A General-Purpose Biotic Index to Measure Changes in Benthic Habitat Quality across Several Pressure Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Computation of the GPBI
2.2. The Four Case Studies
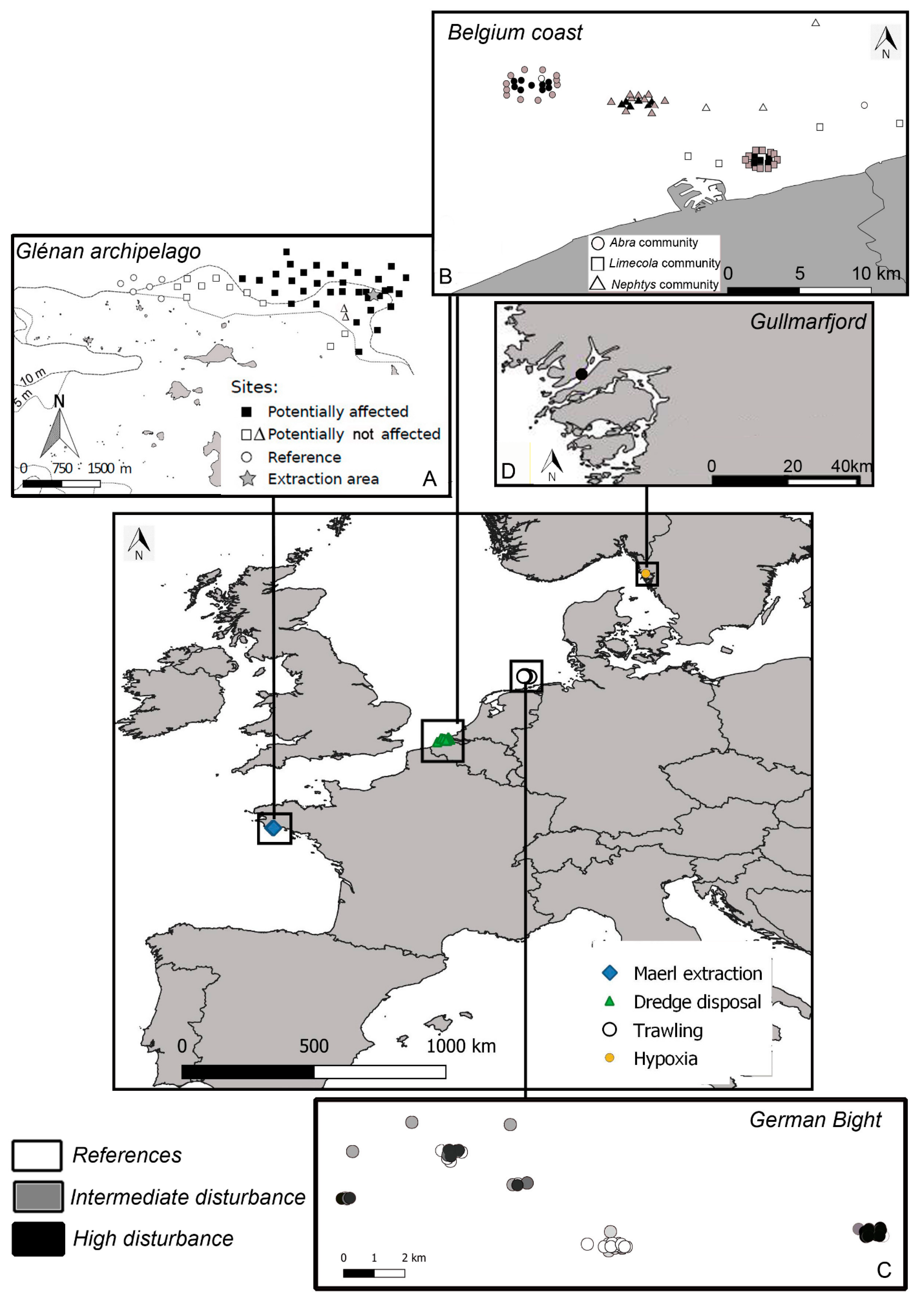
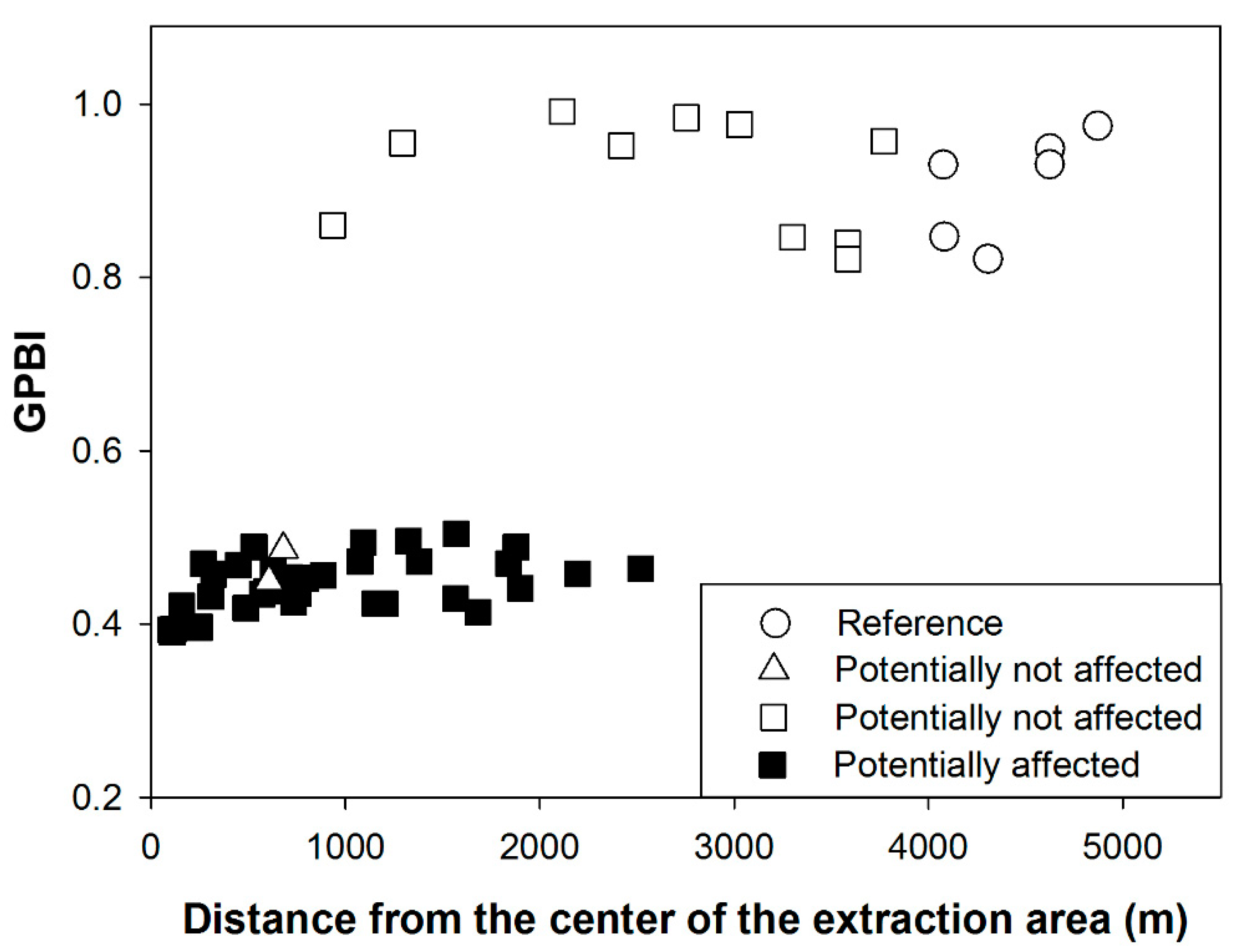
2.2.1. Case Study 1: Maerl Extraction (France, Bay of Biscay)
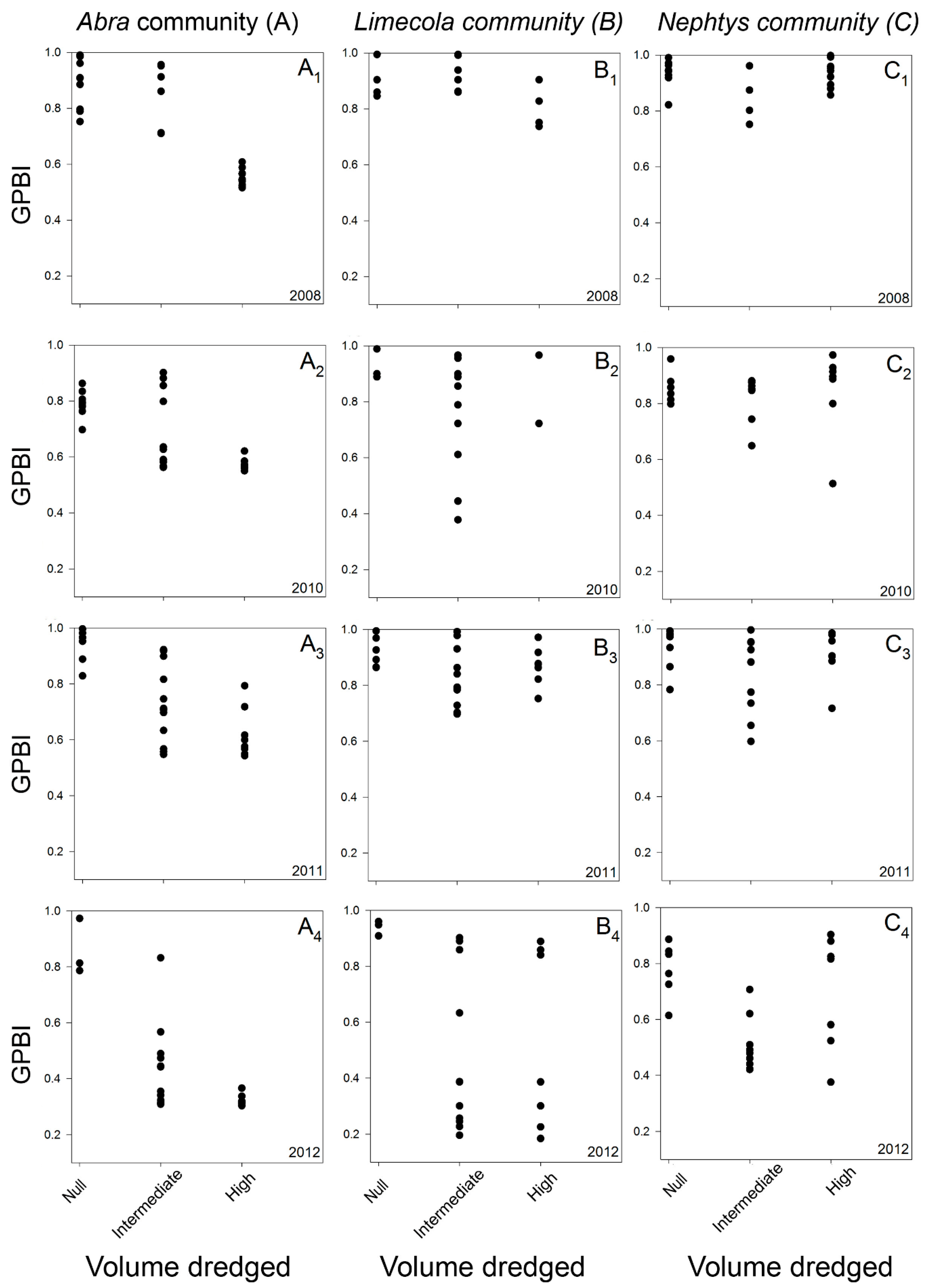
2.2.2. Case Study 2: Dredge Disposal Pressure (Belgium, North Sea)
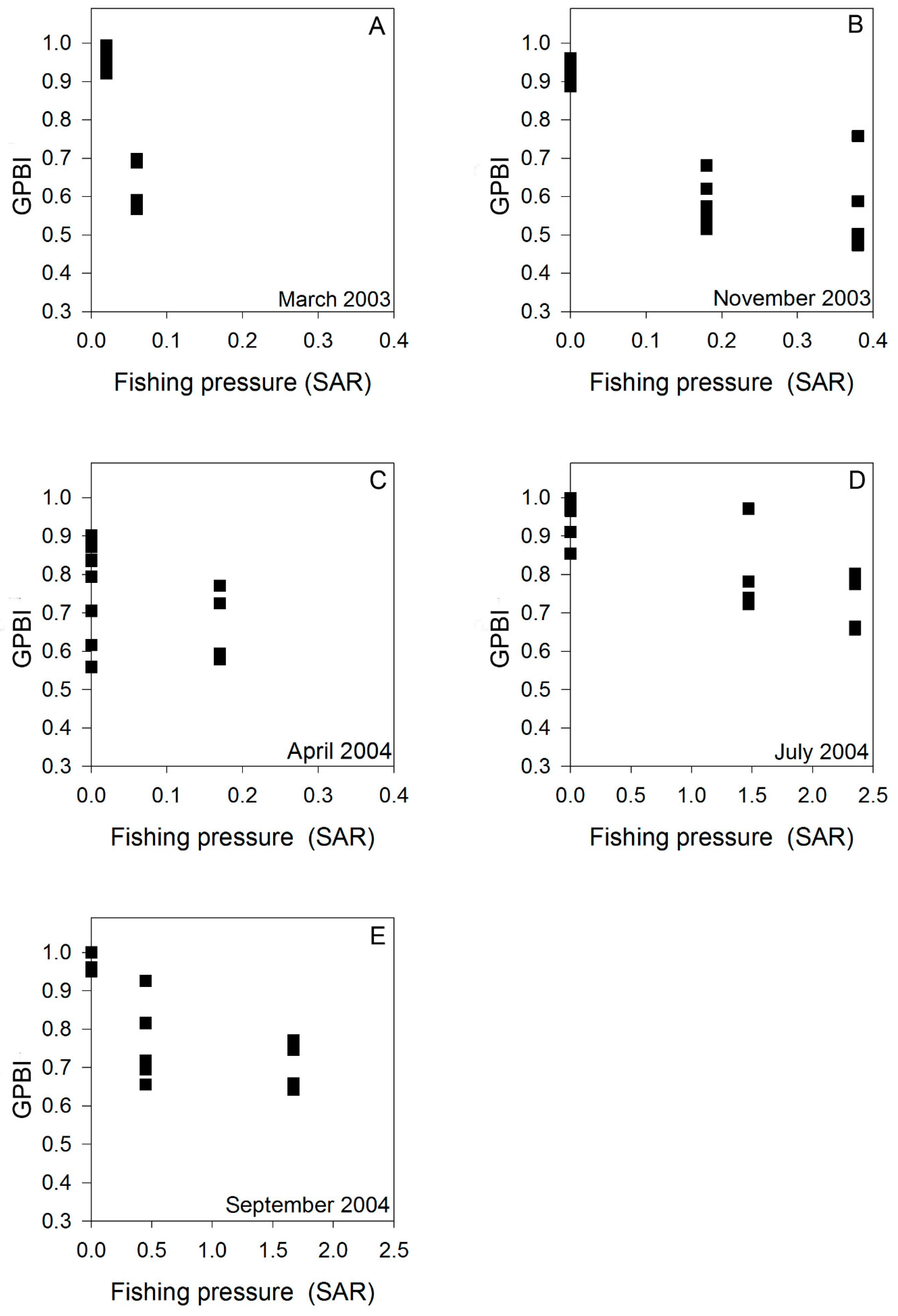
2.2.3. Case Study 3: Trawling Pressure (Germany, North Sea)
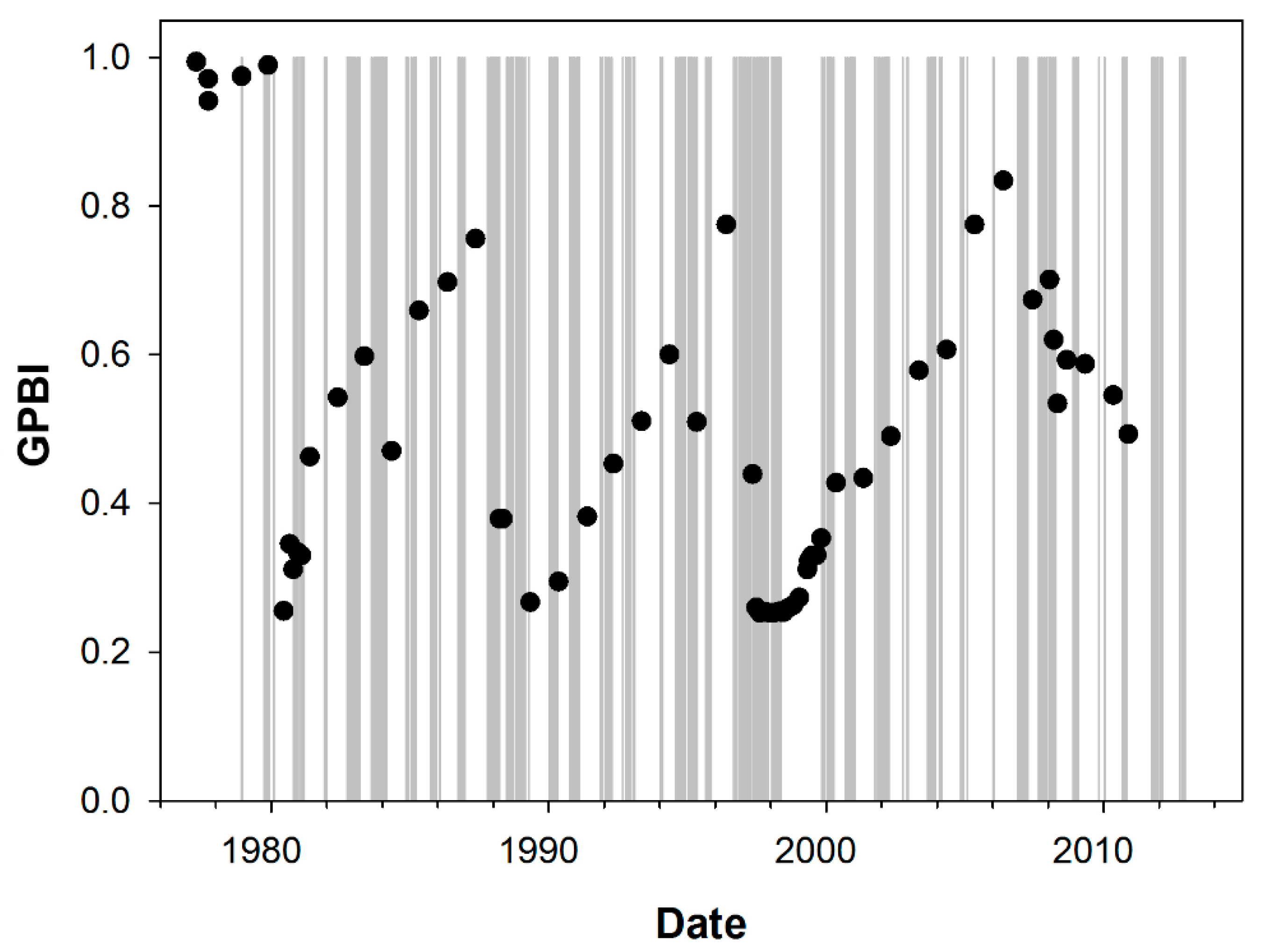
2.2.4. Case Study 4: Hypoxia (Sweden, Gullmarfjord)
2.3. Computation of M-AMBI and TDI
2.4. Performance of the Indices and Calibration of the GPBI
3. Results
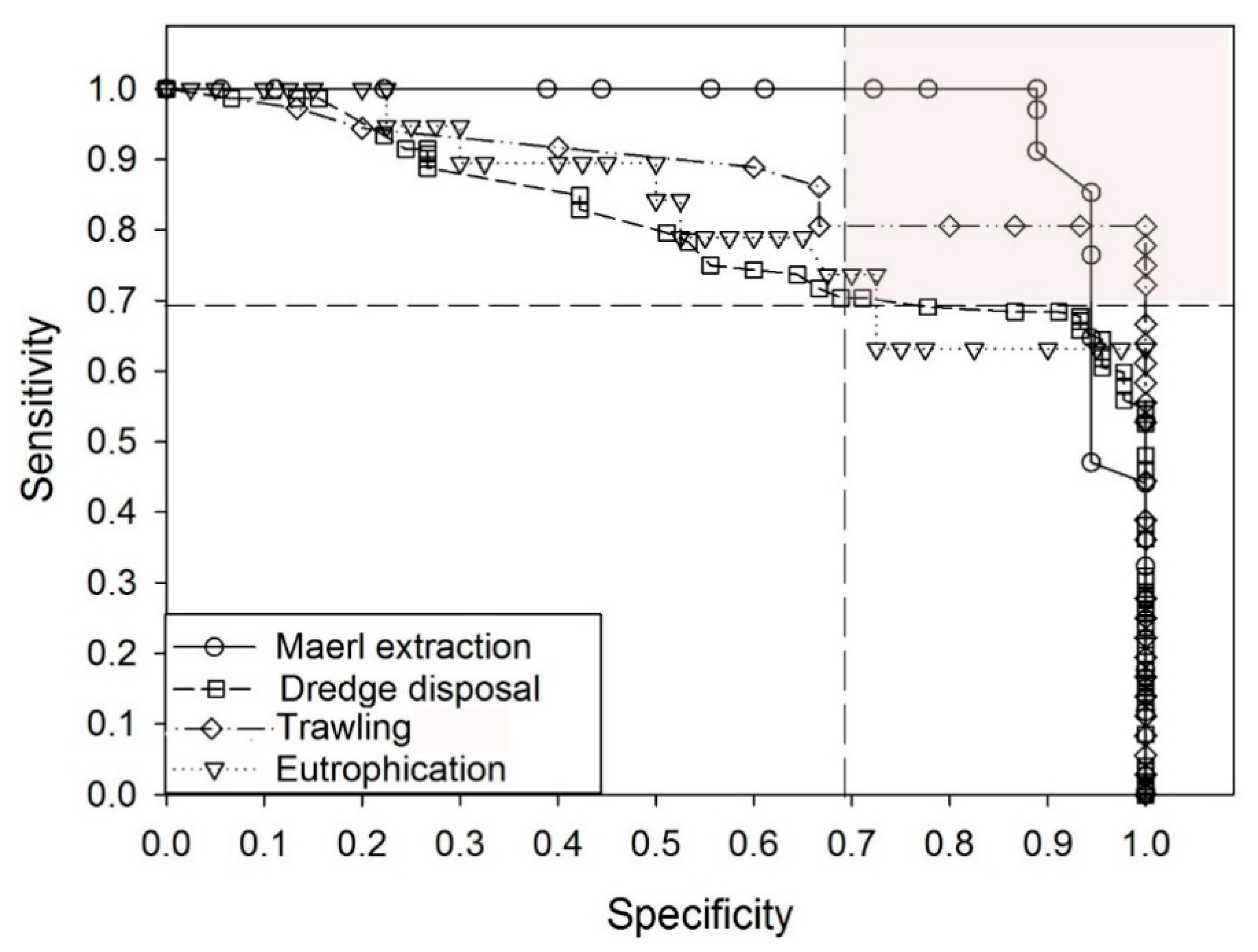
3.1. Performance of the Three Biotic Indices
3.1.1. Case Study 1: Maerl Extraction (France, Bay of Biscay)
