Exploring the State of Machine Learning and Deep Learning in Medicine: A Survey of the Italian Research Community
Abstract
:1. Introduction
- Diagnosis and Prognosis: the analysis of medical data can assist physicians in diagnosing diseases and predicting patient outcomes. Thus, earlier detection and personalized treatment plans could be possible;
- Drug Discovery: this could be accelerated by analyzing large amounts of biological and chemical data. Thus, new drug candidates and new potential treatments for various diseases could be identified;
- Personalized Medicine: treatment plans for individual patients can be tailored by analyzing genetic, molecular, and clinical data. Thus, more precise and effective treatments can be designed;
- Remote Monitoring and Telemedicine: AI-powered devices and applications can monitor patients remotely, enabling timely interventions and reducing the need (and the costs) for frequent in-person visits. Chronic disease management and rural healthcare have a particular benefit;
- Efficiency and Cost Reduction: tasks such as medical coding, billing, and administrative processes can be automated. Furthermore, the processes in a healthcare institution can be analyzed to find possible issues and/or be confronted with gold-standard(s). In this way, it is possible to increase efficiency and reduce costs;
- Patient Engagement: specific applications can provide patients with personalized health information, reminders, and recommendations, promoting proactive healthcare management;
- Clinical Trials: clinical trials can be analyzed, and potential patterns can be identified in large datasets, leading to insights that might not be evident through traditional analysis methods. Thus, the peace of clinical trials and of medical research can be accelerated.
- Integration: integrating AI systems into existing healthcare workflows and electronic health record systems may require significant changes to the infrastructure and processes.
- Lack of Transparency: Some AI models (such as DL models but also some ML models) are very complex and called black boxes due to their inability to provide (clear) explanations for their outputs (i.e., decisions). The lack of transparency can be a relevant barrier to gaining trust and acceptance from medical staff and from patients.
- Bias and Fairness: AI algorithms can inherit biases present in the acquired data used to train them. Thus, their output (i.e., medical decisions) could be affected by biases.
- Data Privacy and Security Concerns: AI in medicine relies heavily on patient data, which raises concerns about privacy and security;
- Regulatory Hurdles: the deployment of AI in medical practice is subject to rigorous regulatory processes to ensure patient safety and efficacy. Moreover, the legal regulation of the utilization of AI is in constant evolution. Navigating these regulatory and legal frameworks can be really time-consuming.
- We review the state of the art in Italy in recent years (i.e., since 2018), focusing on ML/DL in medicine, including all medical areas.
- We present a general map of the ML/DL research in Italy
- We propose a categorization of the ML/DL approaches in medicine
- We comprehensively classify the most relevant medicine-related ML/DL applications
2. Methods
2.1. The Framework
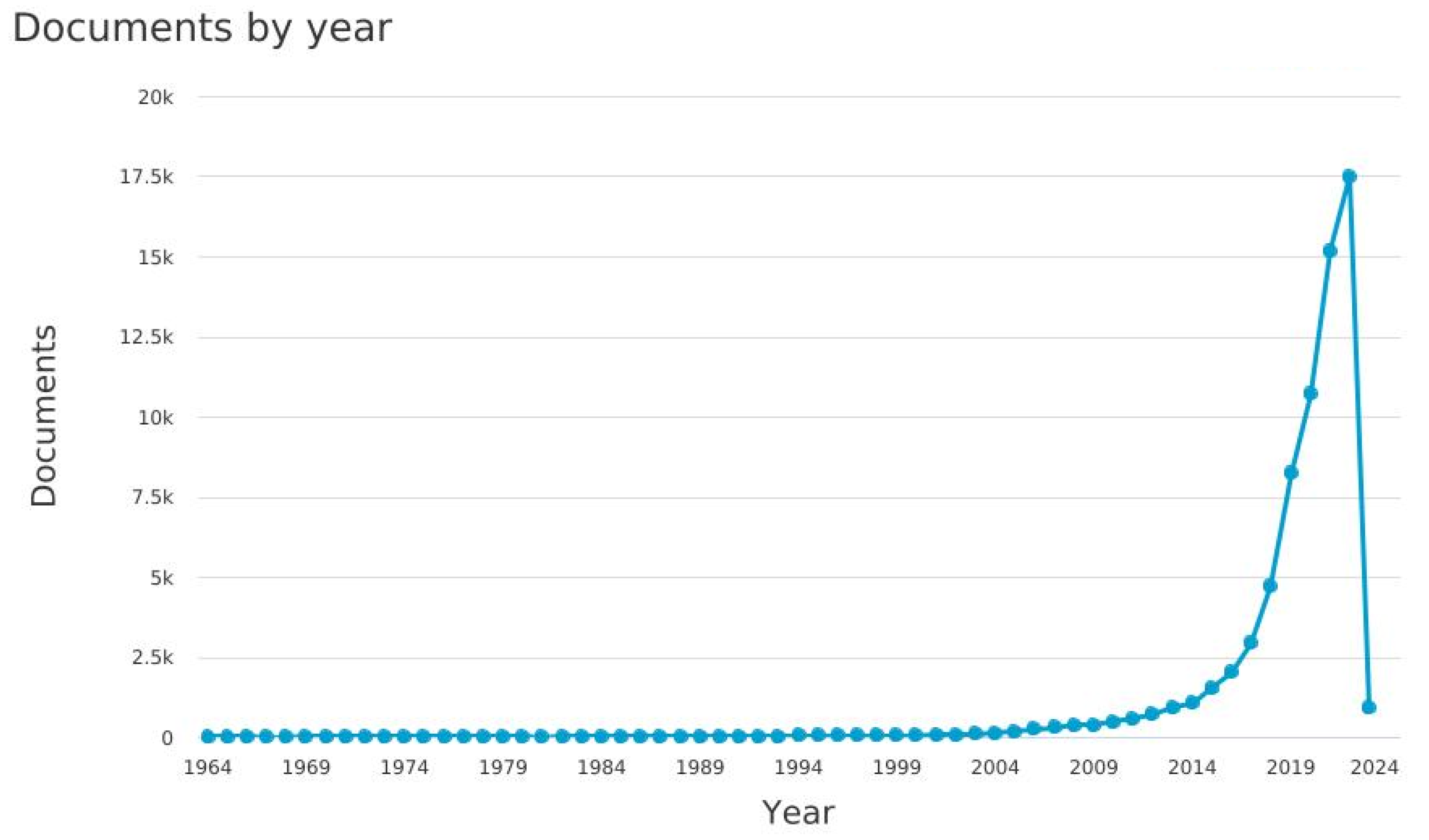
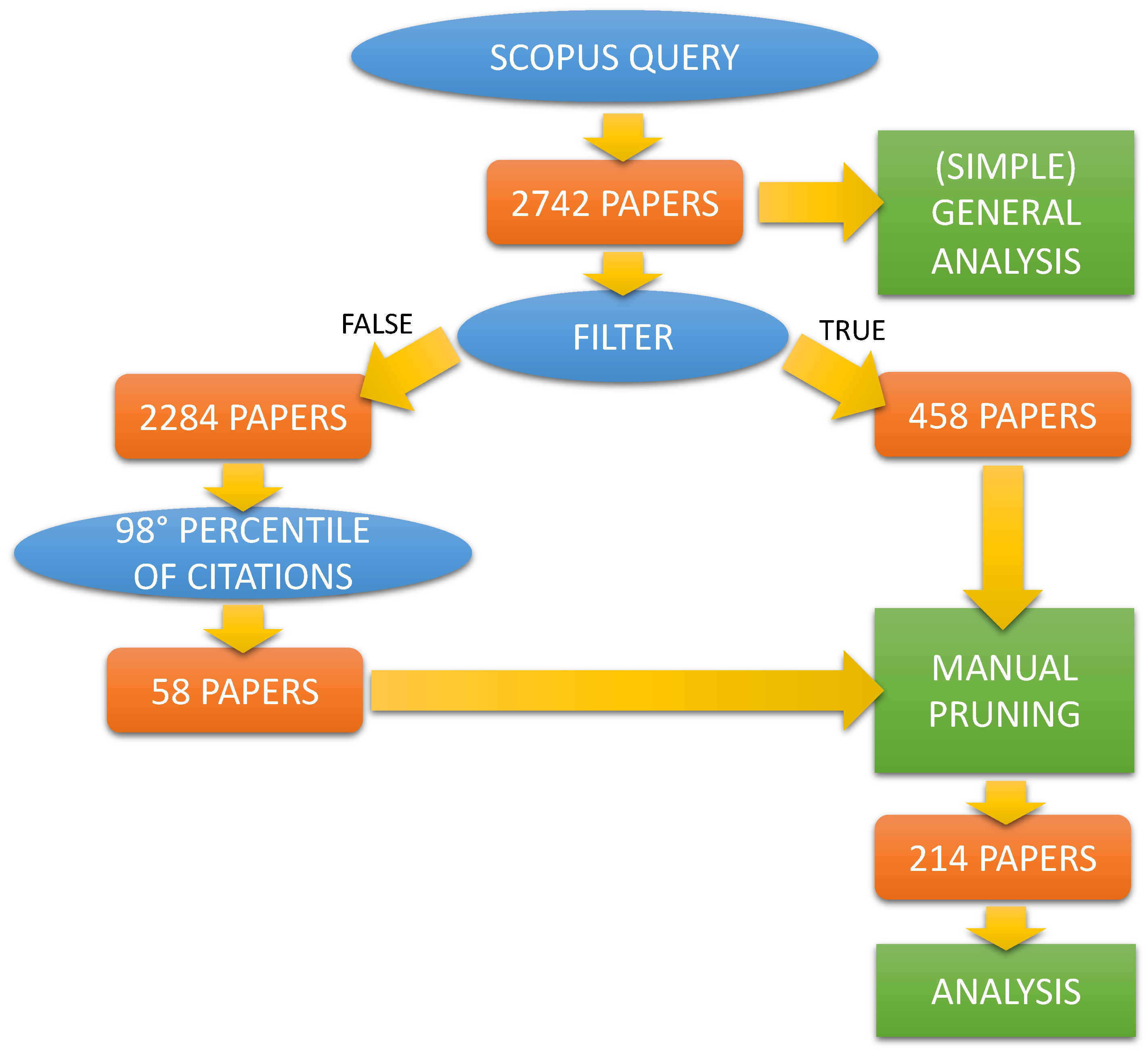
- The starting point of our work is the output of the query in Figure 3, performed (All the queries were performed on 13 January 2023) via SCOPUS (i.e., https://www.scopus.com/search/form.uri?display=advanced (accessed on 13 January 2023)), shown below:
- (( TITLE-ABS-KEY (machine AND learning)
- OR TITLE-ABS-KEY (deep AND learning))
- AND (TITLE-ABS-KEY (medicine)
- OR TITLE-ABS-KEY (medical) OR TITLE-ABS-KEY (health)))
- AND PUBYEAR > 2017
- AND LIMIT-TO ( AFFILCOUNTRY , "Italy" )
- Type of paper: only research journal papers (i.e., we excluded review/survey papers and conference papers);
- Subject area: we have considered only the relevant subject areas in SCOPUS, i.e., medicine, computer science, engineering, biochemistry, genetics and molecular biology, neuroscience, pharmacology, toxicology and pharmaceutics, health profession, nursing, dentistry, immunology and microbiology, and multidisciplinary.
- If the paper includes the author contributions section, the contributions of the Italian researchers are checked to see if their work refers to topics regarding the development of ML/DL methodology, software, implementation, and so on. If such a case does not hold (e.g., the contribution from the Italian researcher is to provide data), the paper is excluded;
- If the paper does not include the author contributions section, for the Italian researchers, we checked both the department/institution they belong and the academic fields and disciplines on which they work (also by checking their track record and CV when available, and past articles they co-authored). If both of them are far outside the scientific fields in which ML and DL methodologies are usually developed and used, such as computer science, engineering, mathematics, statistics, and so on, the paper is excluded.
2.2. Limitations
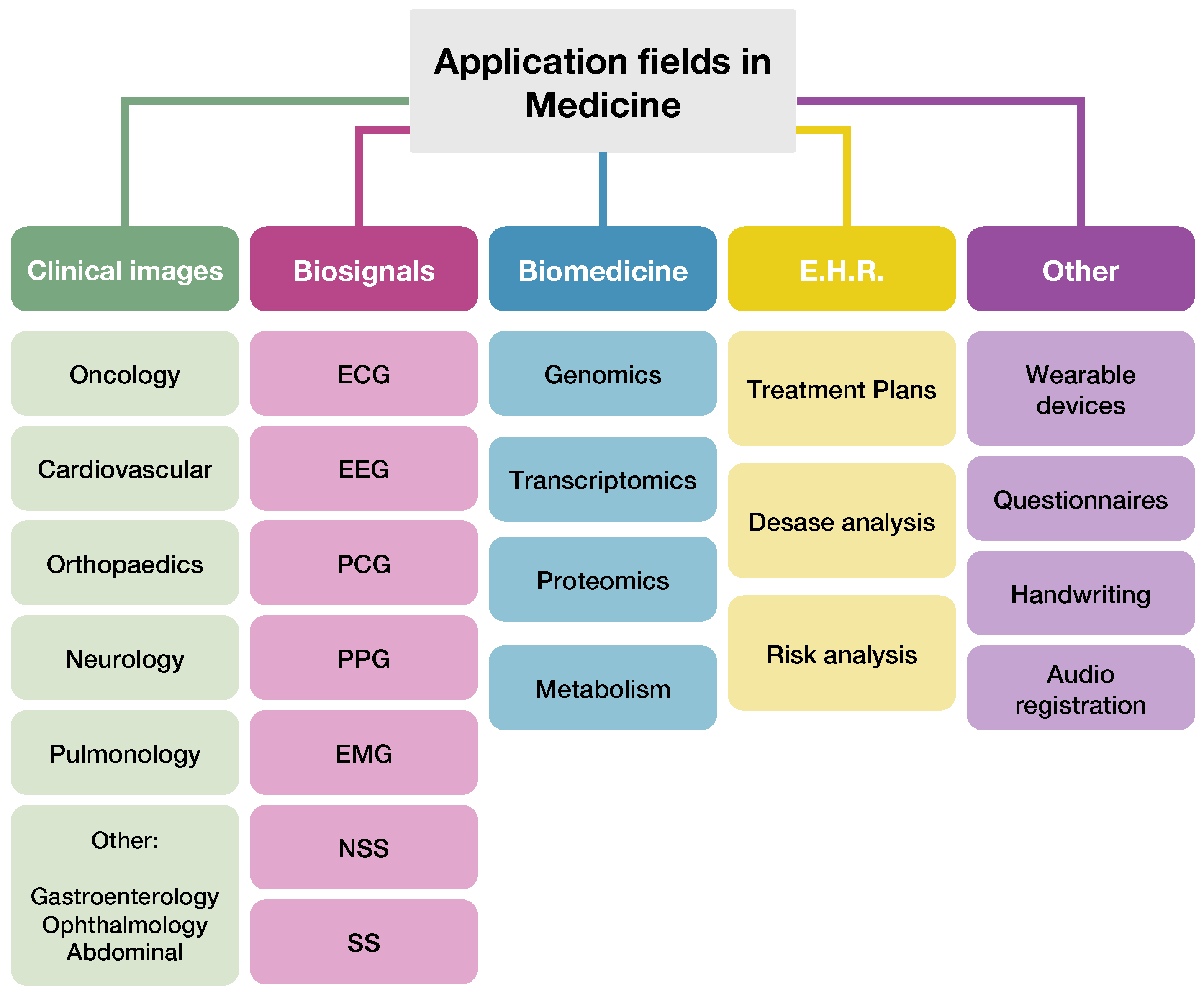
2.3. Analysis Criteria
- the medical topics;
- the type of data;
- the type of pre-processing methods;
- the learning methods;
- the evaluation methods.
- feature selection;
- feature extraction;
- feature reduction;
- data filtering;
- data normalization;
- missing data management;
- undersampling;
- oversampling;
- other.
- ML supervised;
- ML unsupervised;
- ML semisupervised;
- ML reinforcement learning;
- DL supervised;
- DL unsupervised;
- DL semisupervised;
- DL reinforcement learning.
