Low Power EEG Data Encoding for Brain Neurostimulation Implants †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. EEG Data
2.1.2. Encoding Algorithm
2.1.3. Bluetooth Low Energy
2.2. Design Methodology
2.2.1. System Architecture
2.2.2. System Memory
2.2.3. Modelling in MATLAB
|
clear all; close all; clc; %% reading the eeg from edf file file=‘D:\chb01_03.edf’; [record,hdr]=readEDF(file); %% selection of 4 channels %%with duration of 90 s (256 ∗ 90=23,040 samples) channel_1= record(21,23040:46080); channel_2= record(22,23040:46080); channel_3= record(23,23040:46080); channel_4= record(20,23040:46080); %% decimal to binary bin_ch_1=[]; bin_ch_2=[]; bin_ch_3=[]; bin_ch_4=[]; for cols=1:length(channel_1) out=[]; out= dec_2_bin(channel_1(cols)); bin_ch_1= [bin_ch_1; out’]; out= dec_2_bin(channel_2(cols)); bin_ch_2= [bin_ch_2; out’]; out= dec_2_bin(channel_3(cols)); bin_ch_3= [bin_ch_3; out’]; out= dec_2_bin(channel_4(cols)); bin_ch_4= [bin_ch_4; out’]; end bin_ch_1=bin_ch_1’; bin_ch_2=bin_ch_2’; bin_ch_3=bin_ch_3’; bin_ch_4=bin_ch_4’; %% count 0-1 transitions count1_z_2_o=zeros_2_ones(bin_ch_1) count2_z_2_o=zeros_2_ones(bin_ch_2) count3_z_2_o=zeros_2_ones(bin_ch_3) count4_z_2_o=zeros_2_ones(bin_ch_4) |
2.2.4. Buffer Implementation in VHDL
|
FIFO_IMPL: process (clk) is begin if clk’event and clk=‘1’and clk’last_value=‘0’ then if rst = ‘1’ then count <= 0; input <= 0; output <= 0; data_read <= (others => ’0’); else --reduction of count when a value is being read if (en_r = ’1’) then count <= count − 1; end if; --writting process if (en_w = ’1’ and full_i = ’0’) then if input= RAM_L-1 then input<= 0; else input <= (input + 1); end if; ram(input) <= data; count <= count + 1; end if; --reading pointer update if (en_r = ’1’ and empty_i = ’0’) then if output = RAM_L-1 then output <= 0; else data_read <= ram(output); output <= output + 1; end if; end if; end if; end if; end process FIFO_IMPL; full_i <= ’1’ when count= RAM_L else ’0’; empty_i <= ’1’ when count = 0 else ’0’; full <= full_i; empty <= empty_i; |
2.2.5. Data Transmission with BLE
|
void updatevalueLevel() { byte b1,b2,b3,b4; int num1,num2,num3,num4; if (!buff.isFull()){ for (int i=0;i<64;i++){ if (Serial.available()) { combined.parts.firstByte = Serial.read(); while(Serial.available()==0){ } combined.parts.secondByte = Serial.read(); while(Serial.available()==0){ } combined.parts.thirdByte = Serial.read(); while(Serial.available()==0){ } combined.parts.fourthByte = Serial.read(); // combine all the bytes // to create the long integer combined.merged = ((unsigned int) (combined.parts.firstByte)<<24)| (unsigned int) (combined.parts.secondByte<<16)| (unsigned int) (combined.parts.thirdByte<<8)| (unsigned int) (combined.parts.fourthByte); // delta encoding cur_val= combined.merged; delta = cur_val-prev_val; buff.push(delta); prev_val=cur_val; length_buff++; } } |
3. Results
- A0: The channels being studied.
- A1: Transitions from 0 to 1 prior to Delta coding (calculated by multiplying all transitions).
- A2: The transitions from 0 to 1 following Delta coding (estimated by multiplying the total transitions by weight). Comparison results are given in Figure 3.
- A3: Total number of transitions prior to coding ((0-0) + (1-1) + (1-0) + 5 * (0-1)).