3.1.2. Case Study 2: Dredge Disposal Pressure (Belgium, North Sea)
3.1.3. Case Study 3: Trawling Pressure (Germany, North Sea)
3.1.4. Case Study 4: Hypoxia (Sweden, Gullmarfjord)
3.2. Performance of GPBI and Assessment of the Boundary between Good and Moderate EcoQ
4. Discussion
4.1. Performance of the Indices and Ecological Consideration
4.2. Boundary between Good and Moderate EcoQ
4.3. Recommendations for Future Sampling Designs
- Since the computation of GPBI is based on the difference between benthic macrofauna composition and structure at a set of reference and tested stations, it is essential that these are located in the same habitat with similar natural environmental conditions and possibly within a limited geographical range. This condition is indeed necessary for the establishment of a sound relationship between the level of disturbance experienced by the tested station and its faunal composition. Such a habitat-based approach is also required for other biotic indices but is often associated with the stage of conversion of the metric in an EcoQ and not with the computation of the index itself. This issue is particularly important if monitoring areas are characterized by strong “natural” heterogeneity. Future monitoring surveys based on the use of GPBI should adopt a habitat-based sampling design.
- GPBI can be computed using a single reference or a set of reference stations. When using a single reference station, the GPBI of the reference will be 1. When using a set of reference stations, the GPBI of each reference will be 1 minus the difference of its value from the average of the values of GPBI of all reference stations. Results from the present study show that this value is usually slightly smaller than 1 (e.g., between 0.8 and 1 for the maerl case study). This reflects the “natural” variability in benthic macrofauna composition within the habitat of the tested station. Further, monitoring several reference stations allows for the detection of unexpected (local) habitat degradation occurring despite shifts of some of the reference stations. This still enables the assessment of a pressure effect using GPBI based on the remaining monitored references. Moreover, a predisturbance state would serve as a potential temporal reference which could then be compared with the states of the other reference stations. Again, this recommendation is not specific to GPBI, since (1) degradation of a single reference station would affect many biotic indices and (2) current biotic indices based on the assessment of the deviation from a reference state clearly (and explicitly) require a higher number of reference stations [70]. Our recommendation is thus to include several potential reference stations per habitat during future monitoring designs, whenever possible.
