Abstract
To better understand graphene and its interactions with polycyclic aromatic hydrocarbons (PAHs), density-functional-theory (DFT) computations were used. Adsorption energy is likely to rise with the number of aromatic rings in the adsorbates. The DFT results revealed that the distance between the PAH molecules adsorbed onto the G ranged between 2.47 and 3.98 Å depending on the structure of PAH molecule. The Non-Covalent Interactions (NCI) plot supports the concept that van der Waals interactions were involved in PAH adsorption onto the Graphene (G) structure. Based on the DFT-calculated adsorption energy data, a rapid and reliable method employing an empirical model of a quantitative structure–activity relationship (QSAR) was created and validated for estimating the adsorption energies of PAH molecules onto graphene.
1. Introduction
Urban growth has resulted many human activities that pollute the environment with a variety of contaminants, including polycyclic aromatic hydrocarbons (PAHs). PAHs are persistent pollutants with a wide spectrum of biological toxicity due to their intrinsic features. Therefore, removing PAHs from the environment has been a global problem. PAH pollutants are found in both aquatic and terrestrial ecosystems, as well as in the atmosphere [1,2]
PAHs are a vast class of chemical compounds that have two or more fused benzene rings joined in linear, angular, or cluster configurations. They have high melting and boiling temperatures, are generally insoluble in water, and have minimal volatility. Their solubility diminishes as the number of aromatic rings increases [3]. They are lipophilic and extremely soluble in organic solvents. PAHs with more than five rings exist as solids, whereas those with fewer than five rings exist as vapors and particulates. PAH contamination, either directly or indirectly, has a significant impact on human health and well-being, as well as the health and well-being of other creatures across the world [4]. The selection of proper PAH-remediation solutions is always essential since it is heavily reliant on two primary parameters: the contaminated matrix and environmental conditions [5]. For treating PAH-contaminated soils, established remedial techniques include incineration, thermal treatment, solvent extraction/soil washing, chemical oxidation, microorganism or bacterial treatment, phytoremediation, composting/biopiles, and bioreactors [6,7].
Integrating physicochemical and biological approaches to improve PAH-contaminated soil remediation is also widespread [8,9].
Electrokinetic remediation, vermi-remediation, and biocatalyst-assisted remediation are all still in their infancy stage [10,11].
Adsorption is one of the most effective and economically feasible techniques for the removal of PAHs [12,13,14]. The adsorption of PAHs on carbonaceous adsorbents, particularly carbon nanomaterials and their modified forms, has received considerable attention in recent decades [15]. Carbon nanomaterials, such as fullerenes, single- and multi-walled carbon nanotubes (CNTs), and graphene (G), have been shown to have tremendous potential for organic-pollutant adsorption [16,17,18]. Since graphene has a high specific surface area, strong hydrophobicity, and a unique, delocalized, massive π-bond system, it offers a wide range of applications in the adsorption and treatment of aromatic contaminants. Surface-modified graphene has been proven to be an excellent adsorbent with large adsorption capacities and rapid adsorption rates for several PAHs [16,17,19]. So far, only a few theoretical studies in the literature have described the adsorption strength, geometry, and detailed interaction nature of PAHs when adsorbed onto graphene. As a result, in this study, the adsorption mechanisms of six PAHs and G (in mono and sandwiched form) were investigated using density functional theory (DFT) [20].
2. Method
The Dmol3 software from Biovia was used to perform the DFT calculations [21,22]. Generalized Gradient Approximation [23,24] employing the M-11L [25] and the Double Numerical Basis Set plus polarization function (DNP, 3.5) [26,27,28] were used for the geometry optimizations. A lower than 1 × 10−5 Ha convergence standard for the self-consistent field (SCF) was used. The model size was: 7 × 7 (composed of: 126 C and 30 H atoms) layer of graphene to accommodate the PAH molecules (Figure 1).