2.4. Background
- feature selection: is the process of reducing the number of input features (i.e., selection of the relevant features);
- feature extraction: is the process of manipulating and transforming (a subset of) the raw data into (new) features;
- feature reduction: is the process of data transformation from a high-dimensional space into a low-dimensional space, without losing their relevant properties in the transformation;
- data filtering: is the process of removing information that is not useful in a (large) database on a specific criterion;
- data normalization: is the process of transforming data into a standard format;
- missing data management: is the process of managing features that present missing values;
- undersampling and oversampling: is the process of adjusting (i.e., resampling) the class distribution of a data set. In the case of undersampling, the distribution of (observations/instances of) a class is artificially decreased. In the case of oversampling, the distribution of (observations/instances of) a class is artificially increased.
- DL needs a large amount of data, while ML can also work with a small amount of data;
- in DL, the feature engineering phase is eliminated; DL can learn features for improving the output accuracy. On the other side, in ML, the feature engineering phase is application dependent and manually performed;
- DL usually has higher computational requirements that often entail the adoption of specialized hardware with very high performance (i.e., DL has higher time requirements for training than ML);
- DL has mainly black box approaches (explainability is very difficult because of hyper-parameters and complex network design); on the other side, ML provides also white box approaches;
- DL can achieve an accuracy rate higher than ML.
- supervised: is an approach where the learning algorithm is trained on input data labeled with the correct output classes;
- unsupervised: is an approach where the learning algorithm is trained exclusively on unlabeled data. This is particularly useful when the outcome class is not known or the labeling process is too long and expansive;
- semisupervised: is a paradigm where the learning algorithm is trained by using a small amount of labeled data followed by a large amount of unlabeled data. This is particularly useful when a huge amount of data are available, but only a small fraction of them have been labeled;
- reinforcement learning: is a paradigm where the learning algorithm is self-trained on reward and punishment mechanisms (i.e., take actions and learn through trial and error).
3. Results
- in Section 3.1, we provide a general analysis on all papers to provide a (simple) general snapshot of the ML/DL Italian research in the medical area;
- in Section 3.2, we provide a systematic analysis of the selected papers, as described in Section 2.
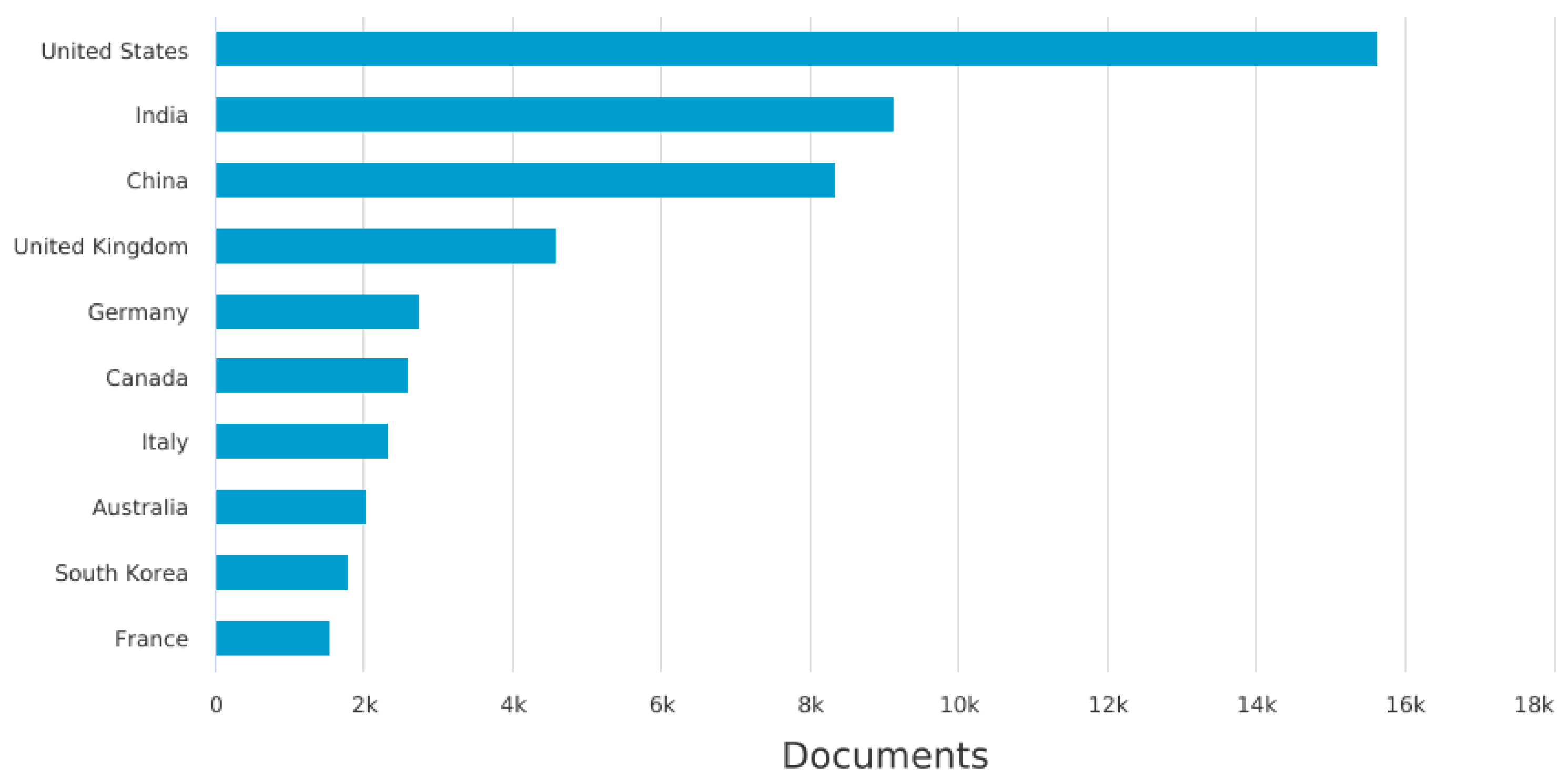
3.1. A Description of Italian Machine Learning/Deep Learning Research in the Medical Area at a Glance
3.2. Systematic Analysis
- how the validation phase is performed;
- the statistical measures used.
- a fixed split of the dataset into a training set and a test set, as well as k-fold cross-validation. The fixed split method was employed in 49 papers, accounting for approximately 22.9%. The most commonly used split values were 90–10%, 80–20%, and 70–30%;
- the k-fold cross-validation method was used in 99 papers, representing approximately 46% of the sample. Various values of k were utilized, but the most frequently used were 10, 5, and 3.
4. Discussion
- Medical and Healthcare Professionals: they could be interested in understanding how ML and DL are used in practice and have reference to specific medical topics that are studied in the research;
- Healthcare institutions: they could find indications about the treatment of specific disease(s) in their interest and consider the adoption of these technologies;
- Technology Companies: when developing AI-based solutions, they may be interested in understanding the needs of the Italian medical sector and in finding possible collaborations in the Italian academic community;
- Patient Associations: they could know how ML and DL are used to improve the quality of care and assistance they receive;
- Academics institutions and Researchers: they could be interested in a detailed review of the current applications of using ML and ML in medical practice to contribute to future research and innovation and to find possible partners for new research projects;
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| AUC-ROC | Area under the curve receiver operating characteristic |
| DL | Deep learning |
| IoT | Internet of Things |
| ML | Machine learning |
References
- Samuel, A.L. Some Studies in Machine Learning Using the Game of Checkers. IBM J. 1959, 3, 535–554. [Google Scholar] [CrossRef]
- Shortliffe, E.H. A rule-based computer program for advising physicians regarding antimicrobial therapy selection. In Proceedings of the 1974 Annual ACM Conference, ACM 1974; Association for Computing Machinery: New York, NY, USA, 1974; p. 739. [Google Scholar] [CrossRef]
- Prodhan, F.A.; Zhang, J.; Hasan, S.S.; Sharma, T.P.P.; Mohana, H.P. A review of machine learning methods for drought hazard monitoring and forecasting: Current research trends, challenges, and future research directions. Environ. Model. Softw. 2022, 149, 105327. [Google Scholar] [CrossRef]
- Mosavi, A.; Ozturk, P.; Chau, K.W. Flood prediction using machine learning models: Literature review. Water 2018, 10, 1536. [Google Scholar] [CrossRef]
- Hossain, E.; Khan, I.; Un-Noor, F.; Sikander, S.S.; Sunny, M.S.H. Application of Big Data and Machine Learning in Smart Grid, and Associated Security Concerns: A Review. IEEE Access 2019, 7, 13960–13988. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Marques, M.R.; Botti, S.; Marques, M.A. Recent advances and applications of machine learning in solid-state materials science. NPJ Comput. Mater. 2019, 5, 83. [Google Scholar] [CrossRef]
- Kamilaris, A.; Prenafeta-Boldú, F.X. Deep learning in agriculture: A survey. Comput. Electron. Agric. 2018, 147, 70–90. [Google Scholar] [CrossRef]
- Chlingaryan, A.; Sukkarieh, S.; Whelan, B. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: A review. Comput. Electron. Agric. 2018, 151, 61–69. [Google Scholar] [CrossRef]
- Young, A.; Marinescu, R.; Oxtoby, N.; Bocchetta, M.; Yong, K.; Firth, N.; Cash, D.; Thomas, D.; Dick, K.; Cardoso, J.; et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat. Commun. 2018, 9, 4273. [Google Scholar] [CrossRef] [PubMed]
- Voulodimos, A.; Doulamis, N.; Doulamis, A.; Protopapadakis, E. Deep Learning for Computer Vision: A Brief Review. Comput. Intell. Neurosci. 2018, 2018, 7068349. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, B.; Jiang, X.; Jia, F.; Li, N.; Nandi, A.K. Applications of machine learning to machine fault diagnosis: A review and roadmap. Mech. Syst. Signal Process. 2020, 138, 106587. [Google Scholar] [CrossRef]
- Pang, G.; Shen, C.; Cao, L.; Hengel, A.V.D. Deep Learning for Anomaly Detection: A Review. ACM Comput. Surv. 2021, 54, 1–38. [Google Scholar] [CrossRef]
- Liu, R.; Yang, B.; Zio, E.; Chen, X. Artificial intelligence for fault diagnosis of rotating machinery: A review. Mech. Syst. Signal Process. 2018, 108, 33–47. [Google Scholar] [CrossRef]
- Mohammadi, M.; Al-Fuqaha, A.; Sorour, S.; Guizani, M. Deep learning for IoT big data and streaming analytics: A survey. IEEE Commun. Surv. Tutor. 2018, 20, 2923–2960. [Google Scholar] [CrossRef]
- Biran, O.; Feder, O.; Moatti, Y.; Kiourtis, A.; Kyriazis, D.; Manias, G.; Mavrogiorgou, A.; Sgouros, N.M.; T. Barata, M.; Oldani, I.; et al. PolicyCLOUD: A prototype of a cloud serverless ecosystem for policy analytics. Data Policy 2022, 4, e44. [Google Scholar] [CrossRef]
- Kyriazis, D.; Autexier, S.; Boniface, M.; Engen, V.; Jimenez-Peris, R.; Jordan, B.; Jurak, G.; Kiourtis, A.; Kosmidis, T.; Lustrek, M.; et al. The CrowdHEALTH project and the Hollistic Health Records: Collective Wisdom Driving Public Health Policies. Acta Inform. Med. 2019, 27, 369–373. [Google Scholar] [CrossRef]
- Piccialli, F.; Somma, V.D.; Giampaolo, F.; Cuomo, S.; Fortino, G. A survey on deep learning in medicine: Why, how and when? Inf. Fusion 2021, 66, 111–137. [Google Scholar] [CrossRef]
- Faouzi, J.; Colliot, O. Classic Machine Learning Methods. In Machine Learning for Brain Disorders; Colliot, O., Ed.; Springer US: New York, NY, USA, 2023; pp. 25–75. [Google Scholar] [CrossRef]
- Du, X.; Cai, Y.; Wang, S.; Zhang, L. Overview of deep learning. In Proceedings of the 2016 31st Youth Academic Annual Conference of Chinese Association of Automation (YAC), Wuhan, China, 11–13 November 2016; pp. 159–164. [Google Scholar] [CrossRef]
- Dargan, S.; Kumar, M.; Ayyagari, M.R.; Kumar, G. A survey of deep learning and its applications: A new paradigm to machine learning. Arch. Comput. Methods Eng. 2020, 27, 1071–1092. [Google Scholar] [CrossRef]
- Tufail, S.; Riggs, H.; Tariq, M.; Sarwat, A.I. Advancements and Challenges in Machine Learning: A Comprehensive Review of Models, Libraries, Applications, and Algorithms. Electronics 2023, 12, 1789. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning (Information Science and Statistics); Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Goodfellow, I.J.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016; Available online: http://www.deeplearningbook.org (accessed on 1 August 2023).