- Finding adequate reference stations clearly constitutes a major difficulty when using biotic indices due to the widespread and long-term alteration by pressures of marine coastal habitats, i.e., the problem of shifting baselines [11]. Sometimes, there is no other choice than choosing a “historical reference”, such as in the present Gullmarfjord case study. This is not the best situation because it is well known that coastal benthic ecosystems are shaped by seasonal and long-term dynamics [68,71,72,73]. Our general recommendation is therefore to monitor both potential reference and tested stations over longer time periods to disentangle natural from anthropogenic drivers for index quantification. The comparison of GPBI values between reference stations and the tested station would then allow for the assessment of the effects of local disturbances, whereas temporal changes in the GPBI values of reference stations would allow for the characterization of the effects of natural disturbances and/or long-term dynamics.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Hoey, G.; Borja, A.; Birchenough, S.; Buhl-Mortensen, L.; Degraer, S.; Fleischer, D.; Kerckhof, F.; Magni, P.; Muxika, I.; Reiss, H.; et al. The use of benthic indicators in Europe: From the Water Framework Directive to the Marine Strategy Framework Directive. Mar. Pollut. Bull. 2010, 60, 2187–2196. [Google Scholar] [CrossRef]
- Diaz, R.J.; Solan, M.; Valente, R.M. A review of approaches for classifying benthic habitats and evaluating habitat quality. J. Environ. Manag. 2004, 73, 165–181. [Google Scholar] [CrossRef]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 1978, 16, 229–311. [Google Scholar]
- Borja, Á.; Marín, S.L.; Muxika, I.; Pino, L.; Rodríguez, J.G. Is there a possibility of ranking benthic quality assessment indices to select the most responsive to different human pressures? Mar. Pollut. Bull. 2015, 97, 85–94. [Google Scholar] [CrossRef]
- Grall, J.; Glémarec, M. Using Biotic Indices to Estimate Macrobenthic Community Perturbations in the Bay of Brest. Estuar. Coast. Shelf Sci. 1997, 44 (Suppl. A), 43–53. [Google Scholar] [CrossRef]
- Borja, A.; Franco, J.; Pérez, V. A Marine Biotic Index to Establish the Ecological Quality of Soft-Bottom Benthos Within European Estuarine and Coastal Environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Borja, A.; Muxika, I.; Franco, J. The application of a Marine Biotic Index to different impact sources affecting soft-bottom benthic communities along European coasts. Mar. Pollut. Bull. 2003, 46, 835–845. [Google Scholar] [CrossRef]
- Muxika, I.; Borja, A.; Bald, J. Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 16–29. [Google Scholar] [CrossRef]
- Dauvin, J.C.; Ruellet, T. Polychaete/amphipod ratio revisited. Mar. Pollut. Bull. 2007, 55, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Dauvin, J.C.; Ruellet, T. The Estuarine Quality Paradox: Is it possible to define an Ecological Quality Status for specific modified and naturally stressed estuarine ecosystems? Mar. Pollut. Bull. 2009, 59, 38–47. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Hiddink, J.G.; Jennings, S.; Kaiser, M.J. Assessing and predicting the relative ecological impacts of disturbance on habitats with different sensitivities. J. Appl. Ecol. 2007, 44, 405–413. [Google Scholar] [CrossRef]
- Labrune, C.; Grémare, A.; Amouroux, J.-M.; Sardá, R.; Gil, J.; Taboada, S. Diversity of polychaete fauna in the Gulf of Lions (NW Mediterranean). Vie Et Milieu 2006, 56, 315–326. [Google Scholar]
- Labrune, C.; Grémare, A.; Amouroux, J.-M.; Sardá, R.; Gil, J.; Taboada, S. Assessment of soft-bottom polychaete assemblages in the Gulf of Lions (NW Mediterranean) based on a mesoscale survey. Estuar. Coast. Shelf Sci. 2007, 71, 133–147. [Google Scholar] [CrossRef]
- De Juan, S.; Demestre, M. A Trawl Disturbance Indicator to quantify large scale fushing impact on benthic ecosystems. Ecol. Indic. 2012, 18, 183–190. [Google Scholar] [CrossRef]
- Foveau, A.; Vaz, S.; Desroy, N.; Kostylev, V.E. Process-driven and biological characterisation and mapping of seabed habitats sensitive to trawling. PLoS ONE 2017, 12, e0184486. [Google Scholar] [CrossRef]
- Certain, G.; Jørgensen, L.L.; Christel, I.; Planque, B.; Bretagnolle, V. Mapping the vulnerability of animal community to pressure in marine systems: Disentangling pressure types and integrating their impact from the individual to the community level. ICES J. Mar. Sci. 2015, 72, 1470–1482. [Google Scholar] [CrossRef]
- Jac, C.; Desroy, N.; Certain, G.; Foveau, A.; Labrune, C.; Vaz, S. Detecting adverse effect on seabed integrity. Part 1: Generic sensitivity indices to measure the effect of trawling on benthic mega-epifauna. Ecol. Indic. 2020, 117, 106631. [Google Scholar] [CrossRef]
- Flåten, G.R.; Botnen, H.; Grung, B.; Kvalheim, O.M. Quantifying disturbances in benthic communities--comparison of the community disturbance index (CDI) to other multivariate methods. Ecol. Indic. 2007, 7, 254–276. [Google Scholar] [CrossRef]
- Johnson, R.L.; Perez, K.T.; Rocha, K.J.; Davey, E.W.; Cardin, J.A. Detecting benthic community differences: Influence of statistical index and season. Ecol. Indic. 2008, 8, 582–587. [Google Scholar] [CrossRef]
- de los Ríos, A.; Pérez, L.; Ortiz-Zarragoitia, M.; Serrano, T.; Barbero, M.C.; Echavarri-Erasun, B.; Juanes, J.A.; Orbea, A.; Cajaraville, M.P. Assessing the effects of treated and untreated urban discharges to estuarine and coastal waters applying selected biomarkers on caged mussels. Mar. Pollut. Bull. 2013, 77, 251–265. [Google Scholar] [CrossRef]
- Ruggiero, A.; Lawton, J.H.; Blackburn, T.M. The geographic ranges of mammalian species in South America: Spatial patterns in environmental resistance and anisotropy. J. Biogeogr. 1998, 25, 1093–1103. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence-absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Murtaugh, P.A. The Statistical Evaluation of Ecological Indicators. Ecol. Appl. 1996, 6, 132–139. [Google Scholar] [CrossRef]
- Grall, J.; Hall-Spencer, J. Problems facing maerl conservation in Brittany. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S55–S64. [Google Scholar] [CrossRef]
- De Grave, S.; Whitaker, A. A census of maerl beds in Irish waters. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 303–311. [Google Scholar] [CrossRef]
- SOGREAH. Modélisation du panache turbide de l’extraction in in vivo. Etude et cartographie du banc de maerl du nord de l’archipel des Glénans. In Rapport Ville de Fouesnant; Rapport Ville de Fouesnant: Fouesnant, France, 2004; pp. 3–38. [Google Scholar]
- Lauwaert, B.; De Witte, B.; Devriese, L.; Fettweis, M.; Martens, C.; Steve, T.; Van Hoey, G.; Vanlede, J. Synthesis Report on the Effects of Dredged Material Dumping on the Marine Environment (Licensing Period 2012–2016). 2016. Available online: https://www.researchgate.net/publication/311653421_Synthesis_report_on_the_effects_of_dredged_material_dumping_on_the_marine_environment_licensing_period_2012-2016 (accessed on 12 June 2021).
