2.1. Thermal Treatment to Obtain Ice XVII
The starting sample is constituted by an H-filled ice in the C-phase, that is, the stable phase at high pressure (about 4 kbar) and low temperature (about 255 K) of the binary system H-HO. The new form of solid water, namely ice XVII, has been realized by exposing the pristine sample to an annealing treatment, which is performed by keeping the sample under dynamic vacuum at a temperature in the range 110–120 K. The whole treatment is done in the Raman optical cell, thus measuring the Raman spectrum of the sample during the annealing process. We had previously verified that the C-structure transforms into ice, probably Ih, at about 130 K with a fast kinetic, hence the annealing temperature is set prudentially at 120 K and the sample is kept under dynamic vacuum for about one hour.
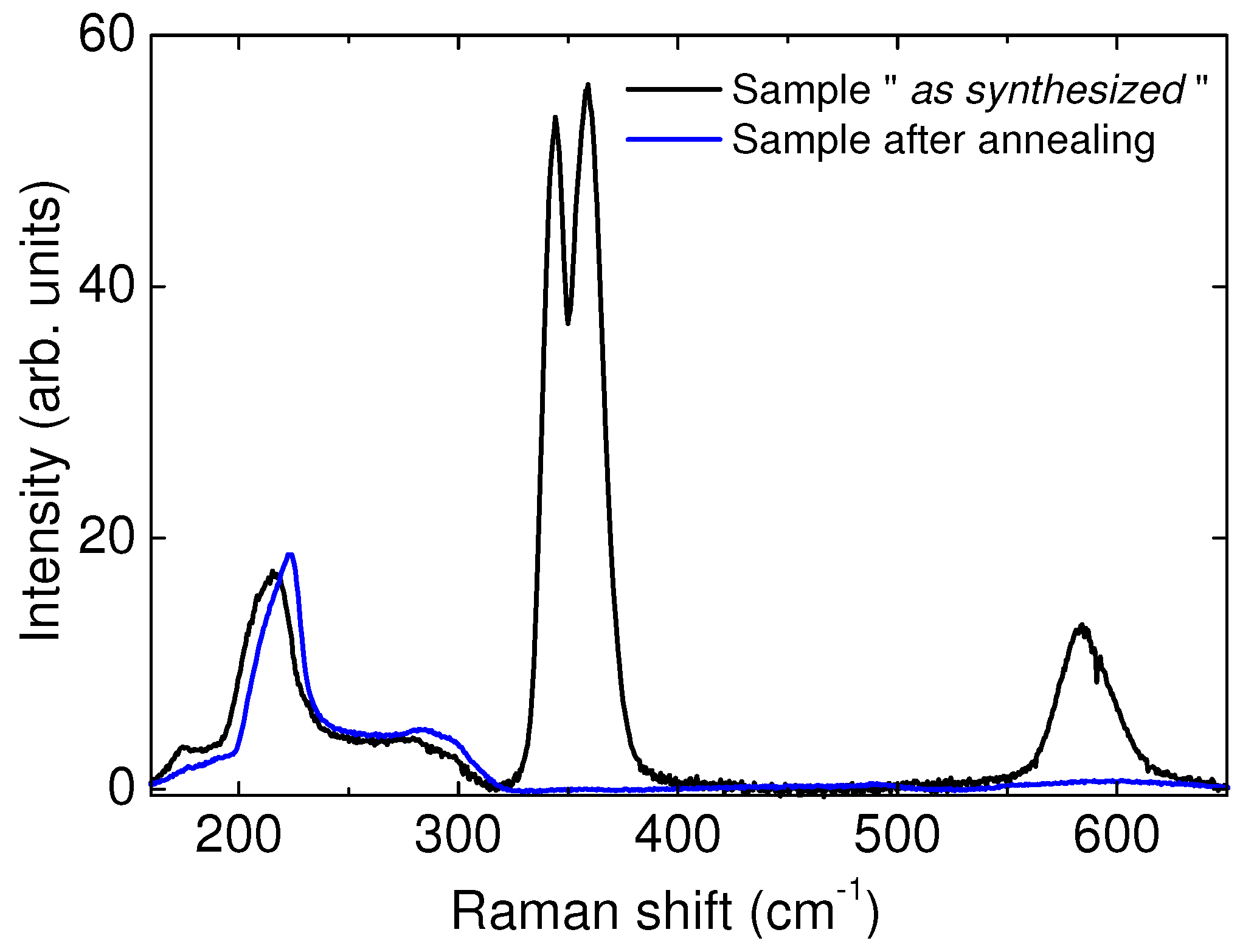
As is shown in
Figure 1, the spectrum of the pristine sample in the region 150–650 cm
contains both the H
O lattice band (from ≃150 to ≃300 cm
) and the two H
rotational bands S
(0) and S
(1) (≃350 and ≃580 cm
, respectively). The shape of the rotational bands is characteristic of the hydrogen molecule experiencing a relatively strong interaction with the host water framework [
19], and is very different from the one observed in the gas. This demonstrates the presence of hydrogen molecules inside the host framework. During the annealing treatment, the lattice band remains similar to the one measured before the treatment, but a gradual decrease of the rotational band intensities is observed, up to their complete undetectability. From these data, it is possible to ascertain that there is a complete release of the hydrogen from the sample, while no evident structural phase transition occurs.
