Spreading and Establishment of the Non Indigenous Species Caprella scaura (Amphipoda: Caprellidae) in the Central Region of the Aegean Sea (Eastern Mediterranean Sea)
Abstract
1. Introduction
2. Materials and Methods
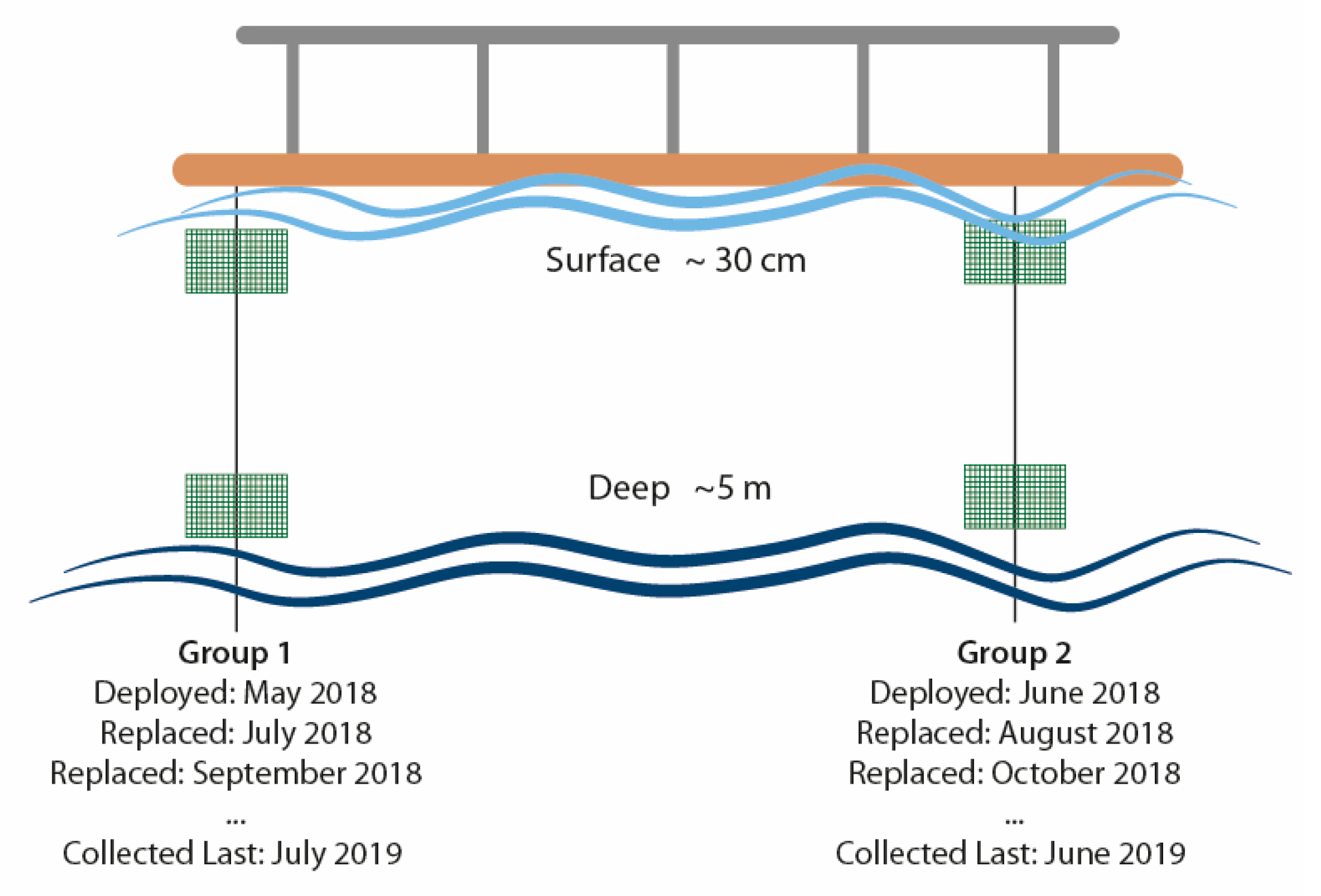
2.1. Sampling Design
2.2. Laboratory Processing
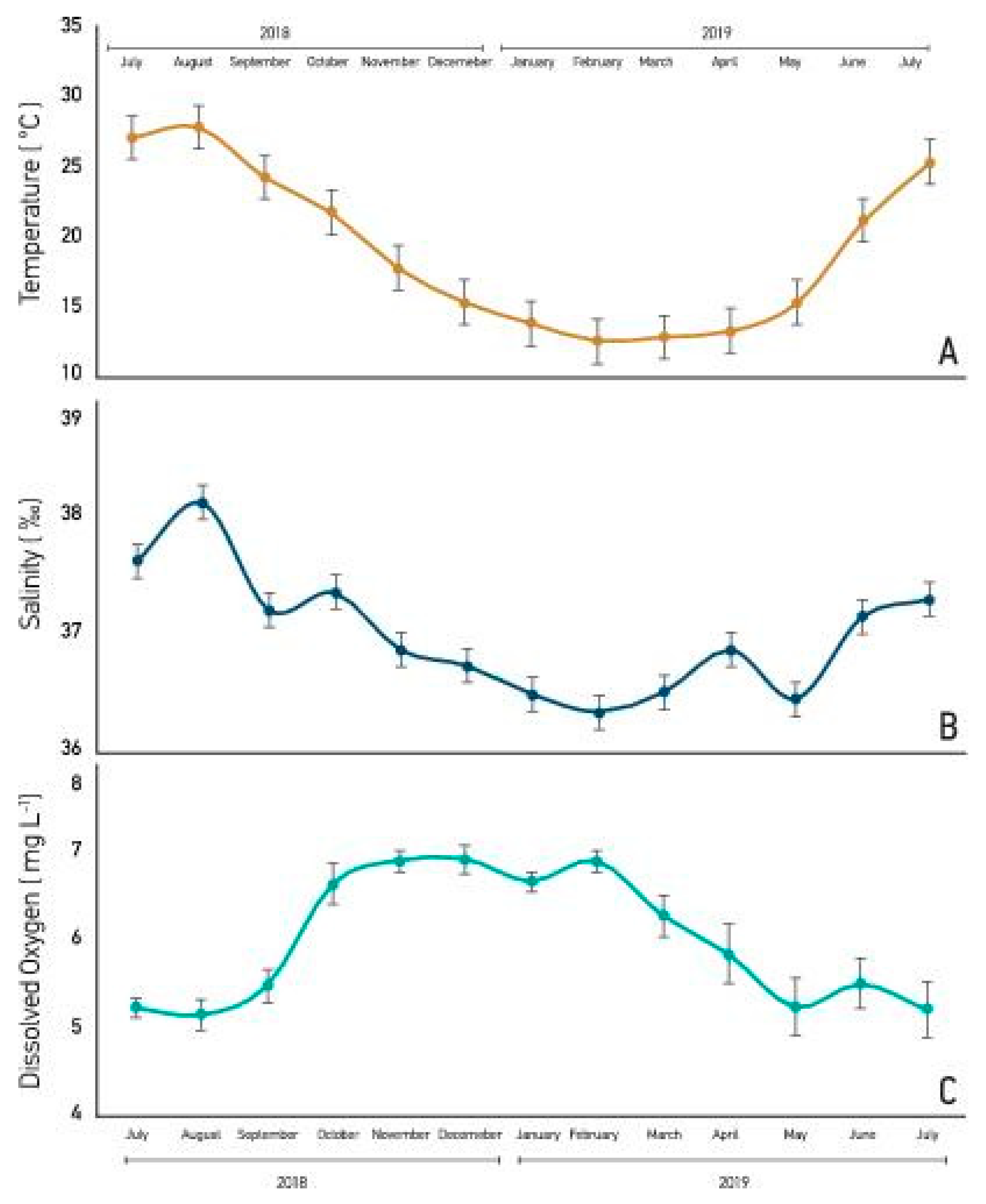
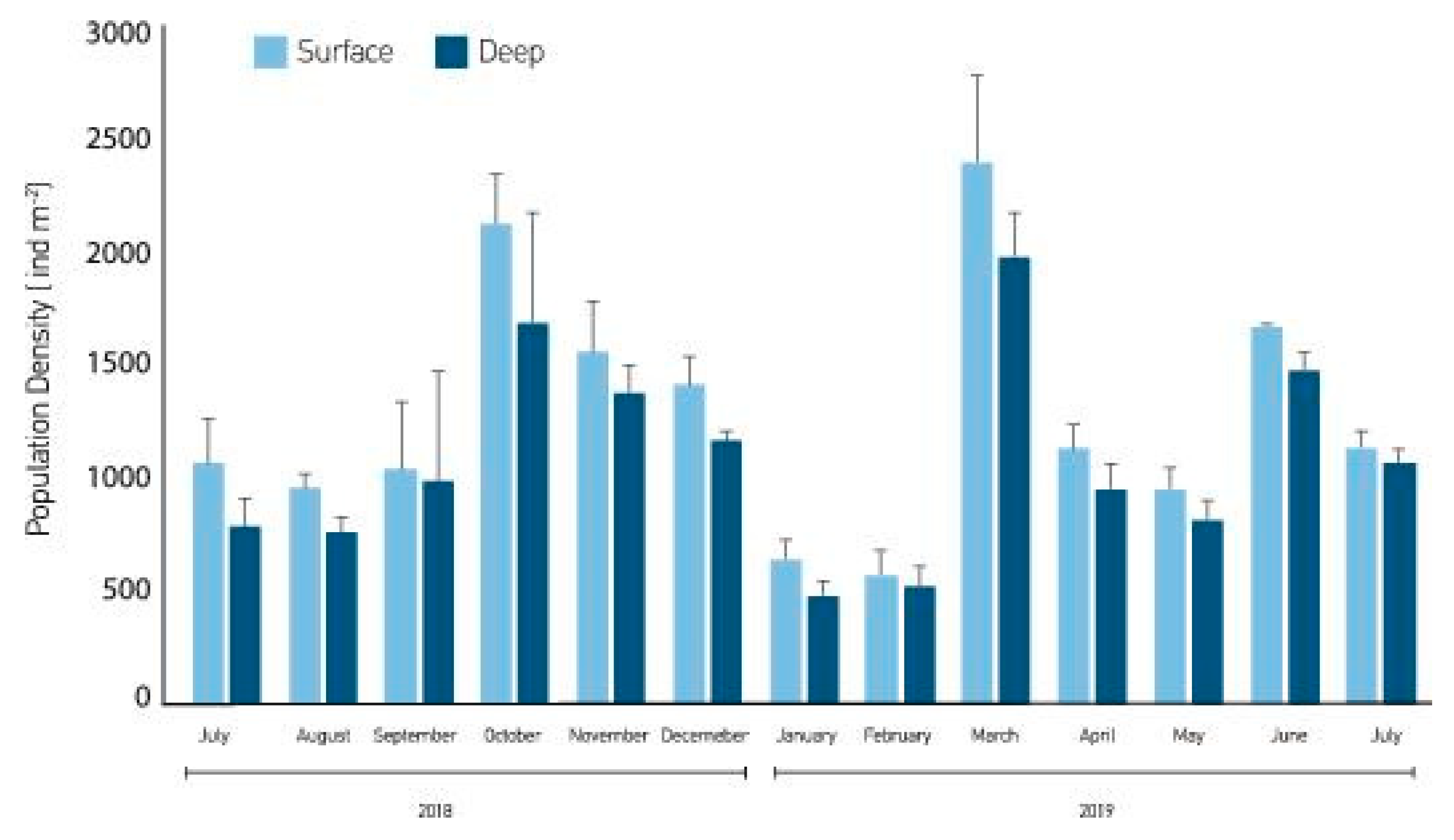
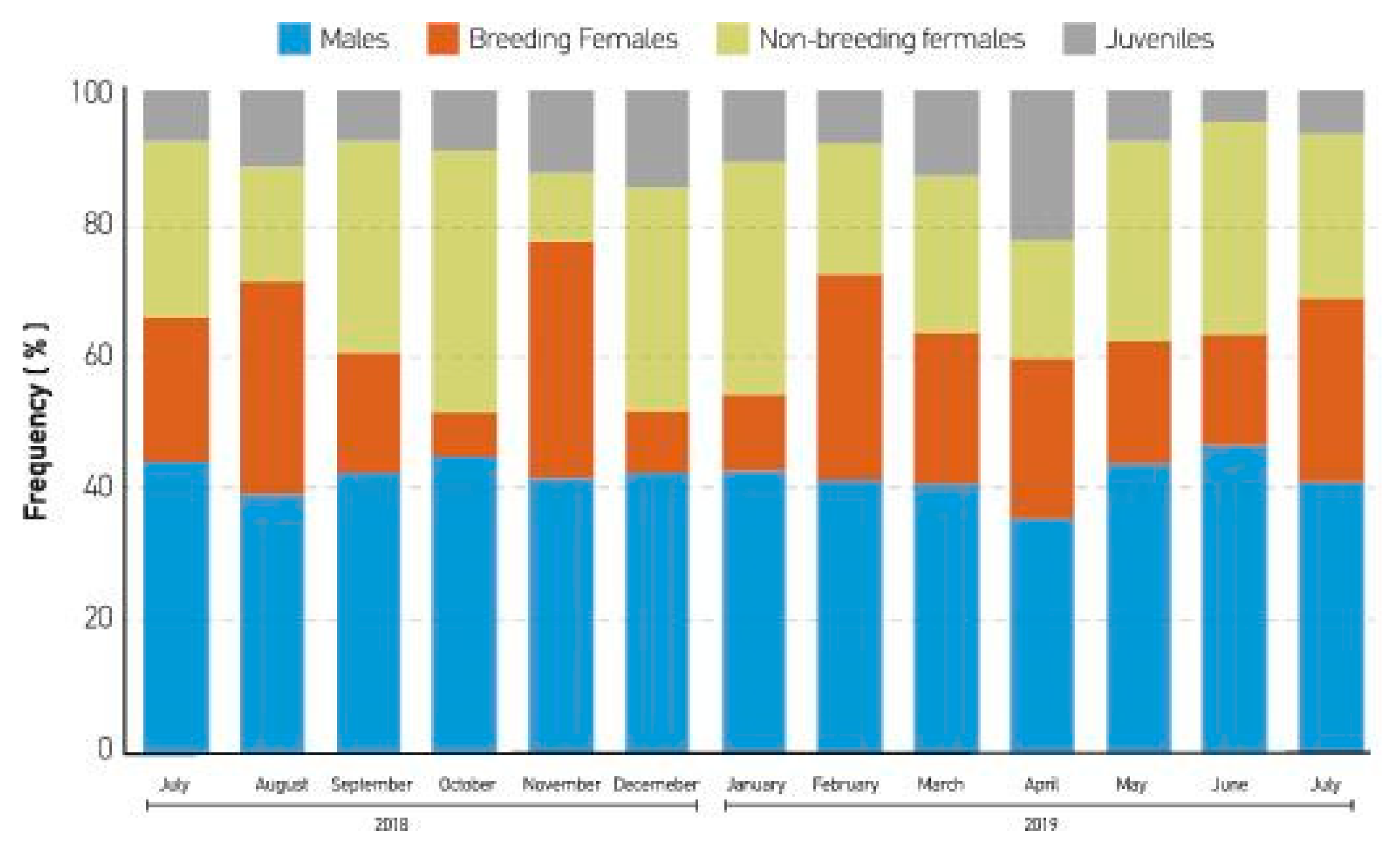
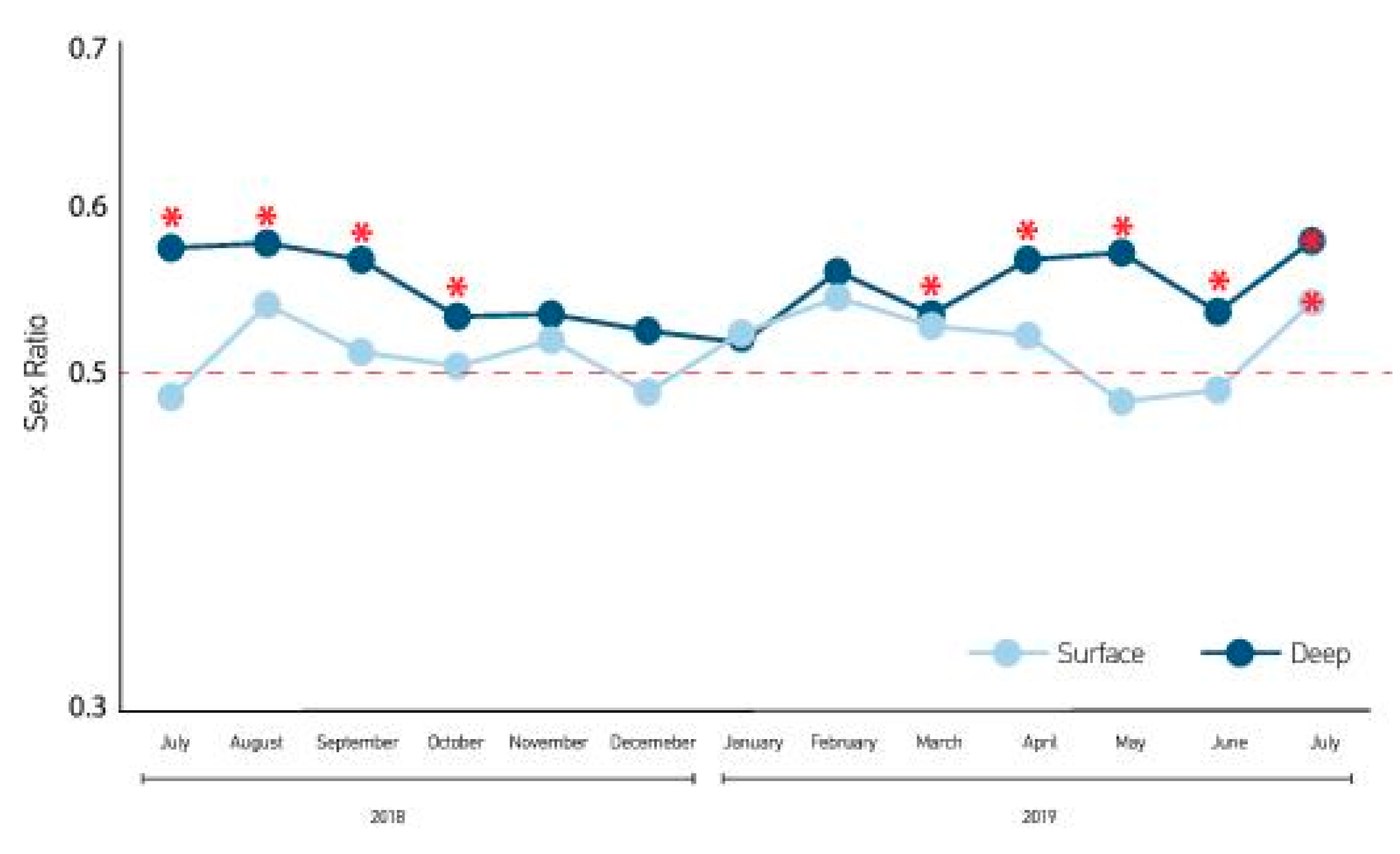
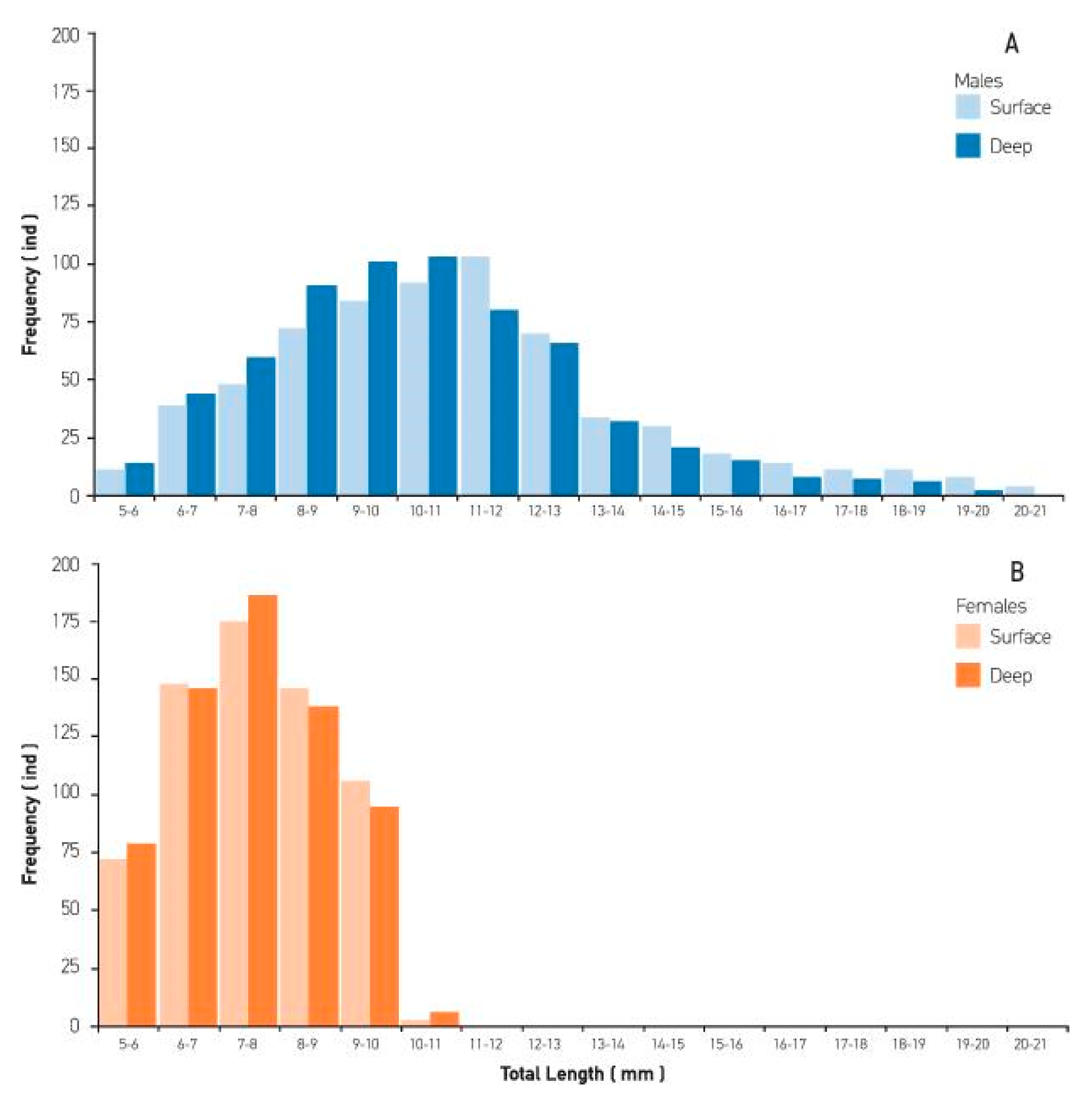
3. Results
4. Discussion
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsiamis, K.; Zenetos, A.; Deriu, I.; Gervasini, E.; Cardoso, A.C. The native distribution range of the European marine non-indigenous species. Aquat. Invasions 2018, 13, 187–198. [Google Scholar] [CrossRef]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2018, 201, 7–16. [Google Scholar] [CrossRef]
- Elliott, M. Biological pollutants and biological pollution––An increasing cause for concern. Mar. Pollut. Bull. 2003, 46, 275–280. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.P.; Geist, J.; Jeschke, J.M.; Kühn, I. Invasive species in Europe: Ecology, status, and policy. Environ. Sci. Eur. 2011, 23, 23. [Google Scholar] [CrossRef]
- Hänfling, B.; Edwards, F.; Gherardi, F. Invasive alien Crustacea: Dispersal, establishment, impact and control. BioControl 2011, 56, 573–595. [Google Scholar] [CrossRef]
- Martínez-Laiz, G.; Ulman, A.; Ros, M.; Marchini, A. Is recreational boating a potential vector for non-indigenous peracarid crustaceans in the Mediterranean Sea? A combined biological and social approach. Mar. Pollut. Bull. 2019, 140, 403–415. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Ros, M.; Dugo-Cota, A.; Burgos, V.; Flores-León, A.M.; Baeza-Rojano, E.; Cabezas, M.P.; Núñez, J. Geographical expansion of the invader Caprella scaura (Crustacea: Amphipoda: Caprellidae) to the East Atlantic coast. Mar. Biol. 2011, 158, 2617–2622. [Google Scholar] [CrossRef]