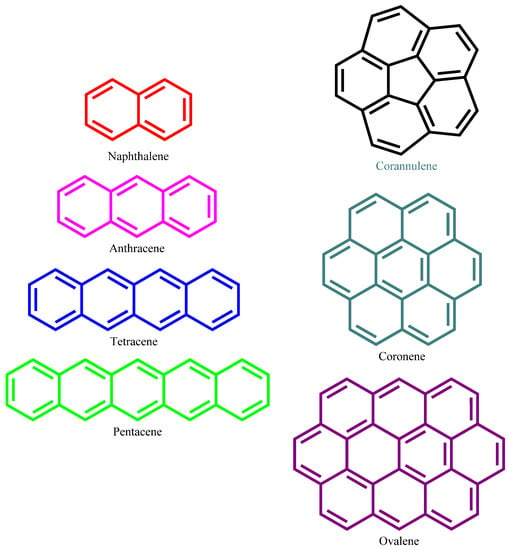
Figure 1.
Structure of the selected PAH molecules for the adsorption study.
The interaction energy (in vacuum) is evaluated as follow [29,30,31]:
where EG/PAH is the total energy of the adsorption system. EG and EPAH are the energies of the isolated PAH and G, respectively.
Eadsorption = EG/PAH − (EG + EPAH)
For the computation of the Non-Covalent Interactions, a PAH molecule (presented in Figure 1) adsorbed onto the 7 × 7 graphene model was used (Figure 2).
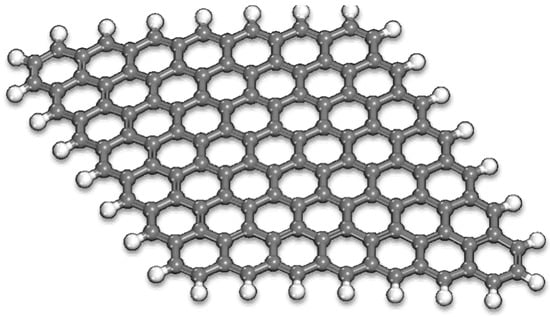
Figure 2.
Optimized structure of the G model used in the adsorption studies (C—grey and H—white spheres).
The geometry was optimized using the M-11L/DNP level of theory. At the density-functional-theory level, using the M06 exchange-correlation functional and the def2-SVP basis set [32], a single-point geometry calculation (using geometry coordinates generated by the Dmol3 software in the preceding phase) was performed using the Orca software [33]. An atom-pair dispersion correction utilizing the zero-damping technique (D30) [34] was used to account for the van der Waals interactions. Using Equation (1) [35,36,37,38], the adsorption energy was calculated. Multiwfn software [39] was used to calculate the Non-Covalent Interaction. Visual Molecular Dynamics [40] software was used to plot the NCI surface.
3. Discussion
The adsorption system, which holds one PAH molecule, was built to study the adsorption behavior of PAHs. It has been previously demonstrated that aromatic rings prefer a paralleled arrangement when adsorbing onto Graphene (G) [15,41]. The adsorption energy of the aforementioned PAHs onto graphene was determined using Equation (1).
The equilibrium distances between the PAH and Graphene as presented in Figure 3 are in range from 2.47 Å to 3.98 Å, and previous studies have shown that the interaction between neutral aromatic molecules and G at these equilibrium distances is determined by the joint interactions of Pauli repulsion, π–π interactions, short-range electrostatic Coulombic interactions, and van der Waals interactions [42,43,44,45]. The distances are in agreement with similar reported studies. Rapacioli et al. reported that the computed equilibrium distance for the adsorption of benzene dimers onto G is in a comparable range of 3.05–3.42 Å [46].