It has been demonstrated that nitrogen molecules can also be trapped in these filled ice samples, as spurious guests [
20], and our Raman measurements have confirmed this. Probably, this is due to the use of liquid nitrogen during the transfer process of the samples from the autoclave (or Dewar container) to the sample cell and for the cryo-conservation of the sample. After the annealing process, the N
Raman line disappears, demonstrating a complete evacuation of the sample also by the N
guest impurity. The nature of the effect of the annealing on the structure of the samples in the C
-phase is due to the combined effect of the removal of both H
and N
from the sample and to the decrease of defects in the structure. The emptied C
-structure has been considered a new form of metastable ice called
Ice XVII, according to the current nomenclature of ice polymorphisms [
16,
17]. The overall similarity of the host Raman band shape before and after the annealing treatment [
16] and the structure refinement of ice XVII by means of neutron diffraction [
17] led us to state that the lattice structures of the C
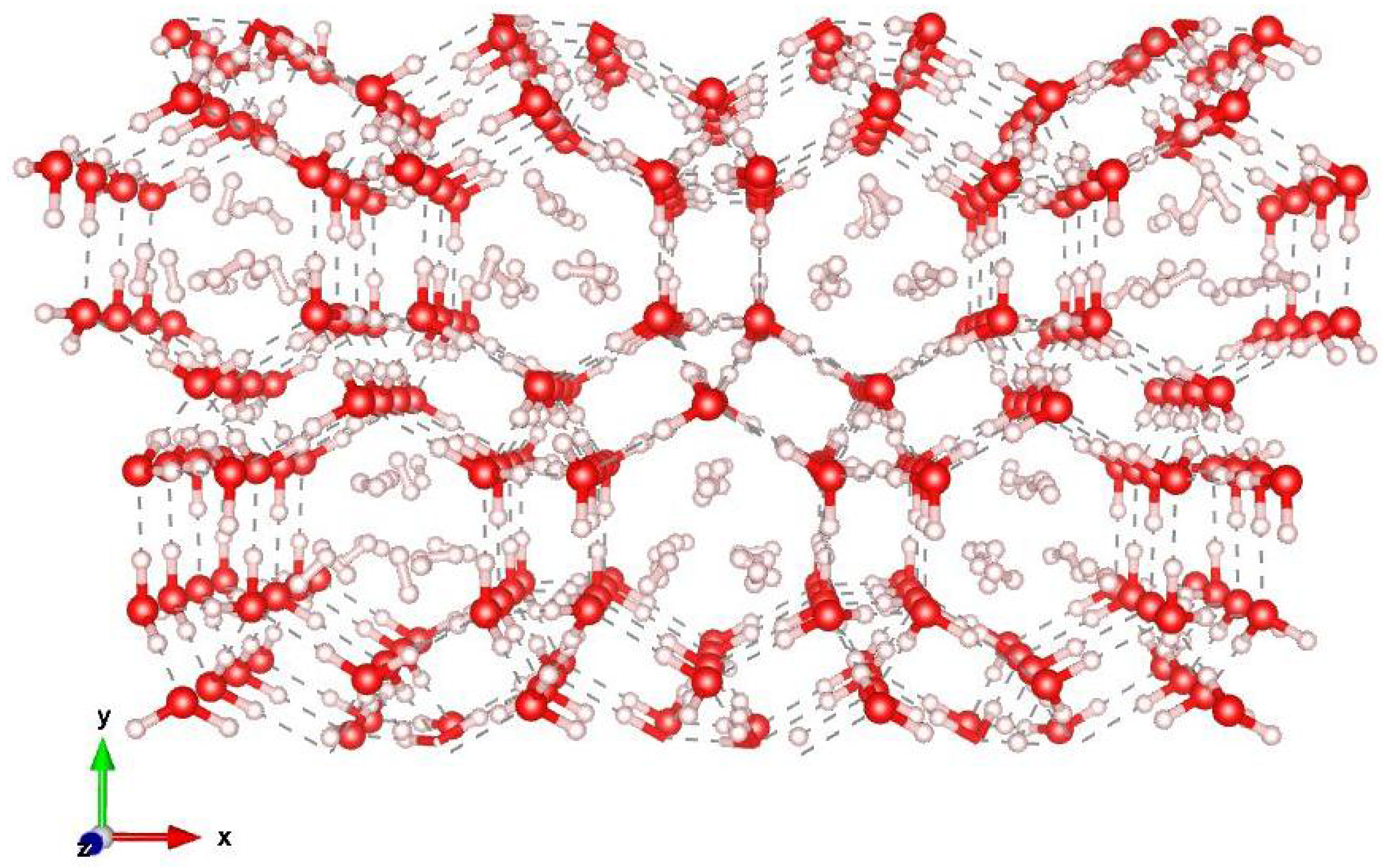
-phase and that of ice XVII are very similar. The water molecules are arranged forming pentagonal rings and giving rise to empty channels, having a diameter of about 6 Å, where the H
molecules can be accommodated, probably in a spiralling configuration. A picture of the arrangements of the water molecules is shown in
Figure 2, where one can observe the existence of channels along the
z-axis, which can host the hydrogen molecules.
2.2. Porosity of Ice XVII
An interesting property of ice XVII is its capability of a very rapid adsorption of hydrogen molecules when exposed to this gas even at a very low pressure (lower than atmospheric one at a temperature below 40 K) [
16]. The fast diffusion of hydrogen in and out of ice XVII makes this system more similar to MOFs than to hydrogen clathrate hydrates, which can efficiently release hydrogen only with a melting process. The amount of hydrogen that is trapped can be deduced measuring the intensity of the rotational Raman bands, which is proportional to the number of adsorbed molecules. The shape of the rotational lines does not change significantly during the hydrogen refilling, demonstrating that the H
molecules penetrate again in the same positions inside the channels as those initially occupied at the time of the C
-phase synthesis.
