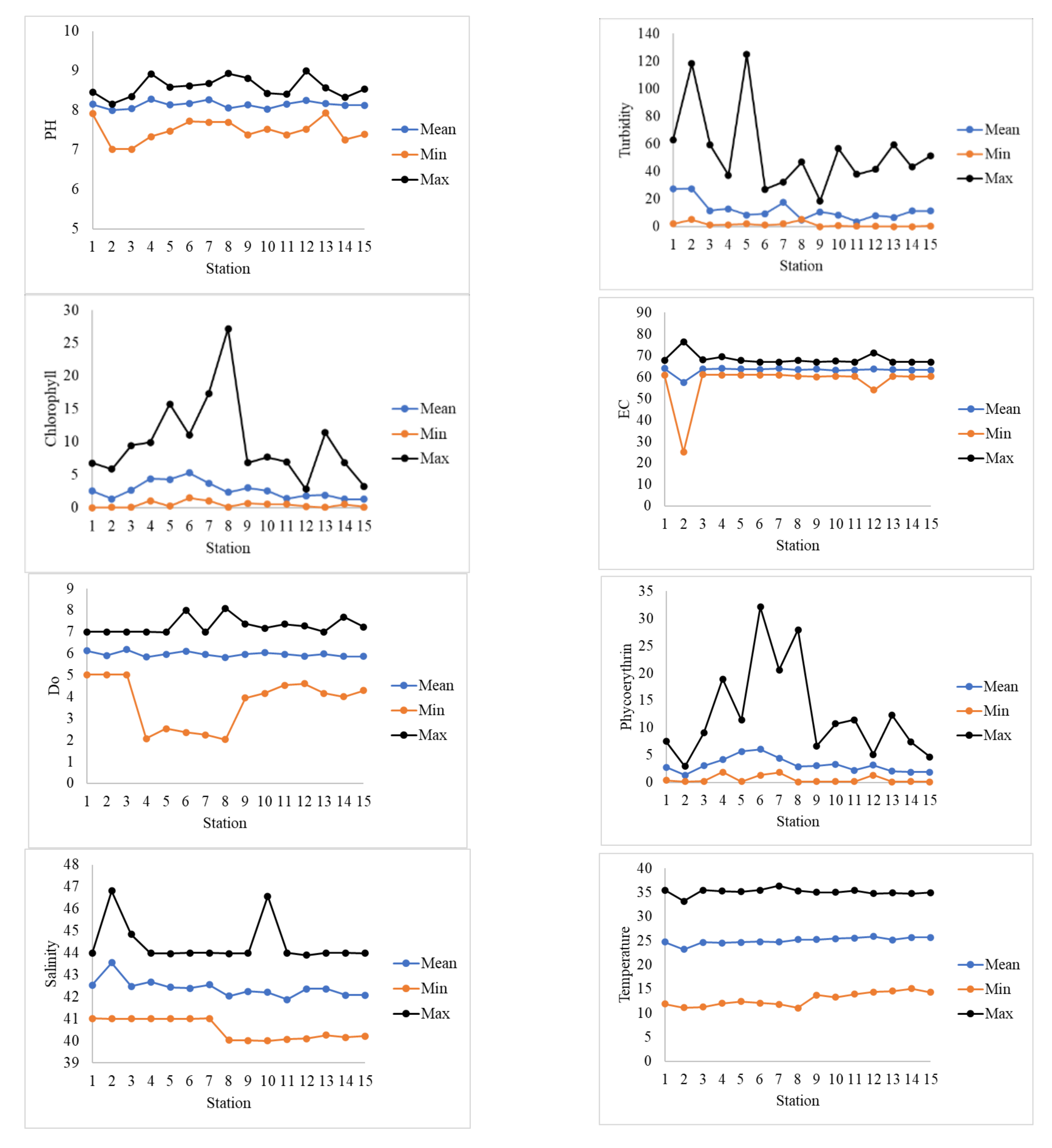
The basic statistics of the water quality parameters of this study are shown in
Figure 2. The figure shows that the maximum, mean, and minimum values of the pH for all stations are within the standard limits of KEPA (
Table 3). These values indicate that the acidity of the water is neutral, and no threat exists to the biological life. The maximum pH value was 8.98 and recorded at station 12, while the minimum value was recorded at two stations, St-02 and St-03, with a value of
.