- Ferrari, E.; Retico, A.; Bacciu, D. Measuring the effects of confounders in medical supervised classification problems: The Confounding Index (CI). Artif. Intell. Med. 2020, 103, 101804. [Google Scholar] [CrossRef] [PubMed]
- Cabitza, F.; Campagner, A.; Albano, D.; Aliprandi, A.; Bruno, A.; Chianca, V.; Corazza, A.; Pietto, F.; Gambino, A.; Gitto, S.; et al. The elephant in the machine: Proposing a new metric of data reliability and its application to a medical case to assess classification reliability. Appl. Sci. 2020, 10, 4014. [Google Scholar] [CrossRef]
- Cabitza, F.; Campagner, A.; Sconfienza, L. As if sand were stone. New concepts and metrics to probe the ground on which to build trustable AI. BMC Med. Inform. Decis. Mak. 2020, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, E.; Daberdaku, S.; Vasta, R.; Calvo, A.; Chiò, A.; Di Camillo, B. Exploiting mutual information for the imputation of static and dynamic mixed-type clinical data with an adaptive k-nearest neighbours approach. BMC Med. Inform. Decis. Mak. 2020, 20, 174. [Google Scholar] [CrossRef]
- Campagner, A.; Ciucci, D.; Svensson, C.M.; Figge, M.; Cabitza, F. Ground truthing from multi-rater labeling with three-way decision and possibility theory. Inf. Sci. 2021, 545, 771–790. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 2021, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- La Gatta, V.; Moscato, V.; Postiglione, M.; Sperlì, G. CASTLE: Cluster-aided space transformation for local explanations. Expert Syst. Appl. 2021, 179, 115045. [Google Scholar] [CrossRef]
- Campagner, A.; Cabitza, F.; Berjano, P.; Ciucci, D. Three-way decision and conformal prediction: Isomorphisms, differences and theoretical properties of cautious learning approaches. Inf. Sci. 2021, 579, 347–367. [Google Scholar] [CrossRef]
- Campagner, A.; Sternini, F.; Cabitza, F. Decisions are not all equal—Introducing a utility metric based on case-wise raters’ perceptions. Comput. Methods Programs Biomed. 2022, 221, 106930. [Google Scholar] [CrossRef]
- Parimbelli, E.; Buonocore, T.; Nicora, G.; Michalowski, W.; Wilk, S.; Bellazzi, R. Why did AI get this one wrong?—Tree-based explanations of machine learning model predictions. Artif. Intell. Med. 2023, 135, 102471. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Panasiti, F.; Stefano, A.; Comelli, A.; Giaccone, P.; Arnone, A.; Minutoli, F.; Quartuccio, N.; Cupidi, C.; et al. Radiomics Analysis of Brain [18 F]FDG PET/CT to Predict Alzheimer’s Disease in Patients with Amyloid PET Positivity: A Preliminary Report on the Application of SPM Cortical Segmentation, Pyradiomics and Machine-Learning Analysis. Diagnostics 2022, 12, 933. [Google Scholar] [CrossRef]
- Dachena, C.; Casu, S.; Fanti, A.; Lodi, M.; Mazzarella, G. Combined use of MRI, fMRIand cognitive data for Alzheimer’s Disease: Preliminary results. Appl. Sci. 2019, 9, 3156. [Google Scholar] [CrossRef]
- De Falco, I.; De Pietro, G.; Sannino, G. Article A Two-Step Approach for Classification in Alzheimer’s Disease. Sensors 2022, 22, 3966. [Google Scholar] [CrossRef]
- Grassi, M.; Rouleaux, N.; Caldirola, D.; Loewenstein, D.; Schruers, K.; Perna, G.; Dumontier, M.; Initiative, A.D.N. A novel ensemble-based machine learning algorithm to predict the conversion from mild cognitive impairment to Alzheimer’s disease using socio-demographic characteristics, clinical information, and neuropsychological measures. Front. Neurol. 2019, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Lella, E.; Lombardi, A.; Amoroso, N.; Diacono, D.; Maggipinto, T.; Monaco, A.; Bellotti, R.; Tangaro, S. Machine learning and DWI brain communicability networks for Alzheimer’s disease detection. Appl. Sci. 2020, 10, 934. [Google Scholar] [CrossRef]
- Redolfi, A.; De Francesco, S.; Palesi, F.; Galluzzi, S.; Muscio, C.; Castellazzi, G.; Tiraboschi, P.; Savini, G.; Nigri, A.; Bottini, G.; et al. Medical Informatics Platform (MIP): A Pilot Study Across Clinical Italian Cohorts. Front. Neurol. 2020, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Bosco, P.; Calderoni, S.; Oliva, P.; Palumbo, L.; Spera, G.; Fantacci, M.; Retico, A. Dealing with confounders and outliers in classification medical studies: The Autism Spectrum Disorders case study. Artif. Intell. Med. 2020, 108, 101926. [Google Scholar] [CrossRef]
- Tartarisco, G.; Cicceri, G.; Di Pietro, D.; Leonardi, E.; Aiello, S.; Marino, F.; Chiarotti, F.; Gagliano, A.; Arduino, G.; Apicella, F.; et al. Use of machine learning to investigate the quantitative checklist for autism in toddlers (Q-CHAT) towards early autism screening. Diagnostics 2021, 11, 574. [Google Scholar] [CrossRef]
- Donelli, M.; Espa, G.; Feraco, P. A Semi-Unsupervised Segmentation Methodology Based on Texture Recognition for Radiomics: A Preliminary Study on Brain Tumours. Electronics 2022, 11, 1573. [Google Scholar] [CrossRef]
- Gonella, G.; Binaghi, E.; Nocera, P.; Mordacchini, C. Investigating the behaviour of machine learning techniques to segment brain metastases in radiation therapy planning. Appl. Sci. 2019, 9, 3335. [Google Scholar] [CrossRef]
- Munir, K.; Frezza, F.; Rizzi, A. Deep Learning Hybrid Techniques for Brain Tumor Segmentation. Sensors 2022, 22, 8201. [Google Scholar] [CrossRef]
- Aljuaid, H.; Alturki, N.; Alsubaie, N.; Cavallaro, L.; Liotta, A. Computer-aided diagnosis for breast cancer classification using deep neural networks and transfer learning. Comput. Methods Programs Biomed. 2022, 223, 106951. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, N.; Pomarico, D.; Fanizzi, A.; Didonna, V.; Giotta, F.; La Forgia, D.; Latorre, A.; Monaco, A.; Pantaleo, E.; Petruzzellis, N.; et al. A roadmap towards breast cancer therapies supported by explainable artificial intelligence. Appl. Sci. 2021, 11, 4881. [Google Scholar] [CrossRef]
- Castaldo, R.; Pane, K.; Nicolai, E.; Salvatore, M.; Franzese, M. The impact of normalization approaches to automatically detect radiogenomic phenotypes characterizing breast cancer receptors status. Cancers 2020, 12, 518. [Google Scholar] [CrossRef]
- Comes, M.; Forgia, D.; Didonna, V.; Fanizzi, A.; Giotta, F.; Latorre, A.; Martinelli, E.; Mencattini, A.; Paradiso, A.; Tamborra, P.; et al. Early prediction of breast cancer recurrence for patients treated with neoadjuvant chemotherapy: A transfer learning approach on dce-mris. Cancers 2021, 13, 2298. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; Flores, A.; Buonanno, G. Machine Learning Approach to Quadratic Programming-Based Microwave Imaging for Breast Cancer Detection. Sensors 2022, 22, 4122. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Gallelli, L.; Marotta, N.; Lippi, L.; Fusco, N.; Calafiore, D.; Cione, E.; Muraca, L.; Maconi, A.; De Sarro, G.; et al. Vitamin D Deficiency in Women with Breast Cancer: A Correlation with Osteoporosis? A Machine Learning Approach with Multiple Factor Analysis. Nutrients 2022, 14, 1586. [Google Scholar] [CrossRef] [PubMed]
- Famouri, S.; Morra, L.; Mangia, L.; Lamberti, F. Breast Mass Detection with Faster R-CNN: On the Feasibility of Learning from Noisy Annotations. IEEE Access 2021, 9, 66163–66175. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Lippi, L.; Antonelli, A.; Calafiore, D.; Ammendolia, V.; Fortunato, L.; Renò, F.; Giudice, A.; et al. Oral Health in Breast Cancer Women with Vitamin D Deficiency: A Machine Learning Study. J. Clin. Med. 2022, 11, 4662. [Google Scholar] [CrossRef]
- Ferro, S.; Bottigliengo, D.; Gregori, D.; Fabricio, A.; Gion, M.; Baldi, I. Phenomapping of patients with primary breast cancer using machine learning-based unsupervised cluster analysis. J. Pers. Med. 2021, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Zanzotto, F.; Riondino, S.; Scarpato, N.; Guadagni, F.; Roselli, M. Breast cancer prognosis using a machine learning approach. Cancers 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Gallivanone, F.; Cava, C.; Corsi, F.; Bertoli, G.; Castiglioni, I. In silico approach for the definition of radiomirnomic signatures for breast cancer differential diagnosis. Int. J. Mol. Sci. 2019, 20, 5825. [Google Scholar] [CrossRef]
- Interlenghi, M.; Salvatore, C.; Magni, V.; Caldara, G.; Schiavon, E.; Cozzi, A.; Schiaffino, S.; Carbonaro, L.; Castiglioni, I.; Sardanelli, F. A Machine Learning Ensemble Based on Radiomics to Predict BI-RADS Category and Reduce the Biopsy Rate of Ultrasound-Detected Suspicious Breast Masses. Diagnostics 2022, 12, 187. [Google Scholar] [CrossRef]
- Militello, C.; Ranieri, A.; Rundo, L.; D’angelo, I.; Marinozzi, F.; Bartolotta, T.; Bini, F.; Russo, G. On unsupervised methods for medical image segmentation: Investigating classic approaches in breast cancer dce-mri. Appl. Sci. 2022, 12, 162. [Google Scholar] [CrossRef]
- Montemezzi, S.; Benetti, G.; Bisighin, M.; Camera, L.; Zerbato, C.; Caumo, F.; Fiorio, E.; Zanelli, S.; Zuffante, M.; Cavedon, C. 3T DCE-MRI Radiomics Improves Predictive Models of Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Front. Oncol. 2021, 11, 630780. [Google Scholar] [CrossRef] [PubMed]
- Pozzoli, S.; Soliman, A.; Bahri, L.; Branca, R.; Girdzijauskas, S.; Brambilla, M. Domain expertise–agnostic feature selection for the analysis of breast cancer data. Artif. Intell. Med. 2020, 108, 101928. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Boudjeltia, K.; Kotsalos, C.; Rousseau, A.; de Sousa, D.; Desmet, J.M.; Van Meerhaeghe, A.; Mira, A.; Chopard, B. Personalized pathology test for Cardiovascular disease: Approximate Bayesian computation with discriminative summary statistics learning. PLoS Comput. Biol. 2022, 18, e1009910. [Google Scholar] [CrossRef] [PubMed]
- De Marco, F.; Ferrucci, F.; Risi, M.; Tortora, G. Classification of QRS complexes to detect Premature Ventricular Contraction using machine learning techniques. PLoS ONE 2022, 17, e0268555. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Gialluisi, A.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.; de Gaetano, G.; et al. Dietary polyphenol intake is associated with biological aging, a novel predictor of cardiovascular disease: Cross-sectional findings from the moli-sani study. Nutrients 2021, 13, 1701. [Google Scholar] [CrossRef]
- Mezzatesta, S.; Torino, C.; De Meo, P.; Fiumara, G.; Vilasi, A. A machine learning-based approach for predicting the outbreak of cardiovascular diseases in patients on dialysis. Comput. Methods Programs Biomed. 2019, 177, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Recenti, M.; Ricciardi, C.; Edmunds, K.; Gislason, M.; Gargiulo, P.; Carraro, U. Healthy Aging within an Image: Using Muscle Radiodensitometry and Lifestyle Factors to Predict Diabetes and Hypertension. IEEE J. Biomed. Health Inform. 2021, 25, 2103–2112. [Google Scholar] [CrossRef]
- Cascarano, G.; Debitonto, F.; Lemma, R.; Brunetti, A.; Buongiorno, D.; De Feudis, I.; Guerriero, A.; Venere, U.; Matino, S.; Rocchetti, M.; et al. A neural network for glomerulus classification based on histological images of kidney biopsy. BMC Med. Inform. Decis. Mak. 2021, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Chicco, D.; Lovejoy, C.; Oneto, L. A Machine Learning Analysis of Health Records of Patients with Chronic Kidney Disease at Risk of Cardiovascular Disease. IEEE Access 2021, 9, 165132–165144. [Google Scholar] [CrossRef]
- Ventrella, P.; Delgrossi, G.; Ferrario, G.; Righetti, M.; Masseroli, M. Supervised machine learning for the assessment of Chronic Kidney Disease advancement. Comput. Methods Programs Biomed. 2021, 209, 106329. [Google Scholar] [CrossRef] [PubMed]
- Angelillo, M.; Balducci, F.; Impedovo, D.; Pirlo, G.; Vessio, G. Attentional Pattern Classification for Automatic Dementia Detection. IEEE Access 2019, 7, 57706–57716. [Google Scholar] [CrossRef]
- Battineni, G.; Chintalapudi, N.; Amenta, F. Machine learning in medicine: Performance calculation of dementia prediction by support vector machines (SVM). Inform. Med. Unlocked 2019, 16, 100200. [Google Scholar] [CrossRef]
- Di Benedetto, M.; Carrara, F.; Tafuri, B.; Nigro, S.; De Blasi, R.; Falchi, F.; Gennaro, C.; Gigli, G.; Logroscino, G.; Amato, G.; et al. Deep networks for behavioral variant frontotemporal dementia identification from multiple acquisition sources. Comput. Biol. Med. 2022, 148, 105937. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F. A novel multi-modal machine learning based approach for automatic classification of EEG recordings in dementia. Neural Netw. 2020, 123, 176–190. [Google Scholar] [CrossRef]
- Aliberti, A.; Pupillo, I.; Terna, S.; MacIi, E.; Di Cataldo, S.; Patti, E.; Acquaviva, A. A Multi-Patient Data-Driven Approach to Blood Glucose Prediction. IEEE Access 2019, 7, 69311–69325. [Google Scholar] [CrossRef]
- Berchialla, P.; Lanera, C.; Sciannameo, V.; Gregori, D.; Baldi, I. Prediction of treatment outcome in clinical trials under a personalized medicine perspective. Sci. Rep. 2022, 12, 4115. [Google Scholar] [CrossRef]
- Bernardini, M.; Romeo, L.; Misericordia, P.; Frontoni, E. Discovering the Type 2 Diabetes in Electronic Health Records Using the Sparse Balanced Support Vector Machine. IEEE J. Biomed. Health Inform. 2020, 24, 235–246. [Google Scholar] [CrossRef]
- Dagliati, A.; Marini, S.; Sacchi, L.; Cogni, G.; Teliti, M.; Tibollo, V.; De Cata, P.; Chiovato, L.; Bellazzi, R. Machine Learning Methods to Predict Diabetes Complications. J. Diabetes Sci. Technol. 2018, 12, 295–302. [Google Scholar] [CrossRef]
- Fiorini, S.; Hajati, F.; Barla, A.; Girosi, F. Predicting diabetes second-line therapy initiation in the Australian population via time span-guided neural attention network. PLoS ONE 2019, 14, e0211844. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Della-Morte, D.; Pastore, D.; Donadel, G.; De Stefano, A.; Palmieri, F. Identifying patterns in multiple biomarkers to diagnose diabetic foot using an explainable genetic programming-based approach. Future Gener. Comput. Syst. 2023, 140, 138–150. [Google Scholar] [CrossRef]
- Tognola, G.; Bonato, M.; Chiaramello, E.; Fiocchi, S.; Magne, I.; Souques, M.; Parazzini, M.; Ravazzani, P. Use of machine learning in the analysis of indoor ELF MF exposure in children. Int. J. Environ. Res. Public Health 2019, 16, 1230. [Google Scholar] [CrossRef] [PubMed]
- Tognola, G.; Chiaramello, E.; Bonato, M.; Magne, I.; Souques, M.; Fiocchi, S.; Parazzini, M.; Ravazzani, P. Cluster analysis of residential personal exposure to ELF magnetic field in children: Effect of environmental variables. Int. J. Environ. Res. Public Health 2019, 16, 4363. [Google Scholar] [CrossRef]
- Della Pepa, G.; Caccavella, V.; Menna, G.; Ius, T.; Auricchio, A.; Chiesa, S.; Gaudino, S.; Marchese, E.; Olivi, A. Machine Learning–Based Prediction of 6-Month Postoperative Karnofsky Performance Status in Patients with Glioblastoma: Capturing the Real-Life Interaction of Multiple Clinical and Oncologic Factors. World Neurosurg. 2021, 149, e866–e876. [Google Scholar] [CrossRef]