From the intensity of the Raman bands, it is possible to obtain a quantitative measurement of the hydrogen content in the hydrate sample, which we express in terms of hydrogen molar fraction
X, i.e., number of H
moles per H
O mole. We can obtain
X from the intensity ratio of the hydrogen rotational lines
and the lattice phonon band
where
f is a calibration factor, and
and
are the Raman cross sections for the
and
rotational lines having intensities
and
, respectively. In this way,
is proportional to the number of H
molecules in the lowest two
J = 0 and
J = 1 rotational states, that are, at low temperature, the sole rotational states with a non-negligible population. The quantity obtained by the ratio of
and
is independent of laser intensity and detector efficiency, and proportional to the H
/H
O molar ratio in the sample, under the hypothesis that the intensities of the bands are proportional to the number of molecules giving rise to them [
22].
The calibration factor
f, that allows for converting the weighted intensity ratio into a hydrogen molar fraction, is calculated by fitting data arising from two independent experimental methods based on our previous Raman results relative to hydrogen sII clathrate [
22] and on volumetric measurements, performed with the apparatus connected to the Raman optical cell, of the gas adsorbed during refilling of the ice XVII sample at different temperatures. A detailed description of this Raman intensity calibration is reported in Ref. [
16].
The C-phase filled ice samples, examined just after the synthesis, are characterized by %, while for the refilled ice XVII in some instances, the measured X value exceeds 40%, reaching higher values than the established theoretical maximum for sII clathrates %. The lower hydrogen content in the pristine samples compared to the refilled ones can be ascribed to the initial presence of nitrogen molecules in the sample that partially fill the channel structure, as well as to unwanted warming of the sample, with consequent H release, during the loading of Raman optical cells.
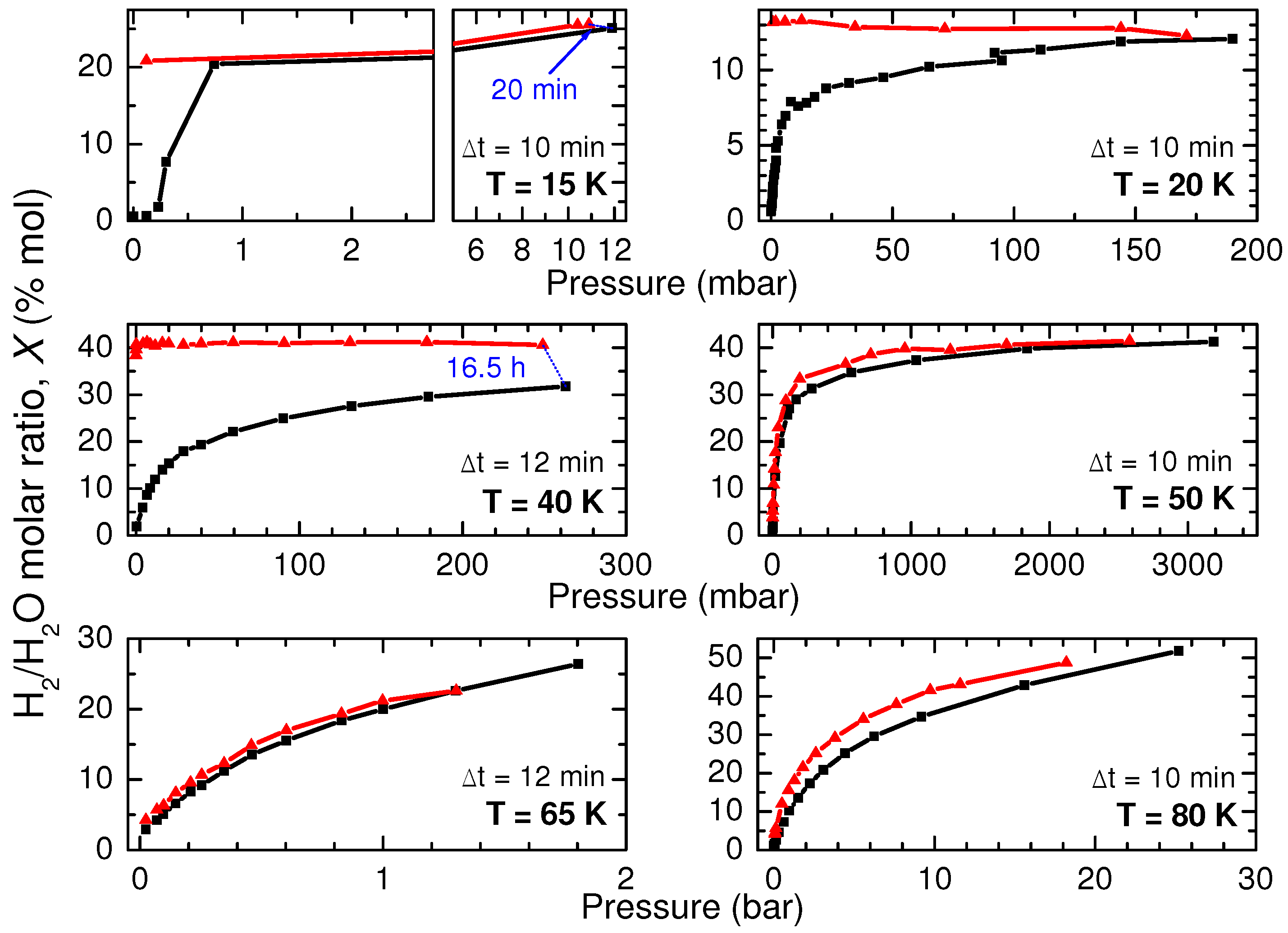
To derive the amount of adsorbed H
as a function of pressure, we have measured several adsorption isotherms at six different temperatures between 15 to 80 K. The results are reported in
Figure 3. Pressure is increased gradually and, when possible, each measurement is taken waiting the same time interval
(indicated in each panel) after each pressure increase step. The pressure at which the saturation is reached is strongly dependent on temperature and ranges between a few millibar at 15 K to several bar at 80 K. In addition, the maximum value of the measured adsorption is strongly temperature-dependent because it depends on the kinetics of hydrogen diffusion.
