Physio-Chemical and Mineralogical Characteristics of Gas Hydrate-Bearing Sediments of the Kerala-Konkan, Krishna-Godavari, and Mahanadi Basins
Abstract
:1. Introduction
1.1. Bottom-Simulating Reflector (BSR)
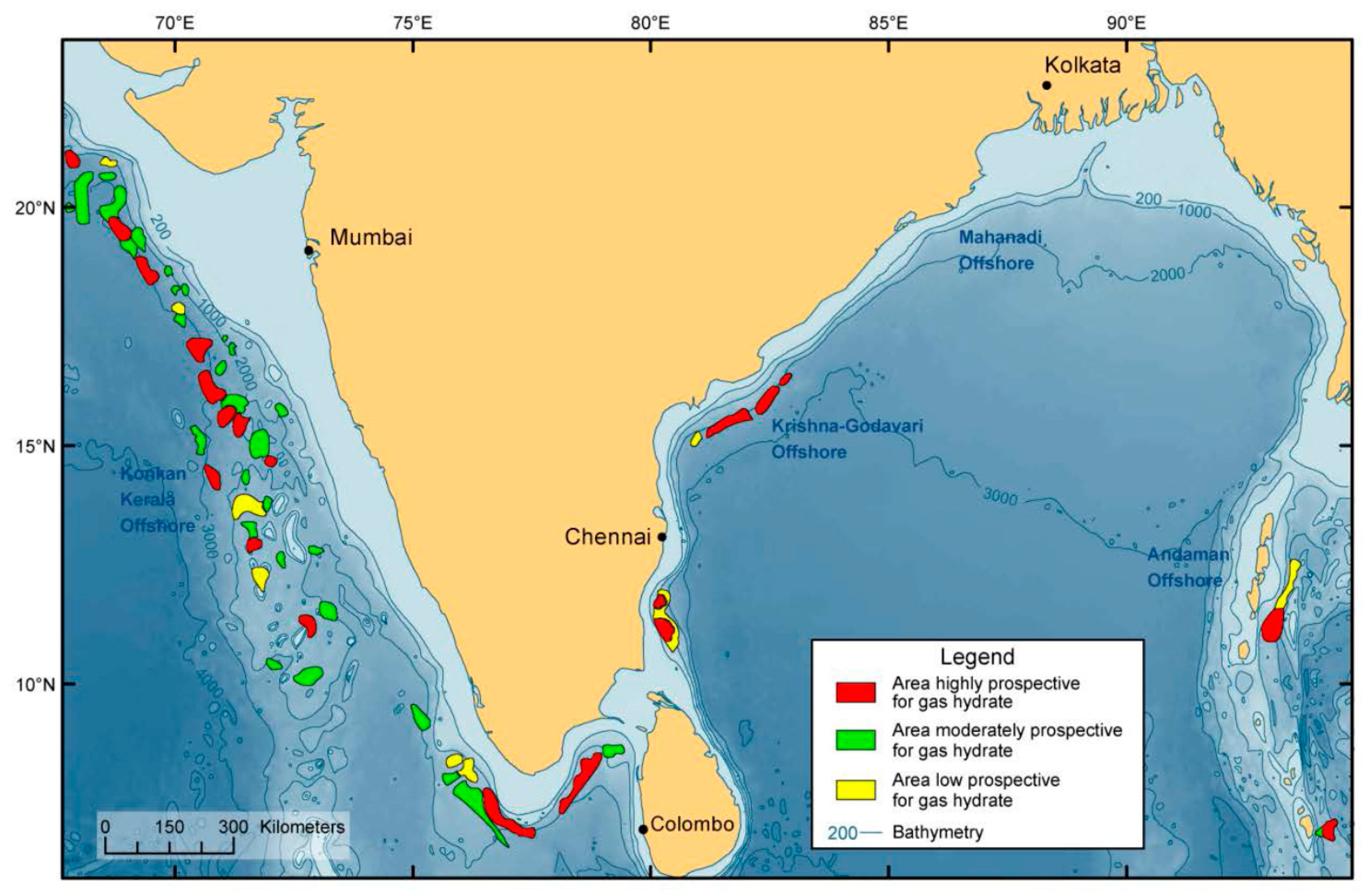
1.2. Geographical Overview
1.2.1. Krishna-Godavari Basin
1.2.2. Mahanadi Basin
1.2.3. Kerala-Konkan Basin
1.2.4. Andaman Basin
2. Material and Methods
2.1. Preparation of Sample
2.2. Experimental Setup
3. Results and Discussion
3.1. Physical Properties of Sediment Samples
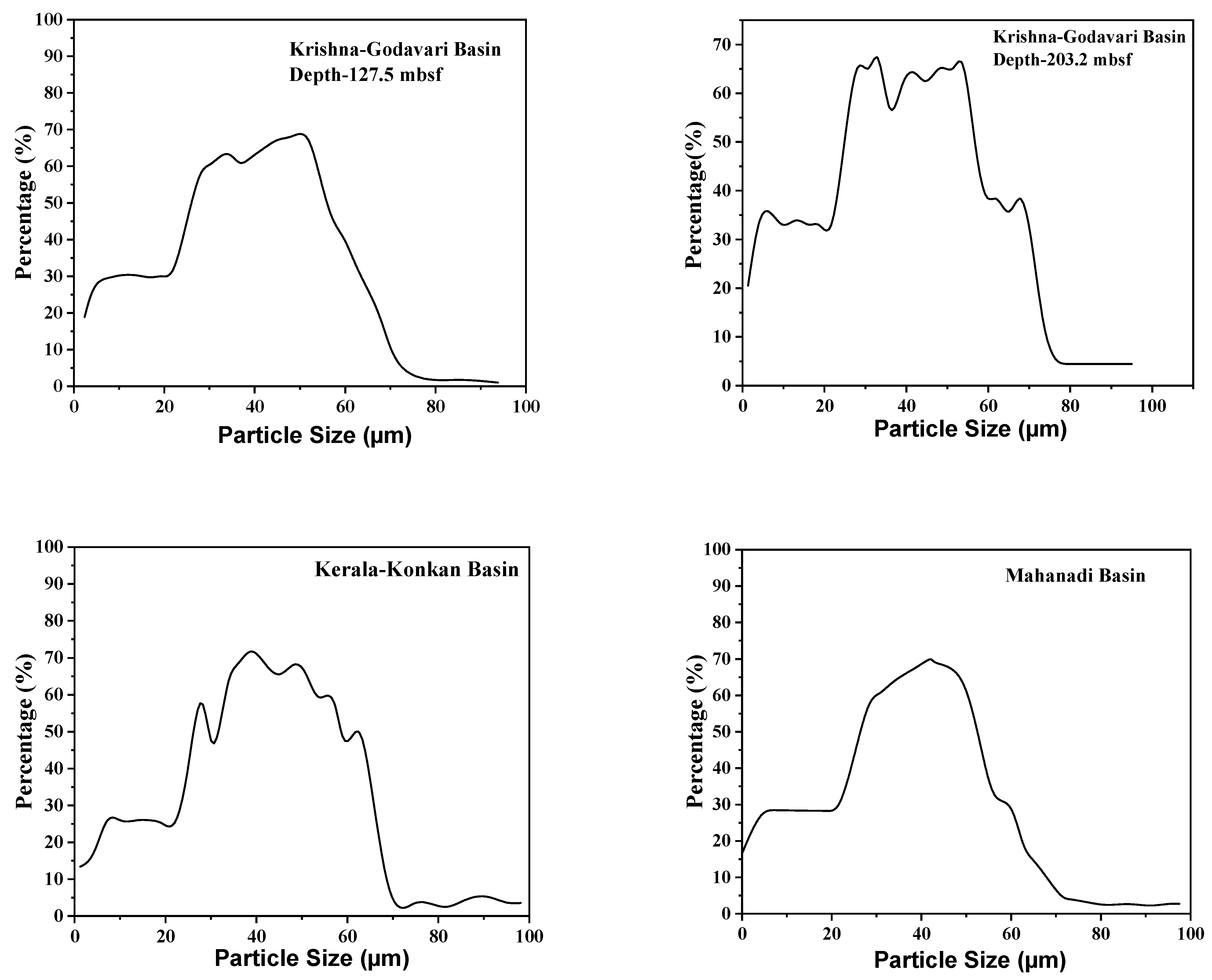
3.2. Particle Size Analyzer (PSA)
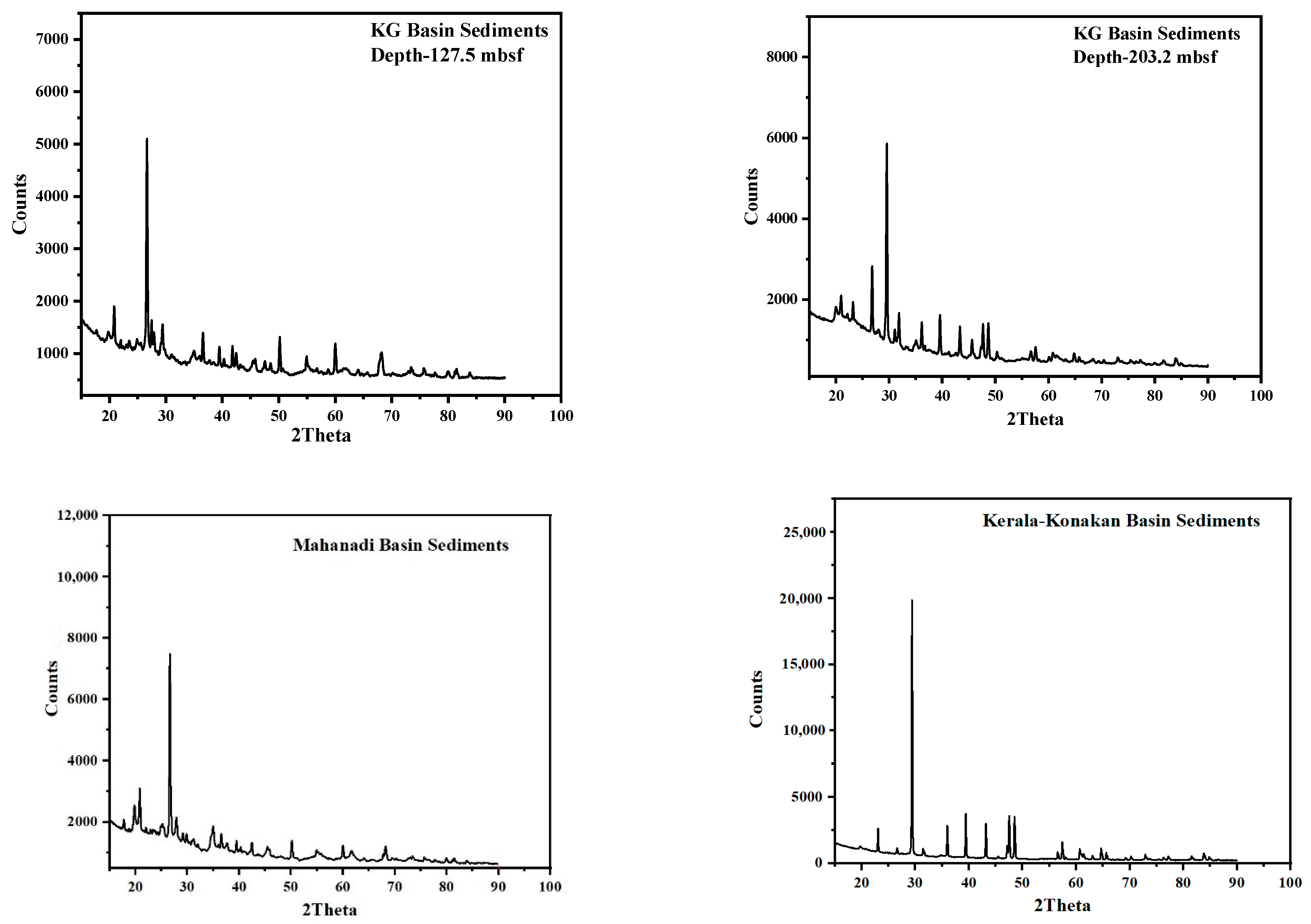
3.3. X-ray Diffraction (XRD)
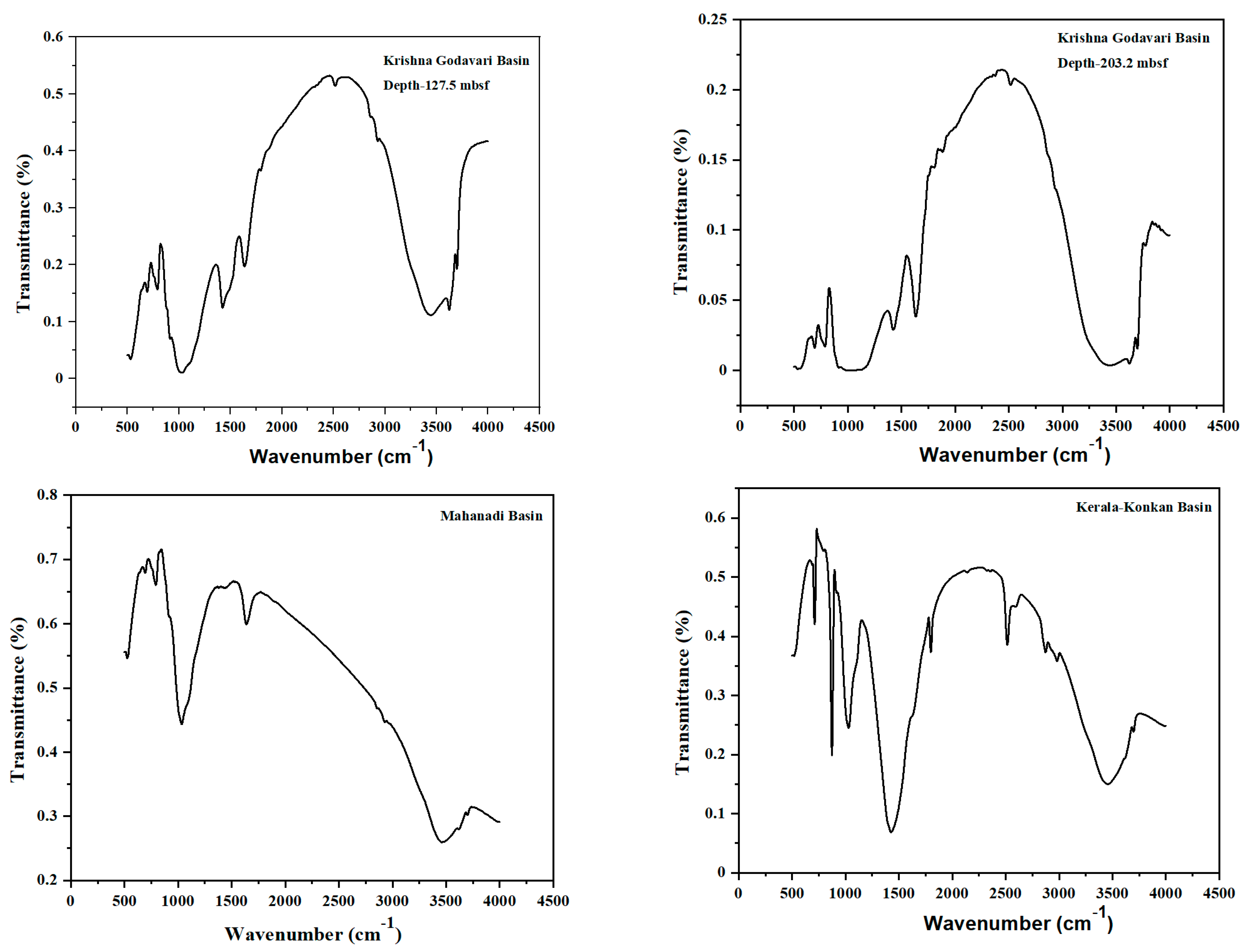
3.4. Fourier Transform Infrared Spectroscopy
3.5. Raman Spectra
3.6. Inductively Coupled Plasma Mass Spectrometry (ICPMS)
3.7. Specific Surface Areas and Cation Exchange Capacities