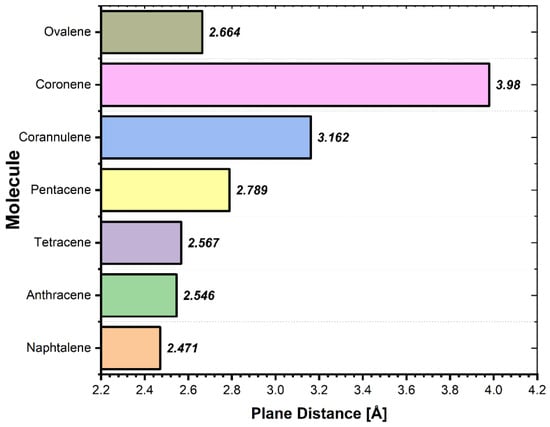
Figure 3.
The equilibrium distances between the PAH molecules and the Graphene surface plane.
Similarly, the adsorption distance on the graphene surface for other aromatic systems, such as 9,10-anthraquinone (AQ) and phenanthraquinone (PQ) are at 3.19 Å and 3.14 Å, respectively, which correlates well with the presented data [47].
The NCI surface plot and the reduced density gradient (RDG) vs. sign (λ) (Figure 4) [22] were used to examine the nature of the interaction between the PAH molecules and the G. In the 2D NCI plot, the greenish-blueish surface and spikes with negative sign (λ) values support the concept that van der Waals interactions are involved in PAH adsorption onto the G structure.
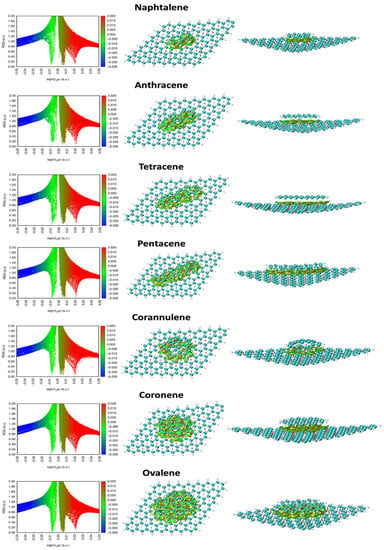
Figure 4.
Surfaces of NCI contacts and the plot of RDG vs. sign(λ)ρ van der Waals interactions between PAH molecules and the G surface.
Adsorption energy is a critical metric when it comes to chemical adsorption onto nanomaterials. Due to the large and growing number of ambient organic compounds, it is impractical and impossible to exhaustively, empirically, or even computationally estimate the values of these parameters using costly approaches such as DFT. Models of quantitative structure–activity relationships (QSARs) may be used in place of empirical parameter values. To the authors’ knowledge, no QSAR models have been developed that can be used to forecast the adsorption characteristics of polycyclic aromatic hydrocarbons on G. By utilizing the correlation between several fast and spatial descriptors (which can be calculated in less than a minute for the structures of PAHs on a simple PC), as illustrated in Table 1, and by employing model building based on multiple linear regression (MLR), a fast estimation of the Eads. of PAHs onto G is proposed.

Table 1.
The values of several fast and spatial descriptors as calculated from QSAR module in Material Studio software.
The term “molecular area” (vdW area) refers to a molecule’s van der Waals area. The molecular surface area indicates how much of a molecule is exposed to the external environment. This descriptor is associated with the binding, transport, and solubility of substances. The term “molecular volume” (vdW volume) refers to the volume contained within a molecule’s van der Waals area. The volume of a molecule is related to its adsorption and transport properties. The molecular density of a molecule is determined by the types of atoms and their arrangement inside it. Ghose’s approach was used to determine the QSAR descriptor ALogP and the molar refractivity [48].
The empirical QSAR equation that best represents the adsorption of the PAH compounds investigated onto G as determined by MLR is as follows:
Eads. [kcal/mol] = −0.501243 ∗ [AC:Chi (2) (Fast Descriptors)] − 1.5027
According to Figure 5, the validity of the equation is checked by comparing the DFT calculated Eads. of PAH with the predicted Eads. values. Figure 5 shows the results. The correlation coefficient provides evidence that the values predicted by the model are accurate.
