Active Pharmaceutical Ingredients Transportation and Release from Aerogel Particles Processes Modeling
Abstract
1. Introduction
2. Materials and Methods
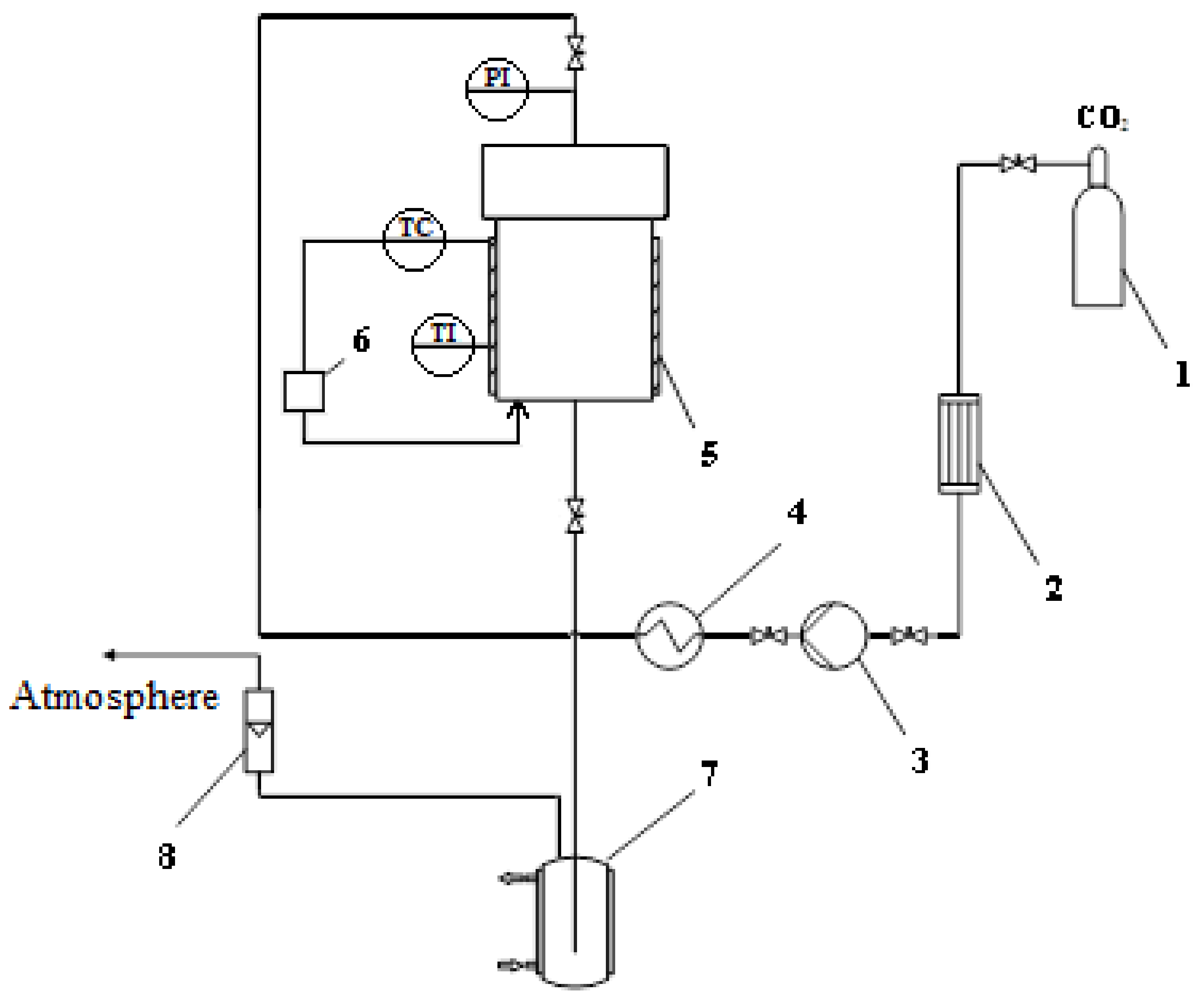
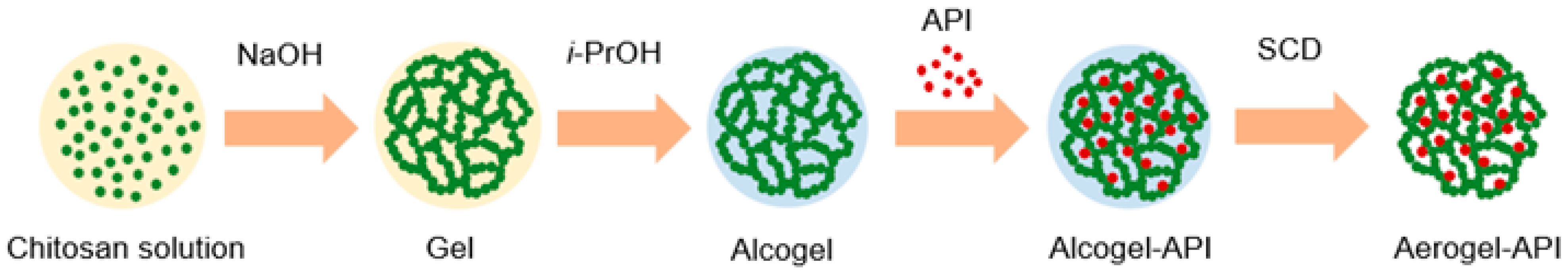
2.1. Chitosan-Based Aerogel Particles Obtaining
2.2. Aerogel–API Pharmaceutical Composition Obtaining
2.3. Experimental Studies of the API Release from Aerogel Particles
- Mobile phase—phosphate buffer: methanol: acetonitrile;
- Volumetric flow rate of the mobile phase—1 mL/min;
- Wavelength—278 nm; and
- Sample volume—20 µL.