- Tariciotti, L.; Caccavella, V.; Fiore, G.; Schisano, L.; Carrabba, G.; Borsa, S.; Giordano, M.; Palmisciano, P.; Remoli, G.; Remore, L.; et al. A Deep Learning Model for Preoperative Differentiation of Glioblastoma, Brain Metastasis and Primary Central Nervous System Lymphoma: A Pilot Study. Front. Oncol. 2022, 12, 816638. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. Survival prediction of patients with sepsis from age, sex, and septic episode number alone. Sci. Rep. 2020, 10, 17156. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Sadiq, S.; Umer, M.; Ullah, S.; Mirjalili, S.; Rupapara, V.; Nappi, M. Improving the Prediction of Heart Failure Patients’ Survival Using SMOTE and Effective Data Mining Techniques. IEEE Access 2021, 9, 39707–39716. [Google Scholar] [CrossRef]
- Lorenzoni, G.; Sabato, S.; Lanera, C.; Bottigliengo, D.; Minto, C.; Ocagli, H.; De Paolis, P.; Gregori, D.; Iliceto, S.; Pisanò, F. Comparison of machine learning techniques for prediction of hospitalization in heart failure patients. J. Clin. Med. 2019, 8, 1298. [Google Scholar] [CrossRef]
- Sbrollini, A.; Barocci, M.; Mancinelli, M.; Paris, M.; Raffaelli, S.; Marcantoni, I.; Morettini, M.; Swenne, C.; Burattini, L. Automatic diagnosis of newly emerged heart failure from serial electrocardiography by repeated structuring & learning procedure. Biomed. Signal Process. Control 2023, 79, 104185. [Google Scholar] [CrossRef]
- Umer, M.; Sadiq, S.; Karamti, H.; Karamti, W.; Majeed, R.; Nappi, M. IoT Based Smart Monitoring of Patients’ with Acute Heart Failure. Sensors 2022, 22, 2431. [Google Scholar] [CrossRef]
- Bellocchio, F.; Carioni, P.; Lonati, C.; Garbelli, M.; Martínez-Martínez, F.; Stuard, S.; Neri, L. Enhanced sentinel surveillance system for covid-19 outbreak prediction in a large european dialysis clinics network. Int. J. Environ. Res. Public Health 2021, 18, 9739. [Google Scholar] [CrossRef]
- Bernardini, M.; Romeo, L.; Frontoni, E.; Amini, M.R. A Semi-Supervised Multi-Task Learning Approach for Predicting Short-Term Kidney Disease Evolution. IEEE J. Biomed. Health Inform. 2021, 25, 3983–3994. [Google Scholar] [CrossRef]
- D’Arnese, E.; Donato, G.W.D.; Sozzo, E.D.; Sollini, M.; Sciuto, D.; Santambrogio, M.D. On the Automation of Radiomics-Based Identification and Characterization of NSCLC. IEEE J. Biomed. Health Inform. 2022, 26, 2670–2679. [Google Scholar] [CrossRef]
- Prelaj, A.; Boeri, M.; Robuschi, A.; Ferrara, R.; Proto, C.; Lo Russo, G.; Galli, G.; De Toma, A.; Brambilla, M.; Occhipinti, M.; et al. Machine Learning Using Real-World and Translational Data to Improve Treatment Selection for NSCLC Patients Treated with Immunotherapy. Cancers 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Dannhauser, D.; Nastri, B.; Ballini, A.; Fiorelli, A.; Santini, M.; Netti, P.; Scacco, S.; Marino, M.; Causa, F.; et al. New trends in precision medicine: A pilot study of pure light scattering analysis as a useful tool for non-small cell lung cancer (nsclc) diagnosis. J. Pers. Med. 2021, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Rundo, L.; Ledda, R.; Noia, C.; Sala, E.; Mauri, G.; Milanese, G.; Sverzellati, N.; Apolone, G.; Gilardi, M.; Messa, M.; et al. A low-dose CT-based radiomic model to improve characterization and screening recall intervals of indeterminate prevalent pulmonary nodules. Diagnostics 2021, 11, 1610. [Google Scholar] [CrossRef]
- Tortora, M.; Cordelli, E.; Sicilia, R.; Miele, M.; Matteucci, P.; Iannello, G.; Ramella, S.; Soda, P. Deep Reinforcement Learning for Fractionated Radiotherapy in Non-Small Cell Lung Carcinoma. Artif. Intell. Med. 2021, 119, 102137. [Google Scholar] [CrossRef]
- Cazzato, G.; Massaro, A.; Colagrande, A.; Lettini, T.; Cicco, S.; Parente, P.; Nacchiero, E.; Lospalluti, L.; Cascardi, E.; Giudice, G.; et al. Dermatopathology of Malignant Melanoma in the Era of Artificial Intelligence: A Single Institutional Experience. Diagnostics 2022, 12, 1972. [Google Scholar] [CrossRef] [PubMed]
- Madonna, G.; Masucci, G.; Capone, M.; Mallardo, D.; Grimaldi, A.; Simeone, E.; Vanella, V.; Festino, L.; Palla, M.; Scarpato, L.; et al. Clinical categorization algorithm (Clical) and machine learning approach (srf-clical) to predict clinical benefit to immunotherapy in metastatic melanoma patients: Real-world evidence from the istituto nazionale tumori irccs fondazione pascale, napoli, italy. Cancers 2021, 13, 4164. [Google Scholar] [CrossRef] [PubMed]
- Barile, B.; Marzullo, A.; Stamile, C.; Durand-Dubief, F.; Sappey-Marinier, D. Data augmentation using generative adversarial neural networks on brain structural connectivity in multiple sclerosis. Comput. Methods Programs Biomed. 2021, 206, 106113. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, G.; Visintainer, R.; Domenici, E.; Lauria, M.; Marchetti, L. MOUSSE: Multi-omics using subject-specific signatures. Cancers 2021, 13, 3423. [Google Scholar] [CrossRef] [PubMed]
- Seccia, R.; Gammelli, D.; Dominici, F.; Romano, S.; Landi, A.; Salvetti, M.; Tacchella, A.; Zaccaria, A.; Crisanti, A.; Grassi, F.; et al. Considering patient clinical history impacts performance of machine learning models in predicting course of multiple sclerosis. PLoS ONE 2020, 15, e0230219. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Borzi, L.; Olmo, G.; Artusi, C.; Imbalzano, G.; Lopiano, L. Speech Impairment in Parkinson’s Disease: Acoustic Analysis of Unvoiced Consonants in Italian Native Speakers. IEEE Access 2021, 9, 166370–166381. [Google Scholar] [CrossRef]
- Buongiorno, D.; Bortone, I.; Cascarano, G.; Trotta, G.; Brunetti, A.; Bevilacqua, V. A low-cost vision system based on the analysis of motor features for recognition and severity rating of Parkinson’s Disease. BMC Med. Inform. Decis. Mak. 2019, 19, 243. [Google Scholar] [CrossRef]
- Caramia, C.; Torricelli, D.; Schmid, M.; Munoz-Gonzalez, A.; Gonzalez-Vargas, J.; Grandas, F.; Pons, J. IMU-Based Classification of Parkinson’s Disease from Gait: A Sensitivity Analysis on Sensor Location and Feature Selection. IEEE J. Biomed. Health Inform. 2018, 22, 1765–1774. [Google Scholar] [CrossRef]
- Borzì, L.; Mazzetta, I.; Zampogna, A.; Suppa, A.; Irrera, F.; Olmo, G. Predicting Axial Impairment in Parkinson’s Disease through a Single Inertial Sensor. Sensors 2022, 22, 412. [Google Scholar] [CrossRef]
- Impedovo, D.; Pirlo, G.; Vessio, G. Dynamic handwriting analysis for supporting earlier Parkinson’s disease diagnosis. Information 2018, 9, 247. [Google Scholar] [CrossRef]
- Mileti, I.; Germanotta, M.; Di Sipio, E.; Imbimbo, I.; Pacilli, A.; Erra, C.; Petracca, M.; Rossi, S.; Del Prete, Z.; Bentivoglio, A.; et al. Measuring gait quality in Parkinson’s disease through real-time gait phase recognition. Sensors 2018, 18, 919. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Lazzaro, G.; Errico, V.; Pisani, A.; Giannini, F.; Saggio, G. The Impact of Wearable Electronics in Assessing the Effectiveness of Levodopa Treatment in Parkinson’s Disease. IEEE J. Biomed. Health Inform. 2022, 26, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Termine, A.; Fabrizio, C.; Strafella, C.; Caputo, V.; Petrosini, L.; Caltagirone, C.; Cascella, R.; Giardina, E. A Hybrid Machine Learning and Network Analysis Approach Reveals Two Parkinson’s Disease Subtypes from 115 RNA-Seq Post-Mortem Brain Samples. Int. J. Mol. Sci. 2022, 23, 2557. [Google Scholar] [CrossRef]
- Cagni, E.; Botti, A.; Wang, Y.; Iori, M.; Petit, S.; Heijmen, B. Pareto-optimal plans as ground truth for validation of a commercial system for knowledge-based DVH-prediction. Phys. Medica 2018, 55, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gravina, M.; Spirito, L.; Celentano, G.; Capece, M.; Creta, M.; Califano, G.; Ruvolo, C.; Morra, S.; Imbriaco, M.; Di Bello, F.; et al. Machine Learning and Clinical-Radiological Characteristics for the Classification of Prostate Cancer in PI-RADS 3 Lesions. Diagnostics 2022, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, A.; Chiarelli, A.; Chiacchiaretta, P.; d’Annibale, M.; Croce, P.; Rosa, C.; Mastrodicasa, D.; Trebeschi, S.; Lambregts, D.; Caposiena, D.; et al. MRI-based clinical-radiomics model predicts tumor response before treatment in locally advanced rectal cancer. Sci. Rep. 2021, 11, 5379. [Google Scholar] [CrossRef]
- Gatta, R.; Vallati, M.; Dinapoli, N.; Masciocchi, C.; Lenkowicz, J.; Cusumano, D.; Casá, C.; Farchione, A.; Damiani, A.; van Soest, J.; et al. Towards a modular decision support system for radiomics: A case study on rectal cancer. Artif. Intell. Med. 2019, 96, 145–153. [Google Scholar] [CrossRef]
- Filitto, G.; Coppola, F.; Curti, N.; Giampieri, E.; Dall’olio, D.; Merlotti, A.; Cattabriga, A.; Cocozza, M.; Taninokuchi Tomassoni, M.; Remondini, D.; et al. Automated Prediction of the Response to Neoadjuvant Chemoradiotherapy in Patients Affected by Rectal Cancer. Cancers 2022, 14, 2231. [Google Scholar] [CrossRef]
- Adamo, S.; Ambrosino, P.; Ricciardi, C.; Accardo, M.; Mosella, M.; Cesarelli, M.; D’addio, G.; Maniscalco, M. A Machine Learning Approach to Predict the Rehabilitation Outcome in Convalescent COVID-19 Patients. J. Pers. Med. 2022, 12, 328. [Google Scholar] [CrossRef]
- Ahmad, M.; Sadiq, S.; Eshmawi, A.; Alluhaidan, A.; Umer, M.; Ullah, S.; Nappi, M. Industry 4.0 technologies and their applications in fighting COVID-19 pandemic using deep learning techniques. Comput. Biol. Med. 2022, 145, 105418. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, M.; Jeon, G.; Piccialli, F. A Framework for Pandemic Prediction Using Big Data Analytics. Big Data Res. 2021, 25, 100190. [Google Scholar] [CrossRef]
- Bartolucci, M.; Benelli, M.; Betti, M.; Bicchi, S.; Fedeli, L.; Giannelli, F.; Aquilini, D.; Baldini, A.; Consales, G.; Di Natale, M.; et al. The incremental value of computed tomography of COVID-19 pneumonia in predicting ICU admission. Sci. Rep. 2021, 11, 15619. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, F.; Lonati, C.; Titapiccolo, J.; Nadal, J.; Meiselbach, H.; Schmid, M.; Baerthlein, B.; Tschulena, U.; Schneider, M.; Schultheiss, U.; et al. Validation of a novel predictive algorithm for kidney failure in patients suffering from chronic kidney disease: The prognostic reasoning system for chronic kidney disease (PROGRES-CKD). Int. J. Environ. Res. Public Health 2021, 18, 12649. [Google Scholar] [CrossRef] [PubMed]
- Bertacchini, F.; Bilotta, E.; Pantano, P. On the temporal spreading of the SARS-CoV-2. PLoS ONE 2020, 15, e0240777. [Google Scholar] [CrossRef] [PubMed]
- Biondi, R.; Curti, N.; Coppola, F.; Giampieri, E.; Vara, G.; Bartoletti, M.; Cattabriga, A.; Cocozza, M.; Ciccarese, F.; De Benedittis, C.; et al. Classification performance for covid patient prognosis from automatic ai segmentation—A single-center study. Appl. Sci. 2021, 11, 5438. [Google Scholar] [CrossRef]
- Bottrighi, A.; Pennisi, M.; Roveta, A.; Massarino, C.; Cassinari, A.; Betti, M.; Bolgeo, T.; Bertolotti, M.; Rava, E.; Maconi, A. A machine learning approach for predicting high risk hospitalized patients with COVID-19 SARS-Cov-2. BMC Med. Inform. Decis. Mak. 2022, 22, 340. [Google Scholar] [CrossRef]
- Bougourzi, F.; Contino, R.; Distante, C.; Taleb-Ahmed, A. Recognition of COVID-19 from CT scans using two-stage deep-learning-based approach: CNR-IEMN. Sensors 2021, 21, 5878. [Google Scholar] [CrossRef]
- Chieregato, M.; Frangiamore, F.; Morassi, M.; Baresi, C.; Nici, S.; Bassetti, C.; Bnà, C.; Galelli, M. A hybrid machine learning/deep learning COVID-19 severity predictive model from CT images and clinical data. Sci. Rep. 2022, 12, 4329. [Google Scholar] [CrossRef] [PubMed]
- De Falco, I.; De Pietro, G.; Sannino, G. Classification of Covid-19 chest X-ray images by means of an interpretable evolutionary rule-based approach. Neural Comput. Appl. 2023, 35, 16061–16071. [Google Scholar] [CrossRef]
- Fadja, A.; Fraccaroli, M.; Bizzarri, A.; Mazzuchelli, G.; Lamma, E. Neural-Symbolic Ensemble Learning for early-stage prediction of critical state of Covid-19 patients. Med. Biol. Eng. Comput. 2022, 60, 3461–3474. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Milic, J.; Tonelli, R.; Ghinelli, F.; Meschiari, M.; Volpi, S.; Faltoni, M.; Franceschi, G.; Iadisernia, V.; Yaacoub, D.; et al. Machine learning in predicting respiratory failure in patients with COVID-19 pneumonia—Challenges, strengths, and opportunities in a global health emergency. PLoS ONE 2020, 15, e0239172. [Google Scholar] [CrossRef] [PubMed]
- Flesia, L.; Monaro, M.; Mazza, C.; Fietta, V.; Colicino, E.; Segatto, B.; Roma, P. Predicting perceived stress related to the covid-19 outbreak through stable psychological traits and machine learning models. J. Clin. Med. 2020, 9, 3350. [Google Scholar] [CrossRef]
- Giotta, M.; Trerotoli, P.; Palmieri, V.; Passerini, F.; Portincasa, P.; Dargenio, I.; Mokhtari, J.; Montagna, M.; De Vito, D. Application of a Decision Tree Model to Predict the Outcome of Non-Intensive Inpatients Hospitalized for COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 13016. [Google Scholar] [CrossRef] [PubMed]
- Gumaei, A.; Ismail, W.; Rafiul Hassan, M.; Hassan, M.; Mohamed, E.; Alelaiwi, A.; Fortino, G. A Decision-Level Fusion Method for COVID-19 Patient Health Prediction. Big Data Res. 2022, 27, 100287. [Google Scholar] [CrossRef]
- Sîrbu, A.; Barbieri, G.; Faita, F.; Ferragina, P.; Gargani, L.; Ghiadoni, L.; Priami, C. Early outcome detection for COVID-19 patients. Sci. Rep. 2021, 11, 18464. [Google Scholar] [CrossRef] [PubMed]
- Iori, M.; Di Castelnuovo, C.; Verzellesi, L.; Meglioli, G.; Lippolis, D.; Nitrosi, A.; Monelli, F.; Besutti, G.; Trojani, V.; Bertolini, M.; et al. Mortality Prediction of COVID-19 Patients Using Radiomic and Neural Network Features Extracted from a Wide Chest X-ray Sample Size: A Robust Approach for Different Medical Imbalanced Scenarios. Appl. Sci. 2022, 12, 3903. [Google Scholar] [CrossRef]
- Kumar, S.; Chaube, M.; Alsamhi, S.; Gupta, S.; Guizani, M.; Gravina, R.; Fortino, G. A novel multimodal fusion framework for early diagnosis and accurate classification of COVID-19 patients using X-ray images and speech signal processing techniques. Comput. Methods Programs Biomed. 2022, 226, 107109. [Google Scholar] [CrossRef] [PubMed]
- Loddo, A.; Meloni, G.; Pes, B. Using Artificial Intelligence for COVID-19 Detection in Blood Exams: A Comparative Analysis. IEEE Access 2022, 10, 119593–119606. [Google Scholar] [CrossRef]
- Monaco, A.; Pantaleo, E.; Amoroso, N.; Bellantuono, L.; Stella, A.; Bellotti, R. Country-level factors dynamics and ABO/Rh blood groups contribution to COVID-19 mortality. Sci. Rep. 2021, 11, 24527. [Google Scholar] [CrossRef]
- Schiaffino, S.; Codari, M.; Cozzi, A.; Albano, D.; Alì, M.; Arioli, R.; Avola, E.; Bnà, C.; Cariati, M.; Carriero, S.; et al. Machine learning to predict in-hospital mortality in covid-19 patients using computed tomography-derived pulmonary and vascular features. J. Pers. Med. 2021, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, M.; Marzullo, A.; De Momi, E.; Calimeri, F. Lesion segmentation in lung CT scans using unsupervised adversarial learning. Med. Biol. Eng. Comput. 2022, 60, 3203–3215. [Google Scholar] [CrossRef]