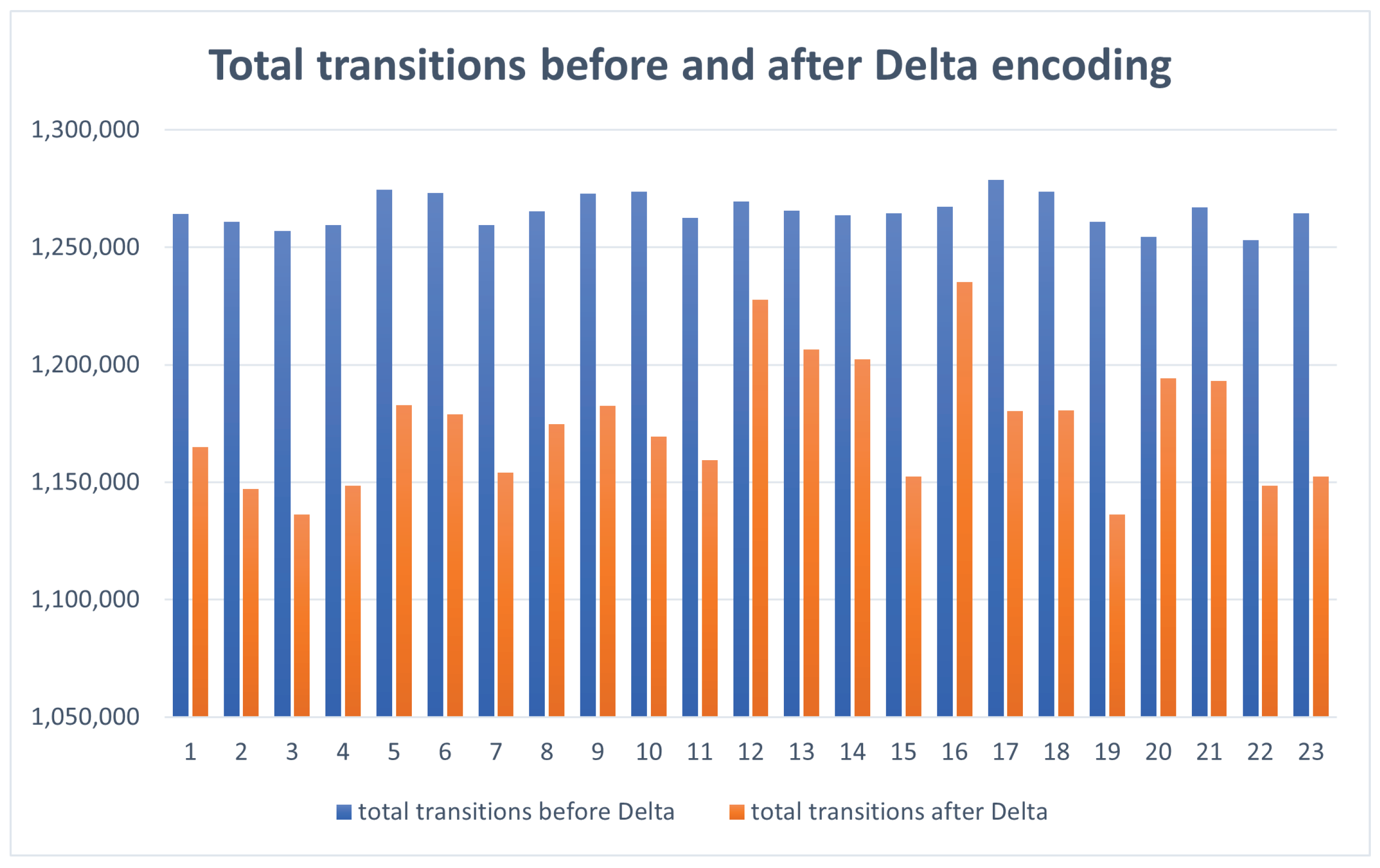
- A4: The total number of transitions following coding ((0-0) + (1-1) + (1-0) + 5 * (0-1)). Comparison results are given in Figure 4.
