018 |
| BNP-2 | −6.97 | −1.11 | −5.26 | 4.15 | 0.017 |
| BNP-3 | −15.31 | −0.95 | −6.53 | 5.58 | −0.041 |
| System | BCP | Density of All Electrons (ρb) | Laplacian (∇2ρb) | Kinetic Electron Density Gb | Potential Energy Density Vb | Energy Density Hb | −Gb/Vb |
|---|---|---|---|---|---|---|---|
| BNP-1 | 67 | 0.34 | −0.96 | 0.32 | −0.89 | −0.56 | 0.36 |
| BNP-2 | 52 | 0.03 | 0.07 | 0.02 | −0.02 | −0.0004 | 0.97 |
| BNP-3 | 52 | 0.09 | 0.28 | 0.12 | −0.17 | −0.05 | 0.70 |
| System | Eads (Gas Medium) | Eads (Water Medium) | DM | ∆H | ∆G |
|---|---|---|---|---|---|
| B12N12 | - | - | 0.000010 | - | - |
| BNP-1 | −27.94 | −30.69 | 11.14 | −21.52 | −19.13 |
| BNP-2 | −6.97 | −4.98 | 1.62 | −2.41 | −1.03 |
| BNP-3 | −15.31 | −15.60 | 7.72 | −10.31 | −7.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kainat; Gul, S.; Ali, Q.; Khan, M.; Rehman, M.U.; Ibrahim, M.; AlAsmari, A.F.; Alasmari, F.; Alharbi, M. Theoretical Modeling of B12N12 Nanocage for the Effective Removal of Paracetamol from Drinking Water. Computation 2023, 11, 183. https://doi.org/10.3390/computation11090183
Kainat, Gul S, Ali Q, Khan M, Rehman MU, Ibrahim M, AlAsmari AF, Alasmari F, Alharbi M. Theoretical Modeling of B12N12 Nanocage for the Effective Removal of Paracetamol from Drinking Water. Computation. 2023; 11(9):183. https://doi.org/10.3390/computation11090183
Chicago/Turabian StyleKainat, Sana Gul, Qaisar Ali, Momin Khan, Munir Ur Rehman, Mohammad Ibrahim, Abdullah F. AlAsmari, Fawaz Alasmari, and Metab Alharbi. 2023. "Theoretical Modeling of B12N12 Nanocage for the Effective Removal of Paracetamol from Drinking Water" Computation 11, no. 9: 183. https://doi.org/10.3390/computation11090183
APA StyleKainat, Gul, S., Ali, Q., Khan, M., Rehman, M. U., Ibrahim, M., AlAsmari, A. F., Alasmari, F., & Alharbi, M. (2023). Theoretical Modeling of B12N12 Nanocage for the Effective Removal of Paracetamol from Drinking Water. Computation, 11(9), 183. https://doi.org/10.3390/computation11090183