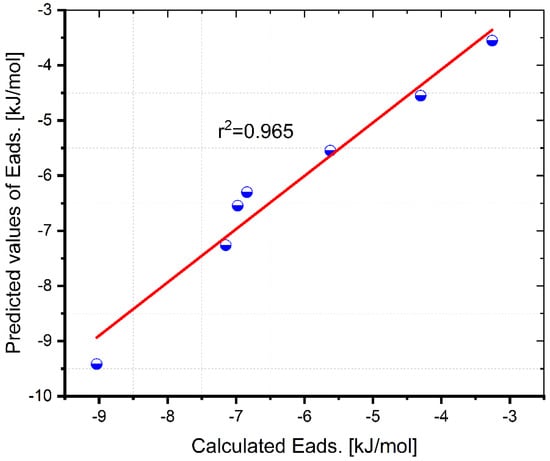
Figure 5.
The DFT computed Eads. of PAH/G vs. the QSAR predicted values of Eads.
The suggested QSAR approach for calculating the adsorption of PAH onto G is intended to be extended and utilized to calculate the adsorption of molecules on other two-dimensional nanomaterials [49].
4. Conclusions
For the prediction of the adsorption of PAH molecules onto the surface of graphene, an empirical QSAR model was developed and tested in this study. It is important to employ the correlation between multiple fast and spatial descriptors, as well as model-building approaches based on multiple linear regression (MLR), in order to design the model. Additionally, a positive association was discovered between the adsorption energies of PAH molecules onto graphene and the number of rings that they include within the structure, which is in accordance with previous findings. The NCI plot is consistent with the idea that van der Waals interactions are involved in the adsorption of PAH onto the G structure.
Author Contributions
Conceptualization, V.M. and M.S.; methodology, V.M. and M.S.; software, V.M.; writing—original draft preparation, M.S. and V.M.; writing—review and editing, V.M. and M.S.; resources, M.S. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the support from the Ministry of Education, Science and Technology of Kosovo (Nr.2-5069) for providing the computing resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 2675. [Google Scholar] [CrossRef] [PubMed]
- Manzetti, S. Polycyclic Aromatic Hydrocarbons in the Environment: Environmental Fate and Transformation. Polycycl. Aromat. Compd. 2013, 33, 311–330. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, H.A. Nanoparticles used for extraction of polycyclic aromatic hydrocarbons. J. Chem. 2019, 2019, 4816849. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Košnář, Z.; Mercl, F.; Aranda, E.; Tlustoš, P. A comparative study to evaluate natural attenuation, mycoaugmentation, phytoremediation, and microbial-assisted phytoremediation strategies for the bioremediation of an aged PAH-polluted soil. Ecotoxicol. Environ. Saf. 2018, 147, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Cofield, N.; Schwab, A.P.; Banks, M.K. Phytoremediation of polycyclic aromatic hydrocarbons in soil: Part I. Dissipation of target contaminants. Int. J. Phytoremediation 2007, 9, 355–370. [Google Scholar] [CrossRef]
- Jeelani, N.; Yang, W.; Xu, L.; Qiao, Y.; An, S.; Leng, X. Phytoremediation potential of Acorus calamus in soils co-contaminated with cadmium and polycyclic aromatic hydrocarbons. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Guo, G.; Liu, C.; Tian, F.; Ding, K.; Wang, H.; Zhang, C.; Yang, F.; Xu, J. Bioaugmentation treatment of polycyclic aromatic hydrocarbon-polluted soil in a slurry bioreactor with a bacterial consortium and hydroxypropyl-β-cyclodextrin. Environ. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Forján, R.; Lores, I.; Sierra, C.; Baragaño, D.; Gallego, J.L.R.; Peláez, A.I. Bioaugmentation Treatment of a PAH-Polluted Soil in a Slurry Bioreactor. Appl. Sci. 2020, 10, 2837. [Google Scholar] [CrossRef] [Green Version]
- Alcántara, M.T.; Gómez, J.; Pazos, M.; Sanromán, M.A. Electrokinetic remediation of PAH mixtures from kaolin. J. Hazard. Mater. 2010, 179, 1156–1160. [Google Scholar] [CrossRef]
