1. Introduction
In 1986, John Haugeland [
1] introduced the term “synthetic intelligence” as a class of simulated intelligence that is able to generate its own knowledge. At present, generative artificial intelligence is a form of synthetic intelligence that uses deep learning algorithms to generate new data from a dataset. For example, ChatGPT, which uses large language models or LLMs, is able to return text in response to an input or prompt. The popularity of AI has led most people to consider that the expression “an Artificial Intelligence” evokes in their personal imagination is a model often drawn from science-fiction movies, which is used as a reference model. The result of this perception is the popular belief that advances in AI will lead to a “cold and nihilistic world” with cities of neo-noir aesthetics densely populated by both humans and “intelligent” programs, machines and robots. In the late 1980s, Carver Mead [
2] proposed the design of algorithms, analog software and bio-inspired integrated circuits that would mimic the behavior of the nervous system of animals. From this idea, neuromorphic computing [
3] was born, with the aim of emulating the behavior of the animal nervous system, in general, and the brain in particular. However, in order to emulate human beings, it is important to bear in mind that human beings experience emotions, i.e., we generate brain and, therefore, physiological responses to stimuli. In other words, we not only process the information contained in stimuli, e.g., braking the vehicle we are driving when the traffic light is turning red, but we also feel emotions. Therefore, it is not uncommon that when we see scenes in the street, e.g., children crossing the street or an old man fallen on the sidewalk, we feel some emotion that modifies our behavior to adapt ourselves to the situation. The interpretation of the emotions experienced and their memory over time lead human beings to have feelings. Obviously, and from a biological point of view, our ability to perceive the feelings of those around us will contribute to the survival of our species [
4].
Neuromorphic computing has the value of recovering interest in some of the underlying principles and objectives with which many of the simulations in artificial life were conceived in the last century [
5,
6]. Although it is not the subject of epistemological analysis, research or review in this paper, the interest in creating an artificial living being, such as the myth of Golem or the romantic idea underlying the novel Frankenstein, has survived into the present century. The survival of this romantic idea is the result of advances in synthetic biology, a discipline that has made possible the design of the first artificial cells in the laboratory [
7]. For instance,
M. mycoides JCVI-syn3.0 [
8] currently represents one of the most outstanding examples. However, all of these achievements fall far short of the qualities that endow the human body with the capacity to feel emotions, making our species an “intelligent and sensitive organic matter”. In our opinion, neuromorphic computing will promote the study and design of materials that mimic some of the “emotional” qualities of organic matter, such as pain, the effect on the immune system of caresses, the beneficial effect of skin-to-skin contact, or other emotional responses such as blushing. Consequently, in the future, “intelligent” programs, machines and robots will be built displaying “emotional” traits or will have been constructed using “sentient” hardware capable of emulating the arousal of such emotions.
Similar to neuromorphic computing, a research area that focuses its efforts on the design of AI models that emulate the nervous system, we propose the concept of hormone-morphic computing in this work. We propose, with this term, the design of bio-inspired AI models, i.e., synthetic intelligent systems that display the main features of the hormonal system. That is, while the control, and, therefore, the output or response of an AI system to a certain input resides, for example, in an artificial neural network or other equivalent model, the modulation or adjustment of the output could be performed through a model that simulates the hormonal system. Therefore, for any AI system with these features, we would not only have implemented pattern recognition and learning but also the expression of emotions, one of the fundamental ingredients of human beings.
Indeed, the possibility of simulating the hormonal system with differential equations is not something new [
9], nor is the application of sentiment analysis techniques [
10] to a conversation, but their incorporation into an AI system could introduce some degree of originality. For instance, we could design a chatbot, i.e., a conversational bot simulating a conversation with a person but exhibiting hormone-morphic traits.
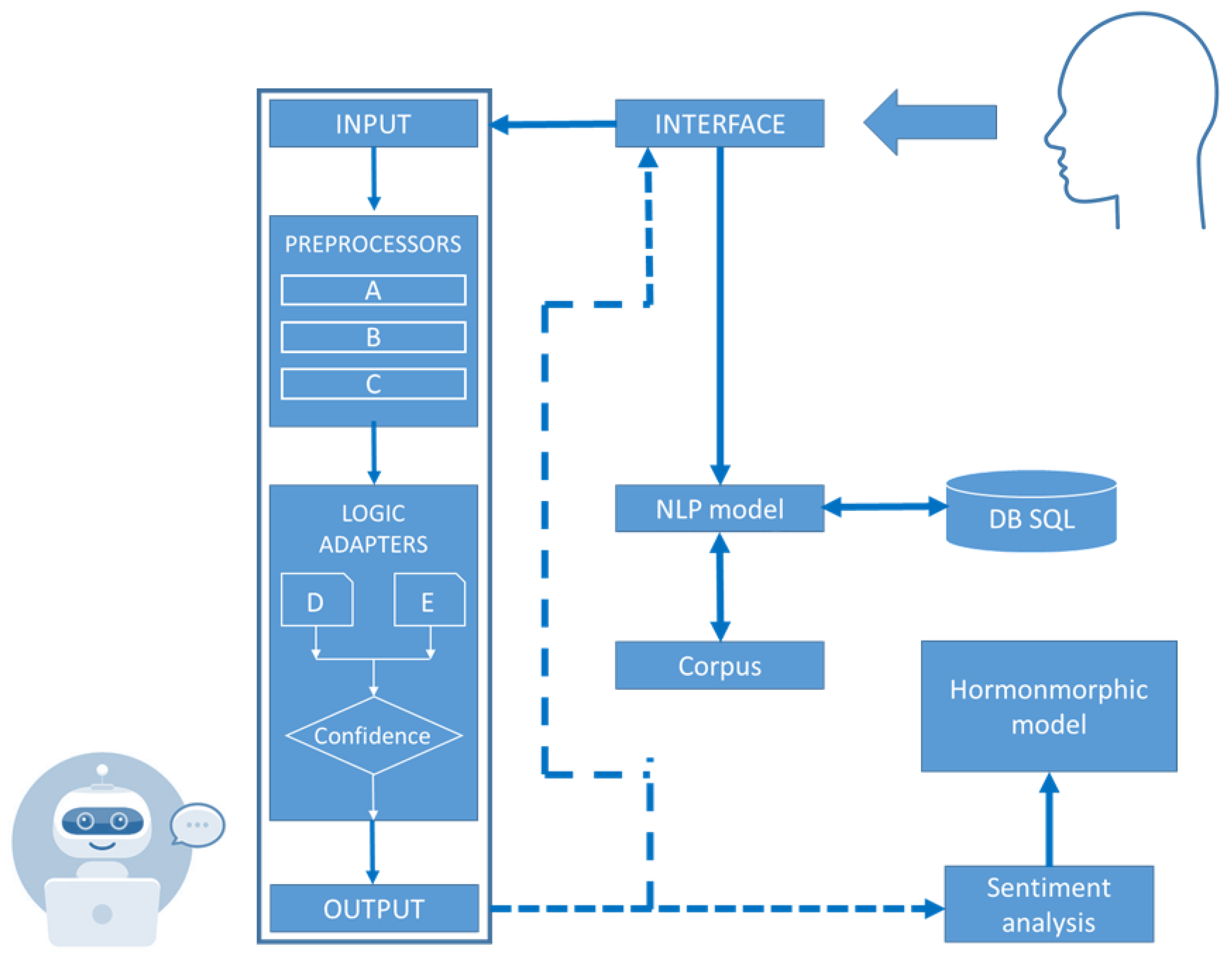