3.8. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.Y.; Kim, G.Y.; Kang, N.K.; Yi, B.Y.; Jung, J.W.; Im, J.H.; Son, B.K.; Bahk, J.J.; Chun, J.H.; Ryu, B.J.; et al. Physical properties of sediments from the Ulleung Basin, East Sea: Results from Second Ulleung Basin Gas Hydrate Drilling Expedition, East Sea (Korea). Mar. Pet. Geol. 2013, 47, 43–55. [Google Scholar] [CrossRef]
- Arora, A.; Cameotra, S.S.; Kumar, R.; Balomajumder, C.; Singh, A.K.; Santhakumari, B.; Kumar, P.; Laik, S. Biosurfactant as a Promoter of Methane Hydrate Formation: Thermodynamic and Kinetic Studies. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.H.; Kumari, A.; Chandrajit, G.D.; Amit, B.M. Thermodynamic modeling and correlations of CH4, C2H6, CO2, H2S, and N2 hydrates with cage occupancies. J. Pet. Explor. Prod. Technol. 2020. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. A primer on the geological occurrence of gas hydrate. Geol. Soc. Lond. Spec. Publ. 1998, 137, 9–30. [Google Scholar] [CrossRef]
- Bei, K.; Xu, T.; Shang, S.; Wei, Z.; Yuan, Y.; Tian, H. Numerical Modeling of Gas Migration and Hydrate Formation in Heterogeneous Marine Sediments. J. Mar. Sci. Eng. 2019, 7, 348. [Google Scholar] [CrossRef] [Green Version]
- Ecker, C.; Dvorkin, J.; Nur, A. Sediments with gas hydrates: Internal structure from seismic AVO. Geophysics 1998, 63, 1659–1669. [Google Scholar] [CrossRef]
- Arora Swaranjit Singh, A.; Chandrajit, C. Natural Gas Hydrate (Clathrates) as an Untapped Resource of Natural Gas. 2015, Volume 6. Available online: https://www.longdom.org/open-access/natural-gas-hydrate-clathrates-as-an-untapped-resource-of-natural-gas-2157-7463-1000234.pdf (accessed on 26 July 2021).