3. Theory
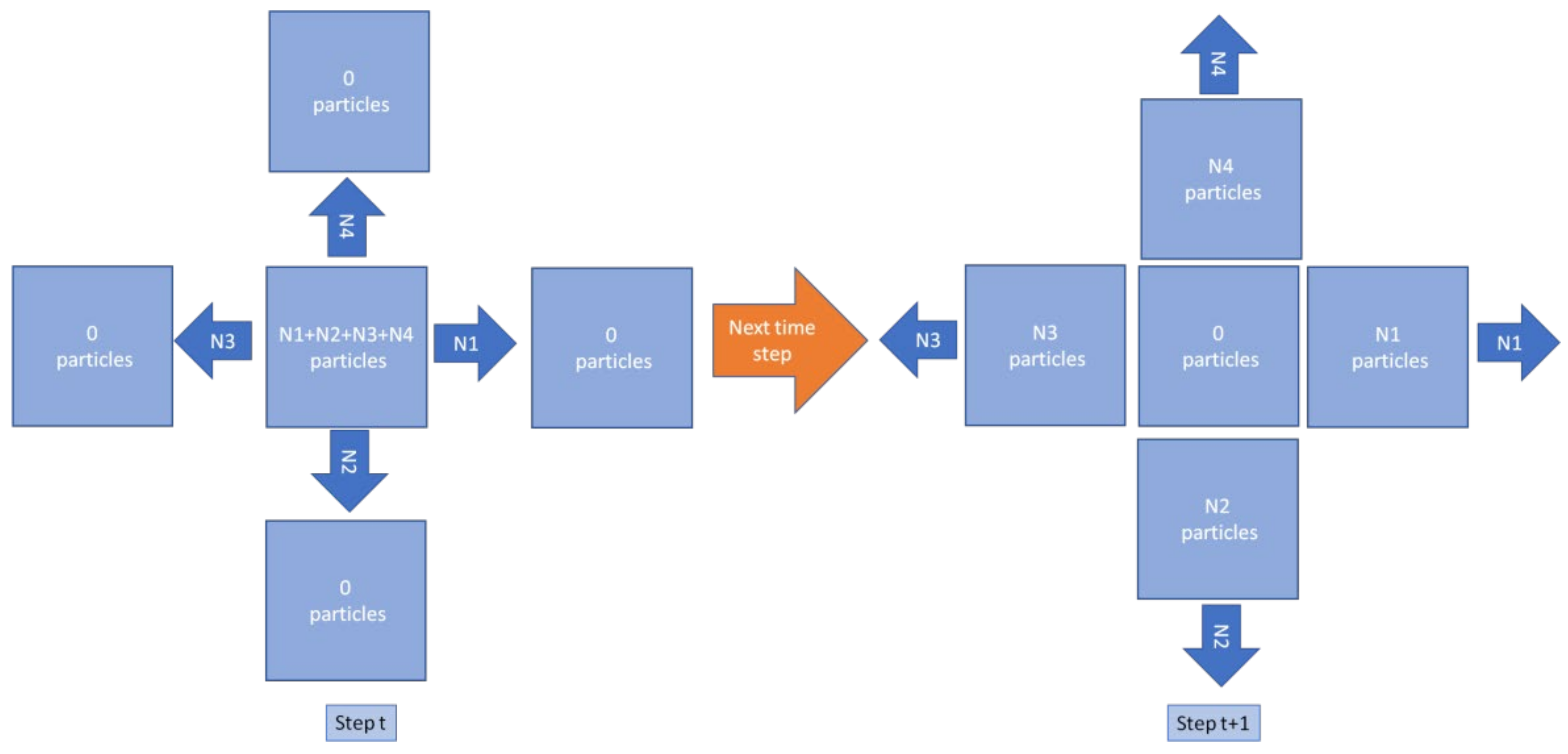
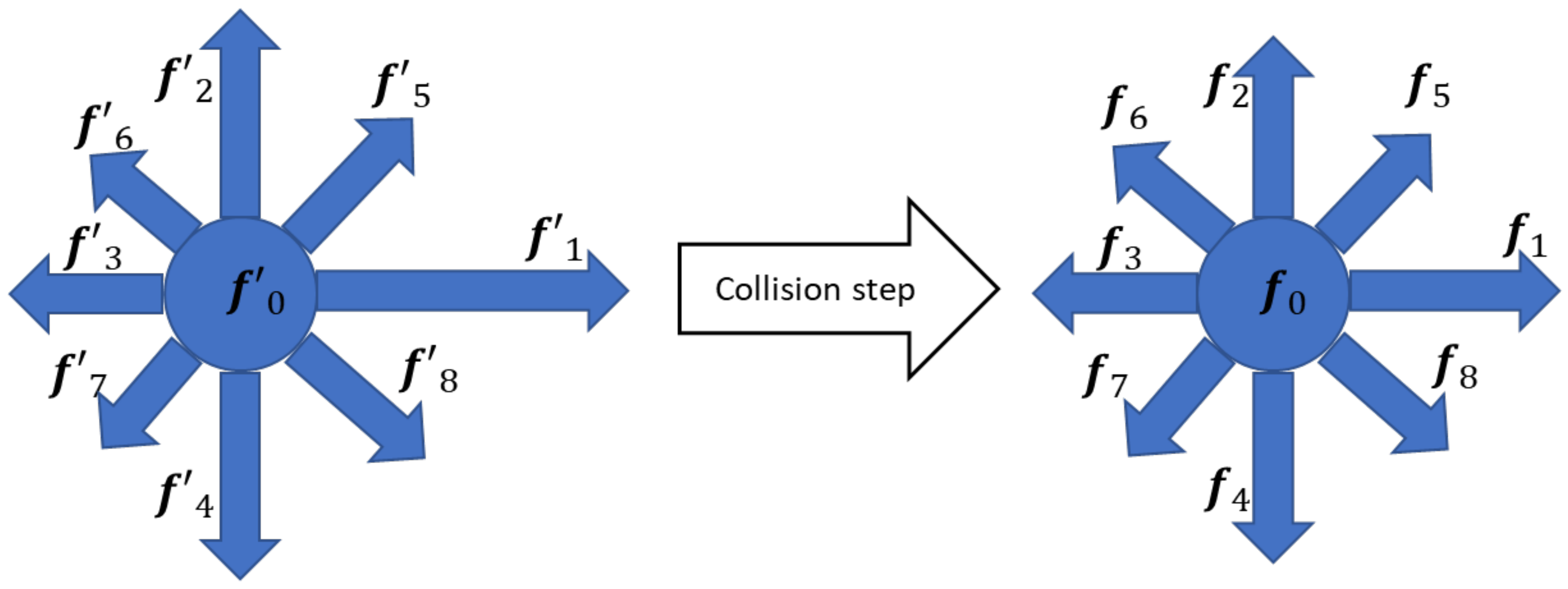
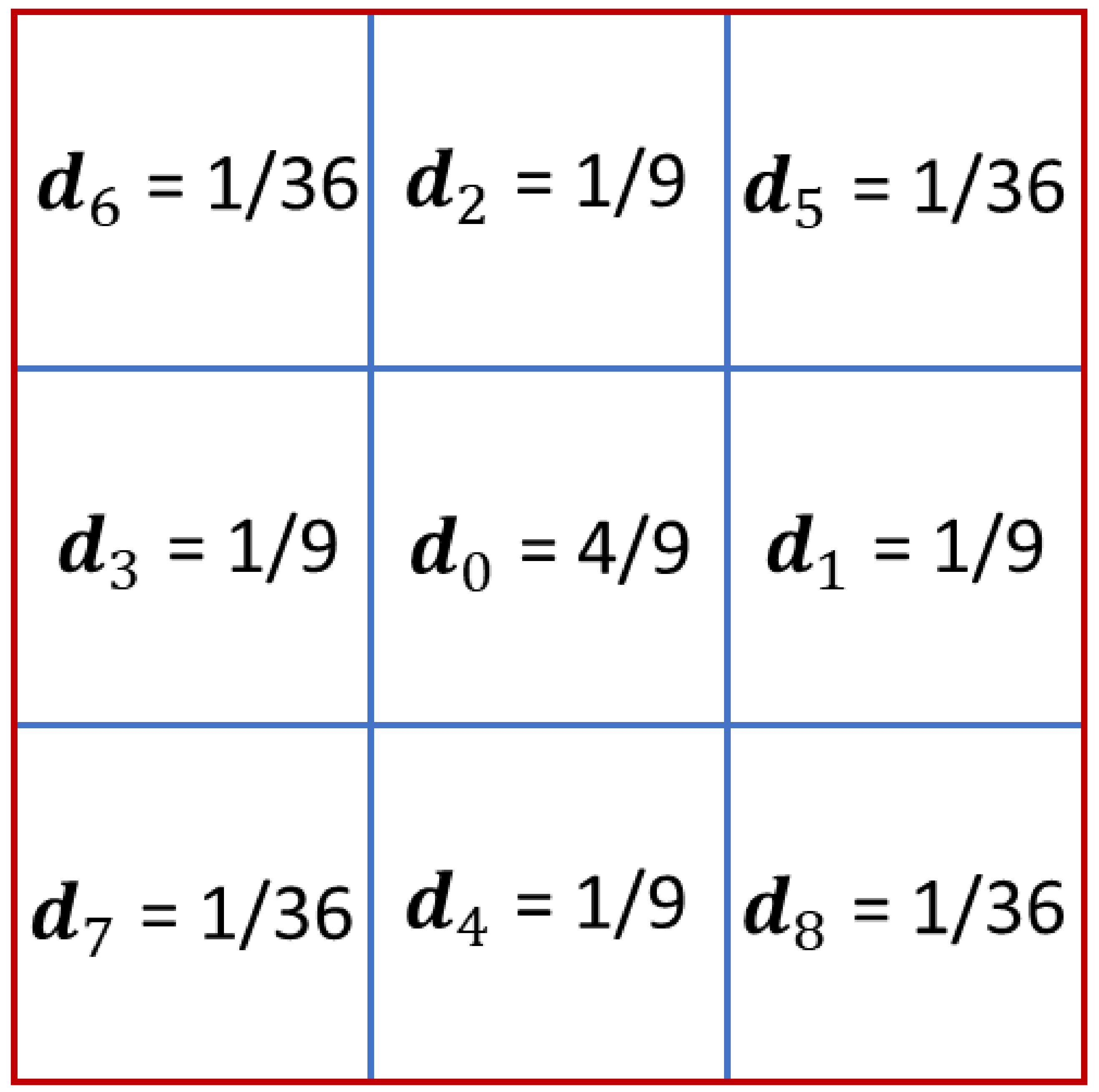
3.1. Hydrodynamics Modeling
- Calculation of the density and particle distribution in the cell in accordance with the streaming step.
- Calculation of the density and particle distribution in the cell in accordance with the collision phase.