In this paper, we present a chatbot that we have named “Fatty”. The goal of the work has been to design an empathic chatbot in which the bot “gets fat” as a consequence of a conversation with its interlocutor, with “eating food” relieving its discomfort. The chatbot exhibits an eating behavior disorder which is triggered when an interlocutor talks to the bot about a topic to which Fatty is particularly sensitive, particularly when the conversation is about soccer or junk food. Fattybot’s “emotions” are expressed during the conversation as a consequence of having included in the bot’s architecture a model of the hormonal system and another of the immune system. These models emulate the molecular and physiological mechanisms responsible for the accumulation of energy and increase in the volume of adipocytes or adipose tissue cells. Consequently, the bot would put on weight as a consequence of the inflammation experienced by the “fat cells” of its “body”.
The work is a further step of the previous models conducted earlier. In [
11], we introduced a minimally viable prototype of an empathic chatbot that we named LENNA. The chatbot was used to evaluate the feasibility of Shannon entropy as a measure of the emotional state changes experienced by the chatbot during a conversation. The conversations were then analyzed by applying sentiment analysis techniques combined with multivariate statistical methods and Fourier analysis [
10]. In a previous work [
12], we simulated a virtual patient by hybridization with the chatbot of a genetic algorithm, stochastic networks, differential equations, etc. The patient presented an altered mental state, whether depressed, stressed or similar. The result of this impaired mental state was the alteration of hormone levels and chronic inflammation of the colon, leading to the development of a cancerous tumor.
2. Materials and Methods
2.5. Emotion Model in Fattybot
In this section, we describe the model whereby ChatterBot simulated to express emotions of depression, anxiety or similar, as a consequence of the conversation held with an interlocutor. The algorithm we used involved a routine in the loop that maintained the conversation in which it performed the sentiment analysis of the response provided by the bot. Sentiment analysis [
10] is an AI technique in which the emotional tone of a series of words is evaluated, extracting significant information about attitudes, emotions and opinions—in this case, from the ChatterBot’s responses. The implementation of this capability in the bot required the installation of Python’s TextBlob library [
15].
The emotions expressed by the bot in words can be positive or negative. The positive emotions are, e.g., joy, gratitude, serenity, interest in the world, hope, pride, amusement, inspiration, amazement and love, while the negative emotions are fear, disgust, anxiety, frustration, guilt, etc. In the analysis, the polarity of emotions P was obtained, i.e., a value between [−1, 1]. Values below 0 refer to a negative emotion and above 0 to a positive emotion, with −1 being very negative emotions and +1 being very positive emotions. Subjectivity S was also obtained; this is a measure of the emotion ranging from being objective to subjective, when its value is 0 or 1, respectively. From these values, it was detected when the ChatterBot experiences negative or positive emotions during the course of the conversation with the interlocutor. However, the value of subjectivity was not used in this model.
Afterwards, and based on the polarity resulting from the conversation, if it was negative, then Fattybot felt depressed. This led it to find itself in a situation whose form of relief was through the compulsive ingestion of food. The result is weight gain due to the accumulation of body fat. Note that the topics that alter Fattybot’s emotional state are football and junk food.
2.5.1. Model of Energy “Accumulation” in Adipose Tissue
The model of energy accumulation in adipose tissue (
Figure 3) is the model described in [
16].
Table 1 shows the differential equations of the model. Equation (1) expresses the rate of the change of plasma leptin (
Lp) where k
s and k
c are the parameters modeling its synthesis and elimination, respectively. In Equation (2),
is the fraction of leptin in blood plasma that reaches the brain. Note that
value is calculated with the Hill equation (
n = 1), where
k2 is the dissociation constant.
A part of the leptin circulating in plasma will reach the brain (Lb), an event that is captured by Expression (3). Entry into the brain is both via receptors (coefficient k1) and diffusion (coefficient k3), k1 and k3 being the input parameters of both mechanisms, respectively.
The ingested food FI and the energy input EI to the organism are represented in Expressions (4) and (5), respectively. Note that FI food intake is related (k4) to brain leptin levels Lb. Thus, leptine Lb stimulates the hypothalamus to suppress food intake decreasing body adiposity. In (4), the parameter k5 models the level of food intake and physical activity or sport performed by a subject. In other words, the higher the k5, the more food that is ingested and the less physical exercise is performed.
