Incorporating Species-Conditional Co-Occurrence When Selecting Indicator Species to Monitor Restoration after Mangrove Removal from the Siangshan Wetland, Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mangrove Removal
2.3. Biological Survey and Experimental Design Experimental Design
2.4. Statistical Analysis
2.5. Model Building
3. Results and Discussion
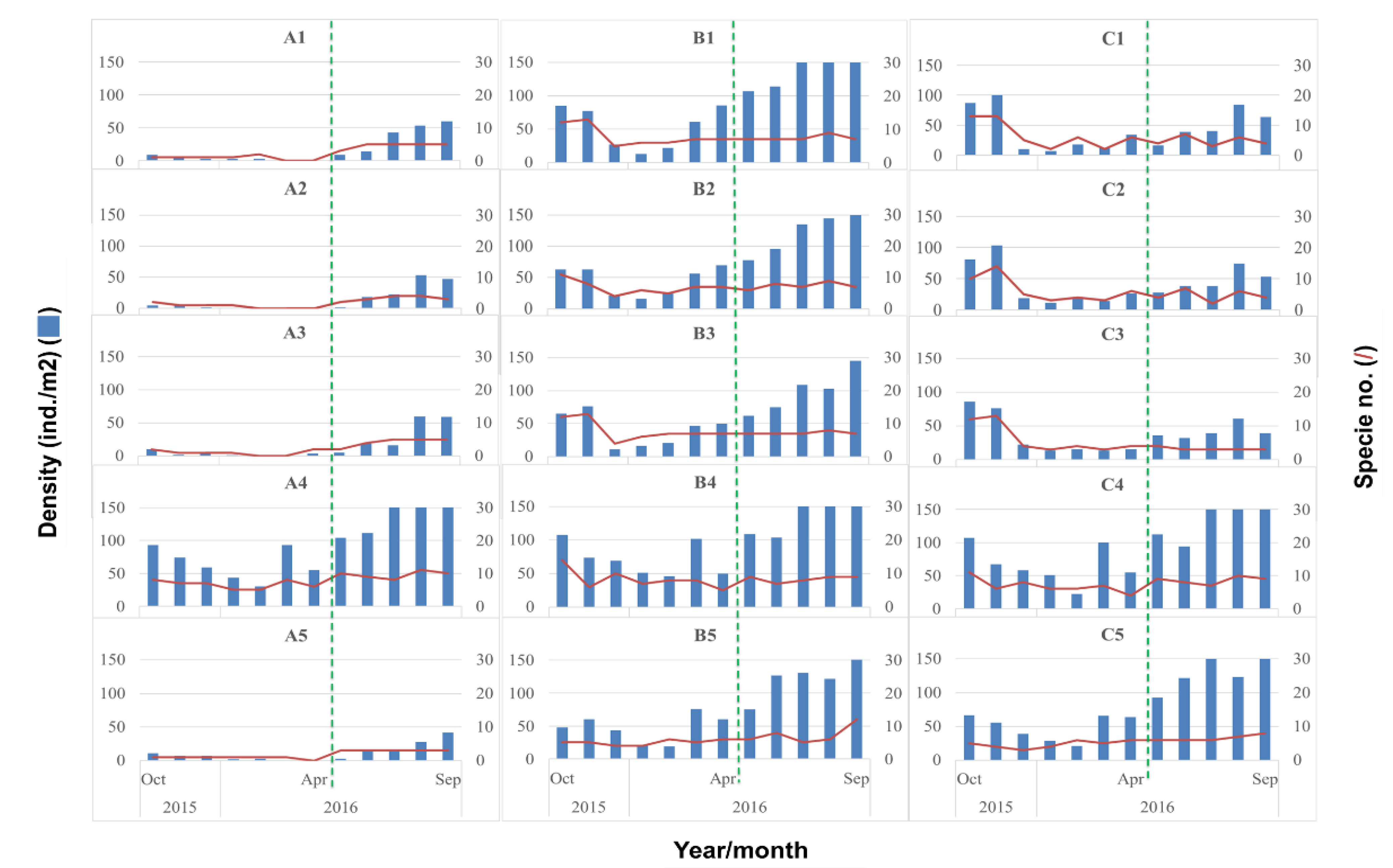