- Breine, N.; Backer, A.; Van Colen, C.; Moens, T.; Hostens, K.; Van Hoey, G. Structural and functional diversity of soft-bottom macrobenthic communities in the Southern North Sea. Estuar. Coast. Shelf Sci. 2018, 214. [Google Scholar] [CrossRef]
- Dannheim, J. Macrozoobenthic Response to Fishery—Trophic Interactions in Highly Dynamic Coastal Ecosystems. Ph.D. Dissertation, University of Bremen, Bremen, Germany, 2007. Available online: http://nbn-resolving.de/urn:nbn:de:gbv:46-diss000108658 (accessed on 31 July 2020).
- Rijnsdorp, A.D.; Buys, A.M.; Storbeck, F.; Visser, E.G. Micro-scale distribution of beam trawl effort in the southern North Sea between 1993 and 1996 in relation to the trawling frequency of the sea bed and the impact on benthic organisms. ICES J. Mar. Sci. 1998, 55, 403–419. [Google Scholar] [CrossRef]
- Dannheim, J.; Brey, T.; Schröder, A.; Mintenbeck, K.; Knust, R.; Arntz, W.E. Trophic look at soft-bottom communities—Short-term effects of trawling cessation on benthos. J. Sea Res. 2014, 85, 18–28. [Google Scholar] [CrossRef]
- Josefson, A.B.; Blomqvist, M.; Hansen, J.L.S.; Rosenberg, R.; Rygg, B. Assessment of marine benthic quality change in gradients of disturbance: Comparison of different Scandinavian multi-metric indices. Mar. Pollut. Bull. 2009, 58, 1263–1277. [Google Scholar] [CrossRef]
- Josefson, A.B.; Widbom, B. Differential response of benthic macrofauna and meiofauna to hypoxia in the Gullmar Fjord basin. Mar. Biol. 1988, 100, 31–40. [Google Scholar] [CrossRef]
- Nilsson, H.C.; Rosenberg, R. Succession in marine benthic habitats and fauna in response to oxygen deficiency: Analysed by sediment profile-imaging and by grab samples. Mar. Ecol. Prog. Ser. 2000, 197, 139–149. [Google Scholar] [CrossRef]
- Rosenberg, R.; Agrenius, S.; Hellman, B.; Nilsson, H.C.; Norling, K. Recovery of marine benthic habitats and fauna in a Swedish fjord following improved oxygen conditions. Mar. Ecol. Prog. Ser. 2002, 234, 43–53. [Google Scholar] [CrossRef]
- Polovodova Asteman, I.; Nordberg, K. Foraminiferal fauna from a deep basin in Gullmar Fjord: The influence of seasonal hypoxia and the North Atlantic Oscillation. J. Sea Res. 2013, 79, 40–49. [Google Scholar] [CrossRef]
- Gogina, M.; Darr, A.; Zettler, M.L. Approach to assess consequences of hypoxia disturbance events for benthic ecosystem functioning. J. Mar. Syst. 2014, 129, 203–213. [Google Scholar] [CrossRef]
- Bald, J.; Borja, A.; Muxika, I.; Franco, J.; Valencia, V. Assessing reference conditions and physico-chemical status according to the European Water Framework Directive: A case-study from the Basque Country (Northern Spain). Mar. Pollut. Bull. 2005, 50, 1508–1522. [Google Scholar] [CrossRef]
- Foveau, A.; Jac, C.; Llpasset, M.; Guillerme, C.; Desroy, N.; Vaz, S. Updated Biological Traits’ Scoring and Protection Status to Calculate sensItivity to Trawling on Mega-Epibenthic Fauna. 2019. Available online: https://agris.fao.org/agris-search/search.do?recordID=QN2019001269067 (accessed on 12 June 2021).
- Conover, W.J.; Iman, R.L. On Multiple-Comparisons Procedures; Tech. Rep. LA-7677-MS; Los Alamos Scientific Laboratory: New Mexico, NM, USA, 1979. [Google Scholar]
- Hale, S.S.; Heltshe, J.F. Signals from the benthos: Development and evaluation of a benthic index for the nearshore Gulf of Maine. Ecol. Indic. 2008, 8, 338–350. [Google Scholar] [CrossRef]
- Hosmer, D.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley Sons Inc.: New York, NY, USA, 2000; 375p. [Google Scholar] [CrossRef]
- Fitch, J.E.; Cooper, K.M.; Crowe, T.P.; Hall-Spencer, J.M.; Phillips, G. Response of multi-metric indices to anthropogenic pressures in distinct marine habitats: The need for recalibration to allow wider applicability. Mar. Pollut. Bull. 2014, 87, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Labrune, C.; Amouroux, J.M.; Sardá, R.; Dutrieux, E.; Thorin, S.; Rosenberg, R.; Grémare, A. Characterization of the ecological quality of the coastal Gulf of Lions (NW Mediterranean). A comparative approach based on three biotic indices. Mar. Pollut. Bull. 2006, 52, 34–47. [Google Scholar] [CrossRef]
- Teixeira, H.; Salas, F.; Neto, J.M.; Patrício, J.; Pinto, R.; Veríssimo, H.; García-Charton, J.A.; Marcos, C.; Pérez-Ruzafa, A.; Marques, J.C. Ecological indices tracking distinct impacts along disturbance-recovery gradients in a temperate NE Atlantic Estuary-Guidance on reference values. Estuar. Coast. Shelf Sci. 2008, 80, 130–140. [Google Scholar] [CrossRef]
- Barbera, C.; Bordehore, C.; Borg, J.; Glémarec, M.; Grall, J.; Hall-Spencer, J.M.; de la Huz, C.; Lanfranco, E.; Lastra, M.; Moore, P.; et al. Conservation and management of northeast Atlantic and Mediterranean maerl beds. Aquat. Conserv Mar. Freshw. Ecosyst. 2003, 13, S65–S76. [Google Scholar] [CrossRef]