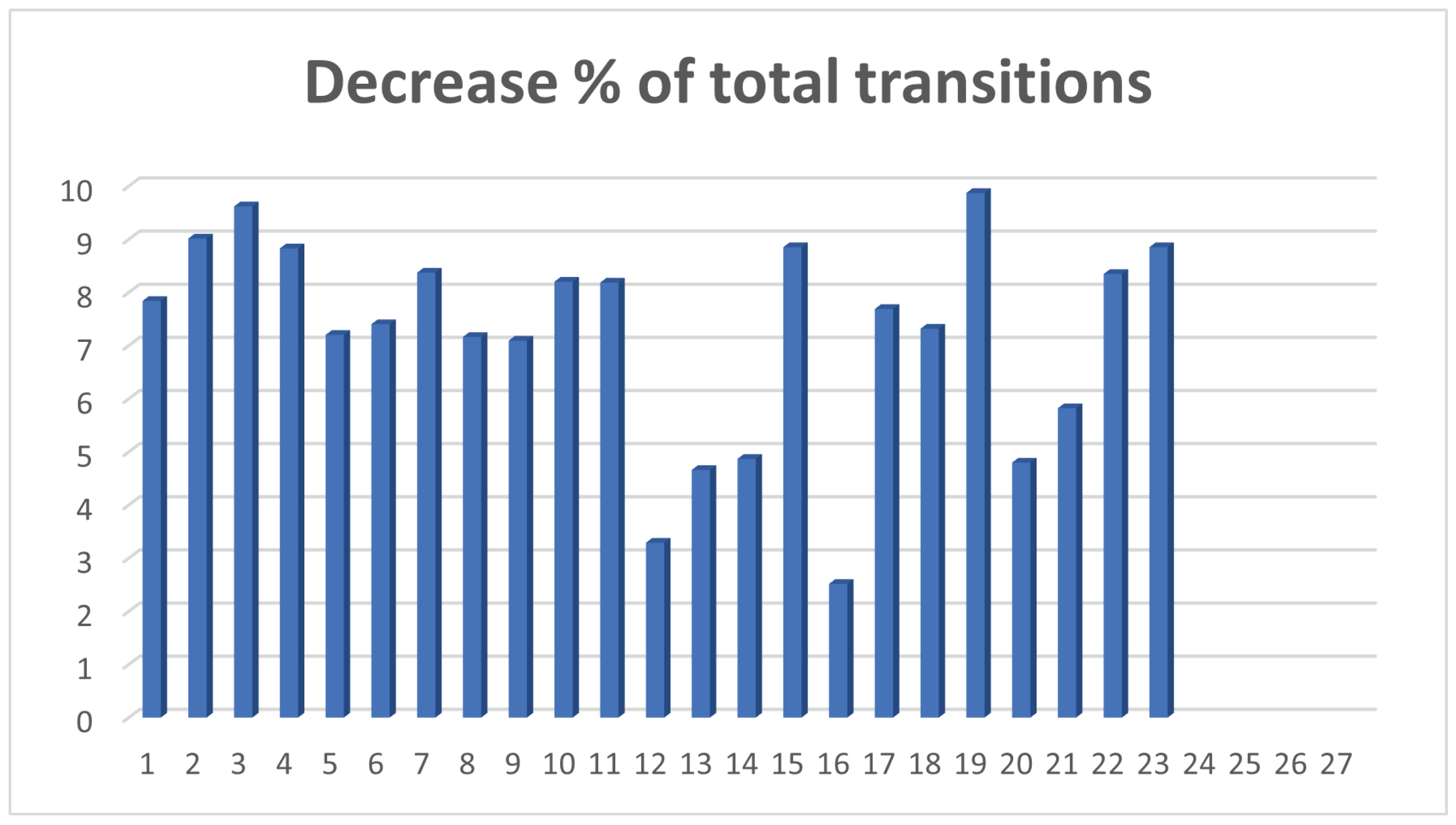
- A5: The percentage of reduction of total transitions.