- The system is divided into square cells of the same size.
- D2Q9 type was chosen as the lattice model—the system is two-dimensional and each cell has eight neighbors.
- Each cell has the following properties: discrete solvent (nasal mucus) density and API discrete density .
- In all left boundary cells, the macroscopic discrete velocity has a constant value .
- Boundary conditions along the vertical axis are periodic (toroidal)—liquid and API particles, when they move beyond the top boundary, move to the bottom boundary cells and move to the top boundary cells, and move beyond the lower ones.
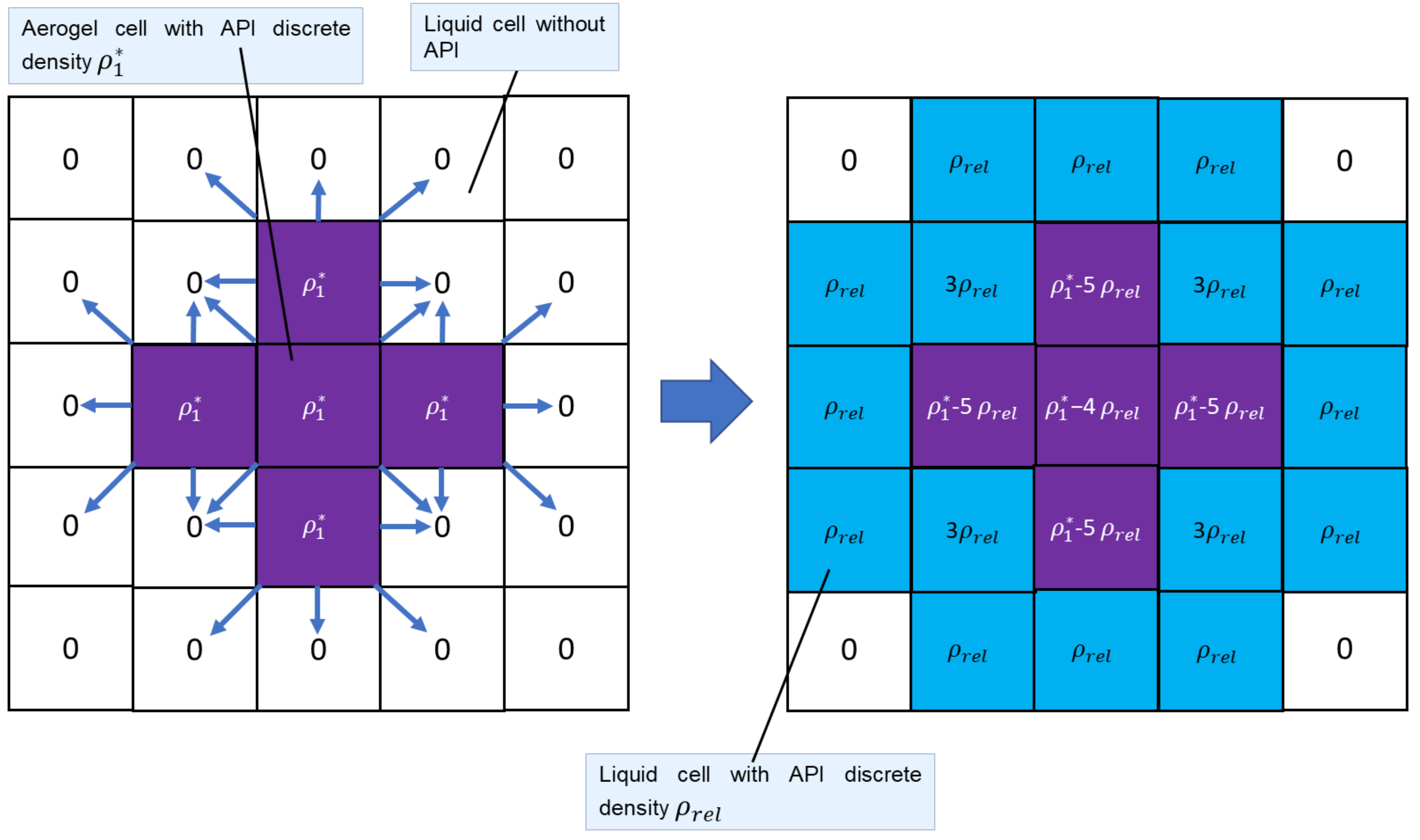
3.2. API Release Process Modeling
- The system is represented as a lattice consisting of square cells.
- In each cell, only one API and one solvent (nasal mucus) are considered.
- The cell is described by the discrete density of the solvent (nasal mucus) and the discrete density of the API .
- The cell at each time step can be in one of two states: “Liquid” or “Aerogel with impregnated API.”
- Each cell has eight neighbors.