- Kumari, A.; Khan, S.H.; Misra, A.K.; Majumder, C.B.; Arora, A. Hydrates of Binary Guest Mixtures: Fugacity Model Development and Experimental Validation. J. Non-Equilib. Thermodyn. 2020, 45, 39–58. [Google Scholar] [CrossRef] [Green Version]
- Miyawaki, J.; Kanda, T.; Suzuki, T.; Okui, T.; Maeda, Y.; Kaneko, K. Macroscopic evidence of enhanced formation of methane nanohydrates in hydrophobic nanospaces. J. Phys. Chem. B 1998, 102, 2187–2192. [Google Scholar] [CrossRef]
- Arora, A.; Cameotra, S.S. Techniques for exploitation of gas hydrate (clathrates) an untapped resource of methane gas. J. Microb. Biochem. Technol. 2015, 7. [Google Scholar] [CrossRef]
- Arora, A.; Kumar, A.; Bhattacharjee, G.; Kumar, P.; Balomajumder, C. Effect of different fixed bed media on the performance of sodium dodecyl sulfate for hydrate based CO2 capture. Mater. Des. 2016, 90, 1186–1191. [Google Scholar] [CrossRef]
- MORK, M.; SCHEI, G.; LARSEN, R. NMR Imaging Study of Hydrates in Sediments. Ann. N. Y. Acad. Sci. 2006, 912, 897–905. [Google Scholar] [CrossRef]
- Bahk, J.J.; Kim, D.H.; Chun, J.H.; Son, B.K.; Kim, J.H.; Ryu, B.J.; Torres, M.E.; Riedel, M.; Schultheiss, P. Gas hydrate occurrences and their relation to host sediment properties: Results from Second Ulleung Basin Gas Hydrate Drilling Expedition, East Sea. Mar. Pet. Geol. 2013, 47, 21–29. [Google Scholar] [CrossRef]
- Kwon, T.H.; Lee, K.R.; Cho, G.C.; Lee, J.Y. Geotechnical properties of deep oceanic sediments recovered from the hydrate occurrence regions in the Ulleung Basin, East Sea, offshore Korea. Mar. Pet. Geol. 2011, 28, 1870–1883. [Google Scholar] [CrossRef]
- Winters, W.J.; Wilcox-Cline, R.W.; Long, P.; Dewri, S.K.; Kumar, P.; Stern, L.; Kerr, L. Comparison of the physical and geotechnical properties of gas-hydrate-bearing sediments from offshore India and other gas-hydrate-reservoir systems. Mar. Pet. Geol. 2014, 58, 139–167. [Google Scholar] [CrossRef] [Green Version]
- Nair, V.C.; Prasad, S.K.; Sangwai, J.S. Characterization and Rheology of Krishna-Godavari basin Sediments. Mar. Pet. Geol. 2019, 110, 275–286. [Google Scholar] [CrossRef]
- Jang, J.; Waite, W.F.; Stern, L.A.; Collett, T.S.; Kumar, P. Physical property characteristics of gas hydrate-bearing reservoir and associated seal sediments collected during NGHP-02 in the Krishna-Godavari Basin, in the offshore of India. Mar. Pet. Geol. 2019, 108, 249–271. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Liao, H.; Liu, C.; Chen, Q.; Hu, G.; Liu, L.; Meng, Q. Strength estimation for hydrate-bearing sediments based on triaxial shearing tests. J. Pet. Sci. Eng. 2020, 184, 106478. [Google Scholar] [CrossRef]
- Gabitto, J.F.; Tsouris, C. Physical Properties of Gas Hydrates: A Review. J. Thermodyn. 2010, 2010, 271291. [Google Scholar] [CrossRef] [Green Version]
- Stoll, R.D.; Bryan, G.M. Physical Properties of Sediments Containing Gas Hydrates. J. Geophys. Res. 1979, 84, 1629–1634. [Google Scholar] [CrossRef]
- Mahabadi, N.; Dai, S.; Seol, Y.; Jang, J. Impact of hydrate saturation on water permeability in hydrate-bearing sediments. J. Pet. Sci. Eng. 2019, 174, 696–703. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Wu, P.; Shen, S.; Liu, T.; Leng, S.; Chang, Y.; Zhao, J. Physical and mechanical properties of the overburden layer on gas hydrate-bearing sediments of the South China sea. J. Pet. Sci. Eng. 2020, 189, 107020. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, H.; Lee, J.; Kang, J.M. CO2 hydrate behavior in the deep ocean sediments; phase equilibrium, formation kinetics, and solubility. Geophys. Res. Lett. 2002, 29, 30–31. [Google Scholar] [CrossRef]
- Cha, S.B.; Ouar, H.; Wildeman, T.R.; Sloan, E.D. A third-surface effect on hydrate formation. J. Phys. Chem. 1988, 92, 6492–6494. [Google Scholar] [CrossRef]
- Yuan, Q.; Kong, L.; Xu, R.; Zhao, Y. A State-Dependent Constitutive Model for Gas Hydrate-Bearing Sediments Considering Cementing Effect. J. Mar. Sci. Eng. 2020, 8, 621. [Google Scholar] [CrossRef]