By using the same method, we examined the gas desorption while releasing pressure (red triangles in
Figure 3). A large hysteresis and other kinetic effects are evident at the lower temperatures. As it is shown in the case of 15 K, 20 K and 40 K, after decreasing pressure down to zero, the sample does not sensibly release hydrogen in
= 10 min. The relevant increase of the adsorbed hydrogen observed after the long exposure to hydrogen performed after the completion of the 40 K adsorption isotherm testifies that the system is not at equilibrium conditions, and this is probably due to the slow molecular diffusion inside the host channels that hinders a further adsorption. At the highest temperatures, i.e., 50, 65 and 80 K, a very small hysteresis is observed. Hence, only these three experimental isotherms are taken as reference for the following characterization of the storage capacity of ice XVII.
In order to study the mechanism of adsorption in a porous material, it can be useful to estimate its enthalpy of adsorption. For a given hydrogen uptake
X, the enthalpy of adsorption
is obtained by means of the Van’t Hoff equation
where
R is the gas constant. For the calculation of the temperature derivative, adsorption isotherms at different temperatures are necessary. We have used those measured at 50, 65 and 80 K, estimating the temperature derivative with a straight line fit.
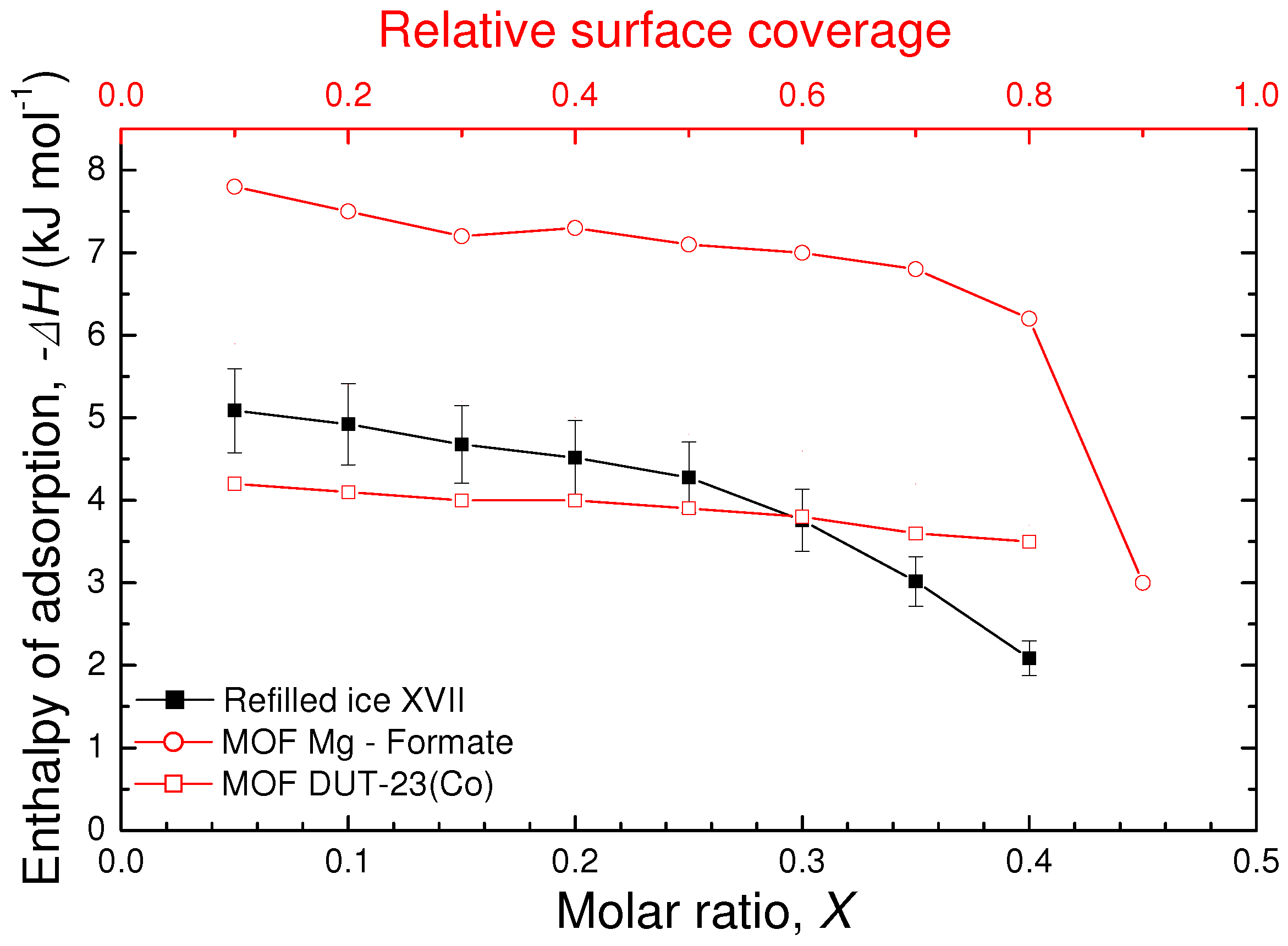
As shown in
Figure 4, the resulting value of
decreases with increasing molar ratio
X starting from about 5 kJ/mol at
10% down to about 2 kJ/mol at
40%. Values in these range are typical for molecular physisorption in porous material like MOFs [
4], whose mechanism of hydrogen adsorption is similar to that in ice XVII and, being driven by van der Waals interactions, does not involve any chemical bond. At lower filling, the intermediate value of the enthalpy of ice XVII compared to those of the DUT-23(Co) and Mg-Formate compounds can be explained with the diameter of the channels in ice XVII (about 0.6 nm) with respect to the MOF compounds considered (about 2 nm [
23] and 0.4 nm [
24], respectively). The decrease of the enthalpy with increasing hydrogen content could suggest the presence of different adsorption sites, that is, different binding energy for molecules placed deep into the channels with respect to those at the entrance of the channels, or even a cooperative effect of the already adsorbed hydrogen molecules on extra adsorbed ones. Moreover, potential little structural changes during the entire filling process could induce the enthalpy behaviour observed above [
17].
