The Use of Psychoacoustics in Marine Mammal Conservation in the United States: From Science to Management and Policy
Abstract
1. Introduction
2. An Early History of Research Involving the Effects of Sound on Marine Mammals and Its Application in Impact Assessments: Up to 2000
3. Advances in Marine Mammal Psychoacoustic Research and the Consideration of Frequency-Based Auditory Responses in Impact Assessments: Since 2000
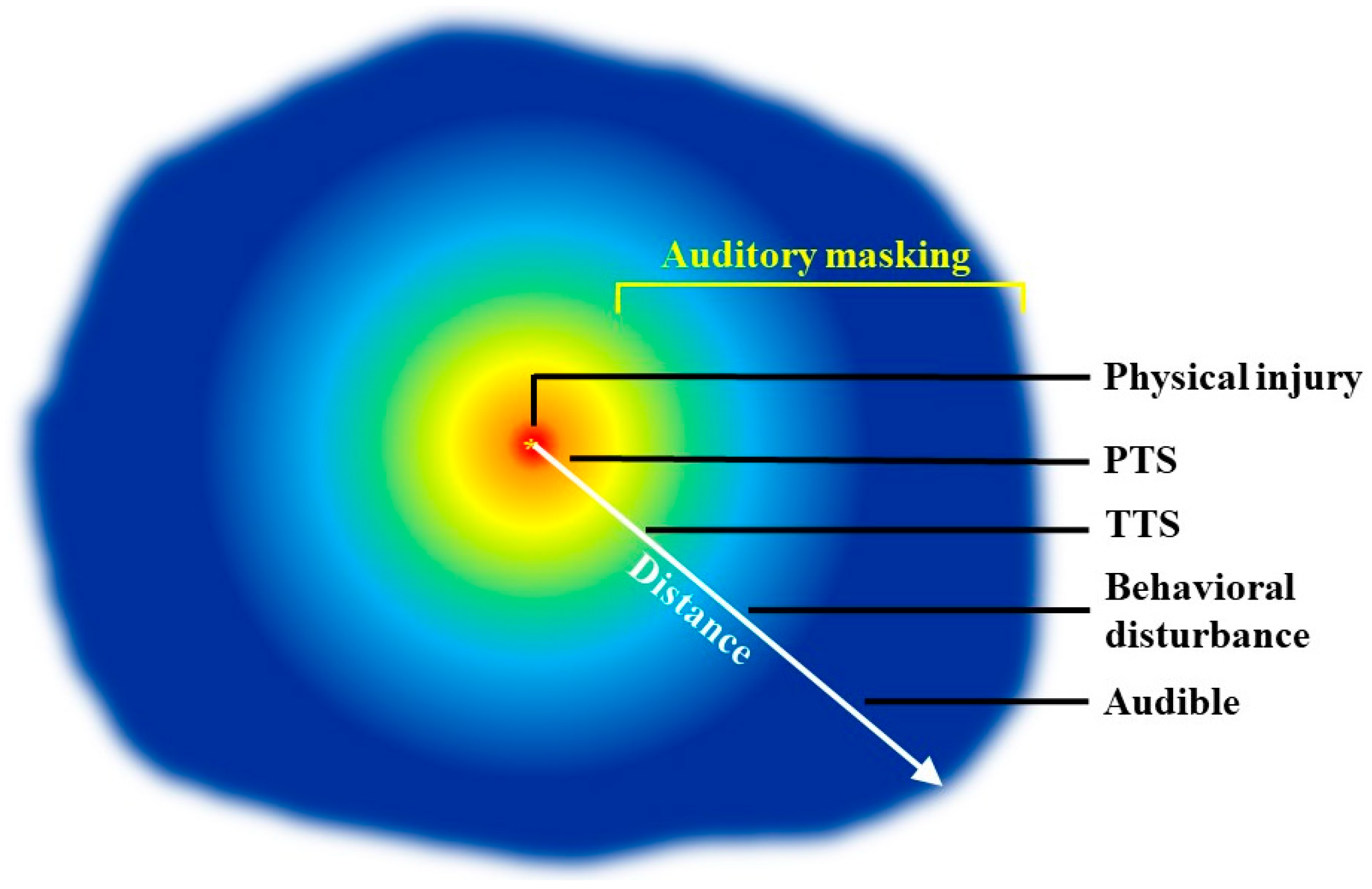
4. Application of Psychoacoustics in the Marine Mammal Regulatory Scheme: Successes and Deficiencies
5. Future Needs in Marine Mammal Psychoacoustic Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The soundscape of the anthropocene ocean. Science 2021, 371. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Marine Mammal. Populations and Ocean. Noise: Determining When Noise Causes Biologically Significant Effects; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Nowacek, D.P.; Thorne, L.H.; Johnston, D.W.; Tyack, P.L. Responses of cetaceans to anthropogenic noise. Mamm. Rev. 2007, 37, 81–115. [Google Scholar] [CrossRef]
- National Research Council. Low-Frequency Sound and Marine Mammals: Current Knowledge and Research Needs; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Shannon, G.; McKenna, M.F.; Angeloni, L.M.; Crooks, K.R.; Fristrup, K.M.; Brown, E.; Warner, K.A.; Nelson, M.D.; White, C.; Briggs, J.; et al. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 2016, 91, 982–1005. [Google Scholar] [CrossRef]
- Richardson, W.J.; Malme, C.I.; Thomson, D.H.; Greene, C.R. Marine Mammals and Noise; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Guan, S.; Brookens, T.; Vignola, J. Use of underwater acoustics in marine conservation and policy: Previous advances, current status, and future needs. J. Mar. Sci. Eng. 2021, 9, 173. [Google Scholar] [CrossRef]
- Lasky, M. Review of undersea acoustics to 1950. J. Acoust. Soc. Am. 1977, 61, 283–297. [Google Scholar] [CrossRef]
- Bjørnø, L. Features of underwater acoustics from Aristotle to our time. Acoust. Phys. 2003, 49, 24–30. [Google Scholar] [CrossRef]
- Schevill, W.E.; Lawrence, B. Underwater listening to the white porpoise (Delphinapterus leucas). Science 1949, 109, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, W.N.; Kohler, R.; Morris, H.N. Porpoise sounds as sonar signals. Science 1953, 117, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Watkins, W.A.; Burns, J.J. The underwater song of Erignathus (bearded seal). Zool. N. Y. Zool. Soc. 1969, 54, 79–83. [Google Scholar]
- Payne, R.S.; McVay, S. Songs of humpback whales. Science 1971, 173, 585–597. [Google Scholar] [CrossRef]
- Payne, R.; Webb, D. Orientation by means of long range acoustic signaling in baleen whales. Ann. N. Y. Acad. Sci. 1971, 188, 110–141. [Google Scholar] [CrossRef] [PubMed]
- Myrberg, A.A., Jr. Ocean noise and the behavior of marine animals: Relationships and implications. In Effects of Noise on Wildlife; Academic Press: New York, NY, USA, 1978; pp. 169–208. [Google Scholar]
- Llewellyn, L.G.; Peiser, C. NEPA and the Environmental Movement: A Brief. History; National Bureau of Standards: Washington, DC, USA, 1973.
- Doub, J.P. The Endangered Species Act.: History, Implementation, Successes, and Controversies, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Roman, J.; Altman, I.; Dunphy-Daly, M.M.; Campbell, C.; Jasny, M.; Read, A.J. The Marine Mammal Protection Act at 40: Status, recovery, and future of U.S. marine mammals. Ann. N. Y. Acad. Sci. 2013, 1286, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, N.; Suckling, K.F.; Hartl, B.; Mehrhoff, L.A. Extinction and the U.S. Endangered Species Act. PeerJ 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Gisiner, R.C. Workshop on the Effects of Anthropogenic Noise in the Marine Environment; Office of Naval Research: Arlington, VA, USA, 1998. [Google Scholar]
- Goertner, J.F. Prediction of Underwater Explosion Safe. Range for Sea Mammals; Naval Surface Weapons Center (CODE R15); White Oak: Silver Spring, MD, USA, 1982. [Google Scholar]
- Richmond, D.; Yelverton, J.; Fletcher, E. Far-Field Underwater-Blast Injuries Produced by Small Charges; Defense Nuclear Agency: Washington, DC, USA, 1973.