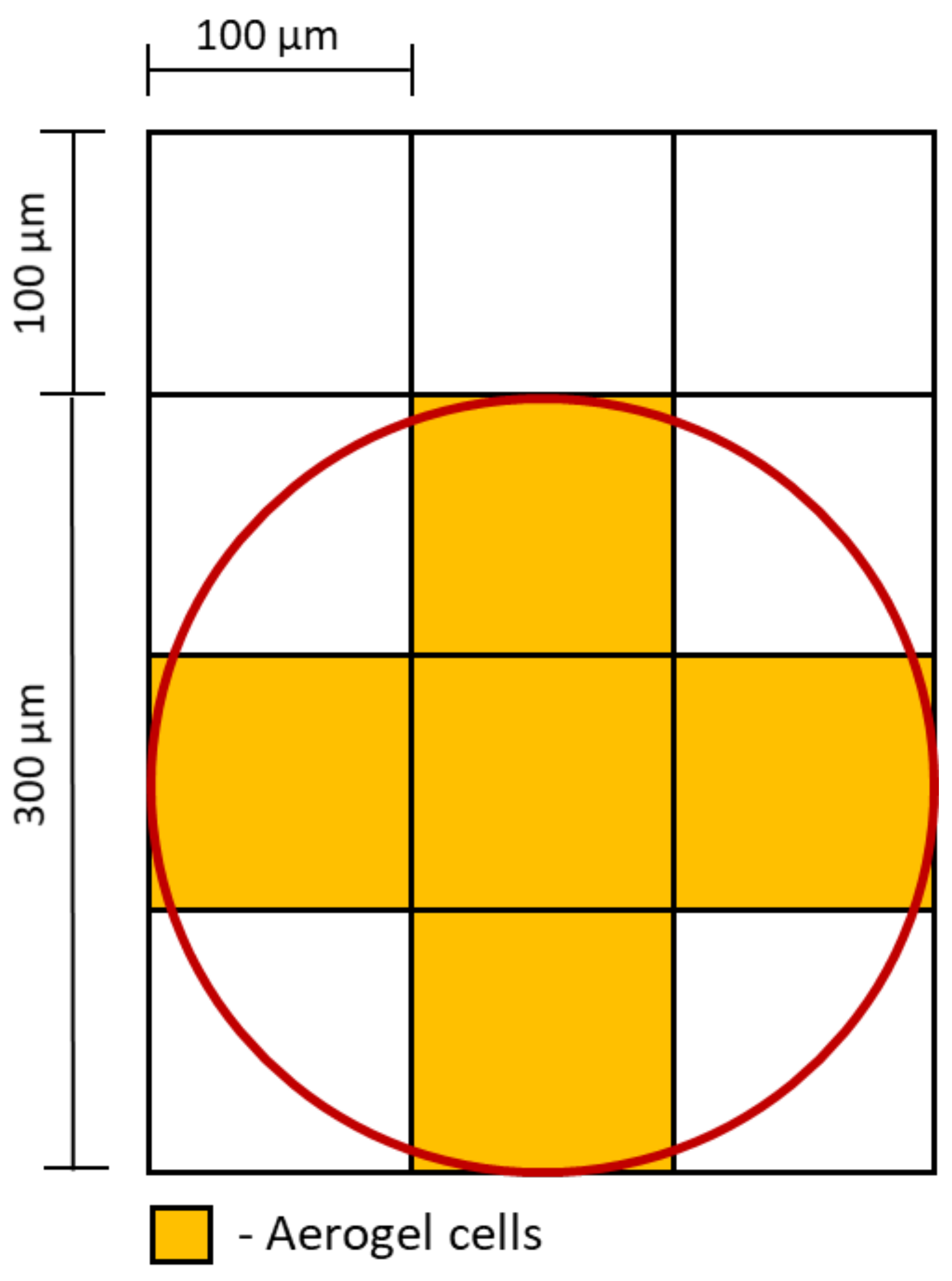
- The internal geometry of the aerogel particle is not considered. The API release from the aerogel particle into the nasal mucus is calculated according to Equation (11).
3.3. Hybrid Model of API Release and Its Flow in the Nasal Mucus Processes
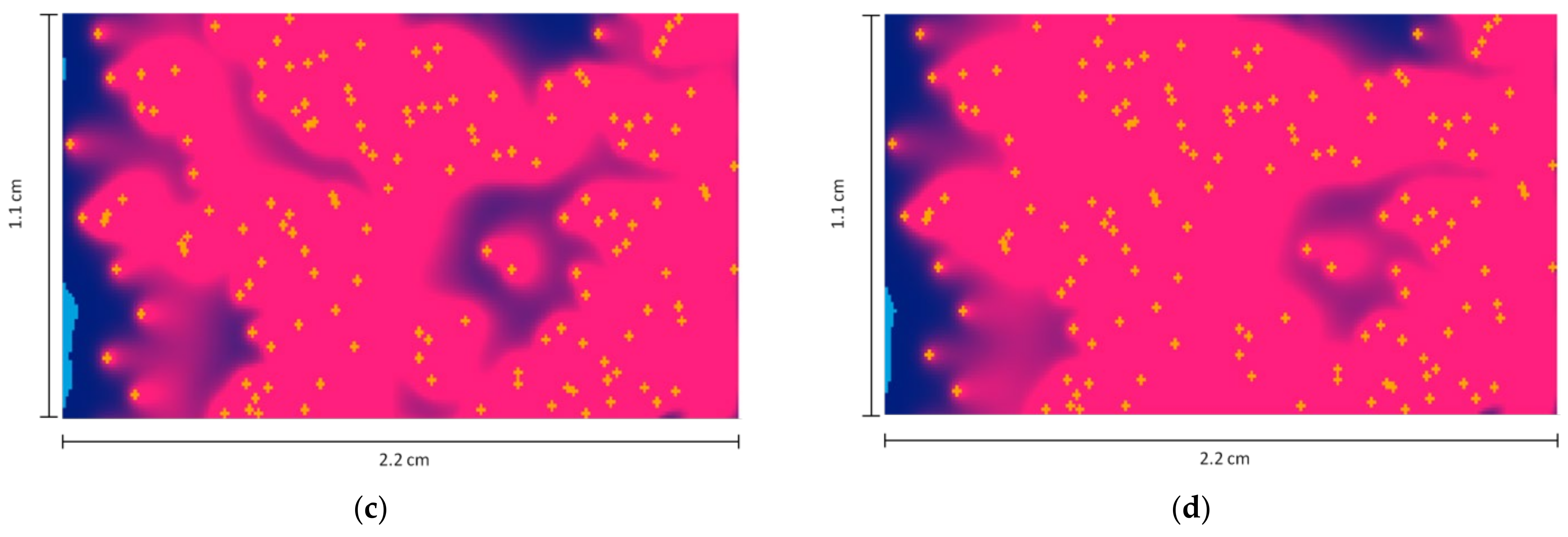
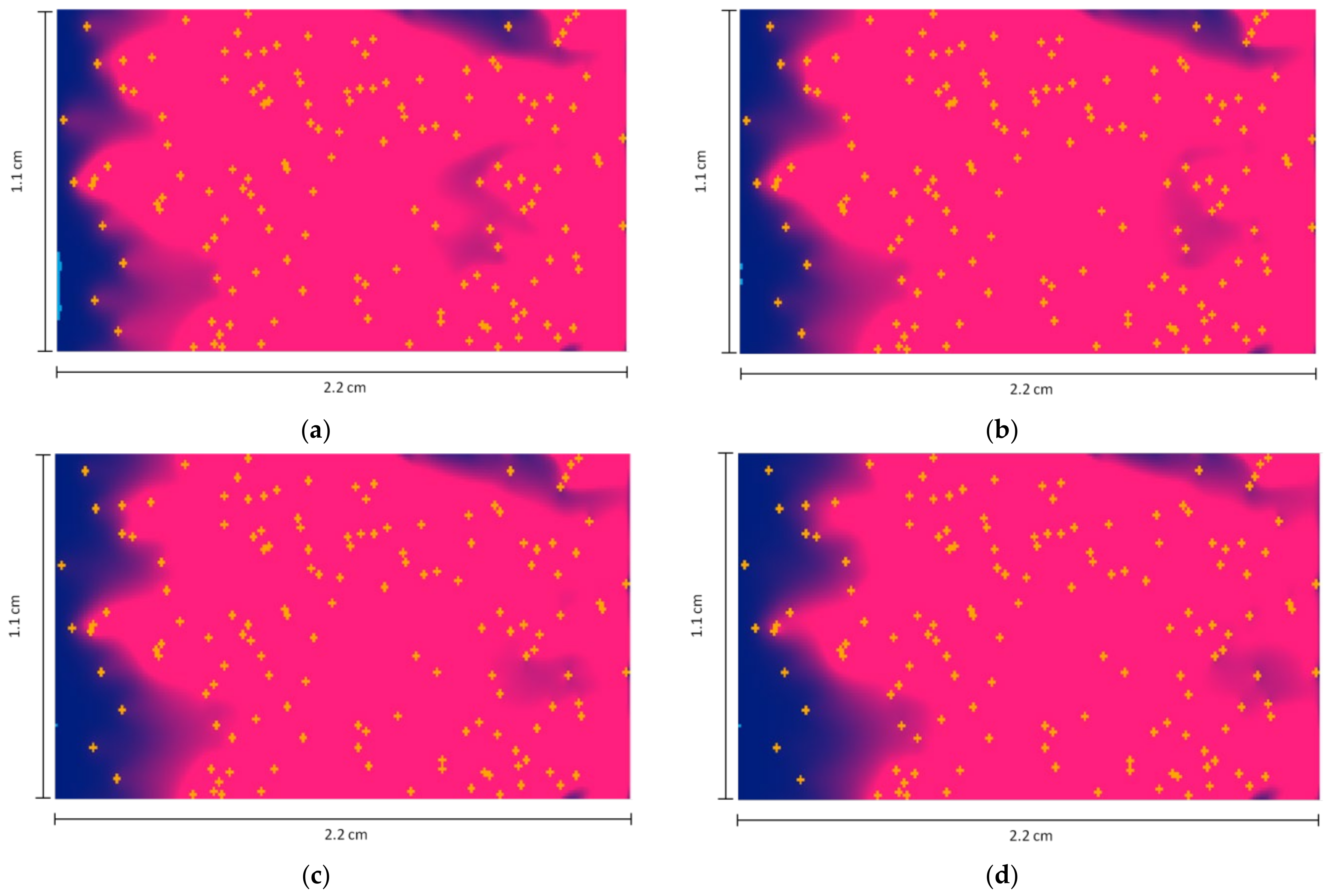
4. Results
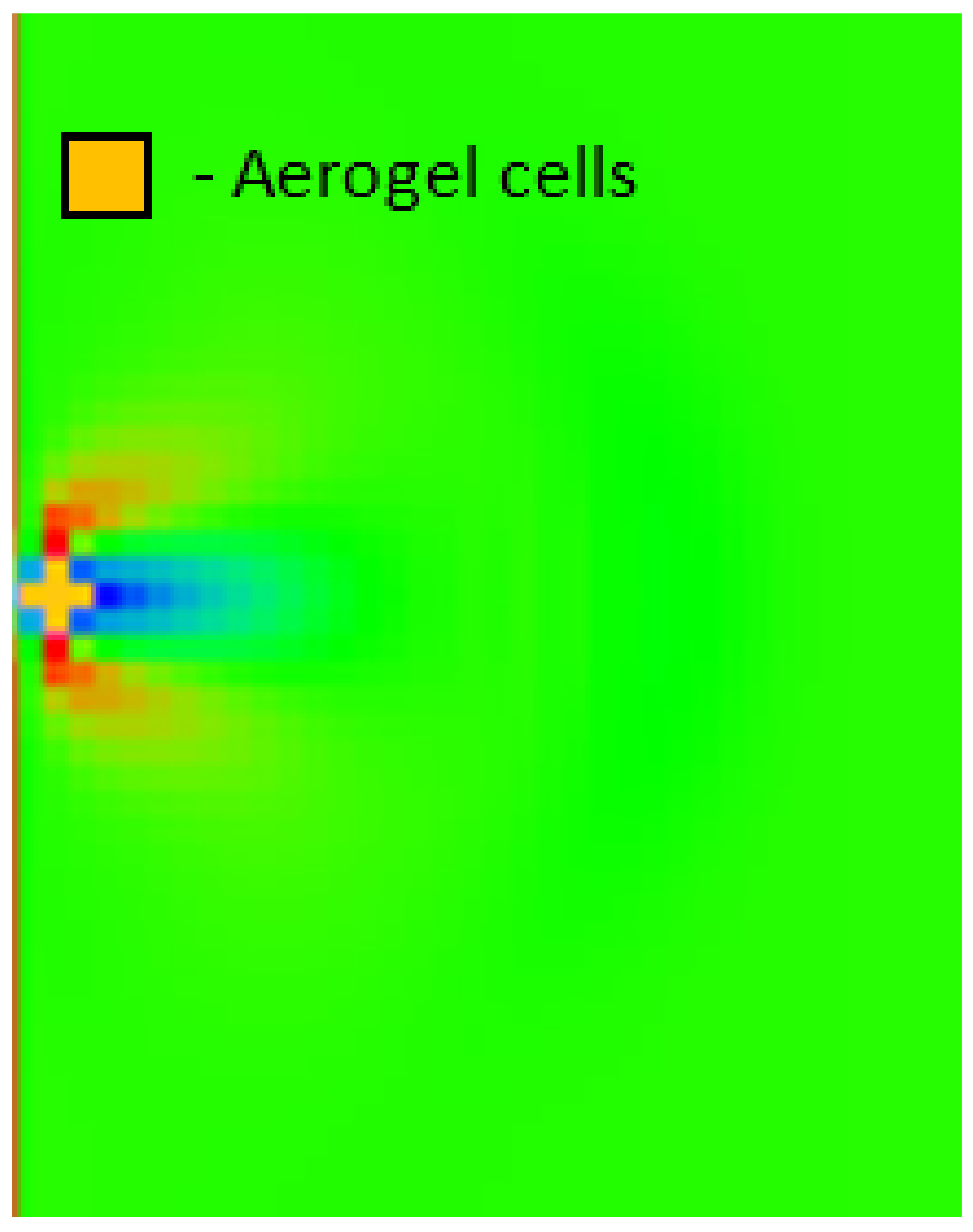
- Length L = 2.2 cm, width H = 1.1 cm.
- The flow velocity inside the cavity = 3 mm/min = 5 × 10−5 m/s, directed from left to right.
- Nasal mucus is considered as a Newtonian fluid, so it has a constant viscosity during the calculation time.
- Nasal mucus is homogeneous.
- The density of nasal mucus ρmucus = 1000 kg/m3.
- The value of dynamic viscosity is the same during the calculation time and is equal to η = 15 Pa∙s. Therefore, the value of kinematic viscosity is υ = η/ρmucus = 15 [N∙s/m2]/1000 [kg/m3] = 15 [kg s∙m/s2∙m2]/1000 [kg/m3] = 0.015 m2/s.
- The speed of sound in the nasal mucus cs is 1500 m/s.
- The number of deposited particles is 141.
- The discrete density of API (melatonin) in each cell is = 7.
- Dissolution constant K = 2 × 10−6.
- Maximum concentration of melatonin = 48 × 10−5 g/mL.
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batista, M.; Gonçalves, V.S.; Gaspar, F.; Nogueira, I.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Lovskaya, D.; Menshutina, N.; Mochalova, M.; Nosov, A.; Grebenyuk, A. Chitosan-based aerogel particles as highly effective local hemostatic agents. production process and in vivo evaluations. Polymers 2020, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Amorphization of drugs by adsorptive precipitation from supercritical solutions: A review. J. Supercrit. Fluids 2018, 132, 105–125. [Google Scholar] [CrossRef]