The turbidity varies widely between stations and for the same station. The maximum turbidity was at station 5 with a value of 125 NTU (exceeds KEPA maximum limit), and the minimum was nearly 0 at stations 9–15. Stations of high turbidity are either located in areas of cohesive beds (St-02 and ST-05) or located in areas where there are frequent waste discharges from coastal outfalls. The highest mean turbidity values were observed at stations ST-01 and ST-02, where continuously suspended nutrients and fine sediments are discharged from the northern rivers (e.g., Shat Alarab and Khor Abdullah).
The coastal water of Kuwait and the Arabian Gulf, in general, is known for its high temperature and salinity. The mean salinity at all stations in 2017 was between 41.9 psu (ST-11) and 43.6 psu (ST-02). The maximum salinity level was
47 psu at St-02, and the minimum was ≈40 psu at stations St-08–St-15. These high salinity levels are due to the extensive amount of desalination plant outfalls along the Arabian Gulf coastline, extreme arid weather, and slow flushing characteristics of the Arabian Gulf and specifically within Kuwaits’ coastal water. High salinity contributes significantly to many coastal environmental, health, and economic issues [
35].
The above statistics of some water quality parameters show that the coastal water of Kuwait is subjected to potential environmental pressure. This pressure is mainly due to high levels of temperature and salinity and low levels of DO. The causes of the potential threats are due to either natural processes, such as climate change, or due to human inferences, such as oil spills, waste discharge, desalination plants’ discharge, and aggressive fishing.
It is essential to realize that some of the water parameters are dependant on each other, and they vary seasonally and spatially. As mentioned earlier, cluster analysis (CA) and principal component/factor analysis (PCA/FA), and Pearson correlation (PC) are frequently used in water quality studies in coastal areas to study the seasonal and spatial variations of water properties. The daily average water quality data is analyzed next using the above three analyses.
3.1. CA
The CA identified the data into discrete groups with similar characteristics where they are closely associated. CA handled both locational and seasonal variations as presented below.
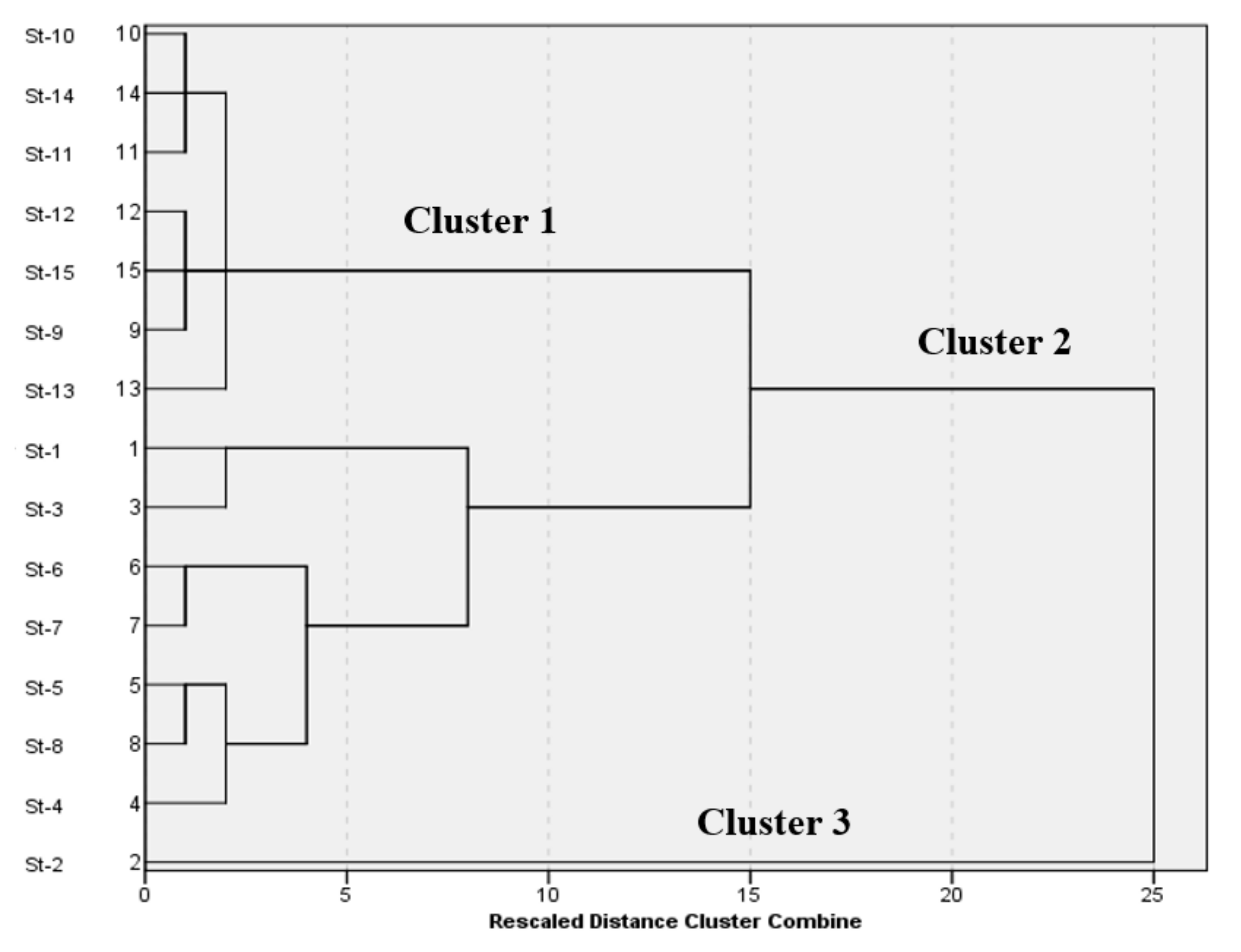
The locational CA characterized the similarity within the 15 stations included in this study. The analyzed data employed the mean values of seawater parameters and resulted in three clusters, as shown in
Figure 3, at (D
link/D
max)
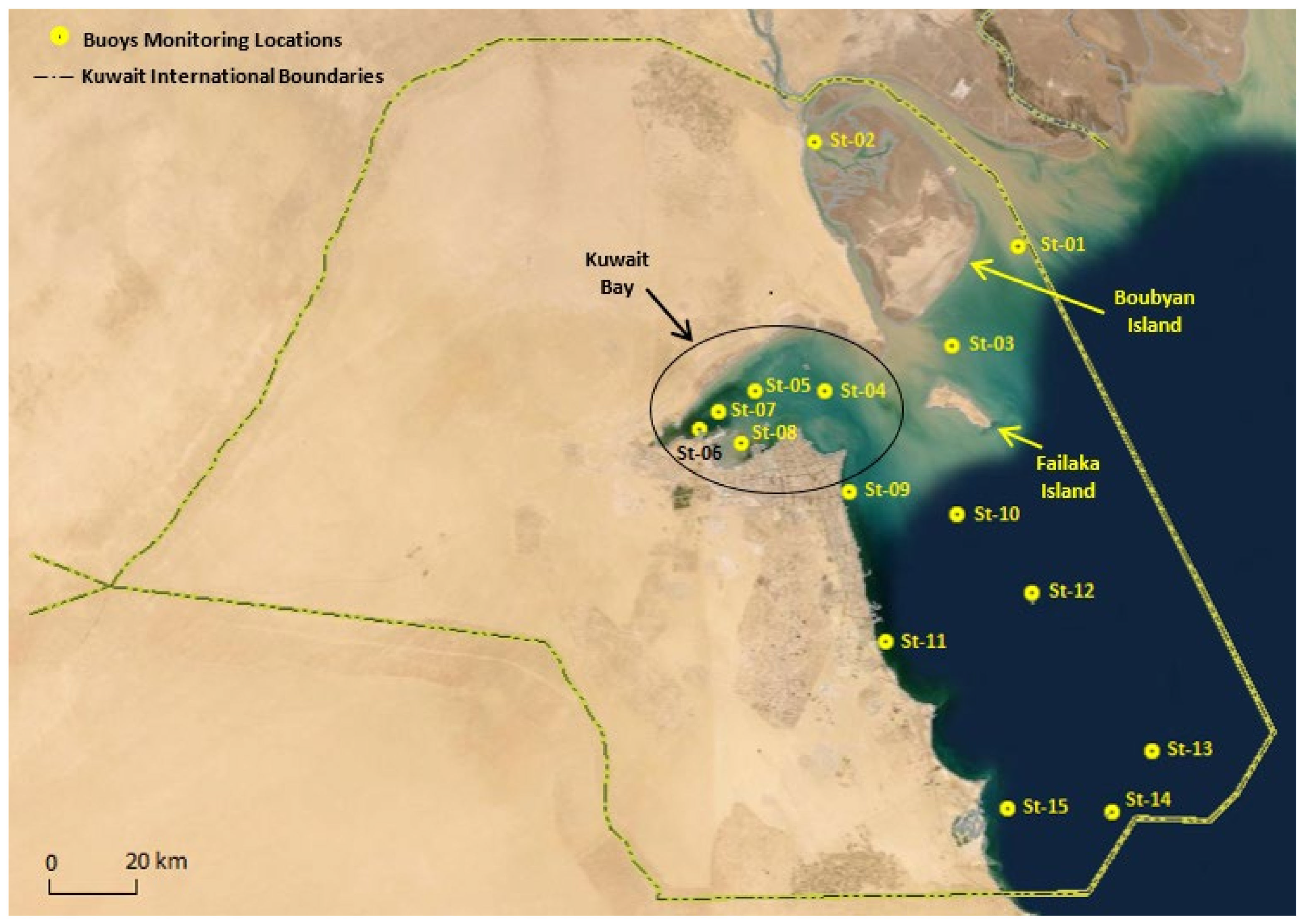
100 < 40. From the mean turbidity values for each station, it is inferred that CA has grouped the stations based on turbidity, and hence, the three groups are classified as high turbidity (HT), medium turbidity (MT), and low turbidity (LT). The