- Yelverton, J.T.; Richmond, D.R.; Fletcher, E.R.; Jones, R.K. Safe. Distances from Underwater Explosions for Mammals and Birds; Lovelace Foundation for Medical Education and Research: Albuquerque, NM, USA, 1973. [Google Scholar]
- Ketten, D.R. Estimates of blast injury and acoustic trauma zones for marine mammals from underwater explosions. In Sensory Systems of Aquatic Mammals; Kastelein, R.A., Thomas, J.A., Nachtigall, P.E., Eds.; De Spil Publishers: Woerden, The Netherlands, 1995; pp. 391–407. ISBN 90-72743-05-9. [Google Scholar]
- Todd, S.; Lien, J.; Marques, F.; Stevick, P.; Ketten, D. Behavioural effects of exposure to underwater explosions in humpback whales (Megaptera novaeangliae). Can. J. Zool. 1996, 74, 1661–1672. [Google Scholar] [CrossRef]
- U.S. Navy. Final Environmental Impact Statement: Shock Testing the Seawolf Submarine; Naval Facilities Engineering Command, Southern Division: North Charleston, SC, USA, 1998. [Google Scholar]
- Richardson, W.J. Behavior, Disturbance Responses and Distribution of Bowhead Whales Balaena mysticetus in the Eastern Beaufort Sea, 1982; U.S. Department of the Interior, Minerals Management Service: Reston, VA, USA, 1983.
- Malme, C.I.; Miles, P.R.; Clark, C.W.; Tyack, P.; Bird, J.E. Investigations of the Potential Effects of Underwater Noise from Petroleum Industry Activities on Migrating Gray Whale Behavior; U.S. Department of the Interior, Minerals Management Service: Anchorage, AK, USA, 1983.
- Malme, C.I.; Miles, P.R.; Clark, C.W.; Tyack, P.; Bird, J.E. Investigations of the Potential Effects of Underwater Noise from Petroleum Industry Activities on Migrating Gray Whale Behavior—Phase II: January 1984 Migration; U.S. Department of the Interior, Minerals Management Service: Anchorage, AK, USA, 1984.
- Ljungblad, D.K.; Würsig, B.; Reeves, R.R.; Clarke, J.; Greene, C.R., Jr. Fall 1983 Beaufort Sea Seismic Monitoring and Bowhead Whale Behavior Studies; Minerals Management Service: Anchorage, AK, USA, 1984.
- Malme, C.I.; Miles, P.; Tyack, P.; Clark, C.; Bird, J.E. Investigations of the Potential Effects of Underwater Noise from Petroleum Industry Activities on Feeding Humpback Whale Behavior; U.S. Department of the Interior, Minerals Management Service: Anchorage, AK, USA, 1985.
- Richardson, W.J.; Fraker, M.A.; Würsig, B.; Wells, R.S. Behaviour of bowhead whales Balaena mysticetus summering in the Beaufort Sea: Reactions to industrial activities. Biol. Conserv. 1985, 32, 195–230. [Google Scholar] [CrossRef]
- Fraker, M.A.; Ljungblad, D.K.; Richardson, W.J.; Van Schoik, D.R. Bowhead Whale Behavior in Relation to Seismic Exploration, Alaska Beaufort Sea, Autumn 1981; U.S. Department of the Interior, Minerals Management Service: Reston, VA, USA, 1985.
- Richardson, W.J. Behavior, Disturbance Responses and Distribution of Bowhead Whales Balaena mysticetus in the Eastern Beaufort Sea. 1980–1984; LGL Ecological Research Associates, Inc.: Bryan, TX, USA, 1985. [Google Scholar]
- Richardson, W.J.; Würsig, B.; Greene, C.R. Reactions of bowhead whales, Balaena mysticetus, to seismic exploration in the Canadian Beaufort Sea. J. Acoust. Soc. Am. 1986, 79, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.J.; Davis, R.A.; Evans, C.R.; Ljungblad, D.K.; Norton, P. Summer distribution of bowhead whales, Balaena mysticetus, relative to oil industry activities in the Canadian Beaufort Sea, 1980–1984. Arctic 1987, 40, 93–104. [Google Scholar] [CrossRef]
- Ljungblad, D.K.; Würsig, B.; Swartz, S.L.; Keene, J.M. Observations on the behavioral responses of bowhead whales (Balaena mysticetus) to active geophysical vessels in the Alaskan Beaufort Sea. Arctic 1988, 41, 183–194. [Google Scholar] [CrossRef]
- Johnson, S.R.; Burns, J.J.; Malme, C.I.; Davis, R.A. Synthesis of Information on the Effects of Noise and Disturbance on Major Haulout Concentrations of Bering Sea Pinnipeds; U.S. Department of the Interior, Minerals Management Service: Anchorage, AK, USA, 1989.
- Richardson, W.J.; Greene, C.R., Jr.; Hanna, J.S.; Koski, W.R.; Miller, G.W.; Patenaude, N.J.; Smultea, M.A. Acoustic Effects of Oil Production Activities on Bowhead and White Whales Visible during Spring Migration Near Pt. Barrow, Alaska—1991 and 1994 Phases: Sound Propagation and Whale Responses to Playbacks of Icebreaker Noise; Mineral Management Service: Herndon, VA, USA, 1995.
- Myrberg, A.A. The effects of man-made noise on the behavior of marine animals. Environ. Int. 1990, 16, 575–586. [Google Scholar] [CrossRef]
- National Research Council. Marine Mammals and Low-Frequency Sound: Progress Since 1994; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Cavanagh, R.C. Criteria and Thresholds for Adverse Effects of Underwater Noise on Marine Animals; Air Force Research Laboratory: Dayton, OH, USA, 2000. [Google Scholar]
- High Energy Seismic Survey. High Energy Seismic Survey Review Process and Interim Operational Guidelines for Marine Surveys Offshore Southern California; The High Energy Seismic Survey Team: Santa Barbara, CA, USA, 1999.
- Scholik-Schlomer, A.R. Where the decibels hit the water: Perspectives on the application of science to real-world underwater noise and marine protected species issues. Acoust. Today 2015, 11, 36–44. [Google Scholar]
- Richardson, W.J.; Würsig, B.; Greene, C.R. Reactions of bowhead whales, Balaena mysticetus, to drilling and dredging noise in the Canadian Beaufort Sea. Mar. Environ. Res. 1990, 29, 135–160. [Google Scholar] [CrossRef]
- Kryter, K.D. Effects of Noise on Man; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Nixon, C.W.; Krantz, D.W.; Johnson, D.L. Human Temporary Threshold Shift and Recovery from 24 Hour Acoustic Exposures; Defense Technical Information Center: Fort Belvoir, VA, USA, 1975.
- Davis, H. Acoustic Trauma in the Guinea Pig. J. Acoust. Soc. Am. 1953, 25, 1180–1189. [Google Scholar] [CrossRef]
- Peters, E.N. Temporary Shifts in auditory thresholds of chinchilla after exposure to noise. J. Acoust. Soc. Am. 1965, 37, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.D.; Eldredge, D.H.; Templer, J.W. Temporary threshold shifts in chinchilla: Electrophysiological correlates. J. Acoust. Soc. Am. 1972, 52, 1115–1123. [Google Scholar] [CrossRef]
- U.S. Navy. Draft Overseas Environmental Impact Statement and Environmental Impact Statement for Surveillance Towed Array Sensor System Low Frequency Active (SURTASS LFA) Sonar; Department of the Navy, Chief of Naval Operations: Arlington, VA, USA, 1999. [Google Scholar]
- Advanced Research Projects Agency. Final Environmental Impact Statement for the Kauai Acoustic Thermometry of Ocean. Climate Project and Its Associated Marine Mammal. Research Program. (Scientific Research Permit Application [P557E]); Advanced Research Projects Agency: Arlington, VA, USA, 1995; Volume 1.
- Kastak, D.; Schusterman, R.J. Temporary threshold shift in a harbor seal (Phoca vitulina). J. Acoust. Soc. Am. 1996, 100, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, S.H.; Carter, D.A.; Smith, R.R.; Kamolnick, T.; Schlundt, C.E. Behavioral Responses and Temporary Shift in Masked Hearing Threshold of Bottlenose Dolphins, Tursiops truncatus, to 1-Second Tones of 141 to 201 dB re 1 µPa; Naval Command, Control, and Ocean Surveillance Center, RDT&E Division: San Diego, CA, USA, 1997.