- Lovskaya, D.; Menshutina, N. Alginate-based aerogel particles as drug delivery systems: Investigation of the supercritical adsorption and in vitro evaluations. Materials 2020, 13, 329. [Google Scholar] [CrossRef]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef]
- Hussein, N.; Omer, H.; Elhissi, A.; Ahmed, W. Advances in nasal drug delivery systems. In Advances in Medical and Surgical Engineering; Ahmed, W., Phoenix, D., Jackson, M., Charalambous, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–311. [Google Scholar]
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef]
- Sun, M.; Yu, X.; Wang, T.; Bi, S.; Liu, Y.; Chen, X. Nasal adaptive chitosan-based nano-vehicles for anti-allergic drug delivery. Int. J. Biol. Macromol. 2019, 135, 1182–1192. [Google Scholar] [CrossRef]
- Mato, Y.L. Nasal route for vaccine and drug delivery: Features and current opportunities. Int. J. Pharm. 2019, 572, 118813. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, W.H., 2nd; Craft, S.; Danielyan, L.; Hallschmid, M.; Schiöth, H.B.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2012, 30, 2475–2484. [Google Scholar] [CrossRef]
- Harshad, P.; Anand, B.; Dushyant, S. Recent techniques in nasal drug delivery: A review. Int. J. Drug Dev. Res 2010, 2, 565–572. [Google Scholar]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef]
- Falahati, M.; Ghoreishi, S. Preparation of Balangu (Lallemantia royleana) seed mucilage aerogels loaded with paracetamol: Evaluation of drug loading via response surface methodology. J. Supercrit. Fluids 2019, 150, 1–10. [Google Scholar] [CrossRef]
- Filali, S.; Bergamelli, C.; Tall, M.L.; Salmon, D.; Laleye, D.; Dhelens, C.; Diouf, E.; Pivot, C.; Pirot, F. Formulation, stability testing, and analytical characterization of melatonin-based preparation for clinical trial. J. Pharm. Anal. 2017, 7, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Yuan, X.-F.; Chen, H. Kinetic theory representation of hydrodynamics: A way beyond the Navier–Stokes equation. J. Fluid Mech. 2006, 550, 413–441. [Google Scholar] [CrossRef]