LT region includes seven stations and is represented by Cluster 1, which combines station 9 to station 15 and is located in Kuwait’s southern region. Additionally, the MT region, called Cluster 2, combines station 1 and station 3 to station 8 and is generally indicated as Kuwait Bay. Lastly, Cluster 3 includes only station 2 while representing the HT area, occupying the northern region of Kuwait and Boubyan Island, as previously shown in
Figure 1.
The high turbidity values in the HT region, including station 2, are due to the shallow water levels that receive silt and clay from Khor Abdallah [
29,
38]. In addition to that, station 2 shows high salinity values related to low water circulation in this region [
39]. The MT region covers the stations near human activities such as fishing and industrial ports. Such activities cause an increase in chlorophyll-a and phycoerythrin values and lead to an increase in the concentration of nutrients in seawater [
40]. Finally, the LT region is located on the south coast of Kuwait and is characterized as open water. In such regions, the water circulation is higher, and the turbidity values are low.
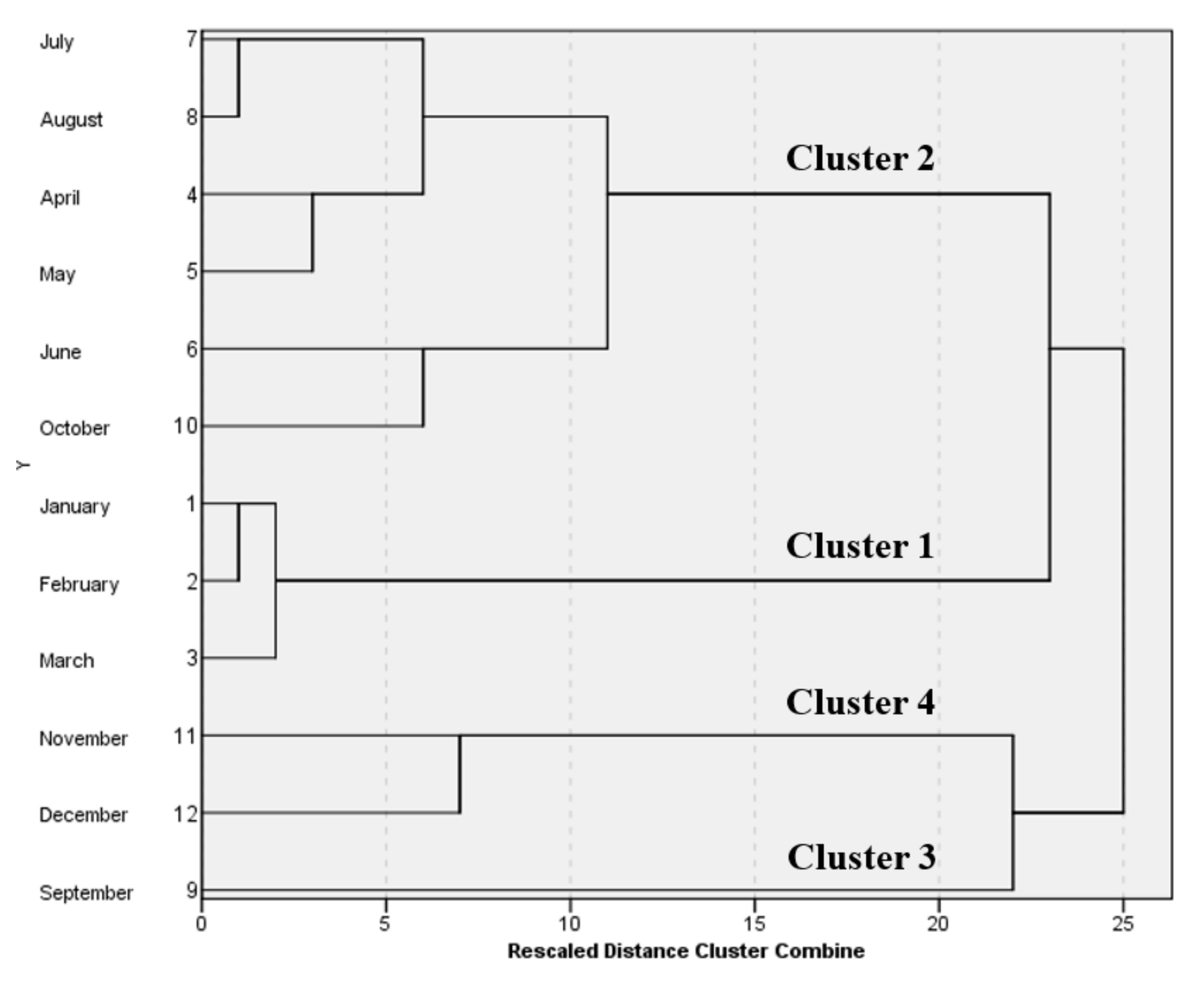
The seasonal variations concerning the coastal water quality values were investigated using the CA method, including the 12 months (January–December) of 2017. The analysis resulted in four significant clusters at (D
link/D
max)
100 < 60, as shown in
Figure 4. Although there are four clusters, it is observed that Cluster 3, which includes the month of September only, lies in between months classified as Cluster 2 (from April to August and October). Such conflict and shifting between October and September result from seasons transition, in which the temperature range in September 2017 was 24–47 °C, while in October 2017, the temperature recorded was in the range of 16–43 °C. Similarly, in 2018 and 2019, the recorded temperature ranged between 25–48 °C and 23–48 °C for September, and 13–43 °C and 20–45 °C for October [
41]. Hence, Cluster 2 and 3 were combined as the analyzed data fitted the values of April–August and October in the summer season. Hence, CA on temporal variation resulted in three seasons, which are winter, summer, and autumn. Spring is very short and overlaps with winter and summer. Cluster 1 combines the months from January to March, referring to the winter season. Additionally, Cluster 4 represents the autumn season which includes November and December.
Kuwait is known for its extreme weather conditions, such as high temperatures in spring and autumn, and the CA resulted in the summer season, ranging from April to October. Similarly, Polikarpov and others [
42] have performed temporal analysis on Kuwait gulf water from October 2005 to September 2006, and their results showed similar clustering with three main seasons: late-winter–spring, summer–early-autumn, and late-autumn–winter.