- Kastak, D.; Schusterman, R.J.; Southall, B.L.; Reichmuth, C.J. Underwater temporary threshold shift induced by octave-band noise in three species of pinniped. J. Acoust. Soc. Am. 1999, 106, 1142–1148. [Google Scholar] [CrossRef]
- Finneran, J.J.; Schlundt, C.E.; Carder, D.A.; Clark, J.A.; Young, J.A.; Gaspin, J.B.; Ridgway, S.H. Auditory and behavioral responses of bottlenose dolphins (Tursiops truncatus) and a beluga whale (Delphinapterus leucas) to impulsive sounds resembling distant signatures of underwater explosions. J. Acoust. Soc. Am. 2000, 108, 417–431. [Google Scholar] [CrossRef]
- Schlundt, C.E.; Finneran, J.J.; Carder, D.A.; Ridgway, S.H. Temporary shift in masked hearing thresholds of bottlenose dolphins, Tursiops truncatus, and white whales, Delphinapterus leucas, after exposure to intense tones. J. Acoust. Soc. Am. 2000, 107, 3496–3508. [Google Scholar] [CrossRef]
- Finneran, J.J.; Schlundt, C.E.; Dear, R.; Carder, D.A.; Ridgway, S.H. Temporary shift in masked hearing thresholds in odontocetes after exposure to single underwater impulses from a seismic watergun. J. Acoust. Soc. Am. 2002, 111, 2929–2940. [Google Scholar] [CrossRef]
- Nachtigall, P.E.; Pawloski, J.L.; Au, W.W.L. Temporary threshold shifts and recovery following noise exposure in the Atlantic bottlenosed dolphin (Tursiops truncatus). J. Acoust. Soc. Am. 2003, 113, 3425–3429. [Google Scholar] [CrossRef]
- Nachtigall, P.E.; Supin, A.Y.; Pawloski, J.; Au, W.W.L. Temporary threshold shifts after noise exposure in the bottlenose dolphin (Tursiops truncatus) measured using evoked auditory potentials. Mar. Mamm. Sci. 2004, 20, 673–687. [Google Scholar] [CrossRef]
- Finneran, J.J.; Schlundt, C.E.; Branstetter, B.; Dear, R.L. Assessing temporary threshold shift in a bottlenose dolphin (Tursiops truncatus) using multiple simultaneous auditory evoked potentials. J. Acoust. Soc. Am. 2007, 122, 1249–1264. [Google Scholar] [CrossRef]
- Lucke, K.; Siebert, U.; Lepper, P.A.; Blanchet, M.-A. Temporary shift in masked hearing thresholds in a harbor porpoise (Phocoena phocoena) after exposure to seismic airgun stimuli. J. Acoust. Soc. Am. 2009, 125, 4060–4070. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Klishin, V.O.; Nechaev, D.I.; Pletenko, M.G.; Rozhnov, V.V.; Supin, A.Y.; Sysueva, E.V.; Tarakanov, M.B. Influence of acoustic noises on the white whale hearing thresholds. Dokl. Biol. Sci. 2011, 440, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Supin, A.Y.; Wang, D.; Wang, K.; Dong, L.; Wang, S. Noise-induced temporary threshold shift and recovery in Yangtze finless porpoises Neophocaena phocaenoides asiaeorientalis. J. Acoust. Soc. Am. 2011, 130, 574–584. [Google Scholar] [CrossRef]
- Finneran, J.J. Noise-induced hearing loss in marine mammals: A review of temporary threshold shift studies from 1996 to 2015. J. Acoust. Soc. Am. 2015, 138, 1702–1726. [Google Scholar] [CrossRef]
- Wartzok, D.; Ketten, D. Marine Mammal Sensory Systems. In Biology of Marine Mammals; Smithsonian Institute Press: Washington, DC, USA, 1999; pp. 117–175. [Google Scholar]
- Houser, D.S.; Helweg, D.A.; Moore, P.W.B. A bandpass filter-bank model of auditory sensitivity in the humpback whale. Aquat. Mamm. 2001, 27, 82–92. [Google Scholar]
- Balcomb, K.C., III; Claridge, D.E. A mass stranding of cetaceans caused by naval sonar in the Bahamas. Bahamas J. Sci. 2001, 8, 1–12. [Google Scholar]
- Evans, D.L.; England, G.R. Joint Interim Report Bahamas Marine Mammal. Stranding Event of 15–16 March 2000; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2001.
- Schrope, M. Whale deaths caused by US Navy’s sonar. Nature 2002, 415, 106. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Gisiner, R.C.; Ketten, D.R.; Hammock, J.A.; Johnson, C.; Tyack, P.L.; Mead, J. Beaked whale strandings and naval exercises. Aquat. Mamm. 2009, 35, 452–472. [Google Scholar] [CrossRef]
- Filadelfo, R.; Pinelis, Y.K.; Davis, S.; Chase, R.; Mintz, J.; Wolfanger, J.; Tyack, P.L.; Ketten, D.; D’Amico, A. Correlating military sonar use with beaked whale mass strandings: What do the historical data show? Aquat. Mamm. 2009, 35, 445–451. [Google Scholar] [CrossRef]
- Jepson, P.D.; Arbelo, M.; Deaville, R.; Patterson, I.A.P.; Castro, P.; Baker, J.R.; Degollada, E.; Ross, H.M.; Herráez, P.; Pocknell, A.M.; et al. Gas-bubble lesions in stranded cetaceans. Nature 2003, 425, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Jepson, P.D.; Deaville, R.; Patterson, I.A.P.; Pocknell, A.M.; Ross, H.M.; Baker, J.R.; Howie, F.E.; Reid, R.J.; Colloff, A.; Cunningham, A.A. Acute and chronic gas bubble lesions in cetaceans stranded in the United Kingdom. Vet. Pathol. 2005, 42, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Edwards, J.F.; Rodríguez, F.; de los Monteros, A.E.; Herráez, P.; Castro, P.; Jaber, J.R.; Martín, V.; Arbelo, M. “Gas and fat embolic syndrome” involving a mass stranding of beaked whales (family Ziphiidae) exposed to anthropogenic sonar signals. Vet. Pathol. 2005, 42, 446–457. [Google Scholar] [CrossRef]
- Southall, B. Biological and Behavioral Response Studies of Marine Mammals in Southern California, 2011 (“SOCAL-11”) Final Project Report; Naval Postgraduate School: Monterey, CA, USA, 2012. [Google Scholar]
- Tyack, P.; Gordon, J.; Thompson, D. Controlled-exposure experiments to determine the effects of noise on marine mammals. Mar. Technol. Soc. J. 2003, 37, 39–51. [Google Scholar] [CrossRef][Green Version]
- Tyack, P.L.; Zimmer, W.M.X.; Moretti, D.; Southall, B.L.; Claridge, D.E.; Durban, J.W.; Clark, C.W.; D’Amico, A.; DiMarzio, N.; Jarvis, S.; et al. Beaked whales respond to simulated and actual Navy sonar. PLoS ONE 2011, 6, e17009. [Google Scholar] [CrossRef]
- Falcone, E.A.; Schorr, G.S.; Watwood, S.L.; DeRuiter, S.L.; Zerbini, A.N.; Andrews, R.D.; Morrissey, R.P.; Moretti, D.J. Diving behaviour of Cuvier’s beaked whales exposed to two types of military sonar. R. Soc. Open Sci. 2017, 4, 170629. [Google Scholar] [CrossRef]
- Sivle, L.D.; Kvadsheim, P.H.; Fahlman, A.; Lam, F.P.A.; Tyack, P.L.; Miller, P.J.O. Changes in dive behavior during naval sonar exposure in killer whales, long-finned pilot whales, and sperm whales. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef]
- DeRuiter, S.L.; Southall, B.L.; Calambokidis, J.; Zimmer, W.M.X.; Sadykova, D.; Falcone, E.A.; Friedlaender, A.S.; Joseph, J.E.; Moretti, D.; Schorr, G.S.; et al. First direct measurements of behavioural responses by Cuvier’s beaked whales to mid-frequency active sonar. Biol. Lett. 2013, 9, 20130223. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Southall, B.L.; DeRuiter, S.L.; Calambokidis, J.; Friedlaender, A.S.; Hazen, E.L.; Falcone, E.A.; Schorr, G.S.; Douglas, A.; Moretti, D.J.; et al. Blue whales respond to simulated mid-frequency military sonar. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130657. [Google Scholar] [CrossRef]
- Stimpert, A.K.; DeRuiter, S.L.; Southall, B.L.; Moretti, D.J.; Falcone, E.A.; Goldbogen, J.A.; Friedlaender, A.; Schorr, G.S.; Calambokidis, J. Acoustic and foraging behavior of a Baird’s beaked whale, Berardius bairdii, exposed to simulated sonar. Sci. Rep. 2014, 4, 7031. [Google Scholar] [CrossRef] [PubMed]