The relationship between the emotional state of the chatbot, i.e., Fattybot, and the input energy EI coming from the “food ingested” by the chatbot is simulated in Expression (5) with parameter. This is a parameter modeling the amount of metabolizable energy by the organism, i.e., the chatbot, being value equal to . Thus, is the total polarity value, a value that is calculated as the sum of the partial polarities throughout the conversation between the chatbot and its human interlocutor.
Once a certain intake of food has been ingested, the energy output EO or energy consumed, i.e., energy spent during physical activity, is given by the Expression (6). In Expression (6), w is the weight of the subject, i.e., of the chatbot, and k6, k7 and k8 are the parameters of Expression (6). Finally, the rate of the energy change is given by the ordinary differential Equation (7).
In summary, since the energy that is stored in the adipose tissue is given by the difference between the input and output energies, i.e.,
, the lower the value of the parameter
k6 (and, therefore, the lower the output energy
EO), the more energy is stored (in the form of neutral fatty acids) in the adipose tissue. The result is an increase in the volume of adipose tissue, thus leading to a consequent “fattening” of the individual (
Figure 4). If Fattybot was built with hardware, then we would reflect the discomfort it feels, whether feeling anxious or depressed, as a result of having a conversation about soccer or junk food by simulating some distinctive trait that would affect the chatbot’s outward appearance.
Table 1.
Energy accumulation model in adipose tissue.
| Ordinary Differential Equation | |
|---|
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
2.5.2. Adipose Tissue Inflammation Model
Obesity is the result of the inflammation of adipose tissue due to an increase in its volume caused by an abnormal response of the immune system (
Figure 4). In order to simulate the inflammation of the adipose tissue due to the intervention of the immune system, we have applied the model [
17].
Table 2 shows the differential equations of the model.
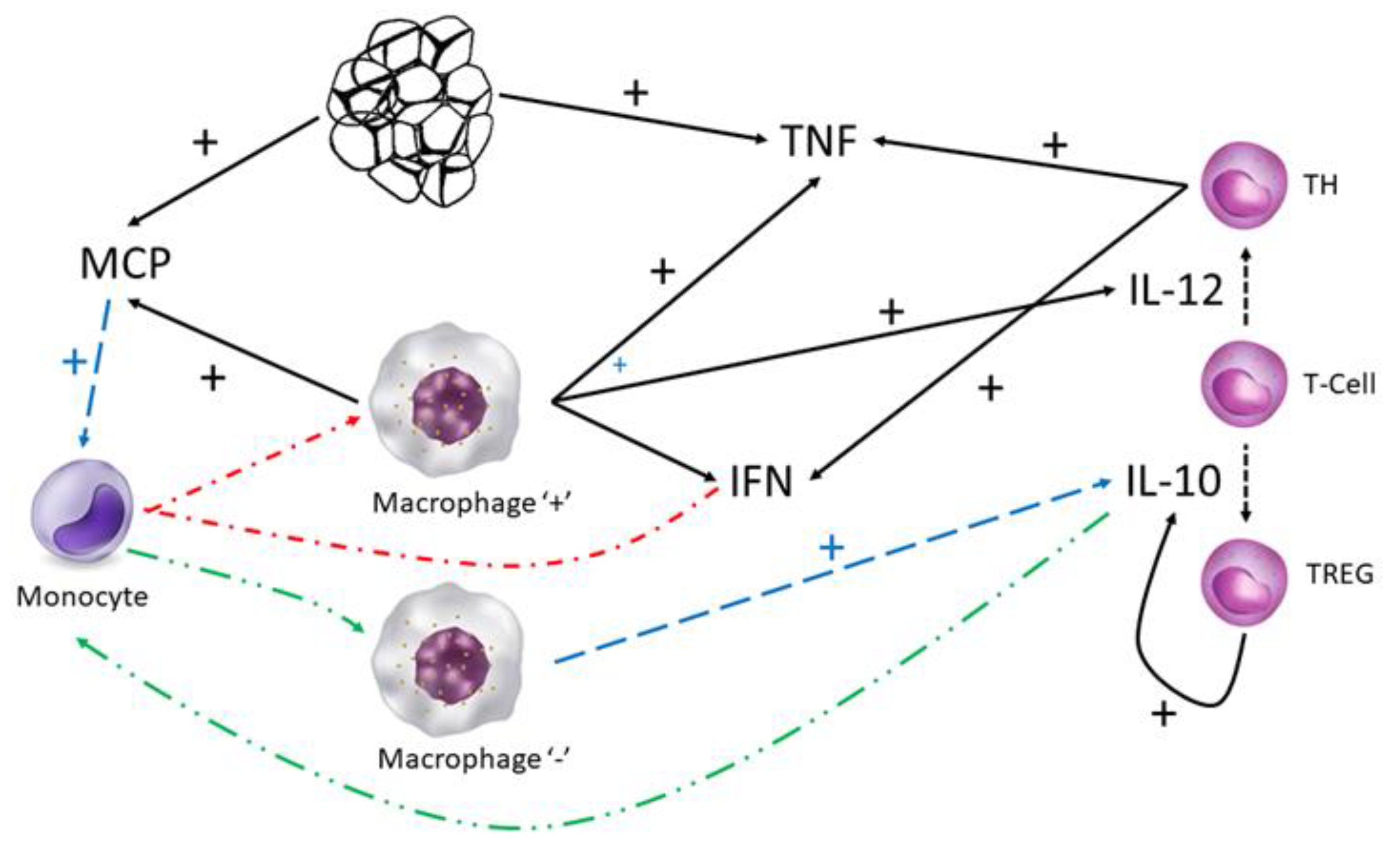
In particular, adipose tissue inflammation is the result of the secretion of the tissues themselves, those of pro-inflammatory cytokines, such as TNF- (tumor necrosis factor-), which promotes insulin resistance (cells cannot take sugar from the blood because they do not recognize insulin properly, affecting the health of the subject) or MCP-1 (monocyte chemoattractant protein 1), which attracts monocytes (a type of white blood cell or leukocyte that indicates inflammation, infection, etc.).