- Rorat, A.; Wloka, D.; Grobelak, A.; Grosser, A.; Sosnecka, A.; Milczarek, M.; Jelonek, P.; Vandenbulcke, F.; Kacprzak, M. Vermiremediation of polycyclic aromatic hydrocarbons and heavy metals in sewage sludge composting process. J. Environ. Manag. 2017, 187, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Satouh, S.; Martín, J.; Del Mar Orta, M.; Medina-Carrasco, S.; Messikh, N.; Bougdah, N.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of polycyclic aromatic hydrocarbons by natural, synthetic and modified clays. Environments 2021, 8, 124. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ou, P.; Wei, Y.; Zhang, X.; Song, J. Polycyclic aromatic hydrocarbons adsorption onto graphene: A DFT and AIMD Study. Materials 2018, 11, 726. [Google Scholar] [CrossRef] [Green Version]
- Perreault, F.; Fonseca De Faria, A.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, S.; Zhao, G.; Wang, Q.; Wang, X. Adsorption of polycyclic aromatic hydrocarbons on graphene oxides and reduced graphene oxides. Chem. Asian J. 2013, 8, 2755–2761. [Google Scholar] [CrossRef]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy Graphene as a Highly Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Zhao, G.; Jiang, L.; He, Y.; Li, J.; Dong, H.; Wang, X.; Hu, W. Sulfonated Graphene for Persistent Aromatic Pollutant Management. Adv. Mater. 2011, 23, 3959–3963. [Google Scholar] [CrossRef]
- Buragohain, M.; Pathak, A.; Sakon, I.; Onaka, T. DFT study on interstellar PAH molecules with aliphatic side groups. Astrophys. J. 2020, 892, 11. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Andzelm, J.; King-Smith, R.D.; Fitzgerald, G. Geometry optimization of solids using delocalized internal coordinates. Chem. Phys. Lett. 2001, 335, 321–326. [Google Scholar] [CrossRef]
- Azizi, E.; Tehrani, Z.A.; Jamshidi, Z. Interactions of small gold clusters, Aun (n=1-3), with graphyne: Theoretical investigation. J. Mol. Graph. Model. 2014, 54, 80–89. [Google Scholar] [CrossRef]
- Hu, L.; Hu, X.; Wu, X.; Du, C.; Dai, Y.; Deng, J. Density functional calculation of transition metal adatom adsorption on graphene. Phys. B Condens. Matter 2010, 405, 3337–3341. [Google Scholar] [CrossRef]
- Peverati, R.; Truhlar, D.G. Performance of the M11 and M11-L density functionals for calculations of electronic excitation energies by adiabatic time-dependent density functional theory. Phys. Chem. Chem. Phys. 2012, 14, 11363–11370. [Google Scholar] [CrossRef] [PubMed]
- Molhi, A.; Hsissou, R.; Damej, M.; Berisha, A.; Bamaarouf, M.; Seydou, M.; Benmessaoud, M.; El Hajjaji, S. Performance of two epoxy compounds against corrosion of C38 steel in 1 M HCl: Electrochemical, thermodynamic and theoretical assessment. Int. J. Corros. Scale Inhib. 2021, 10, 812–837. [Google Scholar] [CrossRef]
- Berisha, A. Experimental, Monte Carlo and molecular dynamic study on corrosion inhibition of mild steel by pyridine derivatives in aqueous perchloric acid. Electrochem 2020, 1, 188–199. [Google Scholar] [CrossRef]
- Berisha, A. The influence of the grafted aryl groups on the solvation properties of the graphyne and graphdiyne- A MD study. Open Chem. 2019, 17, 703–710. [Google Scholar] [CrossRef]
- Berisha, A. First principles details into the grafting of aryl radicals onto the free-standing and borophene/Ag (1 1 1) surfaces. Chem. Phys. 2021, 544, 111124. [Google Scholar] [CrossRef]
- Berisha, A. Ab inito exploration of nanocars as potential corrosion inhibitors. Comput. Theor. Chem. 2021, 1201, 113258. [Google Scholar] [CrossRef]