- Sivle, L.D.; Kvadsheim, P.H.; Curé, C.; Isojunno, S.; Wensveen, P.J.; Lam, F.-P.A.; Visser, F.; Kleivane, L.; Tyack, P.L.; Harris, C.M.; et al. Severity of expert-identified behavioural responses of humpback whale, minke whale, and northern bottlenose whale to naval sonar. Aquat. Mamm. 2015, 41, 469–502. [Google Scholar] [CrossRef]
- Southall, B.; Nowacek, D.; Miller, P.; Tyack, P. Experimental field studies to measure behavioral responses of cetaceans to sonar. Endang. Species. Res. 2016, 31, 293–315. [Google Scholar] [CrossRef]
- Curé, C.; Isojunno, S.; Visser, F.; Wensveen, P.; Sivle, L.; Kvadsheim, P.; Lam, F.; Miller, P. Biological significance of sperm whale responses to sonar: Comparison with anti-predator responses. Endang. Species. Res. 2016, 31, 89–102. [Google Scholar] [CrossRef]
- Southall, B.L.; DeRuiter, S.L.; Friedlaender, A.; Stimpert, A.K.; Goldbogen, J.A.; Hazen, E.; Casey, C.; Fregosi, S.; Cade, D.E.; Allen, A.N.; et al. Behavioral responses of individual blue whales (Balaenoptera musculus) to mid-frequency military sonar. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [PubMed]
- Southall, B.L.; Bowles, A.E.; Ellison, W.T.; Finneran, J.J.; Gentry, R.L.; Greene, C.R., Jr.; Kastak, D.K.; Ketten, D.R.; Miller, J.H.; Nachtigall, P.E.; et al. Marine mammal noise exposure criteria: Initial scientific recommendations. Aquat. Mamm. 2007, 33, 411–521. [Google Scholar] [CrossRef]
- Harris, C.M. Handbook of Acoustical Measurements and Noise Control, 3rd ed.; American Institute of Physics: Woodbury, NY, USA, 1998; ISBN 978-1-56396-774-0. [Google Scholar]
- Wood, J.; Southall, B.; Tollit, D. PG&E Offshore 3-D Seismic Survey Project EIR—Marine Mammal. Technical Report; SMRU Ltd.: Fife, Scotland, UK, 2012. [Google Scholar]
- National Marine Fisheries Service. Taking and importing marine mammals; Taking marine mammals incidental to geophysical surveys related to oil and gas activities in the Gulf of Mexico. Fed. Reg. 2021, 86, 5322–5450. [Google Scholar]
- Finneran, J.J.; Jenkins, A.K. Criteria and Thresholds for U.S. Navy Acoustic and Explosive Effects Analysis; Space and Naval Warfare Systems Center Pacific: San Diego, CA, USA, 2012.
- Finneran, J.J.; Schlundt, C.E. Subjective loudness level measurements and equal loudness contours in a bottlenose dolphin (Tursiops truncatus). J. Acoust. Soc. Am. 2011, 130, 3124–3136. [Google Scholar] [CrossRef]
- Feller, W. An Introduction to Probability Theory and Its Applications, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1968; Volume 1. [Google Scholar]
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of Regulations to the U.S. Navy to Take Marine Mammals Incidental to Conducting Training, Testing, and Routine Military Operations that Use Surveillance Towed Array Sensor System Low Frequency Active Sonar; Marine Mammal Commission: Bethesda, MD, USA, 2019. Available online: https://www.mmc.gov/wp-content/uploads/19-04-01-Harrison-Navy-SURTASS-LFA-PR.pdf (accessed on 20 March 2021).
- Finneran, J.J. Auditory Weighting Functions and TTS/PTS Exposure Functions for Marine Mammals Exposed to Underwater Noise; Space and Naval Warfare Systems Center Pacific: San Diego, CA, USA, 2016.
- National Marine Fisheries Service. Technical Guidance for Assessing the Effects of Anthropogenic Sound on Marine Mammal. Hearing: Underwater Acoustic Thresholds for Onset of Permanent and Temporary Threshold Shifts; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2016; p. 178.
- National Marine Fisheries Service. 2018 Revision to: Technical Guidance for Assessing the Effects of Anthropogenic Sound on Marine Mammal. Hearing (Version 2.0): Underwater Thresholds for Onset of Permanent and Temporary Threshold Shifts; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2018; p. 167. Available online: https://www.fisheries.noaa.gov/national/marinemammal-protection/marine-mammal-acoustic-technical-guidance (accessed on 29 January 2021).
- Southall, B.L.; Finneran, J.J.; Reichmuth, C.; Nachtigall, P.E.; Ketten, D.R.; Bowles, A.E.; Ellison, W.T.; Nowacek, D.P.; Tyack, P.L. Marine mammal noise exposure criteria: Updated scientific recommendations for residual hearing effects. Aquat. Mamm. 2019, 45, 125–232. [Google Scholar] [CrossRef]
- U.S. Navy. Criteria and Thresholds for U.S. Navy Acoustic and Explosive Effects Analysis (Phase III); SSC Pacific: San Diego, CA, USA, 2017. [Google Scholar]
- Ellison, W.T.; Southall, B.L.; Clark, C.W.; Frankel, A.S. A new context-based approach to assess marine mammal behavioral responses to anthropogenic sounds. Conserv. Biol. 2012, 26, 21–28. [Google Scholar] [CrossRef]
- National Marine Fisheries Service. Manual for Optional USER SPREADSHEET TOOL (Version 2.2, December) for 2018 Revision to: Technical Guidance for Assessing the Effects of Anthropogenic Sound on Marine Mammal. Hearing (Version 2.0): Underwater Thresholds for Onset of Permanent and Temporary Threshold Shifts; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2020. Available online: https://media.fisheries.noaa.gov/2020-12/User_Manual%20_DEC_2020_508.pdf?null (accessed on 13 February 2021).
- Sivle, L.D.; Kvadsheim, P.H.; Ainslie, M.A. Potential for population-level disturbance by active sonar in herring. ICES J. Mar. Sci. 2015, 72, 558–567. [Google Scholar] [CrossRef]
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of the Technical Guidance for Assessing the Effects of Anthropogenic Sound on Marine Mammal. Hearing: Underwater Acoustic Thresholds for Onset of Permanent and Temporary Threshold Shifts; Marine Mammal Commission: Bethesda, MD, USA, 2017. Available online: https://www.mmc.gov/wp-content/uploads/17-07-11-Bettridge-NMFS-Technical-Guidance.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of an Incidental Harassment Authorization to Vineyard Wind, LLC, to Take Marine Mammals Incidental to Construction of Commercial Wind Energy Turbines and Associated Facilities off Massachusetts; Marine Mammal Commission: Bethesda, MD, USA, 2019. Available online: https://www.mmc.gov/wp-content/uploads/19-06-03-Harrison-NMFS-Vineyard-Wind-wind-farm-construction-IHA.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of an Incidental Harassment Authorization to Chesapeake Tunnel Joint Venture to take marine mammals incidental to conducting construction activities for the Parallel Thimble Shoal Tunnel Bridge. Project in Virginia; Marine Mammal Commission: Bethesda, MD, USA, 2019. Available online: https://www.mmc.gov/wp-content/uploads/19-12-26-Harrison-Chesapeake-Tunnel-Joint-Venture-IHA.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of an Incidental Harassment Authorization to Virginia Electric and Power Company d/b/a/ Dominion Energy Virginia to Take Marine Mammals Incidental to Construction of Wind Energy Turbines off the Coast. of Virginia; Marine Mammal Commission: Bethesda, MD, USA, 2020. Available online: https://www.mmc.gov/wp-content/uploads/20-04-15-Harrison-Dominion-wind-energy-construction-IHA.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Incidental Harassment Authorization Renewal to Port. of Kalama to Renew. its Authorization to Take Marine Mammals Incidental to Construction of the Kalama Manufacturing and Marine Export Facility on the Columbia River in Washington; Marine Mammal Commission: Bethesda, MD, USA, 2020. Available online: https://www.mmc.gov/wp-content/uploads/20-11-05-Harrison-Port-of-Kalama-IHA-renewal.pdf (accessed on 20 March 2021).