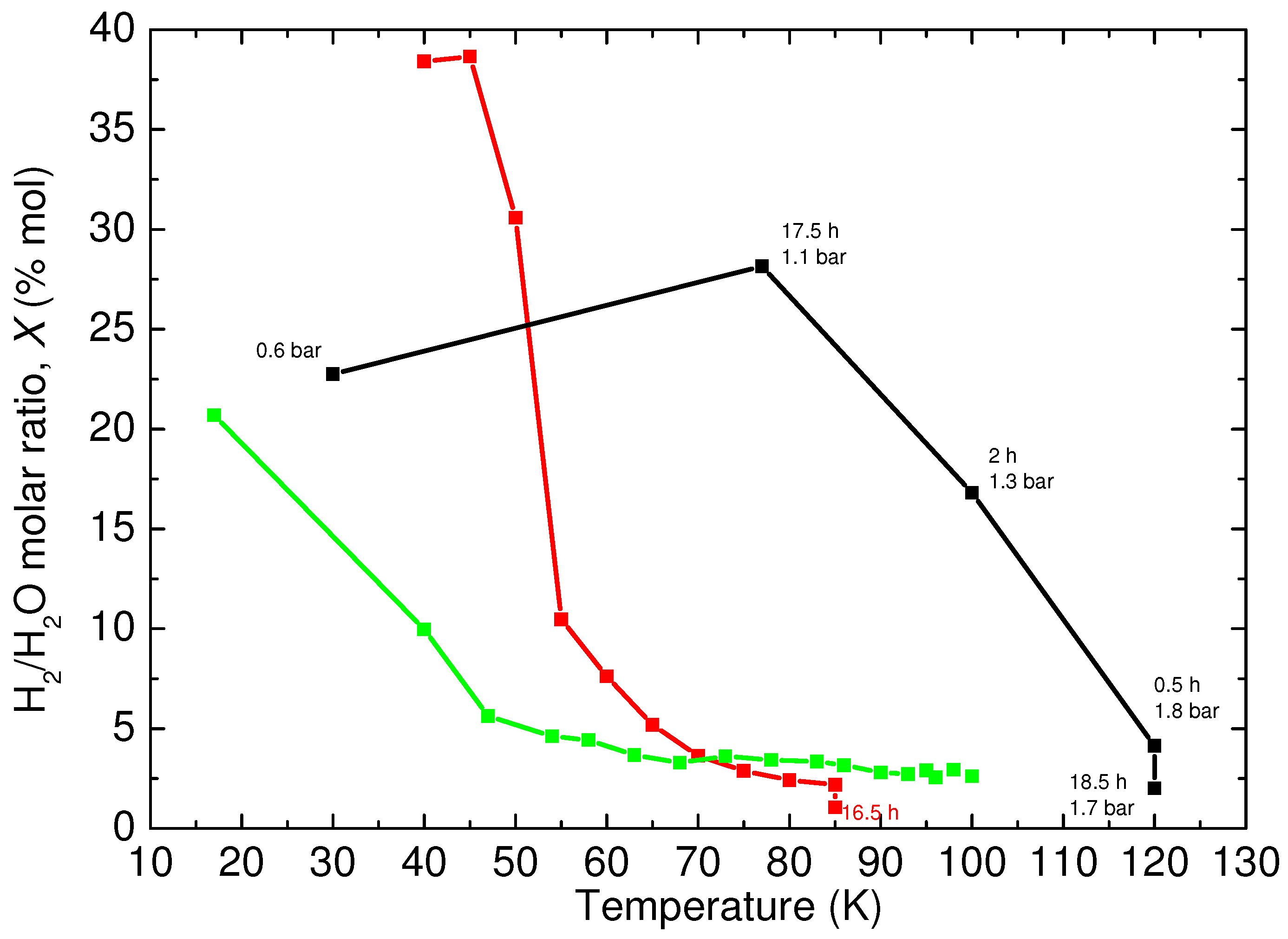
To describe the stability of the sample with respect to H
release, for both the C
-structure filled ice and the H
-refilled ice XVII, it can be useful to measure the amount of hydrogen trapped in the samples as a function of increasing temperature. We have performed this analysis on one pristine sample and on two samples of the refilled ice XVII. As is shown in
Figure 5, the C
-structure filled ice seems to have a wider region of thermal stability, considering that it starts to lose hydrogen at a temperature higher than 75 K and reaches an almost complete evacuation at 120 K.