The connection between the differential equations (
Table 1) modeling
E energy storage in adipose tissue and the differential equations (
Table 2) modeling adipocyte inflammation was conducted as explained below.
In
Table 2, the differential Equation (8) includes
A, which value is obtained by multiplying the energy
E (see Equation (7),
Table 1) stored in the adipose tissue (
Figure 3) by a proportionality constant
. The value
E is the final energy value at the maximum time
t when solving the differential Equation (7).
Once monocytes encounter adipose tissue, they differentiate into macrophages (a type of white blood cell that swallows and eliminates microorganisms, pathogens, cancer cells, etc., attracting the attention of other immune system cells). Pro-inflammatory or “+” macrophages are promoted by the presence of IFN-
(a molecule known as interferon), which, in turn, is secreted by the macrophages themselves and T helper or TH cells (white cells with preventive function). The “+” macrophages secrete interleukin-12 (IL-12), TNF-
and MCP-1 (
Figure 4).
Table 2.
Inflammation model in adipose tissue.
| Ordinary Differential Equation | |
|---|
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
Regulatory T cells or TREGs (another type of white T-type cells) secrete interleukin-10 (IL-10), whose interaction with MCP-1 results in the differentiation of monocytes into anti-inflammatory or “-” macrophages, a phenomenon that we have not included in this model.
The rate of change of pro-inflammatory cytokines (signaling proteins that alert the immune system) is given by the differential Equations (8) and (9) (
Table 2). The rate of change of TNF-
(8) is the result of its synthesis by “+” macrophages (
k9), adipose tissue (
k10) and T cells (
k11) and its subsequent denaturation (
kTNF). Similarly, the rate of change of MCP-1 (9) depends on its synthesis by “+” macrophages (
k12) and adipose tissue (
k13) and its subsequent denaturation (
kMCP). Note how MCP does not depend on T cells.
Equation (10) simply represents the change in the rate of the differentiation of T cells into T helper or TH cells. The number of TH cells depends on the effect of interleukin-12 (IL-12) secreted by “+” macrophages (k14) as the main responsible entity for such differentiation, being the mortality rate of TH cells.
Once TH cells are present, these cells (k16) are together with the “+” macrophages (k15) responsible for the synthesis of IFN- (11), whose concentration decreases () due to denaturation or other biological factors.
The presence in the medium of IL-10 favors the differentiation of T cells (k17) into regulatory T cells or TREG (12), being the mortality rate of this class of T cells.
The secretion of IL-10 (13) depends on TREG cells (k18) and anti-inflammatory macrophages or “-”, although the latter have not been included in the differential Equation (13), being the denaturation rate of interleukin-10.
2.7. Simulation Experiments
Once Fattybot was trained according to the protocols described above, several conversations were held with the chatbot under different experimental conditions. In all of the conversations, unless otherwise indicated, the common values of the parameters described below were chosen.
In the adipose-tissue energy-accumulation model (
Table 1), the simulation experiments were conducted by setting the following parameter values. First, the
ks and
kc rates of leptin synthesis and elimination were equal to 0.5 and 0.1, respectively. The parameters
k1 and
k3 modeling leptin uptake and diffusion in the brain were both equal to 0.5. Additionally, the dissociation constant (
k2) of the Hill Equation (2) was set equal to 0.5. Other parameters of the model received the values
k4 = 0.8, and
k7 and
k5 were both equal to 0.5. We assumed an arbitrary Fattybot weight equal to 100 units and the following initial conditions:
Lp(0) = 50,
Lb(0) = 0 and
E(0) = 15.
Next, we proceeded to set the values of the parameters corresponding to the adipose-tissue inflammation model: k9 = 0.8, k10 = 0.3, k11 = 0.8, kTNF = 0.02, k12 = 0. 8, k13 = 0.3, kMCP = 0.02, k14 = 0.01, kTH = 0.01, k15 = 0.8, k16 = 0.6, kIFN = 0.02, k17 = 0.02, kTREG = 0.01, k18 = 0.8 and kIL = 0.7. The initial conditions chosen to simulate the immune system were: TNF(0) = 5, MCP(0) = 5, TH(0) = 500, IFN(0) = 5, TREG(0) = 50 and IL(0) = 500.
In all of the simulation experiments, the parameter , which links the hormonal system (7) to the immune system (8) through E-energy in the adipose tissue, was equal to 10.
On the basis of these initial conditions and parameter values, we performed the following simulation experiments:
Experiment 1. Suppose we have a conversation with Fattybot, and the chatbot experiences anxiety or any other altered condition during the conversation, being the final value of polarity equal to −4.5999. We will simulate a sedentary or physically inactive chatbot with a high food intake. We will simulate the hormone-morphic scenario described above by assigning k5 = 0.5 and k6 = 0.0000001.
Experiment 2. In this experiment, we simulate a hormone-morphic scenario for a chatbot that experiences anxiety or any other altered condition during a conversation but to a somewhat lesser scale than in the previous experiment. In consequence, the total polarity after the conversation with its interlocutor is equal to −0.3500. In addition, and unlike the previous chatbot, the current one plays sports or leads an active life. In this case, we will assign the values 0.05 and 0.001 to the parameters k5 and k6, respectively.
Experiment 3. We will simulate a scenario where Fattybot suffers from leptin resistance, i.e., a pathology in which the brain does not respond to leptin. Consequently, since leptin is the satiety hormone, the chatbot will ingest food continuously without feeling satisfied. This pathological scenario is simulated by setting the values of k1 and k3 equal to 0.00001. We will assume that, except for this pathology, Fattybot practices sports or leads an active life by setting k5 = 0.05 and k6 = 0.001. In addition, the conversation with its interlocutor has not produced any anxiety or any other altered condition, being the total polarity after the conversation equal to 3.2083.