- Uchida, T.; Takeya, S.; Chuvilin, E.M.; Ohmura, R.; Nagao, J.; Yakushev, V.S.; Istomin, V.A.; Minagawa, H.; Ebinuma, T.; Narita, H. Decomposition of methane hydrates in sand, sandstone, clays and glass beads. J. Geophys. Res. Solid Earth 2004, 109, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Winters, W.J.; Pecher, I.A.; Waite, W.F.; Mason, D.H. Physical properties and rock physics models of sediment containing natural and laboratory-formed methane gas hydrate. Am. Mineral. 2004, 89, 1221–1227. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Luo, H.; Song, Y.; Li, Y. Numerical modeling for the mechanical behavior of marine gas hydrate-bearing sediments during hydrate production by depressurization. J. Pet. Sci. Eng. 2019, 177, 971–982. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Kajiyama, S.; Nishimura, A.; Song, Y. A comparative analysis of the mechanical behavior of carbon dioxide and methane hydrate-bearing sediments. Am. Mineral. 2014, 99, 178–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, F.G.; Sun, C.Y.; Li, Q.P.; Wu, X.Y.; Liu, B.; Chen, G.J. Compressional wave velocity measurements through sandy sediments containing methane hydrate. Am. Mineral. 2011, 96, 1425–1432. [Google Scholar] [CrossRef]
- Collett, T.S.; Boswell, R.; Cochran, J.R.; Kumar, P.; Lall, M.; Mazumdar, A.; Ramana, M.V.; Ramprasad, T.; Riedel, M.; Sain, K.; et al. Geologic implications of gas hydrates in the offshore of India: Results of the National Gas Hydrate Program Expedition 01. Mar. Pet. Geol. 2014, 58, 3–28. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yang, M.; Zheng, J.N.; Chen, B. Production characteristics of two class water-excess methane hydrate deposits during depressurization. Fuel 2018, 232, 99–107. [Google Scholar] [CrossRef]
- Hyndman, R.D.; Spence, G.D. A seismic study of methane hydrate marine bottom simulating reflectors. J. Geophys. Res. 1992, 97, 6683–6698. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; Chemical Industries Series pdf; CRC Press: Boca Raton, FL, USA, 1998; Available online: https://www.routledge.com/Clathrate-Hydrates-of-Natural-Gases/Sloan-Jr-Koh-Koh/p/book/9780849390784 (accessed on 26 July 2021).
- Tinivella, U.; Giustiniani, M. Variations in BSR depth due to gas hydrate stability versus pore pressure. Glob. Planet. Chang. 2013, 100, 119–128. [Google Scholar] [CrossRef]
- Shankar, U.; Sain, K.; Riedel, M. Geothermal modeling for the base of gas hydrate stability zone and saturation of gas hydrate in the Krishna-Godavari basin, eastern Indian margin. J. Geol. Soc. India 2012, 79, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Nur, A. BSR and Methane Hydrates: New Challenges for Geophysics and Rock Physics. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 1996; pp. 429–435. [Google Scholar]
- Krishna Godavari–Basin | NDR (National Data Repository)-Directorate General of Hydrocarbons(DGH) | Ministry of Petroleum and Natural Gas, Government of India. 2015. Available online: https://www.ndrdgh.gov.in/NDR/?page_id=647 (accessed on 27 July 2021).
- Kumar, P.; Collett, T.S.; Boswell, R.; Cochran, J.R.; Lall, M.; Mazumdar, A.; Ramana, M.V.; Ramprasad, T.; Riedel, M.; Sain, K.; et al. Geologic implications of gas hydrates in the offshore of India: Krishna-Godavari Basin, Mahanadi Basin, Andaman Sea, Kerala-Konkan Basin. Mar. Pet. Geol. 2014, 58, 29–98. [Google Scholar] [CrossRef] [Green Version]
- Campanile, D.; Nambiar, C.G.; Bishop, P.; Widdowson, M.; Brown, R. Sedimentation record in the Konkan-Kerala Basin: Implications for the evolution of the Western Ghats and the Western Indian passive margin. Basin Res. 2008, 20, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Verma, R.; Silva, K.D.; Banerjee, S.; Bastia, R.; Nathaniel, D.M. Sub-Basalt Hydrocarbon Prospectivity in Kerala-Konkan Offshore Basin, India: A Basin Modeling Approach. Search Discov. 2011, 30157, 1–7. [Google Scholar]
- Kamesh Raju, K.A.; Ramprasad, T.; Rao, P.S.; Ramalingeswara Rao, B.; Varghese, J. New insights into the tectonic evolution of the Andaman basin, northeast Indian Ocean. Earth Planet. Sci. Lett. 2004, 221, 145–162. [Google Scholar] [CrossRef]
- Winters, W.J.; Novosel, I.; Boldina, O.; Waite, W.F.; Kelsey, S.A.; Hallett, B.W. Physical properties of sediment obtained during the IMAGES VIII/PAGE 127 gas hydrate and paleoclimate cruise on the RV Marion Dufresne in the Gulf of Mexico, 2–18 July 2002. In Initial Report of the IMAGES VIII/PAGE 127 Gas Hydrate and Paleoclimate Cruise on the RV Marion Dufresne in the Gulf of Mexico, 2–18 July 2002; US Geological Survey: Boulder, CO, USA, 2002; pp. 1–65. Available online: https://cmgds.marine.usgs.gov/publications/of2004-1358/pdf_chapters/Chapter4.pdf (accessed on 27 July 2021).