- Phal, S.; Nguyễn, H.; Berisha, A.; Tesfalidet, S. In situ Bi/carboxyphenyl-modified glassy carbon electrode as a sensor platform for detection of Cd2+ and Pb2+ using square wave anodic stripping voltammetry. Sens. Bio-Sens. Res. 2021, 34, 100455. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Pino-Rios, R.; Chigo-Anota, E.; Shakerzadeh, E.; Cárdenas-Jirón, G. B12N12 cluster as a collector of noble gases: A quantum chemical study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 115, 113697. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [Green Version]
- Jessima, S.J.H.M.; Berisha, A.; Srikandan, S.S.; Subhashini, S. Preparation, characterization, and evaluation of corrosion inhibition efficiency of sodium lauryl sulfate modified chitosan for mild steel in the acid pickling process. J. Mol. Liq. 2020, 320, 114382. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Safi, Z.; Hamed, O.; Jodeh, S.; Verma, C.; Ebenso, E.E.E.; El Harfi, A. DGEBA-polyaminoamide as effective anti-corrosive material for 15CDV6 steel in NaCl medium: Computational and experimental studies. J. Appl. Polym. Sci. 2020, 137, 48402. [Google Scholar] [CrossRef]
- Hsissou, R.; Dagdag, O.; Abbout, S.; Benhiba, F.; Berradi, M.; El Bouchti, M.; Berisha, A.; Hajjaji, N.; Elharfi, A. Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J. Mol. Liq. 2019, 284, 182–192. [Google Scholar] [CrossRef]
- Uppalapati, P.K.; Berisha, A.; Velmurugan, K.; Nandhakumar, R.; Khosla, A.; Liang, T. Salen type additives as corrosion mitigator for Ni–W alloys: Detailed electronic/atomic-scale computational illustration. Int. J. Quantum Chem. 2021, 121, e26600. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Rajesh, C.; Majumder, C.; Mizuseki, H.; Kawazoe, Y. A theoretical study on the interaction of aromatic amino acids with graphene and single walled carbon nanotube. J. Chem. Phys. 2009, 130, 124911. [Google Scholar] [CrossRef] [PubMed]
- Berisha, A. Interactions between the aryldiazonium cations and graphene oxide: A DFT study. J. Chem. 2019, 2019, 5126071. [Google Scholar] [CrossRef]
- Mehmeti, V.; Halili, J.; Berisha, A. Which is better for Lindane pesticide adsorption, graphene or graphene oxide? An experimental and DFT study. J. Mol. Liq. 2022, 347, 118345. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Moe, Y.N. On the performance of local density approximation in describing the adsorption of electron donating/accepting molecules on graphene. Chem. Phys. 2012, 406, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, Y.; Wang, Y.B. Noncovalent π⋅⋅⋅π interaction between graphene and aromatic molecule: Structure, energy, and nature. J. Chem. Phys. 2014, 140, 094302. [Google Scholar] [CrossRef]
- Rapacioli, M.; Tarrat, N. Periodic DFTB for supported clusters: Implementation and application on benzene dimers deposited on graphene. Computation 2022, 10, 39. [Google Scholar] [CrossRef]
- Yu, Y.X. A dispersion-corrected DFT study on adsorption of battery active materials anthraquinone and its derivatives on monolayer graphene and h-BN. J. Mater. Chem. A 2014, 2, 8910–8917. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragmental methods: An analysis of ALogP and ClogP methods. J. Phys. Chem. 1998, 102, 3762–3772. [Google Scholar] [CrossRef]
- Yu, B.; Ren, H.; Piao, X. Towards adsorptive enrichment of flavonoids from honey using h-BN monolayer. ChemPhysChem 2022, 23, e202100828. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).