Experiment 4. In this experiment, Fattybot has hypoleptinemia, a pathology of genetic origin consisting of the presence of low leptin levels. In this case, the adipose tissue hardly produces this hormone. The result is that it has no fat, eats uncontrollably and will consequently suffer from excessive insulin production, dyslipidemia (an imbalance in fats), hypogonadism (low levels of sex hormones) and childhood obesity. We will assume that, except for this pathology, Fattybot practices sports or follows an active lifestyle (k5 = 0.05 and k6 = 0.001) and does not suffer from any personality disorder (total polarity equal to 3.2083).
3. Results
The results obtained after training Fattybot show a chatterbot that is able to simulate empathic behaviors in a natural and convincing manner.
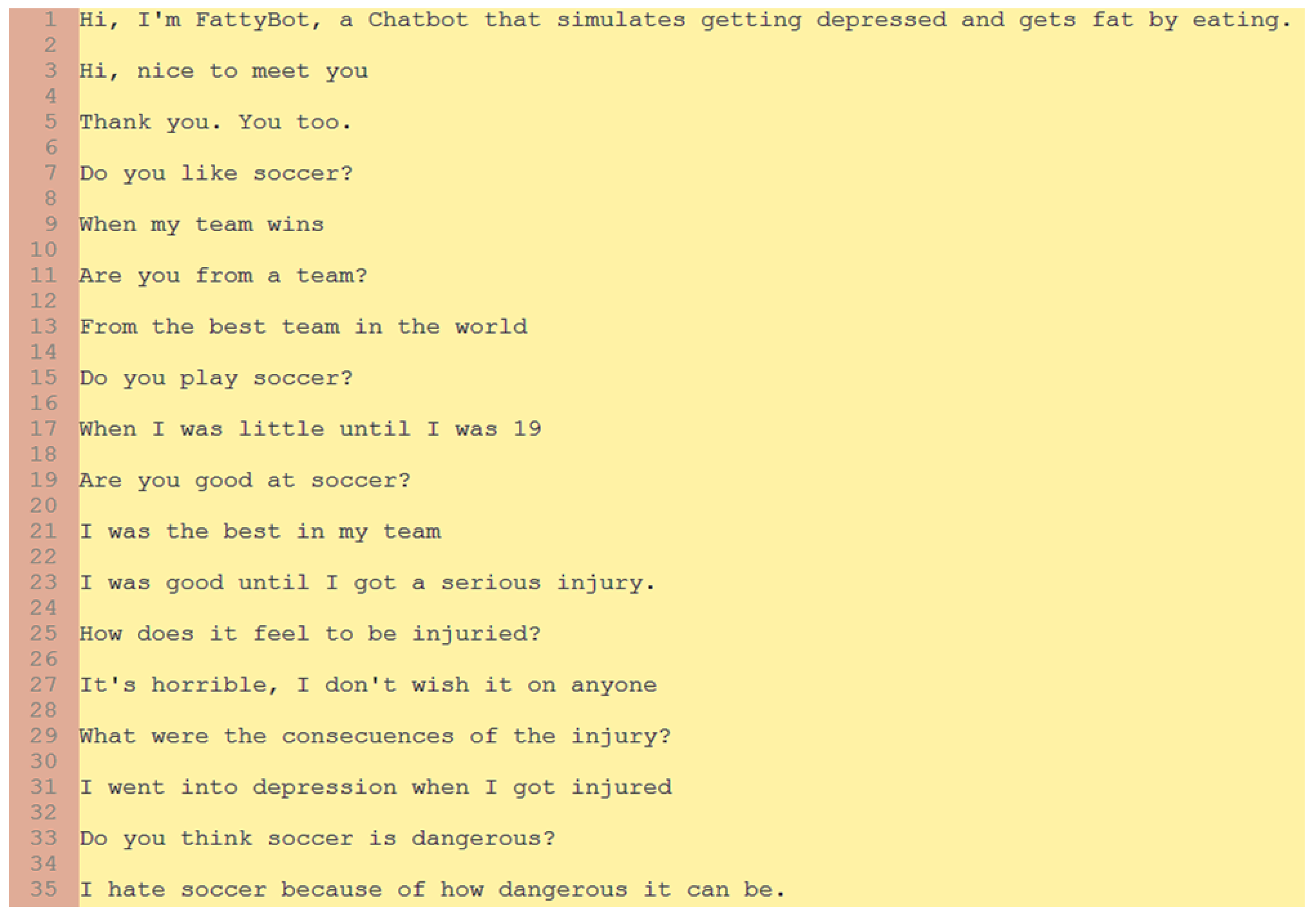
Figure 5 and
Figure 6 show two conversations that are representative of a dialogue with a human interlocutor. While in one of the conversations the topic is junk food (
Figure 5), in the other, it is soccer (
Figure 6), i.e., the two topics to which Fattybot shows a special sensitivity that can trigger its anxiety or any personality disorder, which is then relieved by the chatbot overeating or binge eating.
The hormone-morphic component of the chatbot is illustrated with four representative experiments that illustrate the usefulness and interest of including this component, i.e., including hormonal and immune models to simulate the accumulation of the energy and inflammation of adipose tissue. In other words, and beyond the mere metaphor, we have emulated the physical fattening—in this case, simulated—of the casing, components or body parts with which Fattybot could be “built”. In general, the results of the simulations support the fact that the model parameters and their values are well chosen.