- Kumari, A.; Hasan, S.; Majumder, K.C.B.; Arora, A.; Dixit, G. Physio-chemical and mineralogical analysis of gas hydrate bearing sediments of Andaman Basin. Mar. Geophys. Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Sheard, R.W. Sand, Silt and Clay. Sport. Turf Newsl. Available online: https://www.sportsturfcanada.com/ (accessed on 26 July 2021).
- Chibowski, E. Flocculation and Dispersion Phenomena in Soils BT—Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 301–304. ISBN 978-90-481-3585-1. [Google Scholar]
- Gallo, P.; Ricci, M.A.; Rovere, M. Layer analysis of the structure of water confined in vycor glass. J. Chem. Phys. 2002, 116, 342–346. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, L.; Liu, C.; Zhang, Q.; Wu, Q.; Wu, Q.; Liu, C. Influence of sediment media with different particle sizes on the nucleation of gas hydrate. Nat. Gas Ind. B 2018, 5, 652–659. [Google Scholar] [CrossRef]
- Sly, P.G. Sediment dispersion: Part 1, fine sediments and significance of the silt/clay ratio. Hydrobiologia 1989, 176–177, 99–110. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Roy, S.; Kumar, R. Methane hydrate formation in a test sediment of sand and clay at various levels of water saturation. Can. J. Chem. 2015, 93, 874–881. [Google Scholar] [CrossRef]
- Bello-Palacios, A.; Almenningen, S.; Fotland, P.; Ersland, G. Experimental and Numerical Analysis of the Effects of Clay Content on CH4 Hydrate Formation in Sand. Energy Fuels 2021, 35, 9836–9846. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Newton, E.M.; Russ, J. Russ Raman Spectroscopic Analysis of Pigments and Substrata in Prehistoric Rock Art. J. Mol. Struct. 2000, 550–551, 245–256. [Google Scholar] [CrossRef]
- Dronkers, J.; van den Berg, J. Coastal and Marine Sediments. 2021. Available online: http://coastalwiki.org/wiki/Coastal_and_marine_sediments (accessed on 26 July 2021).
- Jeong, S.W.; Locat, J.; Torrance, J.K.; Leroueil, S. Thixotropic and anti-thixotropic behaviors of fine-grained soils in various flocculated systems. Eng. Geol. 2015, 196, 119–125. [Google Scholar] [CrossRef]




| S.No. | Equipment Name | Company/Model No. | Characteristics of Hydrate-Bearing Sediments |
|---|---|---|---|
| 1 | Multi-Parameter Measuring Device | Model No.-KI-266A, Khera Instruments, Delhi, India | Physical Properties (pH, TDS, Salinity) |
| 2 | TOC Analyzer | Shimadzu, TOC-L, Kyoto, Japan | Total Organic Carbon |
| 3 | Oven Drying and Gas Pycnometer | Ultrapyc 5000, Anton Paar, Graz, Austria | Water Content, Density, Porosity |
| 4 | Inductively Coupled Plasma Spectrometer (ICPMS) | Perkin Elmer, ELAN DRC-e-2004, Boston, MA, USA | Elemental Analysis |
| 5 | Laser Particle Size Analyzer | HORIBA Scientific, nanopartica, Nano, Particle Analyzer, SZ-100, Osaka, Japan | Particle Size Distribution |
| 6 | BET | Micromeritics Asap 2060, Norcross, GA, USA | Specific Surface Area |
| 7 | Scanning Electron Microscope (SEM) | TESCAN MIRA3 FEG-SEM, Brno, Czech Republic | Shape and Surface Texture |