- Ashton, G.V.; Boos, K.; Shucksmith, R.; Cook, E.J. Risk assessment of hull fouling as a vector for marine non-natives in Scotland. Aquat. Invasions 2006, 1, 214–218. [Google Scholar] [CrossRef]
- Ashton, G.V.; Burrows, M.T.; Willis, K.J.; Cook, E.J. Seasonal population dynamics of the non-native Caprella mutica (Crustacea, Amphipoda) on the west coast of Scotland. Mar. Freshw. Res. 2010, 61, 549–559. [Google Scholar] [CrossRef]
- Ros, M.; Guerra-García, J.M.; González-Macías, M.; Saavedra, Á.; López-Fe, C.M. Influence of fouling communities on the establishment success of alien caprellids (Crustacea: Amphipoda) in Southern Spain. Mar. Biol. Res. 2013, 9, 261–273. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, V.; Sanchez-Jerez, P. Fouling assemblages associated with off-coast aquaculture facilities: An overall assessment of the Mediterranean Sea. Mediterr. Mar. Sci. 2017, 18, 87–96. [Google Scholar] [CrossRef]
- Ros, M.; Vázquez-Luis, M.; Guerra-García, J.M. The role of marinas and recreational boating in the occurrence and distribution of exotic caprellids (Crustacea: Amphipoda) in the Western Mediterranean: Mallorca Island as a case study. J. Sea Res. 2013, 83, 94–103. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, V.; Sanchez-Jerez, P. First occurrence of Caprella scaura Templeton, 1836 (Crustacea: Amphipoda) on off-coast fish farm cages in the Mediterranean Sea. Helgol. Mar. Res. 2014, 68, 187–191. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Ros, M.; Baeza-Rojano, E. Seasonal fluctuations and dietary analysis of fouling caprellids (Crustacea: Amphipoda) from marinas of southern Spain. Mar. Biol. Res. 2015, 11, 703–715. [Google Scholar] [CrossRef]