- McDonough, J.M. Lectures in elementary fluid dynamics: Physics, mathematics and applications. In Engineering Textbook Gallery; University of Kentucky: Lexington, KY, USA, 2009; p. 164. [Google Scholar]
- Qian, Y.-H.; d’Humières, D.; Lallemand, P. Lattice BGK models for Navier-Stokes equation. EPL 1992, 17, 479. [Google Scholar] [CrossRef]
- Timm, K.; Kusumaatmaja, H.; Kuzmin, A.; Shardt, O.; Silva, G.; Viggen, E. The lattice Boltzmann method: Principles and practice. Springer Int. Publ. AG Switz. ISSN 2016, 1868–4521. [Google Scholar] [CrossRef]
- Sangoi, M.S.; Todeschini, V.; Steppe, M. Monolithic LC method applied to fesoterodine fumarate low dose extended-release tablets: Dissolution and release kinetics. J. Pharm. Anal. 2015, 5, 137–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elmas, A.; Akyüz, G.; Bergal, A.; Andaç, M.; Andac, O. Mathematical modelling of drug release. Res. Eng. Struct. Mater. 2020, 6, 327–350. [Google Scholar] [CrossRef]
- Ivanov, S.I.; Tiptsova, I.A.; Menshutina, N.V. Modeling dissolution of solids based on cellular automata with changing sizes of cells. Comput. Aided Chem. Eng. 2015, 37, 605–610. [Google Scholar]
- Ganimedov, V.; Muchnaya, M.; Sadovskij, A. The flow of air in the human nasal cavity. Results of mathematical modeling. Russ. J. Biomech. 2015, 37–51. [Google Scholar] [CrossRef]
- Van den Akker, H.E.A. Lattice Boltzmann simulations for multi-scale chemical engineering. Curr. Opin. Chem. Eng. 2018, 21, 67–75. [Google Scholar] [CrossRef]






| Experiment No. | Experiment Time, min | Concentration, g/mL |
|---|---|---|
| 1 | 1.5 | 32.320 × 10−5 |
| 3 | 38.885 × 10−5 | |
| 4.5 | 41.915 × 10−5 | |
| 6 | 47.975 × 10−5 | |
| 2 | 1.5 | 20.343 × 10−5 |