The annealed samples, even with different initial filling, show faster kinetics of hydrogen release, which starts at about 40 K, and the complete emptying is reached at about 100 K. Even if these results are affected by the same uncertainty, it is clear that the filled ice directly quenched in liquid nitrogen after synthesis has a higher capacity of keeping the hydrogen guest trapped. This is probably due to the presence of nitrogen molecules in the pristine crystal, or to structural defects that hinder the diffusive motion of the hydrogen molecules along the channels of the ice structure.
A further increase of the temperature has led to the complete decomposition of ice XVII at 130 K with a relatively fast kinetic (about one hour). The resulting phase is probably ice Ih, according to the Raman HO lattice and O–H stretching bands, even if only diffraction data on this resulting structure would allow a clear determination of its crystal symmetry.
2.3. Applicability in the Hydrogen Storage Field
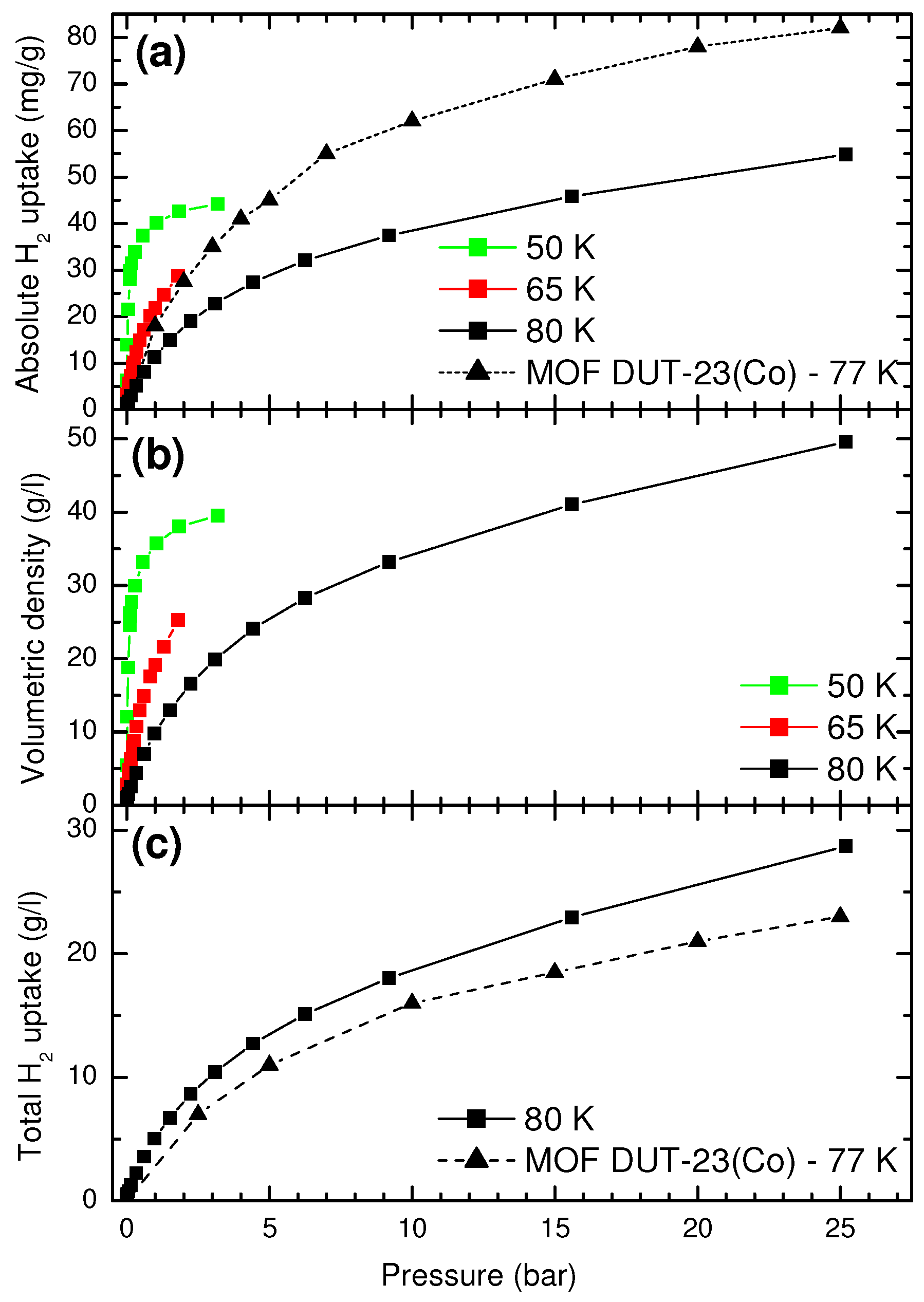
In order to characterize the adsorption capacity of this new material, we have calculated both gravimetric and volumetric capacities. The adsorption properties of ice XVII can be compared with those of materials with highly porous structure, able to realize hydrogen physisorption. Among them, the so-called metal-organic frameworks (MOFs) show the highest hydrogen uptakes and are taken as reference standard in this work.