- Prato, E.; Parlapiano, I.; Biandolino, F. Seasonal fluctuations of some biological traits of the invader Caprella scaura (Crustacea: Amphipoda: Caprellidae) in the Mar Piccolo of Taranto (Ionian Sea, southern Italy). Sci. Mar. 2013, 77, 169–178. [Google Scholar] [CrossRef]
- Weis, J.S. The role of behavior in the success of invasive crustaceans. Mar. Freshw. Behav. Physiol. 2010, 43, 83–98. [Google Scholar] [CrossRef]
- Galil, B.S. The Alien Crustaceans in the Mediterranean Sea: An Historical Review. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 377–401. [Google Scholar]
- Galil, B.S. Alien species in the Mediterranean Sea—which, when, where, why? Hydrobiologia 2008, 606, 105–116. [Google Scholar] [CrossRef]
- Laubitz, D.R.; Mills, E.L. Deep-sea Amphipoda from the western North Atlantic Ocean. Caprellidea. Can. J. Zool. 1972, 50, 371–383. [Google Scholar] [CrossRef]
- Takeuchi, I.; Sawamoto, S. Distribution of caprellid amphipods (Crustacea) in the western North Pacific based on the CSK International Zooplankton Collection. Plankton Biol. Ecol. 1998, 45, 225–230. [Google Scholar]
- Guerra-García, J.M.; García-Gómez, J.C. The Spatial Distribution of Caprellidea (Crustacea: Amphipoda): A Stress Bioindicator in Ceuta (North Africa, Gibraltar Area). Mar. Ecol. 2001, 22, 357–367. [Google Scholar] [CrossRef]
- Díaz, Y.J.; Guerra-García, J.M.; Martín, A. Caprellids (Crustacea: Amphipoda: Caprellidae) from shallow waters of the Caribbean coast of Venezuela. Org. Divers. Evol. 2005, 5, 1–25. [Google Scholar] [CrossRef]
- Caine, E.A. Caprellid amphipods: Fast food for the reproductively active. J. Exp. Mar. Biol. Ecol. 1991, 148, 27–33. [Google Scholar] [CrossRef]
- Woods, C.M.C. Caprellid amphipods: An overlooked marine finfish aquaculture resource? Aquaculture 2009, 289, 199–211. [Google Scholar] [CrossRef]