- Frankel, A.S.; Ellison, W.T.; Buchanan, J. Application of the Acoustic Integration Model (AIM) to predict and minimize environmental impacts. In Proceedings of the Oceans ’02 MTS/IEEE, Biloxi, MI, USA, 29–31 October 2002; pp. 1438–1443. [Google Scholar]
- Houser, D.S. A method for modeling marine mammal movement and behavior for environmental impact assessment. IEEE J. Ocean. Eng. 2006, 31, 76–81. [Google Scholar] [CrossRef]
- U.S. Navy. Determination of Acoustic Effects on Marine Mammals and Sea Turtles for the Hawaii-Southern California Training and Testing Environmental Impact Statement/Overseas Environmental Impact Statement; Naval Undersea Warfare Center Division: Newport, RI, USA, 2012. [Google Scholar]
- Ellison, W.; Racca, R.; Clark, C.; Streever, B.; Frankel, A.; Fleishman, E.; Angliss, R.; Berger, J.; Ketten, D.; Guerra, M.; et al. Modeling the aggregated exposure and responses of bowhead whales Balaena mysticetus to multiple sources of anthropogenic underwater sound. Endang. Species. Res. 2016, 30, 95–108. [Google Scholar] [CrossRef]
- Blackstock, S.A.; Fayton, J.O.; Hulton, P.H.; Moll, T.E.; Jenkins, K.K.; Kotecki, S.; Henderson, E.; Rider, S.; Martin, C.; Bowman, V. Quantifying Acoustic Impacts on Marine Mammals and Sea Turtles: Methods and Analytical Approach for Phase III Training and Testing; Naval Undersea Warfare Center Division: Newport, RI, USA, 2017. [Google Scholar]
- Denes, S.L.; Zeddies, D.G.; Weirathmueller, M.J. Turbine Foundation and Cable Installation at South. Fork Wind Farm: Underwater Acoustic Modeling of Construction Noise; JASCO Applied Sciences: Silver Spring, MD, USA, 2018. [Google Scholar]
- Matthews, M.-N.R.; Ireland, D.S.; Zeddies, D.G.; Brune, R.H.; Pyć, C.D. A modeling comparison of the potential effects on marine mammals from sounds produced by marine vibroseis and air gun seismic sources. J. Mar. Sci. Eng. 2021, 9, 12. [Google Scholar] [CrossRef]
- Shaffer, S.A.; Costa, D.P. A database for the study of marine mammal behavior: Gap analysis, data standardization, and future directions. IEEE J. Ocean. Eng. 2006, 31, 82–86. [Google Scholar] [CrossRef]
- Frankel, A.S.; Vigness-Raposa, K.; Giard, J.; White, A.; Ellison, W.T. Exposures v. individuals: Effects of varying movement patterns and animal behavior on long-term animat model exposure predictions. Proc. Meet. Acoust. 2016, 27, 10038. [Google Scholar] [CrossRef]
- Borcuk, J.R.; Mitchell, G.H.; Watwood, S.L.; Moll, T.E.; Oliveira, E.M.; Robinson, E.R. Dive Distribution and Group Size Parameters for Marine Species Occurring in the U.S. Navy’s Atlantic and Hawaii-Southern California Training and Testing Study Areas; NUWC-NPT Technical Report; Naval Undersea Warfare Center Division: Newport, RI, USA, 2017. [Google Scholar]
- National Marine Fisheries Service. Taking and importing marine mammals; taking marine mammals incidental to Southwest Fisheries Science Center Fisheries Research. Fed. Reg. 2020, 85, 53606–53640. [Google Scholar]
- National Marine Fisheries Service. Takes of marine mammals incidental to specified activities; taking marine mammals incidental to site characterization surveys off the coast of Massachusetts. Fed. Reg. 2021, 86, 11930–11947. [Google Scholar]
- U.S. Fish and Wildlife Service. Marine mammals; incidental take during specified activities; proposed incidental harassment authorization for Pacific walruses and polar bears in Alaska and associated Federal waters. Fed. Reg. 2017, 82, 25304–25322. [Google Scholar]
- U.S. Fish and Wildlife Service. Marine mammals; incidental take during specified activities: Cook Inlet, Alaska. Fed. Reg. 2019, 84, 10224–10251. [Google Scholar]
- U.S. Fish and Wildlife Service. Marine Mammals; Incidental Take During Specified Activities; Proposed Incidental Harassment Authorizations for Northern Sea Otters in Southeast Alaska. Fed. Reg. 2019, 84, 32932–32945. [Google Scholar]
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on U.S. Navy’s Draft Supplemental Environmental Impact Statement/Overseas Environmental Impact Statement for Training Activities Conducted within the Temporary Maritime Activities Area in the Gulf of Alaska; Marine Mammal Commission: Bethesda, MD, USA, 2021. Available online: https://www.mmc.gov/wp-content/uploads/21-01-04-Naval-Facilities-Engineering-Command-Northwest-GOA-Phase-III-DSEIS.pdf (accessed on 20 March 2021).
- Tyack, P.L.; Thomas, L. Using dose–response functions to improve calculations of the impact of anthropogenic noise. Aquat. Conser. Mar. Freshwater Ecosys. 2019, 29, 242–253. [Google Scholar] [CrossRef]
- Danil, K.; St. Leger, J.A. Seabird and dolphin mortality associated with underwater detonation exercises. Mar. Technol. Soc. J. 2011, 45, 89–95. [Google Scholar] [CrossRef]
- National Marine Fisheries Service. Takes of marine mammals incidental to specified activities; taking marine mammals incidental to Washington State Department of Transportation Purdy Bridge Rehabilitation Project, Pierce County, WA. Fed. Reg. 2020, 85, 81886–81904. [Google Scholar]
- National Marine Fisheries Service. Takes of marine mammals incidental to specified activities; taking marine mammals incidental to Gastineau Channel Historical Society Sentinel Island moorage float project, Juneau, Alaska. Fed. Reg. 2020, 85, 18196–18213. [Google Scholar]
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance for an Incidental Harassment Authorization to Lamont-Doherty Earth Observatory to Take Marine Mammals Incidental to Conducting Two Marine Geophysical Surveys in the North. Pacific Ocean; Marine Mammal Commission: Bethesda, MD, USA, 2018. Available online: https://www.mmc.gov/wp-content/uploads/18-07-20-Harrison-LDEO-HI-IHA.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of Regulations to U.S. Navy to take Marine Mammals Incidental to Conducting Training and Testing Activities in the Hawaii-Southern California Training and Testing Study Area; Marine Mammal Commission: Bethesda, MD, USA, 2018. Available online: https://www.mmc.gov/wp-content/uploads/18-07-13-Harrison-Navy-HSTT-PR-Phase-III.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on U.S. Navy’s Draft Supplemental Environmental Impact Statement/Overseas Environmental Impact Statement to Conduct Training and Research, Development, Testing, and Evaluation Activities within the Northwest. Training and Testing Study Area; Marine Mammal Commission: Bethesda, MD, USA, 2019. Available online: https://www.mmc.gov/wp-content/uploads/19-04-15-Naval-Facilities-Engineering-Command-Northwest-NWTT-DSEIS.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of an Incidental Harassment Authorization to U.S. Army Corps of Engineers, Portland District to Take Marine Mammals Incidental to Replacing Dike Markers in the Columbia River. 11 September 2019; Marine Mammal Commission: Bethesda, MD, USA, 2019. Available online: https://www.mmc.gov/wp-content/uploads/19-09-11-Harrision-USACE-CR-marker-IHA.pdf (accessed on 20 March 2021).
- Marine Mammal Commission. Comments and Recommendations from the U.S. Marine Mammal. Commission on National Marine Fisheries Service’s Proposed Issuance of an Incidental Harassment Authorization to Jordan Cove Energy Project, LP to Take Marine Mammals Incidental to Construction of the Jordan Cove Liquefied Natural Gas. (LNG) Facility and Ancillary Activities in Coos Bay, Oregon; Marine Mammal Commission: Bethesda, MD, USA, 2019. Available online: https://www.mmc.gov/wp-content/uploads/19-12-18-Harrison-NMFS-proposed-IHA-Jordan-Cove-LNG_corrected.pdf (accessed on 20 March 2021).