3.2. Principal Component/Factor Analysis (PCA/FA)
This method was applied to the available data set, including eight variables to identify and accentuate the seasonal and locational variability of the water samples’ compositional patterns. As defined by CA, PCA/FA was carried out in three different regions: HT, MT, and LT; and in three seasons: winter, summer, and autumn. The PCA/FA data input matrices (variable x cases) accounted for (8 × 2555) regarding LT and MT regions. However, the data input matrix for the HT region was (8 × 365). Additionally, the analyzed data resulted in three seasons for the seasonal variations: winter, summer, and autumn. Therefore, the data input matrices included (8 × 1260) for winter, (8 × 2996) for summer, and (8 × 854) for autumn.
PCA results were considered accounting for an Eigenvalue greater than 1, and the values of the factor loadings are classified in the ranges of > 0.75, 0.75–0.50, and 0.50–0.30, referred to as strong, moderate, and weak, respectively. Moreover, for the three regions investigated, the LT region yielded four PCs, explaining a total variance of 71.26% in correspondence to the water quality data sets. The MT region resulted in three PCs with a total variance of 57.31%, and the HT region explained a total variance of 64.95%, accounting for the two PCs generated.
Table 4 summarizes the results obtained by PC analysis.
Furthermore, PC1, regarding the LT region, explained 24.38% of the total variance and had strong positive loading on electrical conductivity (EC) and salinity. The relationship between salinity and EC is explained by ions that increase the conductivity of water. Therefore, PC1 might be representative of impact desalination discharge along the southern coast of Kuwait. Moreover, PC2, covering 19.1% of the total variance, has a moderate positive loading on chlorophyll-a and phycoerythrin, which means that this component represents algae or simple aquatic organisms’ impact. Moderate turbidity and positive pH loading are present in PC3, representing water and reef fish aggregation [
43]. PC3 explains 14.88% of the total variance of the LT cluster. The last component in LT is represented by PC4, explains 12.9% of the total variance, and has strong positive loading on DO, indicating the impact of nutrients and biological organisms [
38].
PC analysis on the MT cluster resulted in PC1 and PC2, which explains 43.92% and 21% of the total variance. As the MT region lies in a closed bay area with heavy cargo traffic, which causes increased turbidity, PC1 has a strong negative loading on turbidity and a strong positive loading on chlorophyll-a and phycoerythrin. The negative relationship between turbidity and chlorophyll-a/phycoerythin is explained by large ships’ movement that causes turbidity in water and harms the algae and aquatic life. Additionally, PC2 has strong positive loading on DO, indicating the impact of nutrients and biological organisms.
As for the HT region, PC1 explains 22.4% of the total variance of the data and has moderate positive loading on chlorophyll-a, phycoerythrin, EC, and salinity. Thus, PC1 can indicate the effect of water flow from Shat al Arab delta, reducing the region’s salinity and inversely affecting the aquatic organism population. PC2 explains 18.61% of the total variance of HT region data. Strong positive turbidity and moderate negative temperature loading exist in PC2, indicating the factor representing the impact of nutrients and biological organisms in this region. The inverse relationship between DO and temperature is due to the low solubility of oxygen in water at high temperatures [
44]. Moreover, PC3 explains 16.3% of the total variance and experiences moderate positive loading on salinity and EC, and moderate negative loading on phycoerythrin.
On the other hand, the three seasonal variabilities shown in
Table 5 yielded three PCs for the winter season with a total variance of 60.6% for the water quality datasets. However, a total variance of 57.97% was interpreted for the three PCs obtained regarding the summer season. As for the autumn season, the four PCs explained a total variance of 76.28%. PC1 explains 26.4% of the total variance in the winter data set and experiences strong positive loading on EC and salinity. Thus, this component indicates the effect of water desalination plants and industrial processes on gulf water’s salinity.
Moreover, PC2 explains that 20.2% of the total variance has a moderate positive loading on pH and a moderate negative loading on both chlorophyll and phycoerythrin. The presence of red algae or some cyanobacteria is the cause of the proportional relationship between chlorophyll and phycoerythrin. The inverse relationship between the biological parameters and pH is due to high pH on the species with low maximum pH limits, such as red algae [
45]. Thus, PC2 indicates the effect of pH on red algae in gulf water. The last component (PC3) explains 14% of the total variance, and it was observed that PC3 experiences strong positive loading on DO, representing the effect of microorganisms in seawater.