- Boos, K. Mechanisms of a Successful Immigration from North-East Asia: Population Dynamics, Life History Traits and Interspecific Interactions in the Caprellid Amphipod Caprella Mutica Schurin, 1935 (Crustacea, Amphipoda) in European Coastal Waters; Freie Univertität: Berlin, Germany, 2009. [Google Scholar]
- Guerra-García, J.M. Habitat use of the caprellidea (Crustacea: Amphipoda) from Ceuta, North Africa. Ophelia 2001, 55, 27–38. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Hachero-Cruzado, I.; González-Romero, P.; Jiménez-Prada, P.; Cassell, C.; Ros, M. Towards Integrated Multi-Trophic Aquaculture: Lessons from Caprellids (Crustacea: Amphipoda). PLoS ONE 2016, 11, e0154776. [Google Scholar] [CrossRef]
- González, A.R.; Guerra-García, J.M.; Maestre, M.J.; Ruiz-Tabares, A.; Espinosa, F.; Gordillo, I.; Sánchez-Moyano, J.E.; García-Gómez, J.C. Community structure of caprellids (Crustacea: Amphipoda: Caprellidae) on seagrasses from southern Spain. Helgol. Mar. Res. 2008, 62, 189–199. [Google Scholar] [CrossRef]
- Caine, E.A. Population structures of two species of Caprellid amphipods (Crustacea). J. Exp. Mar. Biol. Ecol. 1979, 40, 103–114. [Google Scholar] [CrossRef]
- Nuñez Velazquez, S.; Rumbold, C.E.; Obenat, S.M. Population dynamics of Caprella dilatata and Caprella equilibra (Peracarida: Amphipoda) in a Southwestern Atlantic harbour. Mar. Biol. Res. 2017, 13, 888–898. [Google Scholar] [CrossRef]
- Krapp, T.; Lang, C.; Libertini, A.; Melzer, R.R. Caprella scaura Templeton, 1836 sensu lato (Amphipoda: Caprellidae) in the Mediterranean. Org. Divers. Evol. 2006, 6, 77–81. [Google Scholar] [CrossRef][Green Version]
- Martínez, J.; Adarraga, I. First record of invasive caprellid Caprella scaura Templeton, 1836 sensu lato (Crustacea: Amphipoda: Caprellidae) from the Iberian Peninsula. Aquat. Invasions 2008, 3, 165–171. [Google Scholar] [CrossRef]