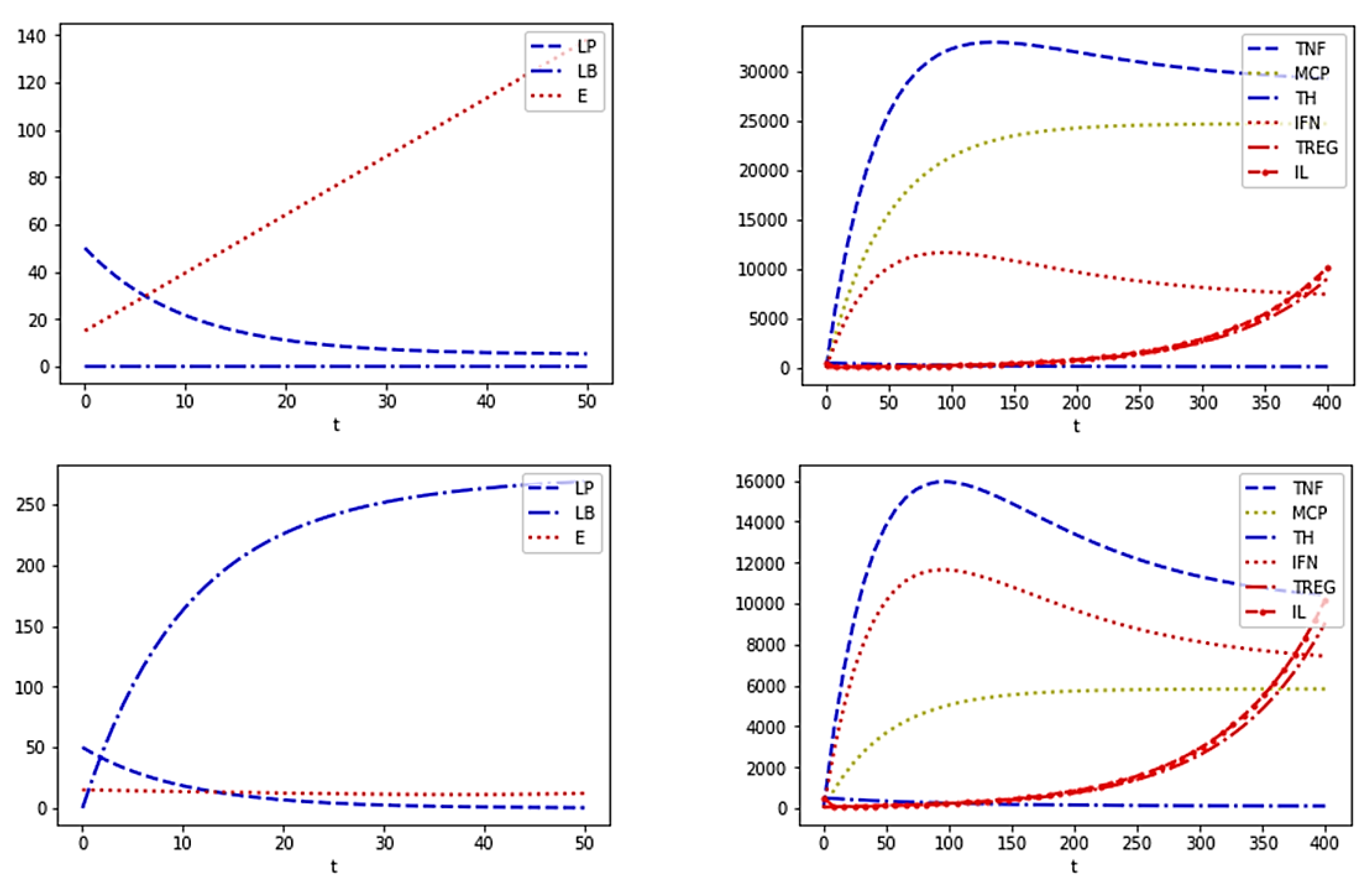
In the first two experiments (
Figure 7), we simulated Fattybot with a well-configured hormone-morphic component but, in one case, with a tendency to experience anxiety after a conversation, which would lead to eating in a compulsive manner. Furthermore, Fattybot would exhibit an inactive mood, e.g., being not very inclined to practice a sporting activity (Experiment 1). In the other experiment (Experiment 2), Fattybot also had depression or exhibited anxiety, but it had a proactive attitude and ate less food when feeling anxious or practicing sports. In both experiments, the blood leptin levels were similar, i.e., 5.3032; however, the energy accumulated in the adipose tissue was about four times higher in the chatbot that exhibited low activity and ate more food (
E = 31.0815) than in the one that ate less food and remained active (
E = 7.8538).
In the next two experiments (
Figure 8), we simulated Fattybot by performing simulations for clinical purposes. In this case, the chatbot suffered from a particular disease, being a chatbot that does not experience anxiety after a conversation and shows an active life.
Figure 8 (Experiment 3) shows the response of the hormonal and immune models when Fattybot suffered from leptin resistance. This is a disease in which the brain responds as if there is no leptin, so that the individual, in this case Fattybot, is never satiated by eating. Note that, although the blood leptin was similar to the two previous experiments, i.e., 5.3032, the energy accumulated in adipose tissue shot well above the usual (
E = 137.9508).
In the other simulation (Experiment 4), Fattybot suffered from hypoleptinemia, a disease in which the adipose tissue barely produces leptin, obtaining a final blood value equal to 0.3369. Although these subjects usually suffer from childhood obesity, in the present scenario, the diseases caused by this pathology go beyond childhood obesity. That is, the chatbot casing, components or body parts would be affected by injuries other than the type of fattening caused by the accumulation of energy in its adipose tissue (E = 12.1726).
Table A1 (see
Appendix A) shows for each of the conversations held with Fattybot, after being trained by different protocols, the average perplexity value scores (APSs), the burstiness scores (BSs) and the maximum perplexity (MP) values. The percentages of human intervention (Real) and AI intervention (Fake) in a conversation were also obtained. It is interesting to note that, according to the percentages of Real obtained (
Table A1), most of the conversations held between the human interlocutor and Fattybot were attributed to two human interlocutors.