- A6: The percentage of reduction of transitions 0-1.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RNS | Responsive Neurostimulation |
| EEG | Electroencephalography |
| FDA | Food and Drug Administration |
| DBS | Deep Brain Stimulation |
| LZ77 | Lempel Ziv 77 |
| LZ78 | Lempel Ziv 78 |
| CS | Compressive Sensing |
| MCEEG | Multichannel EEG |
| RLE | Run Length Encoding |
| FIFO | First-in-First-out |
| BLE | Bluetooth Low Energy |
References
- Dalkilic, E.B. Neurostimulation devices used in treatment of epilepsy. Curr. Treat. Options Neurol. 2017, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Sisterson, N.D.; Wozny, T.A.; Kokkinos, V.; Constantino, A.; Richardson, R.M. Closed-loop brain stimulation for drug-resistant epilepsy: Towards an evidence-based approach to personalized medicine. Neurotherapeutics 2019, 16, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, J.W.; Sillanpaa, M. The natural history and prognosis of epilepsy. In Epilepsy: A Comprehensive Textbook; Engel, P., Pedley, T., Eds.; Raven Press: New York, NY, USA, 1998; pp. 69–86. [Google Scholar]
- Velasco, F.; Saucedo-Alvarado, P.E.; Vazquez-Barron, D.; Trejo, D.; Velasco, A.L. Deep brain stimulation for refractory temporal lobe epilepsy. Current status and future trends. Front. Neurol. 2022, 13, 796846. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Velasco, F.; Velasco, A.L.; Boleaga, B.; Jimenez, F.; Brito, F.; Marquez, I. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures paroxysmal activities. Epilepsia 2000, 41, 158–163. [Google Scholar] [CrossRef]
- Boon, P.; Vonck, K.; De Herdt, V.; Van Dycke, A.; Goethals, M.; Goossens, L.; Van Zandijcke, M.; De Smedt, T.; Dewaele, I.; Achten, R.; et al. Deep Brain Stimulation in patients with refractory temporal lobe epilepsy. Epilepsia 2007, 48, 1551–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentín, A.; García Navarrete, E.; Chelvarajah, R.; Torres, C.; Navas, M.; Vico, L.; Torres, N.; Pastor, J.; Selway, R.; Sola, R.G.; et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 2013, 54, 1823–1833. [Google Scholar] [CrossRef]
- Gregg, N.M.; Marks, V.S.; Sladky, V.; Lundstrom, B.N.; Klassen, B.; Messina, S.A.; Brinkmann, B.H.; Miller, K.J.; Van Gompel, J.J.; Kremen, V.; et al. Anterior nucleus of the thalamus seizure detection in ambulatory humans. Epilepsia 2021, 62, e158–e164. [Google Scholar] [CrossRef]
- Sun, F.T.; Morrell, M.J.; Wharen, R.E. Responsive Cortical Stimulation for the Treatment of Epilepsy. Neurotherapeutics 2008, 5, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Kossoff, E.H.; Ritzl, E.K.; Politsky, J.M.; Murro, A.M.; Smith, J.R.; Duckrow, R.B.; Spencer, D.D.; Bergey, G.K. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia 2004, 45, 1560–1567. [Google Scholar] [CrossRef]
- Sisterson, N.D.; Kokkinos, V. Neuromodulation of Epilepsy Networks. Neurosurg. Clin. N. Am. 2020, 31, 459–470. [Google Scholar] [CrossRef]
- Zawar, I.; Krishnan, B.; Mackow, M.; Alexopoulos, A.; Nair, D.; Punia, V. The Efficacy, Safety, and Outcomes of Brain-responsive Neurostimulation (RNS System) therapy in older adults. Epilepsia Open 2021, 6, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Skarpaas, T.L.; Morrell, M.J. Intracranial Stimulation Therapy for Epilepsy. Neurotherapeutics 2009, 6, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NeuroPace® Patient Data Management System User Manual Model 4340; NeuroPace: Mountain View, CA, USA, 2014; pp. 1–34.