- Mizzan, L. Le specie alloctone del macrozoobenthos della Laguna di Venezia: Il punto della situazione. Boll. Mus. Civ. Stor. Nat. Venezia 1999, 49, 145–177. [Google Scholar]
- Sconfietti, R.; Mangili, F.; Savini, D.; Occhipinti-Ambrogi, A. Diffusion of the alien species Caprella scaura Templeton, 1836 (Amphipoda: Caprellidae) in the northern Adriatic Sea. Biol. Mar. Mediterr. 2005, 12, 335–337. [Google Scholar]
- Bakır, K.; Katağan, T. On the occurrence of Caprella scaura Templeton, 1836 (Crustacea: Amphipoda) in Turkish waters. Zool. Middle East 2011, 52, 125–126. [Google Scholar] [CrossRef]
- Cabezas, M.P.; Xavier, R.; Branco, M.; Santos, A.M.; Guerra-García, J.M. Invasion history of Caprella scaura Templeton, 1836 (Amphipoda: Caprellidae) in the Iberian Peninsula: Multiple introductions revealed by mitochondrial sequence data. Biol. Invasions 2014, 16, 2221–2245. [Google Scholar] [CrossRef]
- Dailianis, T.; Akyol, O.; Babali, N.; Bariche, M.; Crocetta, F.; Gerovasileiou, V.; Chanem, R.; Gökoğlu, M.; Hasiotis, T.; Izquierdo-Muñoz, A.; et al. New Mediterranean Biodiversity Records (July 2016). Mediterr. Mar. Sci. 2016, 12, 608–626. [Google Scholar] [CrossRef]
- Chebaane, S.; Shaiek, M.; Zakhama-Sraieb, R. A new record and range extension of an invasive amphipod Caprella scaura Templeton, 1836 in Tunisia, North Africa. J. Black Sea Mediterr. Environ. 2018, 24, 255–262. [Google Scholar]
- Guerra-García, J.M.; Martínez-Pita, I.; Pita, M.L. Fatty acid composition of the Caprellidea (Crustacea: Amphipoda) from the Strait of Gibraltar. Sci. Mar. 2004, 68, 501–510. [Google Scholar] [CrossRef]
- Cook, E.J.; Shucksmith, R.; Orr, H.; Ashton, G.V.; Berge, J. Fatty acid composition as a dietary indicator of the invasive caprellid, Caprella mutica (Crustacea: Amphipoda). Mar. Biol. 2010, 157, 19–27. [Google Scholar] [CrossRef]