- Ketten, D.R. Structure and function in whale ears. Bioacoustics 1997, 8, 103–135. [Google Scholar] [CrossRef]
- Parks, S.E.; Ketten, D.R.; O’Malley, J.T.; Arruda, J. Anatomical predictions of hearing in the North Atlantic right whale. Anat. Rec. 2007, 290, 734–744. [Google Scholar] [CrossRef]
- Mooney, T.A.; Yamato, M.; Branstetter, B.K. Chapter Four—Hearing in Cetaceans: From Natural History to Experimental Biology. In Advances in Marine Biology; Lesser, M., Ed.; Academic Press: New York, NY, USA, 2012; Volume 63, pp. 197–246. [Google Scholar]
- Cranford, T.W.; Krysl, P. Fin whale sound reception mechanisms: Skull vibration enables low-frequency hearing. PLoS ONE 2015, 10, e0116222. [Google Scholar] [CrossRef]
- Tubelli, A.; Zosuls, A.; Ketten, D.; Mountain, D.C. Prediction of a mysticete audiogram via finite element analysis of the middle ear. In The Effects of Noise on Aquatic Life II; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2015; Volume 875, pp. 57–59. [Google Scholar]
- Tubelli, A.A.; Zosuls, A.; Ketten, D.R.; Mountain, D.C. A model and experimental approach to the middle ear transfer function related to hearing in the humpback whale (Megaptera novaeangliae). J. Acoust. Soc. Am. 2018, 144, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.L.H.; Varela, R.A.; Goldstein, J.D.; McCulloch, S.D.; Bossart, G.D.; Finneran, J.J.; Houser, D.; Mann, D.A. Beaked whale auditory evoked potential hearing measurements. J. Comp. Physiol. A 2006, 192, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Finneran, J.J.; Houser, D.S.; Mase-Guthrie, B.; Ewing, R.Y.; Lingenfelser, R.G. Auditory evoked potentials in a stranded Gervais’ beaked whale (Mesoplodon europaeus). J. Acoust. Soc. Am. 2009, 126, 484–490. [Google Scholar] [CrossRef]
- Pacini, A.F.; Nachtigall, P.E.; Quintos, C.T.; Schofield, T.D.; Look, D.A.; Levine, G.A.; Turner, J.P. Audiogram of a stranded Blainville’s beaked whale (Mesoplodon densirostris) measured using auditory evoked potentials. J. Exp. Biol. 2011, 214, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.D.; Glorig, A.; Sklar, D.L. Temporary threshold shift from octave-band noise: Applications to damage-risk criteria. J. Acoust. Soc. Am. 1959, 31, 522–528. [Google Scholar] [CrossRef]
- Luz, G.A.; Hodge, D.C. Recovery from impulse-noise induced TTS in monkeys and men: A descriptive model. J. Acoust. Soc. Am. 1971, 49, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, W.J.; Lipowczan, A. Impulse noise-induced hearing loss in drop forge operators and the energy concept. Noise Control. Eng. 1982, 18, 24–29. [Google Scholar] [CrossRef]
- Hamernik, R.P.; Patterson, J.H.; Salvi, R.J. The Effect of impulse intensity and the number of impulses on hearing and cochlear pathology in the chinchilla. J. Acoust. Soc. Am. 1987, 81, 1118–1129. [Google Scholar] [CrossRef]
- Dunn, D.E.; Davis, R.R.; Merry, C.J.; Franks, J.R. Hearing loss in the chinchilla from impact and continuous noise exposure. J. Acoust. Soc. Am. 1991, 90, 1979–1985. [Google Scholar] [CrossRef]
- Hamernik, R.P.; Qiu, W. Energy-independent factors influencing noise-induced hearing loss in the chinchilla model. J. Acoust. Soc. Am. 2001, 110, 3163–3168. [Google Scholar] [CrossRef]
- Guan, S.; Miner, R. Underwater noise characterization of down-the-hole pile driving activities off Biorka Island, Alaska. Mar. Pollut. Bull. 2020, 160, 111664. [Google Scholar] [CrossRef]
- Qiu, W.; Davis, B.; Hamernik, R.P. Hearing loss from interrupted, intermittent, and time varying Gaussian noise exposures: The applicability of the equal energy hypothesis. J. Acoust. Soc. Am. 2007, 121, 1613–1620. [Google Scholar] [CrossRef]
- Hamernik, R.P.; Qiu, W.; Davis, B. Hearing loss from interrupted, intermittent, and time Varying non-Gaussian noise exposure: The applicability of the equal energy hypothesis. J. Acoust. Soc. Am. 2007, 122, 2245–2254. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, W.; Zeng, L.; Chen, S.; Cheng, X.; Davis, R.I.; Hamernik, R.P. Application of the kurtosis statistic to the evaluation of the risk of hearing loss in workers exposed to high-level complex noise. Ear Hear. 2010, 31, 527–532. [Google Scholar] [CrossRef]
- Qiu, W.; Hamernik, R.P.; Davis, R.I. The value of a kurtosis metric in estimating the hazard to hearing of complex industrial noise exposures. J. Acoust. Soc. Am. 2013, 133, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Qiu, W.; Heyer, N.J.; Zhang, M.; Zhang, P.; Zhao, Y.; Hamernik, R.P. The use of the kurtosis-adjusted cumulative noise exposure metric in evaluating the hearing loss risk for complex noise. Ear Hear. 2016, 37, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Thomas, L.; Marques, T.; Harwood, J.; Dilley, A.; Neales, B.; Shaffer, J.; McCarthy, E.; New, L.; Jarvis, S.; et al. A risk function for behavioral disruption of Blainville’s beaked whales (Mesoplodon densirostris) from mid-frequency active sonar. PLoS ONE 2014, 9, e85064. [Google Scholar] [CrossRef]
- Miller, P.J.O.; Antunes, R.N.; Wensveen, P.J.; Samarra, F.I.P.; Catarina Alves, A.; Tyack, P.L.; Kvadsheim, P.H.; Kleivane, L.; Lam, F.-P.A.; Ainslie, M.A.; et al. Dose-response relationships for the onset of avoidance of sonar by free-ranging killer whales. J. Acoust. Soc. Am. 2014, 135, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.J.; Miller, G.W.; Greene, C.R. Displacement of migrating bowhead whales by sounds from seismic surveys in shallow waters of the Beaufort Sea. J. Acoust. Soc. Am. 1999, 106, 2281. [Google Scholar] [CrossRef]
- Miller, G.W.; Moulton, V.D.; Davis, R.A.; Holst, M.; Millman, P.; MacGillivray, A.; Hannay, D. Monitoring seismic effects on marine mammals—southeastern Beaufort Sea, 2001–2002. In Offshore Oil and Gas Environmental Effects Monitoring: Approaches and Technologies; Armsworthy, S.L., Cranford, P.J., Lee, K., Eds.; Battelle Press: Columbus, OH, USA, 2005; pp. 511–542. [Google Scholar]
- Miller, P.J.O.; Johnson, M.P.; Madsen, P.T.; Biassoni, N.; Quero, M.; Tyack, P.L. Using at-sea experiments to study the effects of airguns on the foraging behavior of sperm whales in the Gulf of Mexico. Deep Sea Res. Part. I 2009, 56, 1168–1181. [Google Scholar] [CrossRef]
- Blackwell, S.B.; Nations, C.S.; McDonald, T.L.; Thode, A.M.; Mathias, D.; Kim, K.H.; Greene, C.R., Jr.; Macrander, A.M. Effects of airgun sounds on bowhead whale calling rates: Evidence for two behavioral thresholds. PLoS ONE 2015, 10, e0125720. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.M.; Merchant, N.D.; Farcas, A.; Barton, T.R.; Cheney, B.; Bono, S.; Thompson, P.M. Harbour porpoise responses to pile-driving diminish over time. R. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef]
- Russell, D.J.F.; Hastie, G.D.; Thompson, D.; Janik, V.M.; Hammond, P.S.; Scott-Hayward, L.A.S.; Matthiopoulos, J.; Jones, E.L.; McConnell, B.J. Avoidance of wind farms by harbour seals is limited to pile driving activities. J. Appl. Ecol. 2016, 53, 1642–1652. [Google Scholar] [CrossRef]
- Brandt, M.J.; Dragon, A.-C.; Diederichs, A.; Bellmann, M.A.; Wahl, V.; Piper, W.; Nabe-Nielsen, J.; Nehls, G. Disturbance of harbour porpoises during construction of the first seven offshore wind farms in Germany. Mar. Ecol. Prog. Ser. 2018, 596, 213–232. [Google Scholar] [CrossRef]
- New, L.F.; Moretti, D.J.; Hooker, S.K.; Costa, D.P.; Simmons, S.E. Using energetic models to investigate the survival and reproduction of beaked whales (family Ziphiidae). PLoS ONE 2013, 8, e68725. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Amtmann, S.; Schwarz, L.K.; Sumich, J.L.; Costa, D.P. A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Schwarz, L.K.; McHuron, E.; Mangel, M.; Wells, R.S.; Costa, D.P. Stochastic dynamic programming: An approach for modelling the population consequences of disturbance due to lost foraging opportunities. Proc. Meet. Acoust. 2016, 27, 040004. [Google Scholar] [CrossRef]