- Powilleit, M.; Kleine, J.; Leuchs, H. Impacts of experimental dredged material disposal on a shallow, sublittoral macrofauna community in Mecklenburg Bay (western Baltic Sea). Mar. Pollut. Bull. 2006, 52, 386–396. [Google Scholar] [CrossRef]
- Bolam, S.G.; Rees, H.L. Minimizing Impacts of Maintenance Dredged Material Disposal in the Coastal Environment: A Habitat Approach. Environ. Manag. 2003, 32, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Jac, C.; Desroy, N.; Certain, G.; Foveau, A.; Labrune, C.; Vaz, S. Detecting adverse effect on seabed integrity. Part 2: How much of seabed habitats are left in good environmental status by fisheries? Ecol. Indic. 2020, 117, 106617. [Google Scholar] [CrossRef]
- Dayton, P.; Thrush, S.; Agardy, T.; Hofman, R. Environmental effects of marine fishing. Aquat. Conserv. -Mar. Freshw. Ecosyst. 1995, 5, 205–232. [Google Scholar] [CrossRef]
- Hiddink, J.; Jennings, S.; Kaiser, M.; Queiros, A.; Duplisea, D.E.; Piet, G. Cumulative Impacts of Seabed Trawl Disturbance on Benthic Biomass, Production, and Species Richness in Different Habitats. Can. J. Fish. Aquat. Sci. 2006, 63, 721–736. [Google Scholar] [CrossRef]
- Reise, K. Long-term changes in the macrobenthic invertebrate fauna of the Wadden Sea: Are Polychaetes about to take over? Neth. J. Sea Res. 1982, 16, 29–36. [Google Scholar] [CrossRef]
- McLaverty, C.; Eigaard, O.R.; Gislason, H.; Bastardie, F.; Brooks, M.E.; Jonsson, P.; Lehmann, A.; Dinesen, G.E. Using large benthic macrofauna to refine and improve ecological indicators of bottom trawling disturbance. Ecol. Indic. 2020, 110, 105811. [Google Scholar] [CrossRef]
- van de Wolfshaar, K.E.; van Denderen, P.D.; Schellekens, T.; van Kooten, T. Food web feedbacks drive the response of benthic macrofauna to bottom trawling. Fish Fish. 2020, 21, 962–972. [Google Scholar] [CrossRef]
- van Loon, W.M.G.M.; Walvoort, D.J.J.; van Hoey, G.; Vina-Herbon, C.; Blandon, A.; Pesch, R.; Schmitt, P.; Scholle, J.; Heyer, K.; Lavaleye, M.; et al. A regional benthic fauna assessment method for the Southern North Sea using Margalef diversity and reference value modelling. Ecol. Indic. 2018, 89, 667–679. [Google Scholar] [CrossRef]
- Hiddink, J.; Kaiser, M.; Sciberras, M.; McConnaughey, R.; Mazor, T.; Hilborn, R.; Collie, J.; Pitcher, C.; Parma, A.; Suuronen, P.; et al. Selection of indicators for assessing and managing the impacts of bottom trawling on seabed habitats. J. Appl. Ecol. 2020, 57. [Google Scholar] [CrossRef]
- Birchenough, S.N.R.; Frid, C.L.J. Macrobenthic succession following the cessation of sewage sludge disposal. J. Sea Res. 2009, 62, 258–267. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926. [Google Scholar] [CrossRef] [PubMed]
- Hall-Spencer, J.M. Conservation issues concerning the molluscan fauna of maerl beds. J. Conchol. Spec. Publ. 1998, 2, 271–286. [Google Scholar]
- Hall-Spencer, J.; Moore, P. Scallop dredging has profound, long-term impacts on maerl habitats. J. Mater. Sci. 2000, 57, 1407–1415. [Google Scholar] [CrossRef]
- Bernard, G.; Romero-Ramirez, A.; Tauran, A.; Pantalos, M.; Deflandre, B.; Grall, J.; Grémare, A. Declining maerl vitality and habitat complexity across a dredging gradient: Insights from in situ sediment profile imagery (SPI). Sci. Rep. 2019, 9, 16463. [Google Scholar] [CrossRef] [PubMed]
- Alve, E.; Lepland, A.; Magnusson, J.; Backer-Owe, K. Monitoring strategies for re-establishment of ecological reference conditions: Possibilities and limitations. Mar. Pollut. Bull. 2009, 59, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Connors, B.M.; Cooper, A.B. Determining Decision Thresholds and Evaluating Indicators when Conservation Status is Measured as a Continuum. Conserv. Biol. 2014, 28, 1626–1635. [Google Scholar] [CrossRef]
- Erlandsson, C.P.; Stigebrandt, A.; Arneborg, L. The sensitivity of minimum oxygen concentrations in a fjord to changes in biotic and abiotic external forcing. Limnol. Oceanogr. 2006, 51, 631–638. [Google Scholar] [CrossRef]
- Borja, Á.; Dauer, D.M.; Grémare, A. The importance of setting targets and reference conditions in assessing marine ecosystem quality. Ecol. Indic. 2012, 12, 1–7. [Google Scholar] [CrossRef]
- Kröncke, I.; Reiss, H. Influence of macrofauna long-term natural variability on benthic indices used in ecological quality assessment. Mar. Pollut. Bull. 2010, 60, 58–68. [Google Scholar] [CrossRef]
- Chuševė, R.; Nygård, H.; Vaičiūtė, D.; Daunys, D.; Zaiko, A. Application of signal detection theory approach for setting thresholds in benthic quality assessments. Ecol. Indic. 2016, 60, 420–427. [Google Scholar] [CrossRef]
- Lavesque, N.; Blanchet, H.; De Montaudouin, X. Development of a multimetric approach to assess perturbation of benthic macrofauna in Zostera noltii beds. J. Exp. Mar. Biol. Ecol. 2009, 368, 101–112. [Google Scholar] [CrossRef]