- Baeza-Rojano, E.; Hachero-Cruzado, I.; Guerra-García, J.M. Nutritional analysis of freshwater and marine amphipods from the Strait of Gibraltar and potential aquaculture applications. J. Sea Res. 2014, 85, 29–36. [Google Scholar] [CrossRef]
- Lolas, A.; Vafidis, D. Population dynamics of two caprellid species (Crustaceae: Amphipoda: Caprellidae) from shallow hard bottom assemblages. Mar. Biodivers. 2013, 43, 227–236. [Google Scholar] [CrossRef]
- Guerra-García, J.M. The Caprellidea (Crustacea: Amphipoda) from Mauritius Island, Western Indian Ocean. Zootaxa 2003, 232, 1–24. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lolas, A.; Vafidis, D. Population Dynamics, Fishery, and Exploitation Status of Norway Lobster (Nephrops norvegicus) in Eastern Mediterranean. Water 2021, 13, 289. [Google Scholar] [CrossRef]
- Ros Clemente, M. The spreading of the non-native caprellid (Crustacea: Amphipoda) Caprella scaura Templeton, 1836 into southern Europe and northern Africa: A complicated taxonomic history. Mediterr. Mar. Sci. 2014, 15, 145–155. [Google Scholar] [CrossRef]
- Trujillo, P.; Piroddi, C.; Jacquet, J. Fish Farms at Sea: The Ground Truth from Google Earth. PLoS ONE 2012, 7, e30546. [Google Scholar] [CrossRef] [PubMed]
- Caine, E.A. Reproductive Behavior and Sexual Dimorphism of a Caprellid Amphipod. J. Crustacean Biol. 1991, 11, 56–63. [Google Scholar] [CrossRef]
- Ramalhosa, P.; Canning-Clode, J. The invasive caprellid Caprella scaura Templeton, 1836 (Crustacea: Amphipoda: Caprellidae) arrives on Madeira Island, Portugal. BioInvasions Rec. 2015, 4. [Google Scholar] [CrossRef]
- Tim, D.; Pablo, S.-J.; Just, T.B.-S.; Francisca, G.-C.; Carlos, V. Attraction of wild fish to sea-cage fish farms in the south-western Mediterranean Sea: Spatial and short-term temporal variability. Mar. Ecol. Prog. Ser. 2002, 242, 237–252. [Google Scholar] [CrossRef]
- Caine, E.A. Ecology of two littoral species of caprellid amphipods (Crustacea) from Washington, USA. Mar. Biol. 1980, 56, 327–335. [Google Scholar] [CrossRef]