- Youngerman, B.E.; Mahajan, U.V.; Dyster, T.G.; Srinivasan, S.; Halpern, C.H.; McKhann, G.M.; Sheth, S.A. Cost-effectiveness analysis of responsive neurostimulation for drug-resistant focal onset epilepsy. Epilepsia 2021, 62, 2804–2813. [Google Scholar] [CrossRef]
- Fragkou, A.A.; Kakarountas, A.P.; Kokkinos, V. Low-power electroencephalographic data encoding system for implantable brain stimulation systems. In Proceedings of the 2021 6th South-East Europe Design Automation, Computer Engineering, Computer Networks and Social Media Conference (SEEDA-CECNSM), Preveza, Greece, 24–26 September 2021; pp. 1–5. [Google Scholar]
- Moody, G.B.; Mark, R.G.; Goldberger, A.L. PhysioNet: Physiologic signals, time series and related open source software for basic, clinical, and applied research. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 8327–8330. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Information; University of Illinois Press: Champaign, IL, USA, 1949; Volume 97. [Google Scholar]
- Fano, R.M. The Transmission of Information; Massachusetts Institute of Technology, Research Laboratory of Electronics: Cambridge, MA, USA, 1949. [Google Scholar]
- Huffman, D.A. A method for the construction of minimum-redundancy codes. Proc. IRE 1952, 40, 1098–1101. [Google Scholar] [CrossRef]
- Rigler, S.; Bishop, W.; Kennings, A. FPGA-based lossless data compression using Huffman and LZ77 algorithms. In Proceedings of the 2007 Canadian Conference on Electrical and Computer Engineering, Vancouver, BC, Canada, 22–26 April 2007; pp. 1235–1238. [Google Scholar]
- Freschi, V.; Bogliolo, A. A faster algorithm for the computation of string convolutions using LZ78 parsing. Inf. Process. Lett. 2010, 110, 609–613. [Google Scholar] [CrossRef]
- Yan-li, Z.; Xiao-ping, F.; Shao-qiang, L.; Zhe-yuan, X. Improved LZW algorithm of lossless data compression for WSN. In Proceedings of the 2010 3rd International Conference on Computer Science and Information Technology, Chengdu, China, 9–11 July 2010; pp. 523–527. [Google Scholar]
- Zhang, Z.; Jung, T.P.; Makeig, S.; Rao, B.D. Compressed sensing of EEG for wireless telemonitoring with low energy consumption and inexpensive hardware. IEEE Trans. Biomed. Eng. 2012, 60, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Gurve, D.; Delisle-Rodriguez, D.; Bastos-Filho, T.; Krishnan, S. Trends in compressive sensing for EEG signal processing applications. Sensors 2020, 20, 3703. [Google Scholar] [CrossRef]
- Sharma, S.; Chopra, A. The Study: LZW Compression on SEP Protocol.
- Kim, S.; Kim, J.; Chun, H.W. Wave2vec: Vectorizing electroencephalography bio-signal for prediction of brain disease. Int. J. Environ. Res. Public Health 2018, 15, 1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, B.D.; Karipott, S.S.; Wang, Y.; Ong, K.G. Wireless technologies for implantable devices. Sensors 2020, 20, 4604. [Google Scholar] [CrossRef]
- Fisher, R.; Salanova, V.; Witt, T.; Worth, R.; Henry, T.; Gross, R.; Oommen, K.; Osorio, I.; Nazzaro, J.; Labar, D.; et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010, 51, 899–908. [Google Scholar] [CrossRef]
- Jobst, B.C.; Kapur, R.; Barkley, G.L.; Bazil, C.W.; Berg, M.J.; Bergey, G.K.; Boggs, J.G.; Cash, S.S.; Cole, A.J.; Duchowny, M.S. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia 2017, 58, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Geller, E.B.; Skarpaas, T.L.; Gross, R.E.; Goodman, R.R.; Barkley, G.L.; Bazil, C.W.; Berg, M.J.; Bergey, G.K.; Cash, S.S.; Cole, A.J.; et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia 2017, 58, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.R.; Laxer, K.D.; Weber, P.B.; Murro, A.M.; Park, Y.D.; Barkley, G.L.; Smith, B.J.; Gwinn, R.P.; Doherty, M.J.; Noe, K.H. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 2020, 95, e1244–e1256. [Google Scholar] [CrossRef] [PubMed]