- Verde, L.; De Pietro, G.; Ghoneim, A.; Alrashoud, M.; Al-Mutib, K.; Sannino, G. Exploring the Use of Artificial Intelligence Techniques to Detect the Presence of Coronavirus Covid-19 through Speech and Voice Analysis. IEEE Access 2021, 9, 65750–65757. [Google Scholar] [CrossRef]
- Gozzi, N.; Perrotta, D.; Paolotti, D.; Perra, N. Towards a data-driven characterization of behavioral changes induced by the seasonal flu. PLoS Comput. Biol. 2020, 16, e1007879. [Google Scholar] [CrossRef]
- Kalimeri, K.; Delfino, M.; Cattuto, C.; Perrotta, D.; Colizza, V.; Guerrisi, C.; Turbelin, C.; Duggan, J.; Edmunds, J.; Obi, C.; et al. Unsupervised extraction of epidemic syndromes from participatory influenza surveillance self-reported symptoms. PLoS Comput. Biol. 2019, 15, e1006173. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Cogollo, J.; Bonet, I.; Gil, B.; Iadanza, E. Machine Learning Models for Early Prediction of Sepsis on Large Healthcare Datasets. Electronics 2022, 11, 1507. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. Machine learning can predict survival of patients with heart failure from serum creatinine and ejection fraction alone. BMC Med. Inform. Decis. Mak. 2020, 20, 16. [Google Scholar] [CrossRef]
- Wernly, B.; Mamandipoor, B.; Baldia, P.; Jung, C.; Osmani, V. Machine learning predicts mortality in septic patients using only routinely available ABG variables: A multi-centre evaluation. Int. J. Med. Inform. 2021, 145, 104312. [Google Scholar] [CrossRef]
- Iosa, M.; Capodaglio, E.; Pelà, S.; Persechino, B.; Morone, G.; Antonucci, G.; Paolucci, S.; Panigazzi, M. Artificial Neural Network Analyzing Wearable Device Gait Data for Identifying Patients With Stroke Unable to Return to Work. Front. Neurol. 2021, 12, 650542. [Google Scholar] [CrossRef]
- Scrutinio, D.; Ricciardi, C.; Donisi, L.; Losavio, E.; Battista, P.; Guida, P.; Cesarelli, M.; Pagano, G.; D’Addio, G. Machine learning to predict mortality after rehabilitation among patients with severe stroke. Sci. Rep. 2020, 10, 20127. [Google Scholar] [CrossRef]
- Lanera, C.; Berchialla, P.; Baldi, I.; Lorenzoni, G.; Tramontan, L.; Scamarcia, A.; Cantarutti, L.; Giaquinto, C.; Gregori, D. Use of machine learning techniques for case-detection of varicella zoster using routinely collected textual ambulatory records: Pilot observational study. JMIR Med. Inform. 2020, 8, e14330. [Google Scholar] [CrossRef]
- Lanera, C.; Baldi, I.; Francavilla, A.; Barbieri, E.; Tramontan, L.; Scamarcia, A.; Cantarutti, L.; Giaquinto, C.; Gregori, D. A Deep Learning Approach to Estimate the Incidence of Infectious Disease Cases for Routinely Collected Ambulatory Records: The Example ofVaricella-Zoster. Int. J. Environ. Res. Public Health 2022, 19, 5959. [Google Scholar] [CrossRef]
- Verde, L.; De Pietro, G.; Sannino, G. Voice Disorder Identification by Using Machine Learning Techniques. IEEE Access 2018, 6, 16246–16255. [Google Scholar] [CrossRef]
- Verde, L.; De Pietro, G.; Alrashoud, M.; Ghoneim, A.; Al-Mutib, K.; Sannino, G. Leveraging artificial intelligence to improve voice disorder identification through the use of a reliable mobile app. IEEE Access 2019, 7, 124048–124054. [Google Scholar] [CrossRef]
- Bruno, P.; Calimeri, F.; Kitanidis, A.; De Momi, E. Data reduction and data visualization for automatic diagnosis using gene expression and clinical data. Artif. Intell. Med. 2020, 107, 101884. [Google Scholar] [CrossRef]
- Di Santo, R.; Vaccaro, M.; Romanò, S.; Di Giacinto, F.; Papi, M.; Rapaccini, G.; De Spirito, M.; Miele, L.; Basile, U.; Ciasca, G. Machine Learning-Assisted FTIR Analysis of Circulating Extracellular Vesicles for Cancer Liquid Biopsy. J. Pers. Med. 2022, 12, 949. [Google Scholar] [CrossRef]
- Zonta, G.; Malagù, C.; Gherardi, S.; Giberti, A.; Pezzoli, A.; De Togni, A.; Palmonari, C. Clinical validation results of an innovative non-invasive device for colorectal cancer preventive screening through fecal exhalation analysis. Cancers 2020, 12, 1471. [Google Scholar] [CrossRef]
- Celli, F.; Cumbo, F.; Weitschek, E. Classification of Large DNA Methylation Datasets for Identifying Cancer Drivers. Big Data Res. 2018, 13, 21–28. [Google Scholar] [CrossRef]
- Carlini, G.; Curti, N.; Strolin, S.; Giampieri, E.; Sala, C.; Dall’olio, D.; Merlotti, A.; Fanti, S.; Remondini, D.; Nanni, C.; et al. Prediction of Overall Survival in Cervical Cancer Patients Using PET/CT Radiomic Features. Appl. Sci. 2022, 12, 5946. [Google Scholar] [CrossRef]
- Alongi, P.; Stefano, A.; Comelli, A.; Spataro, A.; Formica, G.; Laudicella, R.; Lanzafame, H.; Panasiti, F.; Longo, C.; Midiri, F.; et al. Artificial Intelligence Applications on Restaging [18F]FDG PET/CT in Metastatic Colorectal Cancer: A Preliminary Report of Morpho-Functional Radiomics Classification for Prediction of Disease Outcome. Appl. Sci. 2022, 12, 2941. [Google Scholar] [CrossRef]
- Prezioso, E.; Izzo, S.; Giampaolo, F.; Piccialli, F.; Dell’aversana Orabona, G.; Cuocolo, R.; Abbate, V.; Ugga, L.; Califano, L. Predictive Medicine for Salivary Gland Tumours Identification Through Deep Learning. IEEE J. Biomed. Health Inform. 2022, 26, 4869–4879. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mattavelli, D.; Schreiber, A.; Gualtieri, T.; Rampinelli, V.; Tomasoni, M.; Taboni, S.; Ardighieri, L.; Battocchio, S.; Bozzola, A.; et al. Does Reorganization of Clinicopathological Information Improve Prognostic Stratification and Prediction of Chemoradiosensitivity in Sinonasal Carcinomas? A Retrospective Study on 145 Patients. Front. Oncol. 2022, 12, 799680. [Google Scholar] [CrossRef] [PubMed]
- Buizza, G.; Paganelli, C.; D’ippolito, E.; Fontana, G.; Molinelli, S.; Preda, L.; Riva, G.; Iannalfi, A.; Valvo, F.; Orlandi, E.; et al. Radiomics and dosiomics for predicting local control after carbon-ion radiotherapy in skull-base chordoma. Cancers 2021, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Koprowski, R.; Boccia, R.; Ruggiero, A.; De Rosa, L.; Tortori, A.; Wilczyński, S.; Melillo, P.; Sbordone, S.; Simonelli, F. Classification tree to analyze factors connected with post operative complications of cataract surgery in a teaching hospital. J. Clin. Med. 2021, 10, 5399. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, M.; Meroni, V.; Orlandini, L.; Prandoni, L.; Rossi, N.; Peretti, G.; Dui, L.; Mangiavini, L.; Ferrante, S. Opportunities to improve feasibility, effectiveness and costs associated with a total joint replacements high-volume hospital registry. Comput. Biol. Med. 2020, 121, 103775. [Google Scholar] [CrossRef]
- Marzullo, A.; Moccia, S.; Catellani, M.; Calimeri, F.; Momi, E. Towards realistic image generation using image-domain translation. Comput. Methods Programs Biomed. 2021, 200, 105834. [Google Scholar] [CrossRef]
- Muzio, F.; Rozzi, G.; Rossi, S.; Luciani, G.; Foresti, R.; Cabassi, A.; Fassina, L.; Miragoli, M. Artificial intelligence supports decision making during open-chest surgery of rare congenital heart defects. J. Clin. Med. 2021, 10, 5330. [Google Scholar] [CrossRef]
- Sargos, P.; Leduc, N.; Giraud, N.; Gandaglia, G.; Roumiguié, M.; Ploussard, G.; Rozet, F.; Soulié, M.; Mathieu, R.; Artus, P.; et al. Deep Neural Networks Outperform the CAPRA Score in Predicting Biochemical Recurrence after Prostatectomy. Front. Oncol. 2021, 10, 607923. [Google Scholar] [CrossRef]
- Berjano, P.; Langella, F.; Ventriglia, L.; Compagnone, D.; Barletta, P.; Huber, D.; Mangili, F.; Licandro, G.; Galbusera, F.; Cina, A.; et al. The Influence of Baseline Clinical Status and Surgical Strategy on Early Good to Excellent Result in Spinal Lumbar Arthrodesis: A Machine Learning Approach. J. Pers. Med. 2021, 11, 1377. [Google Scholar] [CrossRef]
- Campagner, A.; Berjano, P.; Lamartina, C.; Langella, F.; Lombardi, G.; Cabitza, F. Assessment and prediction of spine surgery invasiveness with machine learning techniques. Comput. Biol. Med. 2020, 121, 103796. [Google Scholar] [CrossRef]
- Bombieri, M.; Rospocher, M.; Ponzetto, S.; Fiorini, P. Machine understanding surgical actions from intervention procedure textbooks. Comput. Biol. Med. 2023, 152, 106415. [Google Scholar] [CrossRef]
- Kaczmarek-Majer, K.; Casalino, G.; Castellano, G.; Dominiak, M.; Hryniewicz, O.; Kamińska, O.; Vessio, G.; Díaz-Rodríguez, N. PLENARY: Explaining black-box models in natural language through fuzzy linguistic summaries. Inf. Sci. 2022, 614, 374–399. [Google Scholar] [CrossRef]
- Liuzzi, P.; Magliacano, A.; De Bellis, F.; Mannini, A.; Estraneo, A. Predicting outcome of patients with prolonged disorders of consciousness using machine learning models based on medical complexity. Sci. Rep. 2022, 12, 13471. [Google Scholar] [CrossRef]
- Ocagli, H.; Bottigliengo, D.; Lorenzoni, G.; Azzolina, D.; Acar, A.; Sorgato, S.; Stivanello, L.; Degan, M.; Gregori, D. A machine learning approach for investigating delirium as a multifactorial syndrome. Int. J. Environ. Res. Public Health 2021, 18, 7105. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hao, Y.; Yu, Q.; Chen, M.; Humar, I.; Fortino, G. Depression Analysis and Recognition Based on Functional Near-Infrared Spectroscopy. IEEE J. Biomed. Health Inform. 2021, 25, 4289–4299. [Google Scholar] [CrossRef]
- Naseer Qureshi, K.; Din, S.; Jeon, G.; Piccialli, F. An accurate and dynamic predictive model for a smart M-Health system using machine learning. Inf. Sci. 2020, 538, 486–502. [Google Scholar] [CrossRef]
- Betti, S.; Lova, R.; Rovini, E.; Acerbi, G.; Santarelli, L.; Cabiati, M.; Ry, S.; Cavallo, F. Evaluation of an integrated system of wearable physiological sensors for stress monitoring in working environments by using biological markers. IEEE Trans. Biomed. Eng. 2018, 65, 1748–1758. [Google Scholar] [CrossRef]
- Coro, G.; Bardelli, S.; Cuttano, A.; Scaramuzzo, R.; Ciantelli, M. A self-training automatic infant-cry detector. Neural Comput. Appl. 2023, 35, 8543–8559. [Google Scholar] [CrossRef]
- Ragni, F.; Archetti, L.; Roby-Brami, A.; Amici, C.; Saint-Bauzel, L. Intention prediction and human health condition detection in reaching tasks with machine learning techniques. Sensors 2021, 21, 5253. [Google Scholar] [CrossRef]
- Monteriù, A.; Prist, M.; Frontoni, E.; Longhi, S.; Pietroni, F.; Casaccia, S.; Scalise, L.; Cenci, A.; Romeo, L.; Berta, R.; et al. Smart sensing architecture for domestic monitoring: Methodological approach and experimental validation. Sensors 2018, 18, 2310. [Google Scholar] [CrossRef]
- Arpaia, P.; Crauso, F.; De Benedetto, E.; Duraccio, L.; Improta, G.; Serino, F. Soft Transducer for Patient’s Vitals Telemonitoring with Deep Learning-Based Personalized Anomaly Detection. Sensors 2022, 22, 536. [Google Scholar] [CrossRef]
- Sorino, P.; Caruso, M.; Misciagna, G.; Bonfiglio, C.; Campanella, A.; Mirizzi, A.; Franco, I.; Bianco, A.; Buongiorno, C.; Liuzzi, R.; et al. Selecting the best machine learning algorithm to support the diagnosis of Non-Alcoholic Fatty Liver Disease: A meta learner study. PLoS ONE 2020, 15, e0240867. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. An ensemble learning approach for enhanced classification of patients with hepatitis and cirrhosis. IEEE Access 2021, 9, 24485–24498. [Google Scholar] [CrossRef]
- Hassoun, S.; Bruckmann, C.; Ciardullo, S.; Perseghin, G.; Di Gaudio, F.; Broccolo, F. Setting up of a machine learning algorithm for the identification of severe liver fibrosis profile in the general US population cohort. Int. J. Med. Inform. 2023, 170, 104932. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Borrelli, A.; Improta, G. Predictive analysis of lower limb fractures in the orthopedic complex operative unit using artificial intelligence: The case study of AOU Ruggi. Sci. Rep. 2022, 12, 22153. [Google Scholar] [CrossRef] [PubMed]
- Milella, F.; Famiglini, L.; Banfi, G.; Cabitza, F. Application of Machine Learning to Improve Appropriateness of Treatment in an Orthopaedic Setting of Personalized Medicine. J. Pers. Med. 2022, 12, 1706. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, T.; Borrelli, A.; Improta, G. Is It Possible to Predict the Length of Stay of Patients Undergoing Hip-Replacement Surgery? Int. J. Environ. Res. Public Health 2022, 19, 6219. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. Arterial Disease Computational Prediction and Health Record Feature Ranking among Patients Diagnosed with Inflammatory Bowel Disease. IEEE Access 2021, 9, 78648–78657. [Google Scholar] [CrossRef]
- Cavallo, A.; Troisi, J.; Muscogiuri, E.; Cavallo, P.; Rajagopalan, S.; Citro, R.; Bossone, E.; McVeigh, N.; Forte, V.; Di Donna, C.; et al. Cardiac Computed Tomography Radiomics-Based Approach for the Detection of Left Ventricular Remodeling in Patients with Arterial Hypertension. Diagnostics 2022, 12, 322. [Google Scholar] [CrossRef]
- Ricciardi, C.; Cantoni, V.; Improta, G.; Iuppariello, L.; Latessa, I.; Cesarelli, M.; Triassi, M.; Cuocolo, A. Application of data mining in a cohort of Italian subjects undergoing myocardial perfusion imaging at an academic medical center. Comput. Methods Programs Biomed. 2020, 189, 105343. [Google Scholar] [CrossRef]
- Jacob, D.; Unnsteinsdóttir Kristensen, I.; Aubonnet, R.; Recenti, M.; Donisi, L.; Ricciardi, C.; Svansson, H.; Agnarsdóttir, S.; Colacino, A.; Jónsdóttir, M.; et al. Towards defining biomarkers to evaluate concussions using virtual reality and a moving platform (BioVRSea). Sci. Rep. 2022, 12, 8996. [Google Scholar] [CrossRef]
- Montagna, S.; Mariani, S.; Gamberini, E. Augmenting BDI Agency with a Cognitive Service: Architecture and Validation in Healthcare Domain. J. Med. Syst. 2021, 45, 103. [Google Scholar] [CrossRef]
- Falsetti, L.; Rucco, M.; Proietti, M.; Viticchi, G.; Zaccone, V.; Scarponi, M.; Giovenali, L.; Moroncini, G.; Nitti, C.; Salvi, A. Risk prediction of clinical adverse outcomes with machine learning in a cohort of critically ill patients with atrial fibrillation. Sci. Rep. 2021, 11, 18925. [Google Scholar] [CrossRef] [PubMed]
- Durán, C.; Ciucci, S.; Palladini, A.; Ijaz, U.Z.; Zippo, A.G.; Sterbini, F.P.; Masucci, L.; Cammarota, G.; Ianiro, G.; Spuul, P.; et al. Nonlinear machine learning pattern recognition and bacteria-metabolite multilayer network analysis of perturbed gastric microbiome. Nat. Commun. 2021, 12, 1926. [Google Scholar] [CrossRef]
- Nobile, M.; Capitoli, G.; Sowirono, V.; Clerici, F.; Piga, I.; van Abeelen, K.; Magni, F.; Pagni, F.; Galimberti, S.; Cazzaniga, P.; et al. Unsupervised neural networks as a support tool for pathology diagnosis in MALDI-MSI experiments: A case study on thyroid biopsies. Expert Syst. Appl. 2023, 215, 119296. [Google Scholar] [CrossRef]
- Comito, C.; Falcone, D.; Forestiero, A. AI-Driven Clinical Decision Support: Enhancing Disease Diagnosis Exploiting Patients Similarity. IEEE Access 2022, 10, 6878–6888. [Google Scholar] [CrossRef]