| 8 | X-ray Diffraction | Bruker, D8-Advance, 2008 | Mineral Identification |
| 9 | Fourier Transform Infrared Spectroscopy | JASCO, FT/IR-6700 FT-IR Spectrometer, Tokyo, Japan | Mineral Identification |
| 10 | Raman Spectroscopy | Renishaw invia Raman Microscope instrument (Edinburgh, UK) | Structural Identification |
| Sampling Locations | Water Content (%) | Density (g/cm3) | Porosity (%) | Conductivity (mS/cm) | Salinity (ppt) | TDS (ppt) | TOC (ppm) | pH |
|---|---|---|---|---|---|---|---|---|
| KG(1a) | 87.4 | 1.74 | 69.84 | 15.74 | 11.61 | 10.17 | 506.8 | 6.76 |
| KG(1a) | 78.8 | 1.67 | 67.62 | 14.93 | 11.28 | 9.78 | 146.24 | 6.71 |
| Mahanadi | 76.32 | 1.73 | 66.91 | 10.90 | 8.28 | 7.11 | 414.4 | 6.10 |
| KK | 67.2 | 1.61 | 64.03 | 8.78 | 6.52 | 5.63 | 3760.8 | 6.97 |
| Sediments | D10 | D50 | D90 (µm) | Sediments | D10 | D50 | D90 (µm) | ||
|---|---|---|---|---|---|---|---|---|---|
| KG(1a) | min. | 0.52 | 1.63 | 3.52 | KK | min. | 0.48 | 1.79 | 3.81 |
| max. | 2.75 | 58.8 | 678.3 | max. | 2.99 | 13.2 | 278.01 | ||
| KG(1b) | min. | 0.63 | 1.69 | 4.54 | Mahanadi | min. | 0.51 | 1.72 | 3.46 |
| max. | 2.89 | 144.9 | 682.8 | max. | 2.50 | 58.7 | 282.1 | ||
| Sediments | Sand (%) | Silt (%) | Clay-Size (%) | Sediments | Sand (%) | Silt (%) | Clay-Size (%) |
|---|---|---|---|---|---|---|---|
| KG(1a) | 3.35 | 43.76 | 52.01 | KK | 0.43 | 54.09 | 45.42 |
| KG(1b) | 2.37 | 51.23 | 46.29 | Mahanadi | 0.12 | 61.07 | 38.73 |
| Minerals | PDF Index Name | Chemical Formula | 2ϴ | Percentage | |||
|---|---|---|---|---|---|---|---|
| KG(1a) | KG(1a) | Mahanadi | KK | ||||
| Quartz, syn | Silicon Oxide | SiO2 | 26.652 26.640 | 66.34 | 66.88 | 62.81 | 69.37 |
| Calcite, syn | Calcium Carbonate | CaCO3 | 29.406 | 10.9 | 11.09 | 11.2 | 9.87 |
| Halite, syn | Sodium Chloride | NaCl | 31.693 | 7.91 | 8.20 | 8.3 | 6.76 |
| Hematite | Iron Oxide | Fe2O3 | 33.115 | 5.1 | 5.7 | 6.8 | 4.7 |
| Silicon, syn | Silicon | Si | 28.443 | 2.3 | 1.87 | 2.31 | 2.11 |
| Bornite | Copper Iron Sulfide | Cu5FeS4 | 46.945 | 1.9 | 1.63 | 1.89 | 1.01 |
| Eskolaite, syn | Chromium Oxide | Cr2O3 | 33.597 | 2.7 | 1.3 | 1.65 | 1.78 |
| Fluorite, syn | Calcium Fluoride | CaF2 | 47.005 | 1.2 | 1.04 | 2.09 | 1.87 |
| Chromium, syn | Chromium | Cr | 44.393 | 0.7 | 1.01 | 2.1 | 0.91 |
| Corundum, syn | Aluminum Oxide | Al2O3 | 43.363 | 0.5 | 0.3 | 0.6 | 0.67 |
| Wave Number (cm−1) | 792.9 | 1439 | 1150–1270 | 1034 | 875 | 2840–2960 | 490 | 525.8 | 1638 | 3458 |
|---|---|---|---|---|---|---|---|---|---|---|
| Minerals | Quartz | Carbonate | Silica | Kaolinite | Calcite | Smectite | ||||
| Bond | C=O | Si-O-Si | C-H | Si-O | Si-O-Al | Al-O-H |
| Minerals | Chemical Formulation | Color | Characteraistics of Raman Bands (cm−1) |
|---|---|---|---|
| Quartz | SiO2 | Colourless | 148, 357, 465, 1300–1600 |
| Kaolinite | Al2O3 2SiO2·2H2O | White | 130, 143, 245, 270, 336, 394, 418, 431, 463, 3651, 3620, 3669 |
| Hematite | Fe2O3 | Red | 224, 244, 290, 292, 409, 610, 670, 1300 |
| Calcite | CaCO3 | White | 154, 282, 712 1086, 1088 |
| Halite | NaCl | Colourless | 199 |