In summer, PC1 explains 23.6% of the total variance. It has moderate positive loading on chlorophyll-a, phycoerythrin, EC, and salinity, which indicates the effect of desalination plants that may increase capacity during summer and, in turn, increase seawater salinity through brine dumping. Furthermore, increased salinity can affect the red algae growth rate, affecting chlorophyll-a and phycoerythrin concentration [
46]. On the other hand, PC2 in the summer months has a moderate positive loading on EC and salinity, and a weak negative loading on temperature, which indicates that PC2 represents the effect of water temperature on salinity. This effect is demonstrated by the increase of salts solubility in water as temperature increases. Besides, PC2 explains 18.9% of the total variance.
Furthermore, PC3 explains 15.5% of the total variance of the summer season. It has a moderate positive loading on turbidity and DO, and moderate negative loading on temperature. As mentioned above, the inverse relationship between DO and temperature is due to the low solubility of oxygen in water at high temperatures [
44]. Simultaneously, the inverse relationship between temperature and turbidity can be explained by the heavy dust storms that affect the region during the summer seasons [
47]. As dust storms increase turbidity in the seawater, it also decreases water temperature by providing cover from the direct sun exposure.
In autumn, four PCs describe 76.3% of the data set. PC1 explains 21.2% of the total variance of the autumn season data. PC1 has strong positive loading on chlorophyll and phycoerythrin. Similar to the other seasons, it represents the presence of photosynthesis organisms such as red algae. Furthermore, PC2 explains 20.5% of the total variance and has a strong positive loading on salinity.
Additionally, this component represents the effect of desalination and industrial process on seawater. PC3 in autumn has a strong positive loading on pH and moderate positive loading on turbidity. The explanation of the positive correlation between pH and turbidity is due to the dissolution of humic substances in water, reducing the pH [
48]. Lastly, PC4 explains 15.6% of the autumn data set and has a moderate negative loading on DO and a moderate positive loading on temperature. Thus, PC4 represents the impact of nutrients and biological organisms in this season.
3.3. Pearson Correlation
The Pearson correlation matrix for the overall data set is shown in
Table 6. It is noticed that there is no relationship observed between parameters except between chlorophyll-a and phycoerythrin (r = 0.614,
p < 0.05). This correlation was also found in seven stations when performing Pearson’s correlation on each station. Stations 1, 2, 3, 5, 7, 8, and 13 have a Pearson correlation coefficient between chlorophyll-a and phycoerythrin of 0.709, 0.825, 0.640, 0.558, 0.53, and 0.538, respectively. From
Figure 1, most of these stations are located near shorelines and islands, where human activities are high, which increases nutrients in the water that might increase the growth of aquatic organisms such as red algae [
49].
Moreover, stations 1 and 2 exhibited a negative correlation between turbidity and chlorophyll (−0.504 and −0.729, respectively), which is explained by the difficulty of algal growth in harsh turbid environments. In stations 2, 13, and 15, the negative correlation between DO and temperature is given by −0.507, −0.773, and −0.532, respectively. This correlation was only noticed in these three stations as they are away from human activities that can change DO concentrations irrelative to water temperature [
50].
Pearson correlation was also performed on each spatial region, HT, MT, and LT, and the coefficients are summarized in
Table 7,
Table 8 and
Table 9, respectively. For MT and LT regions as a whole, no correlation exists between any of the parameters. As for the HT region, which includes only station 2, some of the relations, such as chlorophyll–phycoerythrin, DO–temperature, and turbidity–chlorophyll, exist, and these relationships represent indications for algal presence, low human activity, and harsh environments for algae growth as discussed above. Additionally, the salinity–phycoerythrin correlation was reported as 0.615, indicating that the phycoerythrin production rate increases with salinity. In previous studies, this positive effect of salinity on phycoerythrin concentration was also discovered while optimizing phycoerythrin production [
51,
52]. However, another study shows no significant difference in phycoerythrin concentration with increasing salinity [
53].
Moreover, analysis of variance (ANOVA) is used to analyze the given factors affecting the dataset and determine whether there are statistically significant differences between them. In this study, ANOVA was applied to investigate the given set of data and check if any similarities in characteristics between the stations may exist, such that they can be merged. However, no significant correlations were found.