- Stacey, W.C.; Litt, B. Technology insight: Neuroengineering and epilepsy-designing devices for seizure control. Nat. Clin. Pract. Neurol. 2008, 4, 190–201. [Google Scholar] [CrossRef]
- Wong, S.; Mani, R.; Danish, S. Comparison and Selection of Current Implantable Anti-Epileptic Devices. Neurotherapeutics 2019, 16, 369–380. [Google Scholar] [CrossRef]




| A0 | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|
| 1 | 673,220 | 549,515 | 1,264,096 | 1,164,980 | 7.8409 | 18.3751 |
| 2 | 669,515 | 527,270 | 1,260,845 | 1,147,182 | 9.0148 | 21.246 |
| 3 | 664,420 | 513,600 | 1,257,039 | 1,136,120 | 9.6194 | 22.6995 |
| 4 | 667,430 | 528,945 | 1,259,597 | 1,148,392 | 8.8286 | 20.749 |
| 5 | 686,040 | 571,775 | 1,274,460 | 1,182,678 | 7.2016 | 16.6557 |
| 6 | 684,600 | 567,005 | 1,273,248 | 1,178,986 | 7.4033 | 17.1772 |
| 7 | 667,360 | 535,950 | 1,259,546 | 1,154,084 | 8.373 | 19.691 |
| 8 | 674,605 | 561,470 | 1,265,401 | 1,174,766 | 7.1626 | 16.7706 |
| 9 | 684,020 | 571,605 | 1,272,830 | 1,182,544 | 7.0933 | 16.4345 |
| 10 | 685,315 | 554,900 | 1,273,815 | 1,169,322 | 8.2032 | 19.0299 |
| 11 | 671,190 | 542,465 | 1,262,620 | 1,159,265 | 8.1858 | 8.1858 |
| 12 | 679,765 | 627,555 | 1,269,519 | 1,227,736 | 3.2912 | 7.6806 |
| 13 | 674,980 | 601,435 | 1,265,526 | 1,206,553 | 4.66 | 10.8959 |
| 14 | 672,665 | 595,725 | 1,263,714 | 1,202,193 | 4.8683 | 11.4381 |
| 15 | 673,250 | 534,130 | 1,264,391 | 1,152,470 | 8.8518 | 20.6639 |
| 16 | 676,825 | 636,815 | 1,267,159 | 1,235,278 | 2.5159 | 5.9114 |
| 17 | 691,235 | 568,575 | 1,278,594 | 1,180,287 | 7.6887 | 17.7451 |
| 18 | 685,225 | 568,765 | 1,273,732 | 1,180,522 | 7.3179 | 16.9959 |
| 19 | 668,810 | 513,760 | 1,260,744 | 1,136,281 | 9.8722 | 23.183 |
| 20 | 661,190 | 586,285 | 1,254,508 | 1,194,316 | 4.7981 | 11.3288 |
| 21 | 676,470 | 584,705 | 1,266,865 | 1,193,110 | 5.8219 | 13.5653 |
| 22 | 659,300 | 528,790 | 1,253,064 | 1,148,467 | 8.3473 | 19.7952 |
| 23 | 673,250 | 534,130 | 1,264,391 | 1,152,470 | 8.8518 | 20.6639 |
| I.I. | Encoder | Memory | Compressor | BLE | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FF | LUT (%) | FF | DSP (%) | BRAM (%) | Power (W) | BRAM (%) | Power (W) | LUT (%) | FF | DSP (%) | BRAM (%) | Power (W) | Power (W) | Power (W) | |
| 1 | 32 | - | - | - | - | - | 40 | 0.072 | - | - | - | - | - | 0.18 | 0.252 |
| 2 | 32 | - | - | - | - | - | 40 | 0.072 | 42 | 20 | 7 | 30 | 0.221 | 0.14 | 0.433 |
| 3 | 32 | 16 | 32 | 0 | 0 | 0.004 | 40 | 0.072 | - | - | - | - | - | 0.16 | 0.236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragkou, A.; Kakarountas, A.; Kokkinos, V. Low Power EEG Data Encoding for Brain Neurostimulation Implants. Information 2022, 13, 194. https://doi.org/10.3390/info13040194
Fragkou A, Kakarountas A, Kokkinos V. Low Power EEG Data Encoding for Brain Neurostimulation Implants. Information. 2022; 13(4):194. https://doi.org/10.3390/info13040194
Chicago/Turabian StyleFragkou, Aikaterini, Athanasios Kakarountas, and Vasileios Kokkinos. 2022. "Low Power EEG Data Encoding for Brain Neurostimulation Implants" Information 13, no. 4: 194. https://doi.org/10.3390/info13040194
APA StyleFragkou, A., Kakarountas, A., & Kokkinos, V. (2022). Low Power EEG Data Encoding for Brain Neurostimulation Implants. Information, 13(4), 194. https://doi.org/10.3390/info13040194