- Molina, S.; Ros, M.; Guerra-García, J.M. Distribution of the Invasive Caprellid Caprella scaura (Crustacea: Amphipoda) in Cádiz Marina, Southern Spain: Implications for its Dispersal. Thalass. Int. J. Mar. Sci. 2017, 33, 81–86. [Google Scholar] [CrossRef]
- Keith, D.E. Substrate selection in caprellid amphipods of Southern California, with emphasis on Caprella californica Stimpson and Caprella equilibra Say (Amphipoda). Pac. Sci. 1971, 25, 387–394. [Google Scholar]
- Conradi, M.; López-González, P.J.; Cervera, J.L.; García-Gómez, J.C. Seasonality and Spatial Distribution of Peracarids Associated with the Bryozoan Bugula neritina in Algeciras Bay, Spain. J. Crustacean Biol. 2000, 20, 334–349. [Google Scholar] [CrossRef]
- Ryland, J.S.; Bishop, J.D.D.; Blauwe, H.D.; El-Nagar, A.; Minchin, D.; Wood, C.A.; Yunnie, A.L.E. Alien species of Bugula (Bryozoa) along the Atlantic coasts of Europe. Aquat. Invasions 2011, 6, 17–31. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive Interactions of Nonindigenous Species: Invasional Meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Manning, T.J.; Land, M.; Rhodes, E.; Chamberlin, L.; Rudloe, J.; Phillips, D.; Lam, T.T.; Purcell, J.; Cooper, H.J.; Emmett, M.R.; et al. Identifying bryostatins and potential precursors from the bryozoan Bugula neritina. Nat. Prod. Res. 2005, 19, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Fehlauer-Ale, K.H.; Mackie, J.A.; Lim-Fong, G.E.; Ale, E.; Pie, M.R.; Waeschenbach, A. Cryptic species in the cosmopolitan Bugula neritina complex (Bryozoa, Cheilostomata). Zool. Scr. 2014, 43, 193–205. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Lefranc, F.; Vieira, L.M.; Kiss, R.; Carbone, M.; van Otterlo, W.A.L.; Lopanik, N.B.; Waeschenbach, A. The Phylum Bryozoa: From Biology to Biomedical Potential. Mar. Drugs 2020, 18, 200. [Google Scholar] [CrossRef]