- Qi, W.; Aliverti, A. A multimodal wearable system for continuous and real-time breathing pattern monitoring during daily activity. IEEE J. Biomed. Health Inform. 2020, 24, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Tarekegn, A.; Ricceri, F.; Costa, G.; Ferracin, E.; Giacobini, M. Predictive modeling for frailty conditions in Elderly People: Machine learning approaches. JMIR Med. Inform. 2020, 8, e16678. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, F.; Guerrini, L.; Gunnarsson, A.; Recenti, M.; Jacob, D.; Cangiano, V.; Tesfahunegn, Y.; Islind, A.; Tortorella, F.; Tsirilaki, M.; et al. CT-and MRI-Based 3D Reconstruction of Knee Joint to Assess Cartilage and Bone. Diagnostics 2022, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Crocamo, C.; Viviani, M.; Bartoli, F.; Carrà, G.; Pasi, G. Detecting binge drinking and alcohol-related risky behaviours from twitter’s users: An exploratory content-and topology-based analysis. Int. J. Environ. Res. Public Health 2020, 17, 1510. [Google Scholar] [CrossRef]
- Spiga, O.; Cicaloni, V.; Visibelli, A.; Davoli, A.; Paparo, M.; Orlandini, M.; Vecchi, B.; Santucci, A. Towards a precision medicine approach based on machine learning for tailoring medical treatment in alkaptonuria. Int. J. Mol. Sci. 2021, 22, 1187. [Google Scholar] [CrossRef]
- Delnevo, G.; Mancini, G.; Roccetti, M.; Salomoni, P.; Trombini, E.; Andrei, F. The prediction of body mass index from negative affectivity through machine learning: A confirmatory study. Sensors 2021, 21, 2361. [Google Scholar] [CrossRef]
- Leoni, J.; Strada, S.; Tanelli, M.; Jiang, K.; Brusa, A.; Proverbio, A. Automatic stimuli classification from ERP data for augmented communication via Brain–Computer Interfaces. Expert Syst. Appl. 2021, 184, 115572. [Google Scholar] [CrossRef]
- Danilov, V.; Skirnevskiy, I.; Manakov, R.; Gerget, O.; Melgani, F. Feature selection algorithm based on PDF/PMF area difference. Biomed. Signal Process. Control 2020, 57, 101681. [Google Scholar] [CrossRef]
- Piccialli, F.; Calabrò, F.; Crisci, D.; Cuomo, S.; Prezioso, E.; Mandile, R.; Troncone, R.; Greco, L.; Auricchio, R. Precision medicine and machine learning towards the prediction of the outcome of potential celiac disease. Sci. Rep. 2021, 11, 5683. [Google Scholar] [CrossRef] [PubMed]
- Ocagli, H.; Lanera, C.; Lorenzoni, G.; Prosepe, I.; Azzolina, D.; Bortolotto, S.; Stivanello, L.; Degan, M.; Gregori, D. Profiling patients by intensity of nursing care: An operative approach using machine learning. J. Pers. Med. 2020, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Bottigliengo, D.; Berchialla, P.; Lanera, C.; Azzolina, D.; Lorenzoni, G.; Martinato, M.; Giachino, D.; Baldi, I.; Gregori, D. The role of genetic factors in characterizing extra-intestinal manifestations in Crohn’s disease patients: Are bayesian machine learning methods improving outcome predictions? J. Clin. Med. 2019, 8, 865. [Google Scholar] [CrossRef]
- Delmastro, F.; Martino, F.; Dolciotti, C. Cognitive Training and Stress Detection in MCI Frail Older People through Wearable Sensors and Machine Learning. IEEE Access 2020, 8, 65573–65590. [Google Scholar] [CrossRef]
- Bernardini, M.; Romeo, L.; Mancini, A.; Frontoni, E. A Clinical Decision Support System to Stratify the Temporal Risk of Diabetic Retinopathy. IEEE Access 2021, 9, 151864–151872. [Google Scholar] [CrossRef]
- Peralta, R.; Garbelli, M.; Bellocchio, F.; Ponce, P.; Stuard, S.; Lodigiani, M.; Matos, J.; Ribeiro, R.; Nikam, M.; Botler, M.; et al. Development and validation of a machine learning model predicting arteriovenous fistula failure in a large network of dialysis clinics. Int. J. Environ. Res. Public Health 2021, 18, 12355. [Google Scholar] [CrossRef]
- Amato, F.; Coppolino, L.; Cozzolino, G.; Mazzeo, G.; Moscato, F.; Nardone, R. Enhancing random forest classification with NLP in DAMEH: A system for DAta Management in eHealth Domain. Neurocomputing 2021, 444, 79–91. [Google Scholar] [CrossRef]
- Montagna, S.; Pengo, M.; Ferretti, S.; Borghi, C.; Ferri, C.; Grassi, G.; Muiesan, M.; Parati, G. Machine Learning in Hypertension Detection: A Study on World Hypertension Day Data. J. Med. Syst. 2023, 47, 1. [Google Scholar] [CrossRef]
- Iadanza, E.; Goretti, F.; Sorelli, M.; Melillo, P.; Pecchia, L.; Simonelli, F.; Gherardelli, M. Automatic Detection of Genetic Diseases in Pediatric Age Using Pupillometry. IEEE Access 2020, 8, 34949–34961. [Google Scholar] [CrossRef]
- Bernardini, M.; Morettini, M.; Romeo, L.; Frontoni, E.; Burattini, L. TyG-er: An ensemble Regression Forest approach for identification of clinical factors related to insulin resistance condition using Electronic Health Records. Comput. Biol. Med. 2019, 112, 103358. [Google Scholar] [CrossRef]
- Patrini, I.; Ruperti, M.; Moccia, S.; Mattos, L.; Frontoni, E.; De Momi, E. Transfer learning for informative-frame selection in laryngoscopic videos through learned features. Med. Biol. Eng. Comput. 2020, 58, 1225–1238. [Google Scholar] [CrossRef]
- Amato, F.; Marrone, S.; Moscato, V.; Piantadosi, G.; Picariello, A.; Sansone, C. HOLMeS: eHealth in the big data and deep learning era. Information 2019, 10, 34. [Google Scholar] [CrossRef]
- Chatterjee, R.; Maitra, T.; Hafizul Islam, S.; Hassan, M.; Alamri, A.; Fortino, G. A novel machine learning based feature selection for motor imagery EEG signal classification in Internet of medical things environment. Future Gener. Comput. Syst. 2019, 98, 419–434. [Google Scholar] [CrossRef]
- Raglio, A.; Imbriani, M.; Imbriani, C.; Baiardi, P.; Manzoni, S.; Gianotti, M.; Castelli, M.; Vanneschi, L.; Vico, F.; Manzoni, L. Machine learning techniques to predict the effectiveness of music therapy: A randomized controlled trial. Comput. Methods Programs Biomed. 2020, 185, 105160. [Google Scholar] [CrossRef]
- Cicirelli, G.; D’Orazio, T. A Low-Cost Video-Based System for Neurodegenerative Disease Detection by Mobility Test Analysis. Appl. Sci. 2023, 13, 278. [Google Scholar] [CrossRef]
- Moro, M.; Pastore, V.; Tacchino, C.; Durand, P.; Blanchi, I.; Moretti, P.; Odone, F.; Casadio, M. A markerless pipeline to analyze spontaneous movements of preterm infants. Comput. Methods Programs Biomed. 2022, 226, 107119. [Google Scholar] [CrossRef]
- Amina, M.; Yazdani, J.; Rovetta, S.; Masulli, F. Toward development of PreVoid alerting system for nocturnal enuresis patients: A fuzzy-based approach for determining the level of liquid encased in urinary bladder. Artif. Intell. Med. 2020, 106, 101819. [Google Scholar] [CrossRef]
- Veneroni, C.; Acciarito, A.; Lombardi, E.; Imeri, G.; Kaminsky, D.; Gobbi, A.; Pompilio, P.; Dellaca’, R. Artificial intelligence for quality control of oscillometry measures. Comput. Biol. Med. 2021, 138, 104871. [Google Scholar] [CrossRef]
- Masi, D.; Risi, R.; Biagi, F.; Vasquez Barahona, D.; Watanabe, M.; Zilich, R.; Gabrielli, G.; Santin, P.; Mariani, S.; Lubrano, C.; et al. Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome. Nutrients 2022, 14, 373. [Google Scholar] [CrossRef]
- De Nunzio, G.; Conte, L.; Lupo, R.; Vitale, E.; Calabrò, A.; Ercolani, M.; Carvello, M.; Arigliani, M.; Toraldo, D.; De Benedetto, L. A New Berlin Questionnaire Simplified by Machine Learning Techniques in a Population of Italian Healthcare Workers to Highlight the Suspicion of Obstructive Sleep Apnea. Front. Med. 2022, 9, 866822. [Google Scholar] [CrossRef]
- Altilio, R.; Rossetti, A.; Fang, Q.; Gu, X.; Panella, M. A comparison of machine learning classifiers for smartphone-based gait analysis. Med. Biol. Eng. Comput. 2021, 59, 535–546. [Google Scholar] [CrossRef]
- Galassi, A.; Fasulo, E.; Ciceri, P.; Casazza, R.; Bonelli, F.; Zierold, C.; Calleri, M.; Blocki, F.; Palmieri, M.; Mastronardo, C.; et al. 1,25-dihydroxyvitamin D as Predictor of Renal Worsening Function in Chronic Kidney Disease. Results From the PASCaL-1,25D Study. Front. Med. 2022, 9, 840801. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Chen, R.; Zheng, J.; Cai, Y.; Fortino, G. Risk Prediction of Renal Failure for Chronic Disease Population Based on Electronic Health Record Big Data. Big Data Res. 2021, 25, 100234. [Google Scholar] [CrossRef]
- Mollalo, A.; Vahedi, B.; Bhattarai, S.; Hopkins, L.; Banik, S.; Vahedi, B. Predicting the hotspots of age-adjusted mortality rates of lower respiratory infection across the continental United States: Integration of GIS, spatial statistics and machine learning algorithms. Int. J. Med. Inform. 2020, 142, 104248. [Google Scholar] [CrossRef]
- Monaco, A.; Amoroso, N.; Bellantuono, L.; Pantaleo, E.; Tangaro, S.; Bellotti, R. Multi-time-scale features for accurate respiratory sound classification. Appl. Sci. 2020, 10, 8606. [Google Scholar] [CrossRef]
- Brancati, N.; Frucci, M.; Gragnaniello, D.; Riccio, D.; Di Iorio, V.; Di Perna, L.; Simonelli, F. Learning-based approach to segment pigment signs in fundus images for Retinitis Pigmentosa analysis. Neurocomputing 2018, 308, 159–171. [Google Scholar] [CrossRef]
- Leone, A.; Rescio, G.; Manni, A.; Siciliano, P.; Caroppo, A. Comparative Analysis of Supervised Classifiers for the Evaluation of Sarcopenia Using a sEMG-Based Platform. Sensors 2022, 22, 2721. [Google Scholar] [CrossRef]
- Rossi, A.; Pappalardo, L.; Cintia, P.; Iaia, F.; Fernàndez, J.; Medina, D. Effective injury forecasting in soccer with GPS training data and machine learning. PLoS ONE 2018, 13, e0201264. [Google Scholar] [CrossRef]
- Martorell-Marugán, J.; Chierici, M.; Jurman, G.; Alarcón-Riquelme, M.E.; Carmona-Sáez, P. Differential diagnosis of systemic lupus erythematosus and Sjögren’s syndrome using machine learning and multi-omics data. Comput. Biol. Med. 2023, 152, 106373. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Caprioli, S.; Tonacci, A.; Billeci, L.; Greco, M.; Negrini, S.; Cittadini, G.; Zentilin, P.; Spagnolo, E.; Gangemi, S. A machine learning application to predict early lung involvement in scleroderma: A feasibility evaluation. Diagnostics 2021, 11, 1880. [Google Scholar] [CrossRef]
- Polignano, M.; Narducci, F.; Iovine, A.; Musto, C.; De Gemmis, M.; Semeraro, G. HealthAssistantBot: A Personal Health Assistant for the Italian Language. IEEE Access 2020, 8, 107479–107497. [Google Scholar] [CrossRef]
- Ferrante, G.; Fasola, S.; Piazza, M.; Tenero, L.; Zaffanello, M.; La Grutta, S.; Piacentini, G. Vitamin D and Healthcare Service Utilization in Children: Insights from a Machine Learning Approach. J. Clin. Med. 2022, 11, 7157. [Google Scholar] [CrossRef]
- Awais, M.; Chiari, L.; Ihlen, E.; Helbostad, J.; Palmerini, L. Classical machine learning versus deep learning for the older adults free-living activity classification. Sensors 2021, 21, 4669. [Google Scholar] [CrossRef]
- Vaccari, I.; Orani, V.; Paglialonga, A.; Cambiaso, E.; Mongelli, M. A generative adversarial network (GAN) technique for internet of medical things data. Sensors 2021, 21, 3726. [Google Scholar] [CrossRef]
- Piccialli, F.; Cuomo, S.; Crisci, D.; Prezioso, E.; Mei, G. A deep learning approach for facility patient attendance prediction based on medical booking data. Sci. Rep. 2020, 10, 14623. [Google Scholar] [CrossRef] [PubMed]
- Gerussi, A.; Verda, D.; Cappadona, C.; Cristoferi, L.; Bernasconi, D.; Bottaro, S.; Carbone, M.; Muselli, M.; Invernizzi, P.; Asselta, R.; et al. LLM-PBC: Logic Learning Machine-Based Explainable Rules Accurately Stratify the Genetic Risk of Primary Biliary Cholangitis. J. Pers. Med. 2022, 12, 1587. [Google Scholar] [CrossRef]
- Kumar, V.; Recupero, D.; Riboni, D.; Helaoui, R. Ensembling Classical Machine Learning and Deep Learning Approaches for Morbidity Identification from Clinical Notes. IEEE Access 2021, 9, 7107–7126. [Google Scholar] [CrossRef]
- Stoianov, I.; Pennartz, C.; Lansink, C.; Pezzulo, G. Model-based spatial navigation in the hippocampus-ventral striatum circuit: A computational analysis. PLoS Comput. Biol. 2018, 14, e1006316. [Google Scholar] [CrossRef]
- Silvestri, S.; Gargiulo, F.; Ciampi, M. Iterative Annotation of Biomedical NER Corpora with Deep Neural Networks and Knowledge Bases. Appl. Sci. 2022, 12, 5775. [Google Scholar] [CrossRef]
- Piantadosi, G.; Sansone, M.; Fusco, R.; Sansone, C. Multi-planar 3D breast segmentation in MRI via deep convolutional neural networks. Artif. Intell. Med. 2020, 103, 101781. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, F.; Urgese, G.; Ficarra, E.; Di Cataldo, S. Dealing with lack of training data for convolutional neural networks: The case of digital pathology. Electronics 2019, 8, 256. [Google Scholar] [CrossRef]
- Mazzoleni, M.; Previdi, F.; Bonfiglio, N. Classification algorithms analysis for brain–computer interface in drug craving therapy. Biomed. Signal Process. Control 2019, 52, 463–472. [Google Scholar] [CrossRef]
- Merone, M.; Sansone, C.; Soda, P. A computer-aided diagnosis system for HEp-2 fluorescence intensity classification. Artif. Intell. Med. 2019, 97, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Son, L.; Ciaramella, A.; Thu Huyen, D.; Staiano, A.; Tuan, T.; Van Hai, P. Predictive reliability and validity of hospital cost analysis with dynamic neural network and genetic algorithm. Neural Comput. Appl. 2020, 32, 15237–15248. [Google Scholar] [CrossRef]
- de Farias, E.; di Noia, C.; Han, C.; Sala, E.; Castelli, M.; Rundo, L. Impact of GAN-based lesion-focused medical image super-resolution on the robustness of radiomic features. Sci. Rep. 2021, 11, 21361. [Google Scholar] [CrossRef]
- Ismail, W.; Hassan, M.; Alsalamah, H.; Fortino, G. CNN-based health model for regular health factors analysis in internet-of-medical things environment. IEEE Access 2020, 8, 52541–52549. [Google Scholar] [CrossRef]
- Chirikhina, E.; Chirikhin, A.; Dewsbury-Ennis, S.; Bianconi, F.; Xiao, P. Skin characterizations by using contact capacitive imaging and high-resolution ultrasound imaging with machine learning algorithms. Appl. Sci. 2021, 11, 8714. [Google Scholar] [CrossRef]
- Dipaola, F.; Gatti, M.; Pacetti, V.; Bottaccioli, A.; Shiffer, D.; Minonzio, M.; Menè, R.; Levra, A.; Solbiati, M.; Costantino, G.; et al. Artificial intelligence algorithms and natural language processing for the recognition of syncope patients on emergency department medical records. J. Clin. Med. 2019, 8, 1677. [Google Scholar] [CrossRef]
- Rizzo, S.; Savastano, A.; Lenkowicz, J.; Savastano, M.; Boldrini, L.; Bacherini, D.; Falsini, B.; Valentini, V. Artificial intelligence and oct angiography in full thickness macular hole. New developments for personalized medicine. Diagnostics 2021, 11, 2319. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.; Le, N.; Tran, T.; Ngo, V.; Nguyen, P. Detection of tuberculosis from chest X-ray images: Boosting the performance with vision transformer and transfer learning. Expert Syst. Appl. 2021, 184, 115519. [Google Scholar] [CrossRef]
- Europe—canceratlas.cancer.org. Available online: https://canceratlas.cancer.org/the-burden/europe/ (accessed on 19 June 2023).