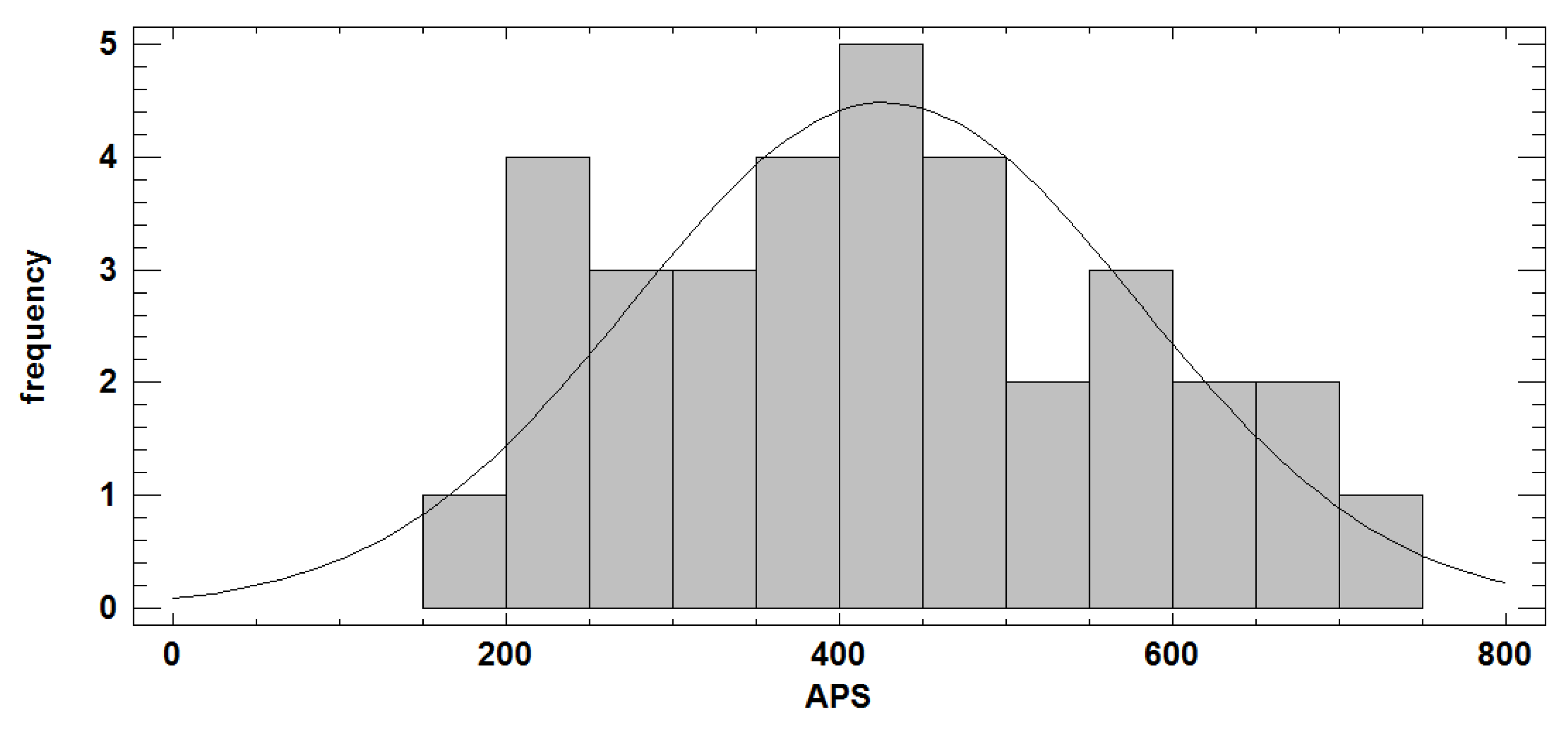
Table 3 shows the descriptive statistics for the APS, BS and MP, suggesting the standardized skewness and kurtosis values, i.e., 0.55324 and −0.762967, that possibly the APS values fit to a normal distribution (
Figure 9). Indeed, according to the Shapiro–Wilk test, we obtained a value of the statistic W = 0.9795 and a
p-value equal to 0.7601, concluding that the APS fits to a normal distribution N (427.674, 151.225).