| TL (mm) | Males | Breeding Females | Non-Breeding Females | Juveniles |
|---|---|---|---|---|
| Surface | ||||
| Minimum | 5.8 | 5.6 | 5.1 | 2.6 |
| Maximum | 21.1 | 10.1 | 10.2 | 4.9 |
| Mean ± SD | 11.1 ± 3.0 | 8.3 ± 1.2 | 7.3 ± 1.1 | 3.8 ± 0.5 |
| Deep | ||||
| Minimum | 5.4 | 5.3 | 5.0 | 2.8 |
| Maximum | 19.0 | 10.2 | 10.3 | 4.9 |
| Mean (SD) | 10.4 ± 2.6 | 8.3 ± 1.2 | 7.3 ± 1.3 | 3.9 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lolas, A.; Karapanagiotidis, I.T.; Panagiotaki, P.; Vafidis, D. Spreading and Establishment of the Non Indigenous Species Caprella scaura (Amphipoda: Caprellidae) in the Central Region of the Aegean Sea (Eastern Mediterranean Sea). J. Mar. Sci. Eng. 2021, 9, 857. https://doi.org/10.3390/jmse9080857
Lolas A, Karapanagiotidis IT, Panagiotaki P, Vafidis D. Spreading and Establishment of the Non Indigenous Species Caprella scaura (Amphipoda: Caprellidae) in the Central Region of the Aegean Sea (Eastern Mediterranean Sea). Journal of Marine Science and Engineering. 2021; 9(8):857. https://doi.org/10.3390/jmse9080857
Chicago/Turabian StyleLolas, Alexios, Ioannis T. Karapanagiotidis, Panagiota Panagiotaki, and Dimitris Vafidis. 2021. "Spreading and Establishment of the Non Indigenous Species Caprella scaura (Amphipoda: Caprellidae) in the Central Region of the Aegean Sea (Eastern Mediterranean Sea)" Journal of Marine Science and Engineering 9, no. 8: 857. https://doi.org/10.3390/jmse9080857
APA StyleLolas, A., Karapanagiotidis, I. T., Panagiotaki, P., & Vafidis, D. (2021). Spreading and Establishment of the Non Indigenous Species Caprella scaura (Amphipoda: Caprellidae) in the Central Region of the Aegean Sea (Eastern Mediterranean Sea). Journal of Marine Science and Engineering, 9(8), 857. https://doi.org/10.3390/jmse9080857