From the previously measured hydrogen molar ratio
X, we have derived, in isotherm conditions, the absolute hydrogen uptake
(milligrams of H
per grams of H
+ H
O) and the volumetric hydrogen density
(grams of H
per liter of H
+ H
O). These isotherms are reported in panels (a,b) of
Figure 6, respectively. To calculate these quantities, we consider only the amount of H
which is adsorbed in the pores, that is, the one that is revealed by Raman spectroscopy.
Since the ice XVII sample has the form of a fine powder, for real tank application, we have also to consider the free gas present between ice grains. Hence, we need to evaluate all hydrogen present in a given volume, independently from its state (gas or adsorbed), that is, the total hydrogen uptake
(grams of H
per liter of H
+ H
O). We know from our measurements that, for a given volume of compacted ice powder, roughly one half is filled by ice crystallites. In this hypothesis, the total hydrogen uptake is given by
where
is the density of the free hydrogen gas at pressure
P [
25]. The total uptake is reported in panel (c) of
Figure 6.
Always in the view of storage applications, e.g., portable tanks, we report the usable capacity
, which is given by
where
is the maximum allowed tank pressure (some tens of bar for standard tanks) and
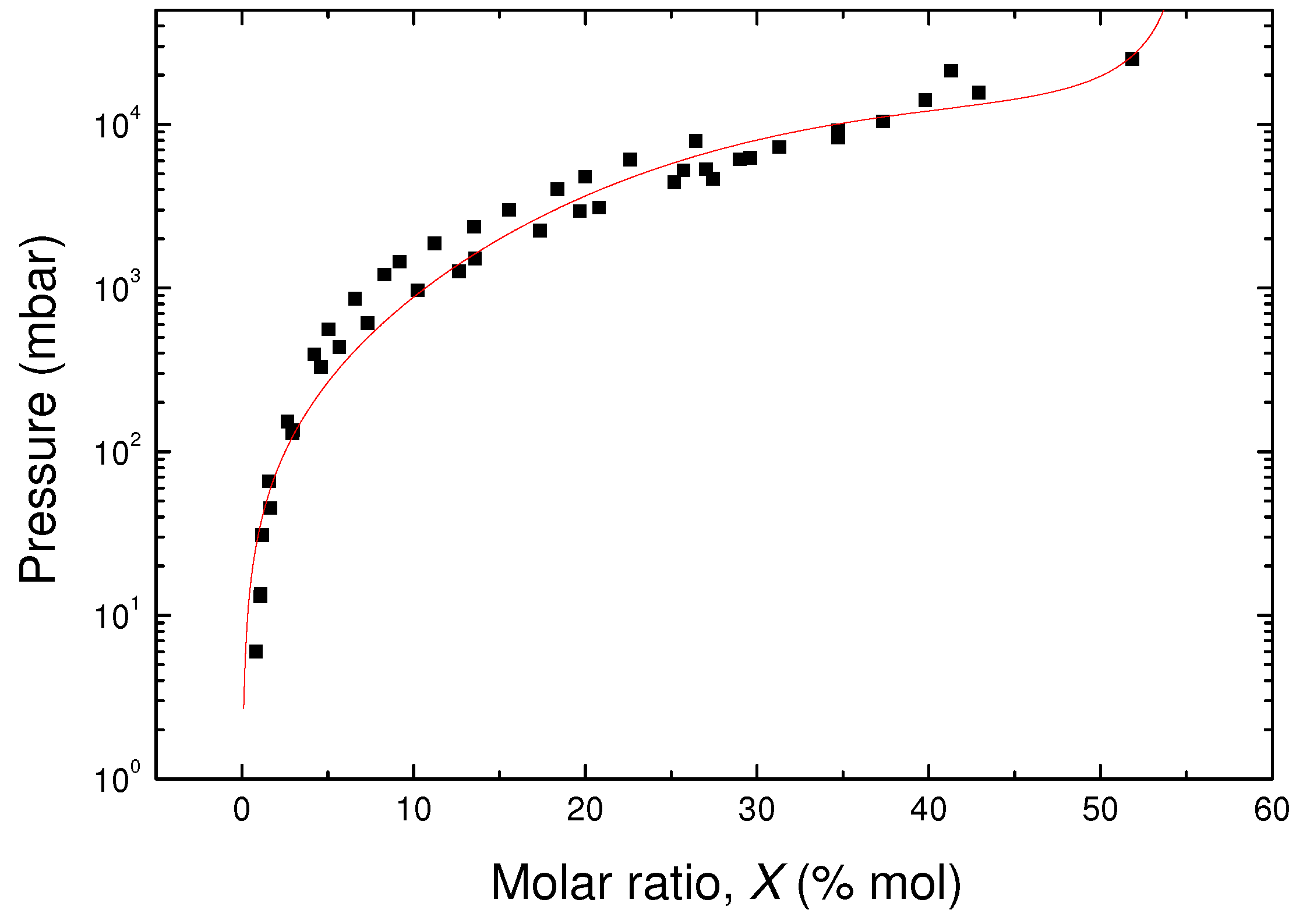
is the minimum pressure required by a hydrogen consumer to operate (e.g., 2 bar for a fuel cell). Due to the experimental limit on the maximum pressure (about 25 bar) of the gas circuit connected to our Raman cell, we have measured the equilibrium isotherms only in a narrow temperature range (50–80 K). Thus, to gain information on the usable capacity and on the general thermodynamic behaviour of our system also outside this temperature range, we have fitted the experimental data and extrapolated them at higher temperatures (up to 110 K) with an equation of state, suitable for type I isotherms, which allow for obtaining the pressure
P as a function of the amount of hydrogen adsorbed
X and temperature
T [
18]
where
K is the Henry’s constant,
m is the saturation capacity,
the arbitrary coefficients and
the reference temperature. The values of the enthalpy of adsorption can be derived by fitting the experimental values (
Figure 4) with an inverse polynomial. The parameters
K,
m and
can be obtained by fitting all at once the three sets of experimental values (50, 65 and 80 K), once normalized to the temperature-dependent exponential factor in Equation (
5), i.e.,
Since we are interested in the extrapolation at temperatures higher than 80 K, we have taken
= 80 K. The resulting best fit is reported with a red line in
Figure 7.