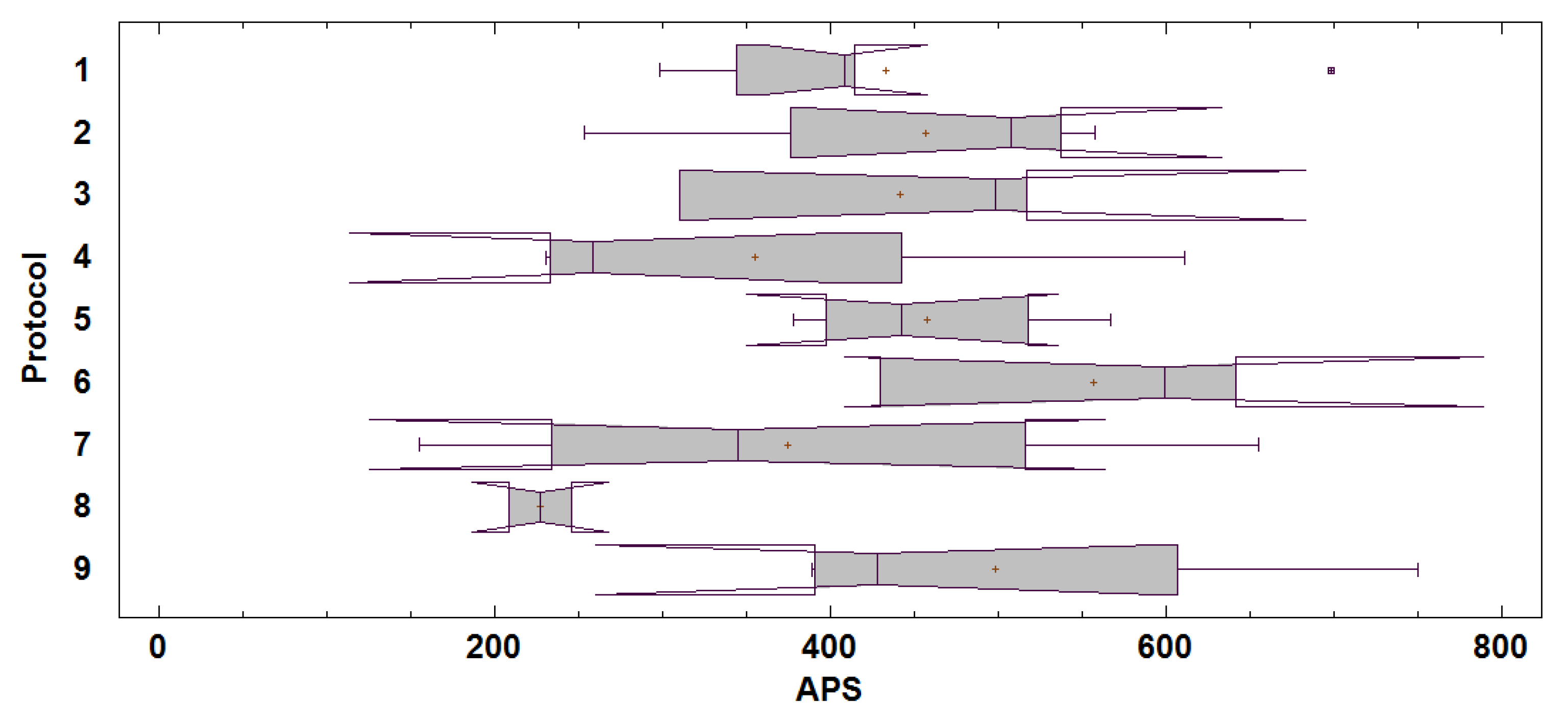
Figure 10 shows the box-and-whisker plot for the APS values in each of the Fattybot training protocols (
Section 2.5). A comparison with a Kruskal–Wallis test of the medians of the APS values among the training protocols leads us to conclude that, with a
p-value of 0.3175 and a confidence level of 95%, there are no significant differences in the training protocols. In other words, the protocol to be followed to train Fattybot has no effect on its ability to hold a conversation with an interlocutor.
4. Discussion
Although it may seem a far-fetched idea now, robots will be built with hardware to mimic the physiological features of organic matter in the future. For years, work has been underway along these lines, and many of the seminal papers have been published on the basis of which it will be possible to build this “organo-inspired hardware” in the future. In this way, machines will not only be endowed with AI but will also share with us many of the characteristics that make us human. Of course, Fatty is an elementary model, a “toy model”, with which we can explore some of these ideas. We think that these kinds of empathic chatbots will not only enable human–machine communication or interaction more like that between humans, but they could also be used for other purposes, such as simulating virtual patients. Another emerging area where AI models with a Fattybot-like architecture could be of interest is what is known as computational psychiatry. Diseases such as schizophrenia, depression or behavioral disorders caused by anxiety are studied under ideas and methodologies coming from computer science and AI. Computational psychiatry interprets under this approach the relationships between the brain, its environment and the symptoms of mental illnesses [
18,
19]. Thus, for example, with NLP techniques, it is possible to measure the coherence of a conversation, the emotionality, the atypical use of grammar, etc.
Nowadays, the convergence between synthetic AI, AI based on neuromorphic computing, the development of NLP techniques and sentiment analysis necessarily leads to the design of AI models with a higher degree of bioinspiration, and, although still far from strong AI, it is already possible to recognize some of the characteristic features of human beings. For example, hallucinations in ChatGPT, i.e., the generation of responses that are not based on real data or on the information provided in the prompts, resemble the lapses or slips that people sometimes have during a conversation.
The possibility of emulating with the electronic circuit systems of biological nature or physiological phenomena is not new. In fact, Carver Mead coined the term neuromorphic within the integration of large-scale circuits [
2]. Thus, e.g., Metal Oxide Semiconductor Field-Effect Transistors (MOSFETs) allow for modeling the characteristics of neuronal ion channels. Another example [
20] is the possibility of emulating the high energy efficiency of the brain with electronic components, even though they contain potentially defective elements. Progress in materials or devices that emulate neuromorphic behavior is rapid, and some examples show applications for people with sensorimotor deficiencies [
21], as well as for the development of robots that interact with their environment, handling and learning as living beings would [
22].
Although neurons, their synapses, neural circuits and the brain have traditionally been the source of inspiration for the design of AI algorithms, the modeling of other systems such as hormonal systems is gradually making its way into AI [
23,
24]. In fact, hormonal computation is already being discussed as one of the novel paradigms in AI [
25]. However, other systems, such as the immune system, which is responsible for homeostasis or internal balance against external aggressions, are also finding their place in the design of algorithms that could very well be incorporated into the vast repertoire of algorithms and models in AI. At present, there are immune system models implemented at the hardware level [
26]. These models are the current version of the classical algorithms of what is known as artificial immune system [
27] and have been worked on for years [
28,
29] with respect to their possible implementation at the hardware level.
In this paper, we have described a chatbot model, Fatty, in which assuming a hypothetical situation—the chatbot eats compulsively, being fattened up when talking to it about soccer or junk food—we show how the involvement of models of the hormonal system and the immune system can result in AI systems with features closer to those of human beings. In the future, AI architectures will be designed in which, while the brain will still be the main element responsible for the AI system’s control, the incorporation of systems such as the hormonal system will allow for the emulation of behaviors and responses of greater complexity than, for example, those currently provided by generative AI systems.