| Silicon | Si | Blue-Grey Metallic | 480, 520 |
| Bornite | Cu5FeS4 | Copper Red, Bronze Brown, Purple | 265, 291, 320, 354, 470, 472 |
| Sediments Sample | Zn ppm | Mg ppm | Fe Ppm | Cu ppm | K ppm | Mn ppm | Al ppm | Si ppm | Ti ppm |
|---|---|---|---|---|---|---|---|---|---|
| KG(1a) | 54.5468 | 429.3818 | 6459.293 | 12.3342 | 1152.639 | 126.0628 | 3025.748 | 0.2338 | 0.6332 |
| KG(1b) | 21.6668 | 361.3846 | 2291.756 | 5.2406 | 582.3844 | 43.349 | 1286.133 | 16.842 | 1.1428 |
| Mahanadi | 48.9012 | 403.0738 | 4259.515 | 122.473 | 777.1196 | 47.5806 | 2258.604 | 6.5728 | 0.859 |
| KK | 18.6542 | 274.9 | 1153.634 | 4.282 | 441.7348 | 99.0292 | 1008.338 | 8.9012 | 3.61 |
| Sediments Sample | Particle Size (µm) | Clay Mineral and Content (%) | Specific Surface Area (m2/g) | Cation Exchange Capacities (meq/100 g) | ||||
|---|---|---|---|---|---|---|---|---|
| Kaolinite | Smectite | Illite | Kaolinite | Smectite | Illite | |||
| KG(1a) | 0.38–0.86 | 0.9–2.4 | 0.03–0.08 | 14.7 | 6.4 | 68.9 | 20.1 | 8.062 |
| KG(1b) | 15.1 | 6.7 | 65.6 | 22.1 | 5.498 | |||
| Mahanadi | 16.3 | 7.4 | 66.3 | 43.2 | 8.588 | |||
| KK | 12.8 | 5.9 | 59.2 | 19.0 | 7.228 | |||
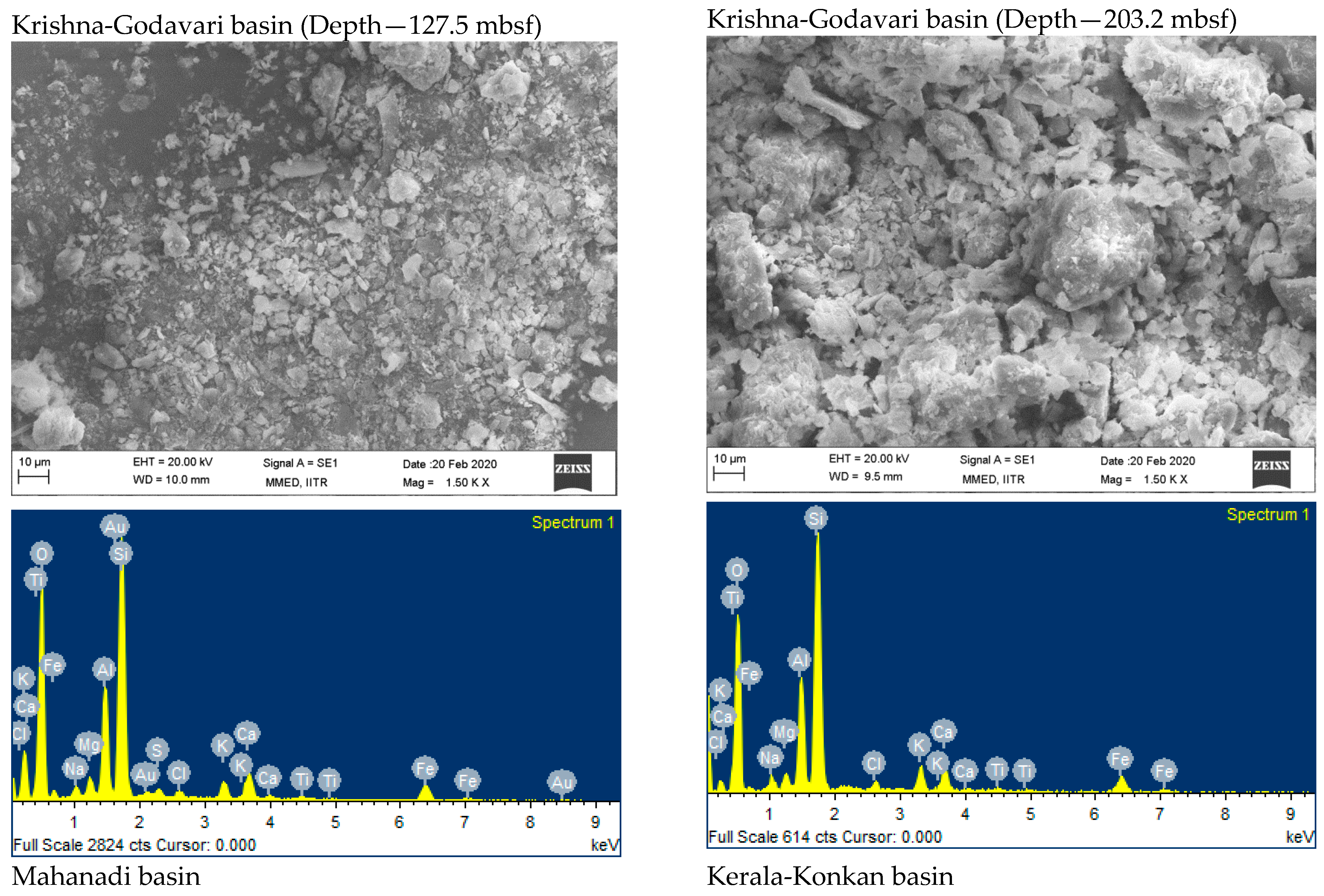
| Elements (wt. %) | Krishna-Godavari Basin Depth—127.5 mbsf | Krishna-Godavari Basin Depth—203.2 mbsf | Mahanadi Basin | Kerala-Konkan Basin |
|---|---|---|---|---|
| O | 60.05 | 53.54 | 67.11 | 67.09 |
| Na | 1.22 | 1.47 | 1.61 | 0.82 |
| Mg | 1.32 | 1.09 | 1.23 | 0.62 |
| Al | 6.89 | 8.82 | 6.23 | 2.03 |
| Si | 16.31 | 23.36 | 18.41 | 5.74 |
| S | 1.02 | ---- | ---- | ----- |
| Cl | 0.69 | 0.90 | 0.66 | 0.42 |
| K | 1.73 | 3.11 | 2.04 | 0.61 |
| Ca | 4.60 | 2.55 | ---- | 21.44 |
| Au | 2.56 | ---- | ----- | ---- |
| Fe | 3.61 | 5.17 | 2.70 | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, A.; Balomajumder, C.; Arora, A.; Dixit, G.; Gomari, S.R. Physio-Chemical and Mineralogical Characteristics of Gas Hydrate-Bearing Sediments of the Kerala-Konkan, Krishna-Godavari, and Mahanadi Basins. J. Mar. Sci. Eng. 2021, 9, 808. https://doi.org/10.3390/jmse9080808
Kumari A, Balomajumder C, Arora A, Dixit G, Gomari SR. Physio-Chemical and Mineralogical Characteristics of Gas Hydrate-Bearing Sediments of the Kerala-Konkan, Krishna-Godavari, and Mahanadi Basins. Journal of Marine Science and Engineering. 2021; 9(8):808. https://doi.org/10.3390/jmse9080808
Chicago/Turabian StyleKumari, Anupama, Chandrajit Balomajumder, Amit Arora, Gaurav Dixit, and Sina Rezaei Gomari. 2021. "Physio-Chemical and Mineralogical Characteristics of Gas Hydrate-Bearing Sediments of the Kerala-Konkan, Krishna-Godavari, and Mahanadi Basins" Journal of Marine Science and Engineering 9, no. 8: 808. https://doi.org/10.3390/jmse9080808
APA StyleKumari, A., Balomajumder, C., Arora, A., Dixit, G., & Gomari, S. R. (2021). Physio-Chemical and Mineralogical Characteristics of Gas Hydrate-Bearing Sediments of the Kerala-Konkan, Krishna-Godavari, and Mahanadi Basins. Journal of Marine Science and Engineering, 9(8), 808. https://doi.org/10.3390/jmse9080808