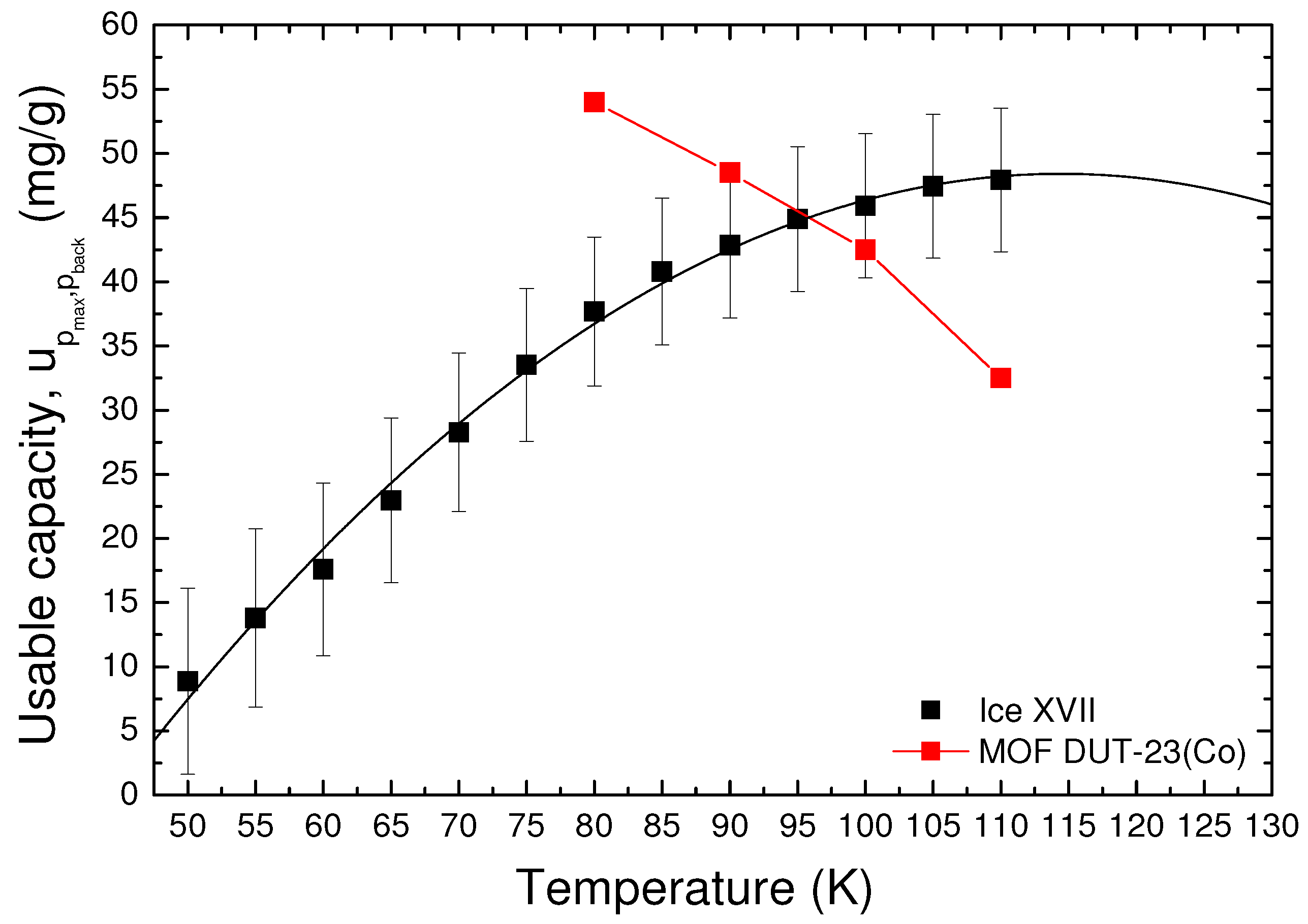
We have calculated the isotherms
for temperatures in the range 50–110 K, so as to be able to derive the usable capacity in this entire range of temperature. The results are reported in
Figure 8. In order to have our system in saturation conditions at the maximum pressure in the entire range of temperature considered, in these calculations, we have considered the maximum tank pressure equal to 40 bar, while, for the reference sample, DUT-23(Co) is 25 bar because this is the maximum pressure at which the isotherms have been measured in this case [
4].
By means of a parabolic fit of the extrapolated data, we have derived the temperature at which the usable capacity should assume the maximum value. This temperature, usually called optimum operating temperature , represents the working temperature at which the storage system has the best performances. For ice XVII, this value is about 114 K.
All the estimated quantities are summarized in
Table 1, where the values belonging to some MOF systems are reported for comparison.