- Dauvin, J.-C. The Muddy Fine Sand Abra alba-Melinna palmata Community of the Bay of Morlaix Twenty Years After the Amoco Cadiz Oil Spill. Mar. Pollut. Bull. 2000, 40, 528–536. [Google Scholar] [CrossRef]
- Labrune, C.; Grémare, A.; Guizien, K.; Amouroux, J.M. Long-term comparison of soft bottom macrobenthos in the Bay of Banyuls-sur-Mer (Northwestern Mediterranean Sea): A reappraisal. J. Sea Res. 2007, 58, 125–143. [Google Scholar] [CrossRef]
- Romero-Ramirez, A.; Bonifácio, P.; Labrune, C.; Sardá, R.; Amouroux, J.M.; Bellan, G.; Duchêne, J.C.; Hermand, R.; Karakassis, I.; Dounas, C.; et al. Long-term (1998–2010) large-scale comparison of the ecological quality status of gulf of lions (NW Mediterranean) benthic habitats. Mar. Pollut. Bull. 2016, 102, 102–113. [Google Scholar] [CrossRef] [PubMed]
| Disturbance | Dataset | Sampling Area (m2) | Subset | Date of Sampling | No. of Sampling Occasions | Depth Range (m) |
|---|---|---|---|---|---|---|
| Maerl extraction | Atlantic (Glénan, France) | 0.3 | April 2003 | 52 | 6–12m | |
| Dredge disposal | North Sea (Belgium) | 0.1 | Abra 2008 | Autumn 2008 | 26 | |
| Limecola 2008 | 18 | |||||
| Nephtys 2008 | 21 | |||||
| Abra 2010 | 32 | |||||
| Limecola 2010 | 22 | |||||
| Nephtys 2010 | 22 | |||||
| Abra 2011 | 29 | |||||
| Limecola 2011 | 25 | |||||
| Nephtys 2011 | 22 | |||||
| Abra 2012 | 26 | |||||
| Limecola 2012 | 22 | |||||
| Nephtys 2012 | 22 | |||||
| Trawling | German Bight (North Sea, Germany) | 0.3 | HE0185 | March 2003 | 10 | 30 |
| HE0200 | November 2003 | 14 | ||||
| HE0205 | April 2004 | 14 | ||||
| HE0214 | July 2004 | 13 | ||||
| HE0219 | September 2004 | 14 | ||||
| Hypoxia | Gullmarfjord (Sweden) | 0.3–0.4 | 1977–2008 | 59 | 118 |
| GPBI | M-AMBI | TDI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dataset | Subset | N | df | χ | p-Value | χ | p-Value | N | df | χ | p-Value |
| Maerl extraction (Glénan) | 52 | 1 | 29.63 | <0.001 *** | 0.04 | 0.847 | 19 | 1 | 5.76 | 0.016 ** | |
| Dredge disposal (Belgium) | Abra 2008 | 26 | 2 | 18.50 | <0.001 *** | 17.61 | <0.001 *** | 25 | 2 | 15.82 | <0.001 *** |
| Limecola 2008 | 18 | 2 | 8.39 | 0.015 * | 0.43 | 0.805 | 6 | 2 | 0.62 | 0.732 | |
| Nephtys 2008 | 21 | 2 | 3.22 | 0.199 | 0.01 | 0.995 | 20 | 2 | 0.42 | 0.809 | |
| Abra 2010 | 32 | 2 | 15.04 | <0.001 *** | 3.80 | 0.150 | 25 | 2 | 14.89 | <0.001 *** | |
| Limecola 2010 | 22 | 2 | 6.38 | 0.041 * | 1.58 | 0.454 | 5 | 2 | 1.407 | 0.496 | |
| Nephtys 2010 | 22 | 2 | 2.38 | 0.304 | 1.68 | 0.432 | 22 | 2 | 10.05 | 0.006 | |
| Abra 2011 | 29 | 2 | 14.77 | <0.001 *** | 6.77 | 0.034 * | 26 | 2 | 4.65 | 0.097 | |
| Limecola 2011 | 25 | 2 | 5.09 | 0.078 | 5.93 | 0.051 | 7 | 1 | 0.04 | 0.845 | |
| Nephtys 2011 | 22 | 2 | 2.06 | 0.357 | 7.50 | 0.023 | 20 | 2 | 2.64 | 0.267 | |
| Abra 2012 | 26 | 2 | 12.82 | 0.002 ** | 10.70 | 0.005 ** | 24 | 2 | 7.85 | 0.020 * | |
| Limecola 2012 | 22 | 2 | 7.61 | 0.022 * | 4.46 | 0.995 | 3 | ||||
| Nephtys 2012 | 22 | 2 | 9.02 | 0.011 * | 0.92 | 0.630 | 20 | 2 | 8.18 | 0.017 * | |
| Trawling (North Sea) | March 2003 | 10 | 1 | 6.82 | 0.009 ** | 4.81 | 0.028 | 10 | 1 | 0.88 | 0.347 |
| November 2003 | 14 | 2 | 9.10 | 0.010 * | 6.80 | 0.033 | |||||
| April 2004 | 14 | 2 | 6.17 | 0.046 * | 6.65 | 0.031 | |||||
| July 2004 | 13 | 2 | 6.86 | 0.032 * | 5.20 | 0.074 | 13 | 2 | 1.70 | 0.427 | |
| September 2004 | 14 | 2 | 9.23 | 0.009 ** | 4.27 | 0.118 | 13 | 2 | 6.201 | 0.045 * | |
| Hypoxia (Gullmarfjord) | 59 | 1 | 15.93 | <0.001 *** | 23.21 | <0.001 *** | 11 | 1 | 4.82 | 0.028 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labrune, C.; Gauthier, O.; Conde, A.; Grall, J.; Blomqvist, M.; Bernard, G.; Gallon, R.; Dannheim, J.; Van Hoey, G.; Grémare, A. A General-Purpose Biotic Index to Measure Changes in Benthic Habitat Quality across Several Pressure Gradients. J. Mar. Sci. Eng. 2021, 9, 654. https://doi.org/10.3390/jmse9060654
Labrune C, Gauthier O, Conde A, Grall J, Blomqvist M, Bernard G, Gallon R, Dannheim J, Van Hoey G, Grémare A. A General-Purpose Biotic Index to Measure Changes in Benthic Habitat Quality across Several Pressure Gradients. Journal of Marine Science and Engineering. 2021; 9(6):654. https://doi.org/10.3390/jmse9060654
Chicago/Turabian StyleLabrune, Céline, Olivier Gauthier, Anxo Conde, Jacques Grall, Mats Blomqvist, Guillaume Bernard, Régis Gallon, Jennifer Dannheim, Gert Van Hoey, and Antoine Grémare. 2021. "A General-Purpose Biotic Index to Measure Changes in Benthic Habitat Quality across Several Pressure Gradients" Journal of Marine Science and Engineering 9, no. 6: 654. https://doi.org/10.3390/jmse9060654
APA StyleLabrune, C., Gauthier, O., Conde, A., Grall, J., Blomqvist, M., Bernard, G., Gallon, R., Dannheim, J., Van Hoey, G., & Grémare, A. (2021). A General-Purpose Biotic Index to Measure Changes in Benthic Habitat Quality across Several Pressure Gradients. Journal of Marine Science and Engineering, 9(6), 654. https://doi.org/10.3390/jmse9060654