| 3 | 32.764 × 10−5 | |
| 4.5 | 35.893 × 10−5 | |
| 6 | 39.836 × 10−5 | |
| 3 | 1.5 | 13.213 × 10−5 |
| 3 | 23.692 × 10−5 | |
| 4.5 | 31.946 × 10−5 | |
| 6 | 37.737 × 10−5 |
| Experiment No. | Time, min | Deviation, % | ||
|---|---|---|---|---|
| 1 | 1.5 | 30.184 × 10−5 | 32.320 × 10−5 | 6.61 |
| 3 | 41.475 × 10−5 | 38.885 × 10−5 | 6.66 | |
| 4.5 | 45.610 × 10−5 | 41.915 × 10−5 | 8.82 | |
| 6 | 46.597 × 10−5 | 47.975 × 10−5 | 0.79 | |
| 2 | 1.5 | 20.343 × 10−5 | 18.683 × 10−5 | 8.46 |
| 3 | 32.764 × 10−5 | 30.210 × 10−5 | 7.80 | |
| 4.5 | 35.893 × 10−5 | 37.209 × 10−5 | 3.67 | |
| 6 | 39.836 × 10−5 | 41.445 × 10−5 | 4.04 | |
| 3 | 1.5 | 13.213 × 10−5 | 13.993 × 10−5 | 5.90 |
| 3 | 23.692 × 10−5 | 24.016 × 10−5 | 1.37 | |
| 4.5 | 31.946 × 10−5 | 31.087 × 10−5 | 2.69 | |
| 6 | 37.737 × 10−5 | 36.073 × 10−5 | 4.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, I.; Uvarova, A.; Mochalova, M.; Menshutina, N. Active Pharmaceutical Ingredients Transportation and Release from Aerogel Particles Processes Modeling. Computation 2022, 10, 139. https://doi.org/10.3390/computation10080139
Lebedev I, Uvarova A, Mochalova M, Menshutina N. Active Pharmaceutical Ingredients Transportation and Release from Aerogel Particles Processes Modeling. Computation. 2022; 10(8):139. https://doi.org/10.3390/computation10080139
Chicago/Turabian StyleLebedev, Igor, Anastasia Uvarova, Maria Mochalova, and Natalia Menshutina. 2022. "Active Pharmaceutical Ingredients Transportation and Release from Aerogel Particles Processes Modeling" Computation 10, no. 8: 139. https://doi.org/10.3390/computation10080139
APA StyleLebedev, I., Uvarova, A., Mochalova, M., & Menshutina, N. (2022). Active Pharmaceutical Ingredients Transportation and Release from Aerogel Particles Processes Modeling. Computation, 10(8), 139. https://doi.org/10.3390/computation10080139