- McHuron, E.A.; Peterson, S.H.; Hückstädt, L.A.; Melin, S.R.; Harris, J.D.; Costa, D.P. The energetic consequences of behavioral variation in a marine carnivore. Ecol. Evol. 2018, 8, 4340–4351. [Google Scholar] [CrossRef]
- Pirotta, E.; Booth, C.G.; Costa, D.P.; Fleishman, E.; Kraus, S.D.; Lusseau, D.; Moretti, D.; New, L.F.; Schick, R.S.; Schwarz, L.K.; et al. Understanding the population consequences of disturbance. Ecol. Evol. 2018, 8, 9934–9946. [Google Scholar] [CrossRef]
- Wilson, L.J.; Harwood, J.; Booth, C.G.; Joy, R.; Harris, C.M. A decision framework to identify populations that are most vulnerable to the population level effects of disturbance. Conserv. Sci. Pract. 2020, 2, e149. [Google Scholar] [CrossRef]
- Pirotta, E.; Booth, C.G.; Cade, D.E.; Calambokidis, J.; Costa, D.P.; Fahlbusch, J.A.; Friedlaender, A.S.; Goldbogen, J.A.; Harwood, J.; Hazen, E.L.; et al. Context-dependent variability in the predicted daily energetic costs of disturbance for blue whales. Conserv. Physiol. 2021, 9. [Google Scholar] [CrossRef]
- Booth, C.G.; Sinclair, R.R.; Harwood, J. Methods for monitoring for the population consequences of disturbance in marine mammals: A review. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Reichmuth, C. Psychophysical Studies of Auditory Masking in Marine Mammals: Key Concepts and New Directions. In The Effects of Noise on Aquatic Life; Popper, A.N., Hawkins, A., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 730, pp. 23–27. [Google Scholar]
- Erbe, C.; Reichmuth, C.; Cunningham, K.; Lucke, K.; Dooling, R. Communication masking in marine mammals: A review and research strategy. Mar. Pollut. Bull. 2016, 103, 15–38. [Google Scholar] [CrossRef]
- Clark, C.; Ellison, W.; Southall, B.; Hatch, L.; Van Parijs, S.; Frankel, A.; Ponirakis, D. Acoustic masking in marine ecosystems: Intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 2009, 395, 201–222. [Google Scholar] [CrossRef]
- Guan, S.; Southall, B.L.; Barlow, J.; Vignola, J.F.; Judge, J.A.; Turo, D. Inter-ping sound field from a simulated mid-frequency active sonar, and its implication to marine mammal tonal masking. Proc. Meet. Acoust. 2016, 27, 070023. [Google Scholar] [CrossRef]
- Pine, M.K.; Hannay, D.E.; Insley, S.J.; Halliday, W.D.; Juanes, F. Assessing vessel slowdown for reducing auditory masking for marine mammals and fish of the western Canadian Arctic. Mar. Pollut. Bull. 2018, 135, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Pine, M.K.; Nikolich, K.; Martin, B.; Morris, C.; Juanes, F. Assessing auditory masking for management of underwater anthropogenic noise. J. Acoust. Soc. Am. 2020, 147, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.J.O.; Biassoni, N.; Samuels, A.; Tyack, P.L. Whale songs lengthen in response to sonar. Nature 2000, 405, 903. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.D.; Osborne, R.W.; Hoelzel, A.R. Whale-call response to masking boat noise. Nature 2004, 428, 910. [Google Scholar] [CrossRef]
- Di Iorio, L.; Clark, C.W. Exposure to seismic survey alters blue whale acoustic communication. Biol. Lett. 2010, 6, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Thode, A.M.; Blackwell, S.B.; Conrad, A.S.; Kim, K.H.; Marques, T.; Thomas, L.; Oedekoven, C.S.; Harris, D.; Bröker, K. Roaring and repetition: How bowhead whales adjust their call density and source level (Lombard effect) in the presence of natural and seismic airgun survey noise. J. Acoust. Soc. Am. 2020, 147, 2061–2080. [Google Scholar] [CrossRef]
- Parks, S.E.; Clark, C.W.; Tyack, P.L. Short- and long-term changes in right whale calling behavior: The potential effects of noise on acoustic communication. J. Acoust. Soc. Am. 2007, 122, 3725–3731. [Google Scholar] [CrossRef]
- Scheifele, P.M.; Andrew, S.; Cooper, R.A.; Darre, M.; Musiek, F.E.; Max, L. Indication of a Lombard vocal response in the St. Lawrence River beluga. J. Acoust. Soc. Am. 2005, 117, 1486–1492. [Google Scholar] [CrossRef]
- Holt, M.M.; Noren, D.P.; Veirs, V.; Emmons, C.K.; Veirs, S. Speaking up: Killer whales (Orcinus orca) increase their call amplitude in response to vessel noise. J. Acoust. Soc. Am. 2009, 125, EL27–EL32. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.E.; Johnson, M.; Nowacek, D.; Tyack, P.L. Individual right whales call louder in increased environmental noise. Biol. Lett. 2011, 7, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.A.; Cato, D.H.; Noad, M.J. Evidence of a Lombard response in migrating humpback whales (Megaptera novaeangliae). J. Acoust. Soc. Am. 2014, 136, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Fournet, M.E.H.; Matthews, L.P.; Gabriele, C.M.; Haver, S.; Mellinger, D.K.; Klinck, H. Humpback whales Megaptera novaeangliae alter calling behavior in response to natural sounds and vessel noise. Mar. Ecol. Prog. Ser. 2018, 607, 251–268. [Google Scholar] [CrossRef]
- Helble, T.A.; Guazzo, R.A.; Martin, C.R.; Durbach, I.N.; Alongi, G.C.; Martin, S.W.; Boyle, J.K.; Henderson, E.E. Lombard effect: Minke whale boing call source levels vary with natural variations in ocean noise. J. Acoust. Soc. Am. 2020, 147, 698–712. [Google Scholar] [CrossRef]
- Guazzo, R.A.; Helble, T.A.; Alongi, G.C.; Durbach, I.N.; Martin, C.R.; Martin, S.W.; Henderson, E.E. The Lombard effect in singing humpback whales: Source levels increase as ambient ocean noise levels increase. J. Acoust. Soc. Am. 2020, 148, 542–555. [Google Scholar] [CrossRef]
- Brumm, H.; Zollinger, S.A. The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 2011, 148, 1173–1198. [Google Scholar] [CrossRef]
- Noren, D.P.; Holt, M.M.; Dunkin, R.C.; Williams, T.M. The metabolic cost of communicative sound production in bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 2013, 216, 1624–1629. [Google Scholar] [CrossRef]
- Holt, M.M.; Noren, D.P.; Dunkin, R.C.; Williams, T.M. Vocal performance affects metabolic rate in dolphins: Implications for animals communicating in noisy environments. J. Exp. Biol. 2015, 218, 1647–1654. [Google Scholar] [CrossRef]
- Noren, D.P.; Holt, M.M.; Dunkin, R.C.; Williams, T.M. The metabolic cost of whistling is low but measurable in dolphins. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef] [PubMed]
- Ising, H.; Kruppa, B. Health effects caused by noise: Evidence in the literature from the past 25 years. Noise Health 2004, 6, 5–13. [Google Scholar] [PubMed]
- Skogstad, M.; Johannessen, H.A.; Tynes, T.; Mehlum, I.S.; Nordby, K.-C.; Lie, A. Systematic review of the cardiovascular effects of occupational noise. Occup. Med. 2016, 66, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Kim, M.; Jang, C.; Choung, T.; Sim, K.-A.; Seo, D.; Chang, S.I. The public health impact of road-traffic noise in a highly-populated city, Republic of Korea: Annoyance and sleep disturbance. Sustainability 2018, 10, 2947. [Google Scholar] [CrossRef]
- Bolm-Audorff, U.; Hegewald, J.; Pretzsch, A.; Freiberg, A.; Nienhaus, A.; Seidler, A. Occupational noise and hypertension risk: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 6281. [Google Scholar] [CrossRef]
- Shin, S.; Bai, L.; Oiamo, T.H.; Burnett, R.T.; Weichenthal, S.; Jerrett, M.; Kwong, J.C.; Goldberg, M.S.; Copes, R.; Kopp, A.; et al. Association between road traffic noise and incidence of diabetes mellitus and hypertension in Toronto, Canada: A population-based cohort study. J. Am. Heart Ass. 2020, 9, e013021. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, B.; Xia, Q.; Ji, X.; Pan, L.; Bao, Y.; Lin, Y.; Zhang, R. The effects of occupational noise exposure on the cardiovascular system: A review. J. Public Health Emerg. 2020, 4. [Google Scholar] [CrossRef]
- Wright, A.J.; Soto, N.A.; Baldwin, A.L.; Bateson, M.; Beale, C.M.; Clark, C.; Deak, T.; Edwards, E.F.; Fernández, A.; Godinho, A.; et al. Do marine mammals experience stress related to anthropogenic noise? Int. J. Comp. Psychol. 2007, 20, 274–316. [Google Scholar]
- Kellar, N.M.; Catelani, K.N.; Robbins, M.N.; Trego, M.L.; Allen, C.D.; Danil, K.; Chivers, S.J. Blubber cortisol: A potential tool for assessing stress response in free-ranging dolphins without effects due to sampling. PLoS ONE 2015, 10, e0115257. [Google Scholar] [CrossRef]