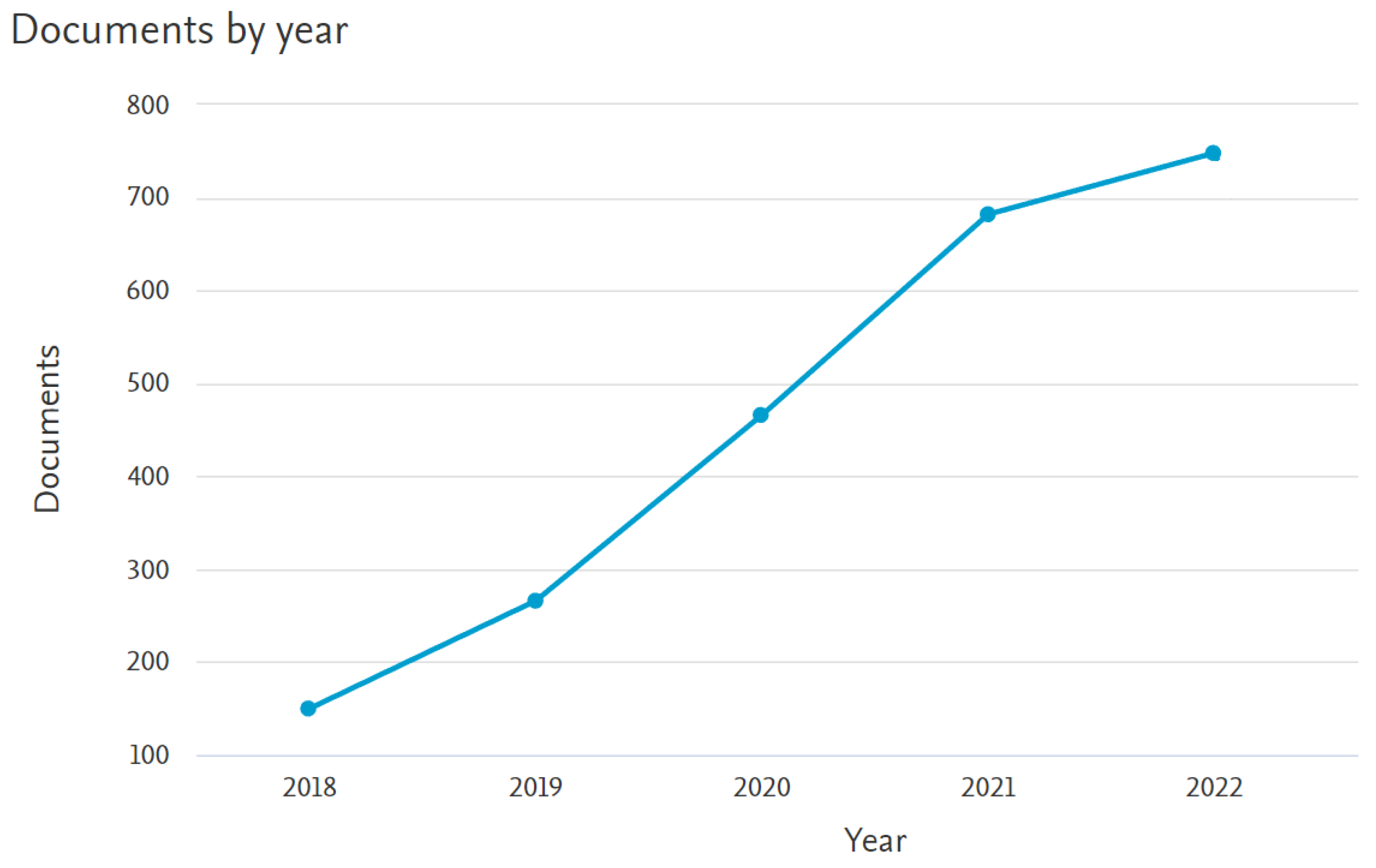
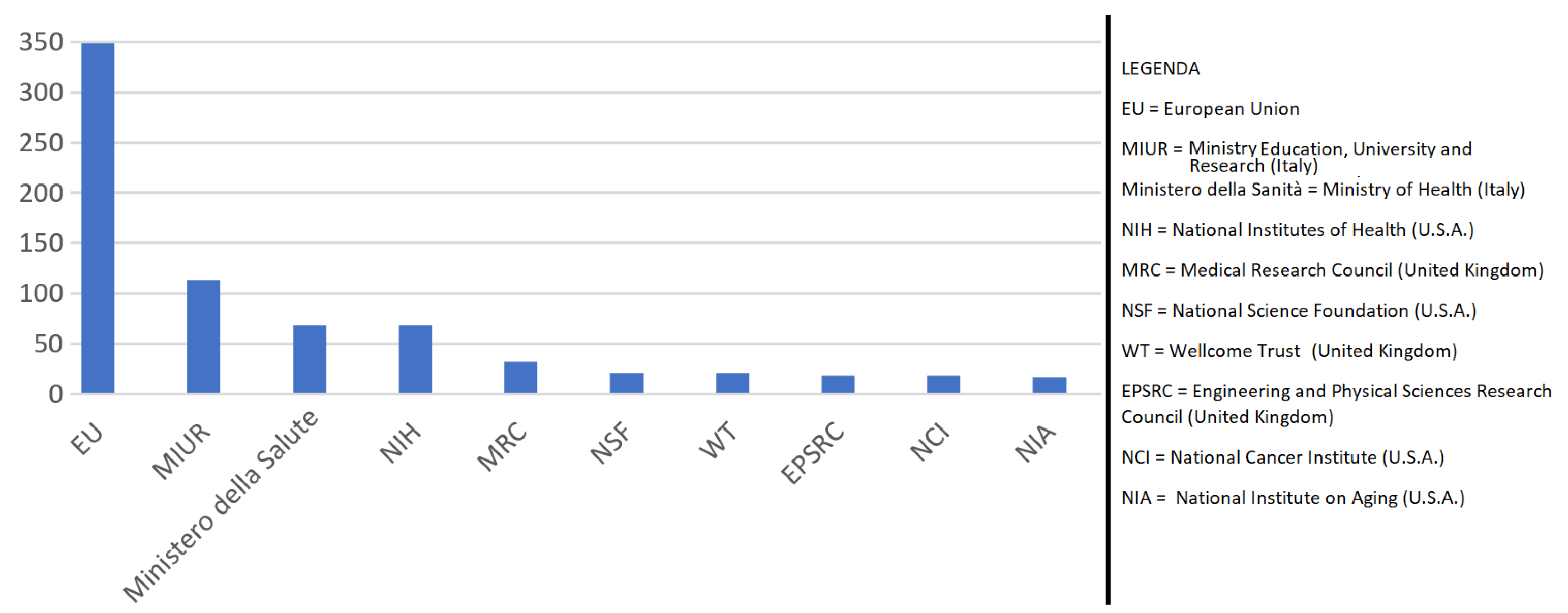
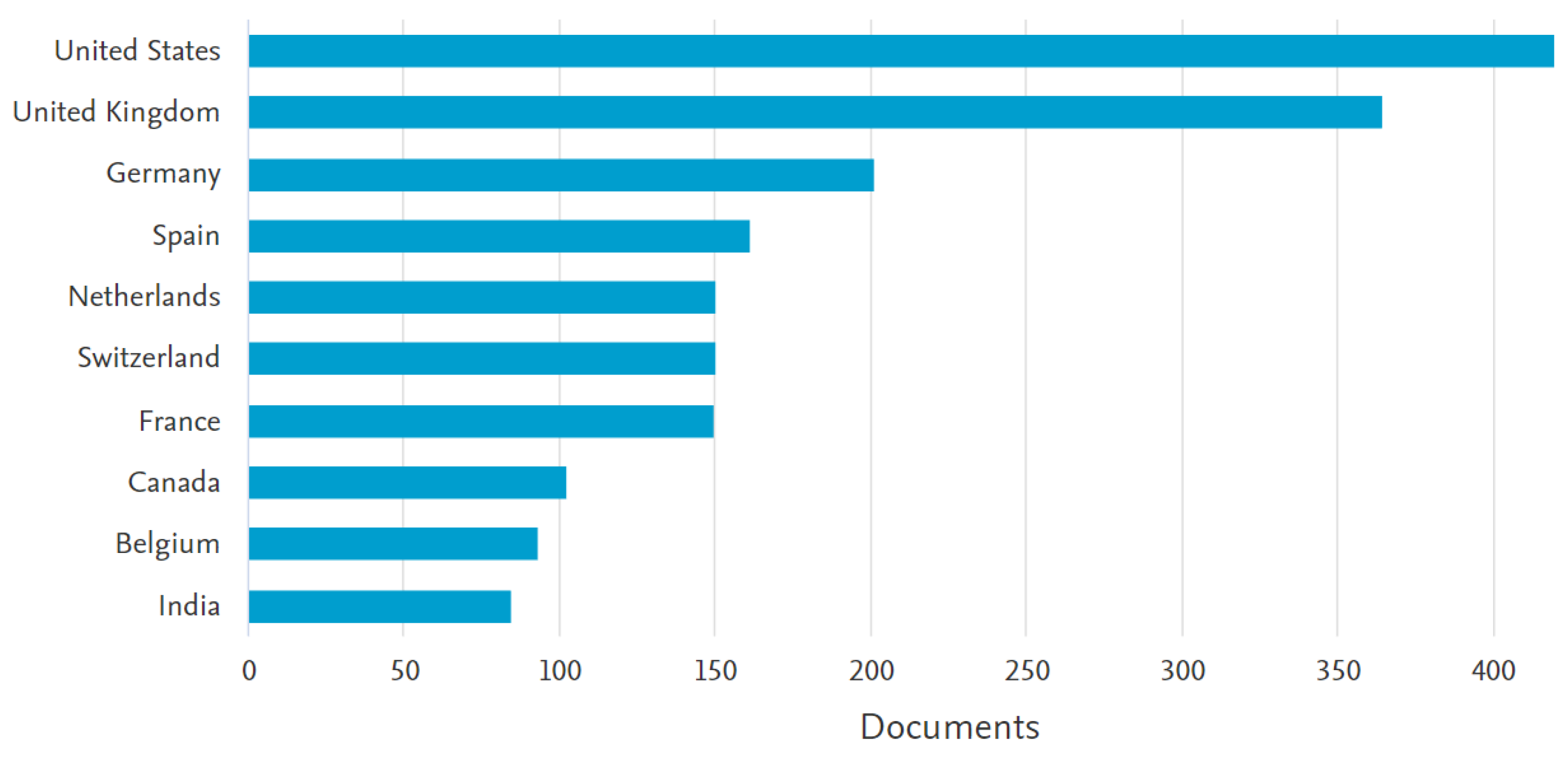
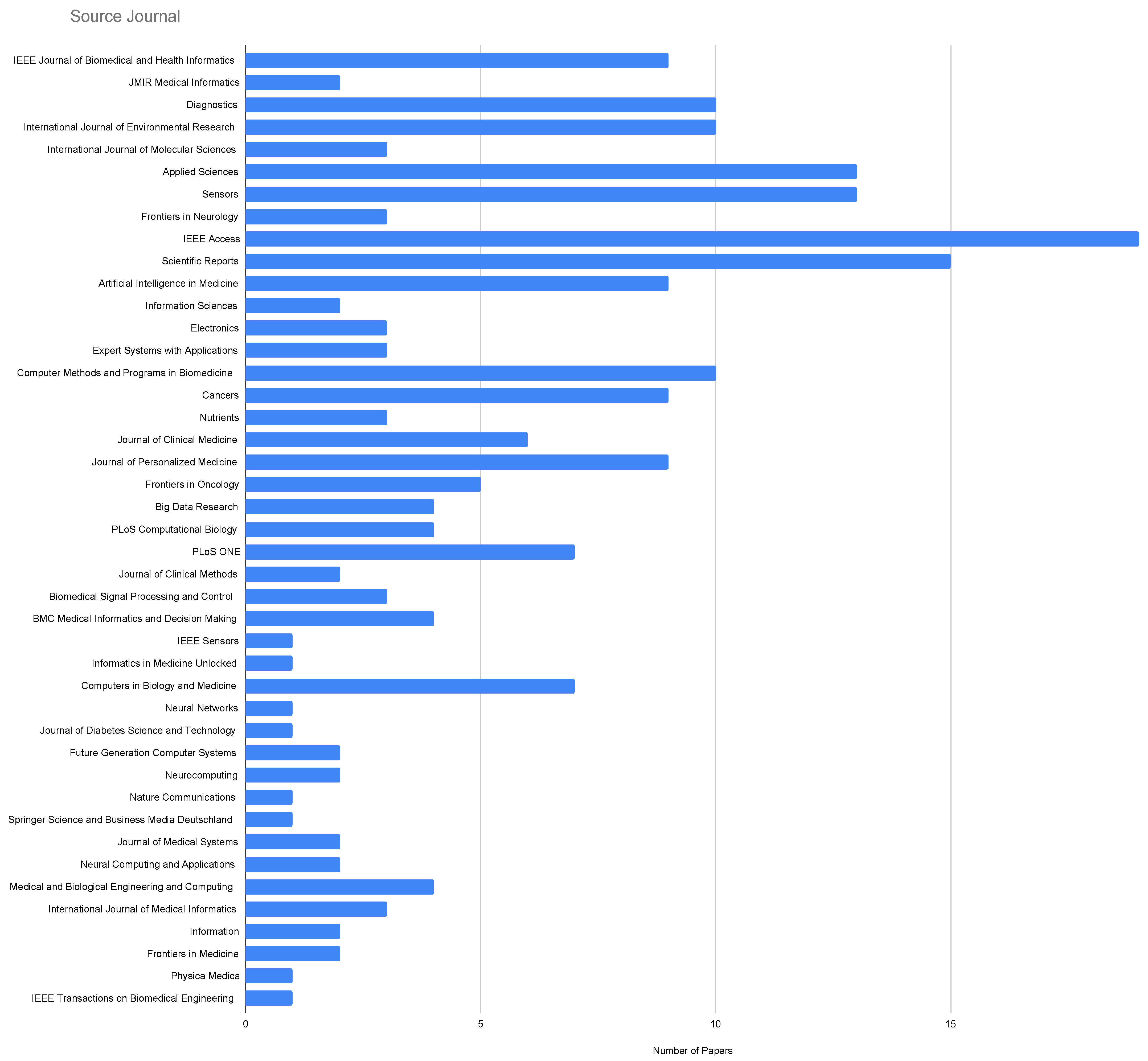
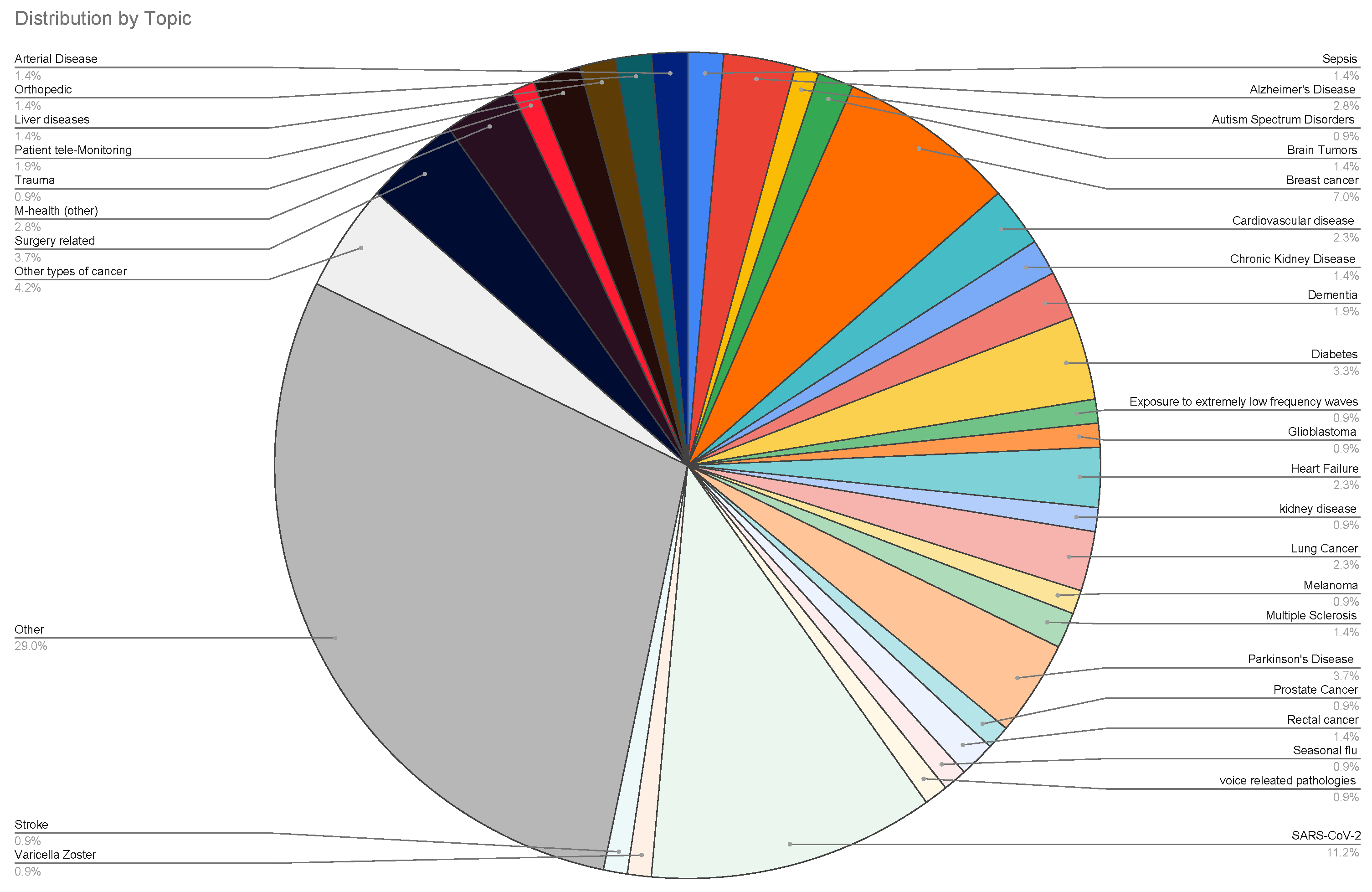
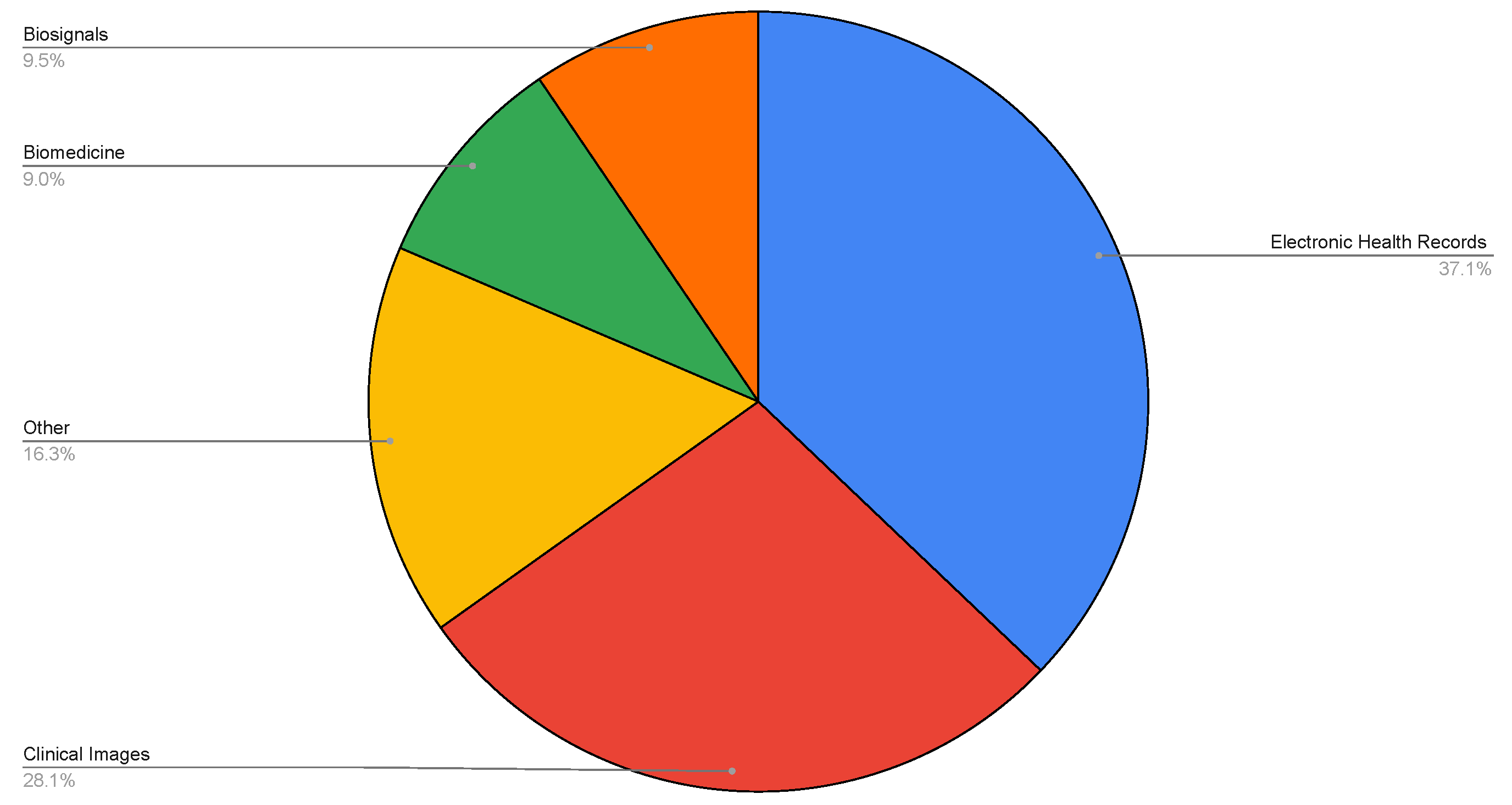
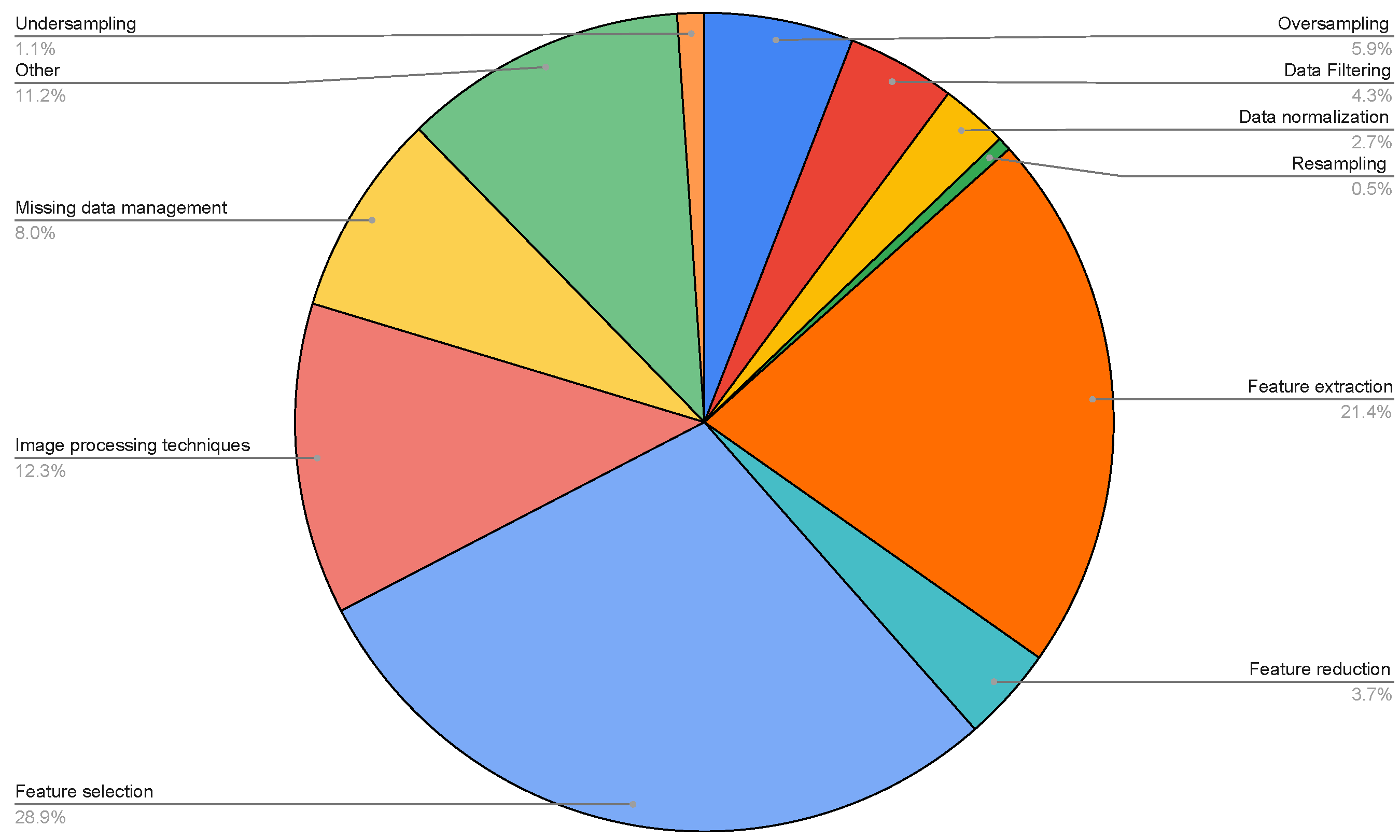
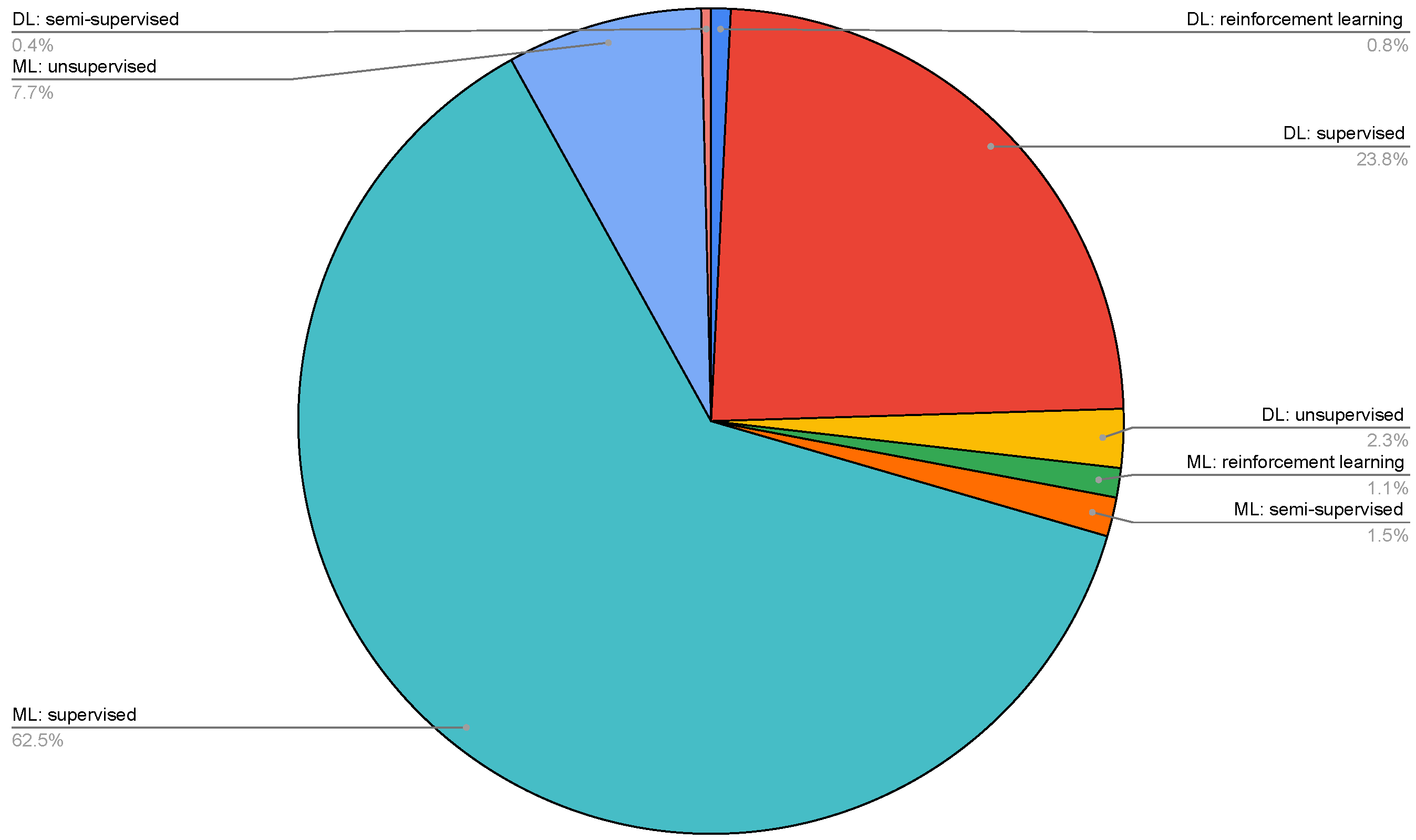
| Topic | Reference |
|---|---|
| Alzheimer’s disease | [35,36,37,38,39,40] |
| Autism Spectrum Disorders | [41,42] |
| Brain Tumors | [43,44,45] |
| Breast Cancer | [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] |
| Cardiovascular disease | [61,62,63,64,65] |
| Chronic Kidney Disease | [66,67,68] |
| Dementia | [69,70,71,72] |
| Diabetes | [73,74,75,76,77,78] |
| Exposure to extremely low frequency waves | [79,80] |
| Glioblastoma | [81,82] |
| Heart Failure | [83,84,85,86,87] |
| Kidney Disease | [88,89] |
| Lung Cancer | [90,91,92,93,94] |
| Melanoma | [95,96] |
| Multiple Sclerosis | [97,98,99] |
| Parkinson’s Disease | [100,101,102,103,104,105,106,107] |
| Prostate Cancer | [108,109] |
| Rectal Cancer | [110,111,112] |
| SARS-CoV-2 | [113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136] |
| Seasonal Flu | [137,138] |
| Sepsis | [139,140,141] |
| Stroke | [142,143] |
| Varicella Zoster | [144,145] |
| Voice-related Pathologies | [146,147] |
| Other Types of Cancer | [148,149,150,151,152,153,154,155,156] |
| Surgery-Related | [157,158,159,160,161,162,163,164] |