- Bechshoft, T.; Wright, A.J.; Weisser, J.J.; Teilmann, J.; Dietz, R.; Hansen, M.; Björklund, E.; Styrishave, B. Developing a new research tool for use in free-ranging cetaceans: Recovering cortisol from harbour porpoise skin. Conserv. Physiol. 2015, 3. [Google Scholar] [CrossRef]
- Keogh, M.J.; Gastaldi, A.; Charapata, P.; Melin, S.; Fadely, B.S. Stress-related and reproductive hormones in hair from three North Pacific otariid species: Steller sea lions, California sea lions and northern fur seals. Conserv. Physiol. 2020, 8. [Google Scholar] [CrossRef]
- Pujade Busqueta, L.; Crocker, D.E.; Champagne, C.D.; McCormley, M.C.; Deyarmin, J.S.; Houser, D.S.; Khudyakov, J.I. A blubber gene expression index for evaluating stress in marine mammals. Conserv. Physiol. 2020, 8, coaa082. [Google Scholar] [CrossRef] [PubMed]
- Bechshoft, T.; Wright, A.J.; Styrishave, B.; Houser, D. Measuring and validating concentrations of steroid hormones in the skin of bottlenose dolphins (Tursiops truncatus). Conserv. Physiol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.E.; Reeves, R.R.; Southall, B.L.; Ragen, T.J.; Suydam, R.S.; Clark, C.W. A new framework for assessing the effects of anthropogenic sound on marine mammals in a rapidly changing Arctic. BioScience 2012, 62, 289–295. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Approaches to Understanding the Cumulative Effects of Stressors on Marine Mammals; National Academies Press: Washington, DC, USA, 2017; ISBN 978-0-309-44048-6. [Google Scholar]
- Faulkner, R.C.; Farcas, A.; Merchant, N.D. Guiding principles for assessing the impact of underwater noise. J. Appl. Ecol. 2018, 55, 2531–2536. [Google Scholar] [CrossRef]
- Pirotta, E.; Mangel, M.; Costa, D.P.; Goldbogen, J.; Harwood, J.; Hin, V.; Irvine, L.M.; Mate, B.R.; McHuron, E.A.; Palacios, D.M.; et al. Anthropogenic disturbance in a changing environment: Modelling lifetime reproductive success to predict the consequences of multiple stressors on a migratory population. Oikos 2019, 128, 1340–1357. [Google Scholar] [CrossRef]
- Houser, D.S.; Finneran, J.J.; Ridgway, S.H. Research with Navy marine mammals benefits animal care, conservation and biology. Int. J. Comp. Psychol. 2010, 23, 249–268. [Google Scholar]
- Fetherston, T. Environment in a (high-tech) box: Navy model simulates undersea sound fields & marine mammal locations to plan training & testing activities. Currents 2011, 42–43. [Google Scholar]
| 1 | Only in-water thresholds are described in detail herein. Details regarding in-air thresholds are beyond the scope of the paper. |
| 2 | The metric considered the total energy of all exposures based on the greatest EFD in any one-third octave band for frequencies greater than 100 Hz for odontocetes and 10 Hz for mysticetes. |
| 3 | Which is termed by the Navy as sonar within the 1–10 kHz range. Other navies, particularly those in Europe, define a portion of the MFAS range as low-frequency active sonar (LFAS), whereas LFAS in the United States is any sonar that operates below 1 kHz. |
| 4 | At the same time, Wood et al. [90] developed simple probabilistic dose response functions for seismic surveys based on a given received level at 90, 50, and 10 percent response rates for porpoises and beaked whales, migrating mysticetes, and all other species—the dose response functions also were intended to be used with the M-weighting functions. Variations of the Wood et al. [90] dose response functions have been used only once by the U.S. regulatory community [91]. |
| 5 | The Navy used many of the same criteria in its EISs for previous shock trials and for training and testing activities analyzed under the Tactical Training Theater Assessment and Planning (TAP I) documents. |
| 6 | Along with Type I weighting functions. |
| 7 | For more than two decades, the Navy also has used a metric it has termed “single ping equivalent” (SPE) to estimate behavioral responses of marine mammals to Surveillance Towed Array Sensor System Low Frequency Active (SURTASS LFA) sonar. SPE is a quasi-metric that the Navy has used to apply its SPE-based behavioral risk function, even though the metric is not based on any sort of physical quantity nor is it recognized by either the American National Standards Institute or the International Organization for Standardization. The U.S. Marine Mammal Commission, an independent oversight agency, has reviewed the deficiencies and inappropriatness of SPE and the underlying behavioral risk function [95]. |
| 8 | The Navy, and thus NMFS, maintained and continues to maintain that the behavior thresholds only apply to multiple underwater detonations, not single detonations regardless of the net explosive weight. |
| 9 | Southall et al. [99] also recommended that the same weighting functions and TTS and PTS thresholds be used. However, the authors termed MF cetaceans as HF cetaceans and HF cetaceans as very high frequency (VHF) cetaceans. Southall et al. [99] developled a modified nomenclature that accounted for additional subdivisions within the LF and HF cetacean functional hearing groups but acknowledged that there were insufficient data to define further the exposure criteria within those subdivisions. |
| 10 | For explosive sources, the Navy again used behavior thresholds that were 5 dB less than the TTS thresholds for each functional hearing group and assumed that the behavior thresholds only apply to multiple underwater detonations. |
| 11 | 3MB is available at http://oalib.hlsresearch.com/Sound%20and%20Marine%20Mammals/3MB%20HTML.htm (accessed on 29 March 2021). |
| 12 | Including drilling, vibratory pile driving and removal, dynamic positioning systems and other vessel sounds. |
| 13 | Furthermore, the distances themselves are unsubstantiated. |
| 14 | Acoustic deterrent devices also are used to deter marine mammals from close ranges, which add a confounding factor to any behavioral response data collected [161]. |
| 15 | |
| 16 | Or groups of similar sources. For example, BRFs could be developed for vibratory pile driving and drilling combined or BRFs for seismic surveys could apply to underwater and confined detonations if data are lacking. |
| 17 | BOEM recently established the Center for Marine Acoustics that is staffed with experts to conduct underwater acoustic and animat modeling in support of its environmental impact assessments. https://www.boem.gov/center-marine-acoustics (accessed on 21 March 2021). |
| Functional Hearing Group | Impulsive | Non-Impulsive | ||||
|---|---|---|---|---|---|---|
| TTS | PTS | TTS | PTS | |||
| SELcum | SPLpeak | SELcum | SPLpeak | SELcum | SELcum | |
| LF | 168 | 213 | 183 | 219 | 179 | 199 |
| MF | 170 | 224 | 185 | 230 | 178 | 198 |
| HF | 140 | 196 | 155 | 202 | 153 | 173 |
| SI | 175 | 220 | 190 | 226 | 186 | 206 |
| OW | 188 | 226 | 203 | 232 | 199 | 219 |
| PW | 170 | 212 | 185 | 218 | 181 | 201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Brookens, T. The Use of Psychoacoustics in Marine Mammal Conservation in the United States: From Science to Management and Policy. J. Mar. Sci. Eng. 2021, 9, 507. https://doi.org/10.3390/jmse9050507
Guan S, Brookens T. The Use of Psychoacoustics in Marine Mammal Conservation in the United States: From Science to Management and Policy. Journal of Marine Science and Engineering. 2021; 9(5):507. https://doi.org/10.3390/jmse9050507
Chicago/Turabian StyleGuan, Shane, and Tiffini Brookens. 2021. "The Use of Psychoacoustics in Marine Mammal Conservation in the United States: From Science to Management and Policy" Journal of Marine Science and Engineering 9, no. 5: 507. https://doi.org/10.3390/jmse9050507
APA StyleGuan, S., & Brookens, T. (2021). The Use of Psychoacoustics in Marine Mammal Conservation in the United States: From Science to Management and Policy. Journal of Marine Science and Engineering, 9(5), 507. https://doi.org/10.3390/jmse9050507