| M-health | [165,166,167,168,169,170] |
| Patient Telemonitoring | [171,172,173,174] |
| Liver Diseases | [175,176,177] |
| Orthopedic | [178,179,180] |
| Arterial Disease | [181,182,183] |
| Trauma | [184,185] |
| Other | [186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247] |
| Number of Data Types | Number of Papers |
|---|---|
| 1 | 176 |
| 2 | 32 |
| 3 | 6 |
| Number of Pre-Processing Methods | Number of Papers |
|---|---|
| 0 | 86 |
| 1 | 87 |
| 2 | 29 |
| 3 | 10 |
| 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottrighi, A.; Pennisi, M. Exploring the State of Machine Learning and Deep Learning in Medicine: A Survey of the Italian Research Community. Information 2023, 14, 513. https://doi.org/10.3390/info14090513
Bottrighi A, Pennisi M. Exploring the State of Machine Learning and Deep Learning in Medicine: A Survey of the Italian Research Community. Information. 2023; 14(9):513. https://doi.org/10.3390/info14090513
Chicago/Turabian StyleBottrighi, Alessio, and Marzio Pennisi. 2023. "Exploring the State of Machine Learning and Deep Learning in Medicine: A Survey of the Italian Research Community" Information 14, no. 9: 513. https://doi.org/10.3390/info14090513
APA StyleBottrighi, A., & Pennisi, M. (2023). Exploring the State of Machine Learning and Deep Learning in Medicine: A Survey of the Italian Research Community. Information, 14(9), 513. https://doi.org/10.3390/info14090513