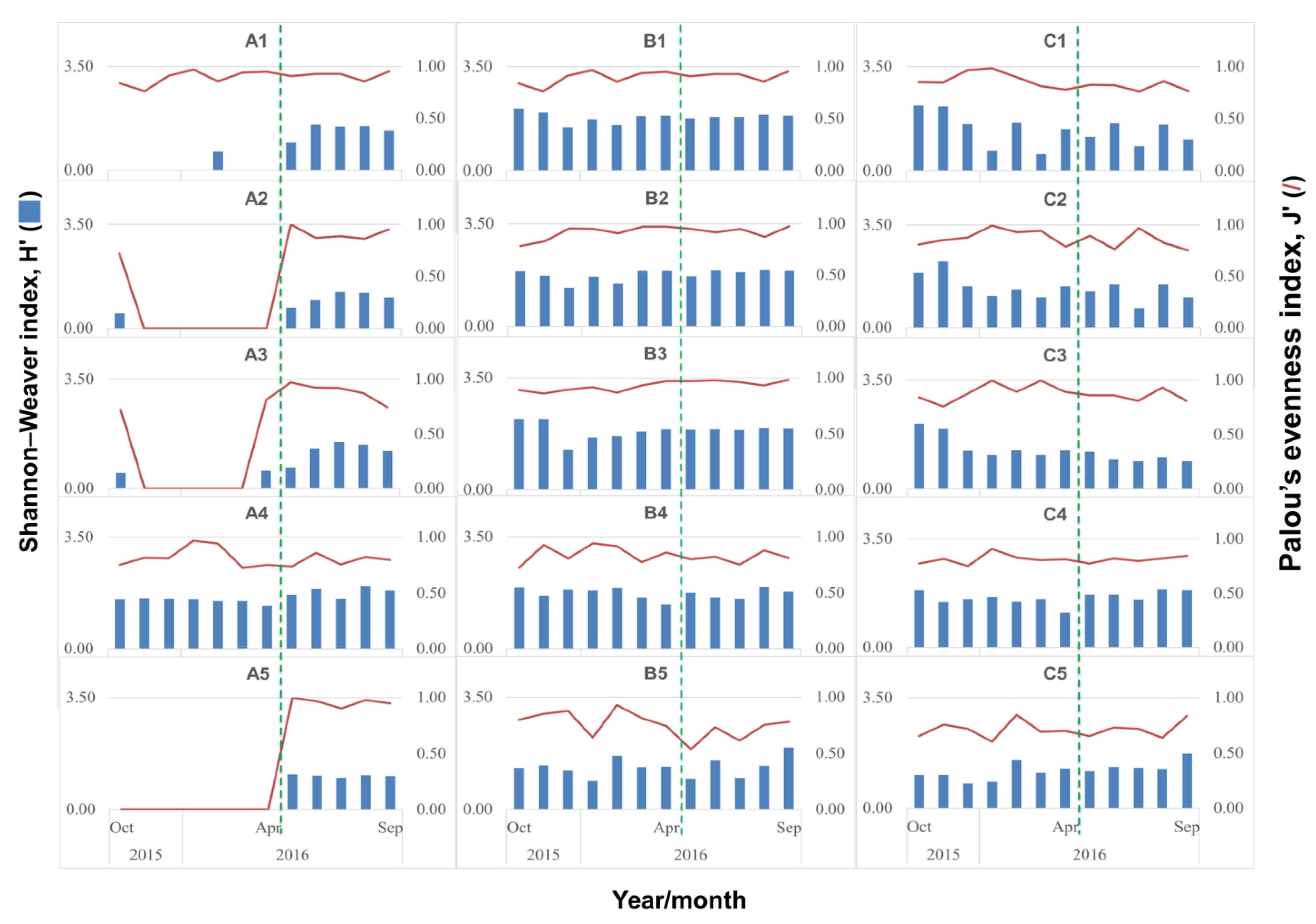
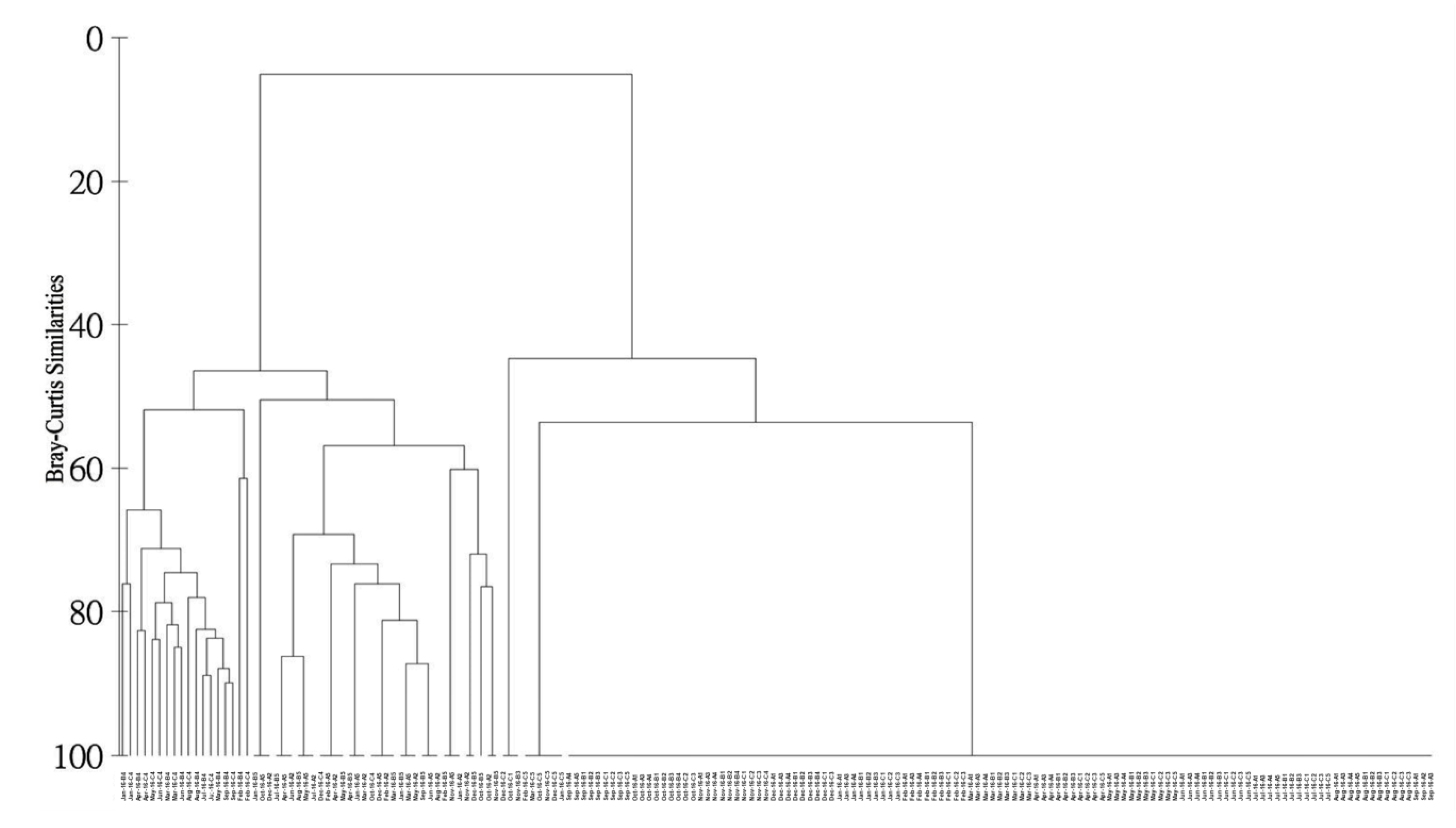
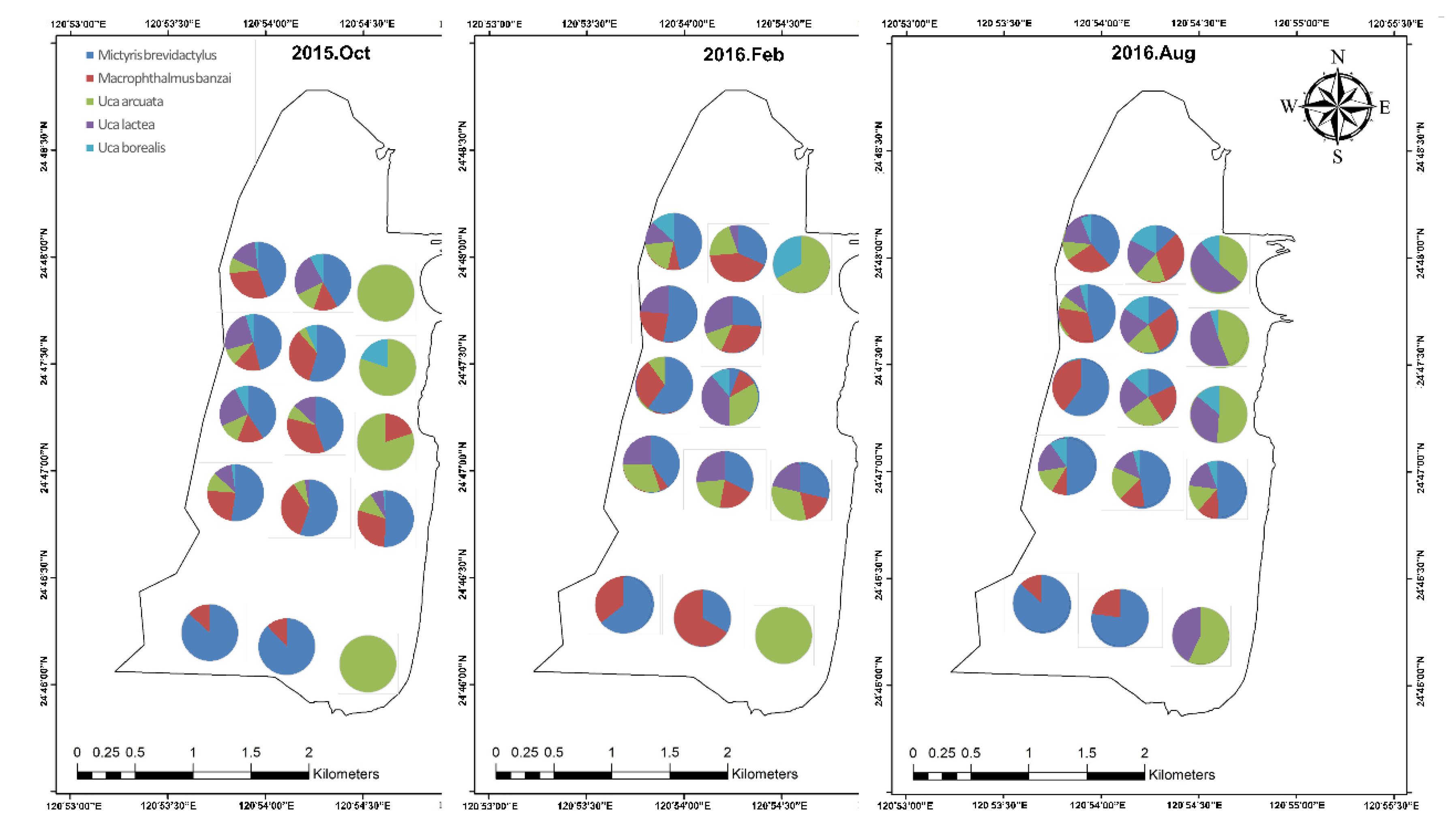
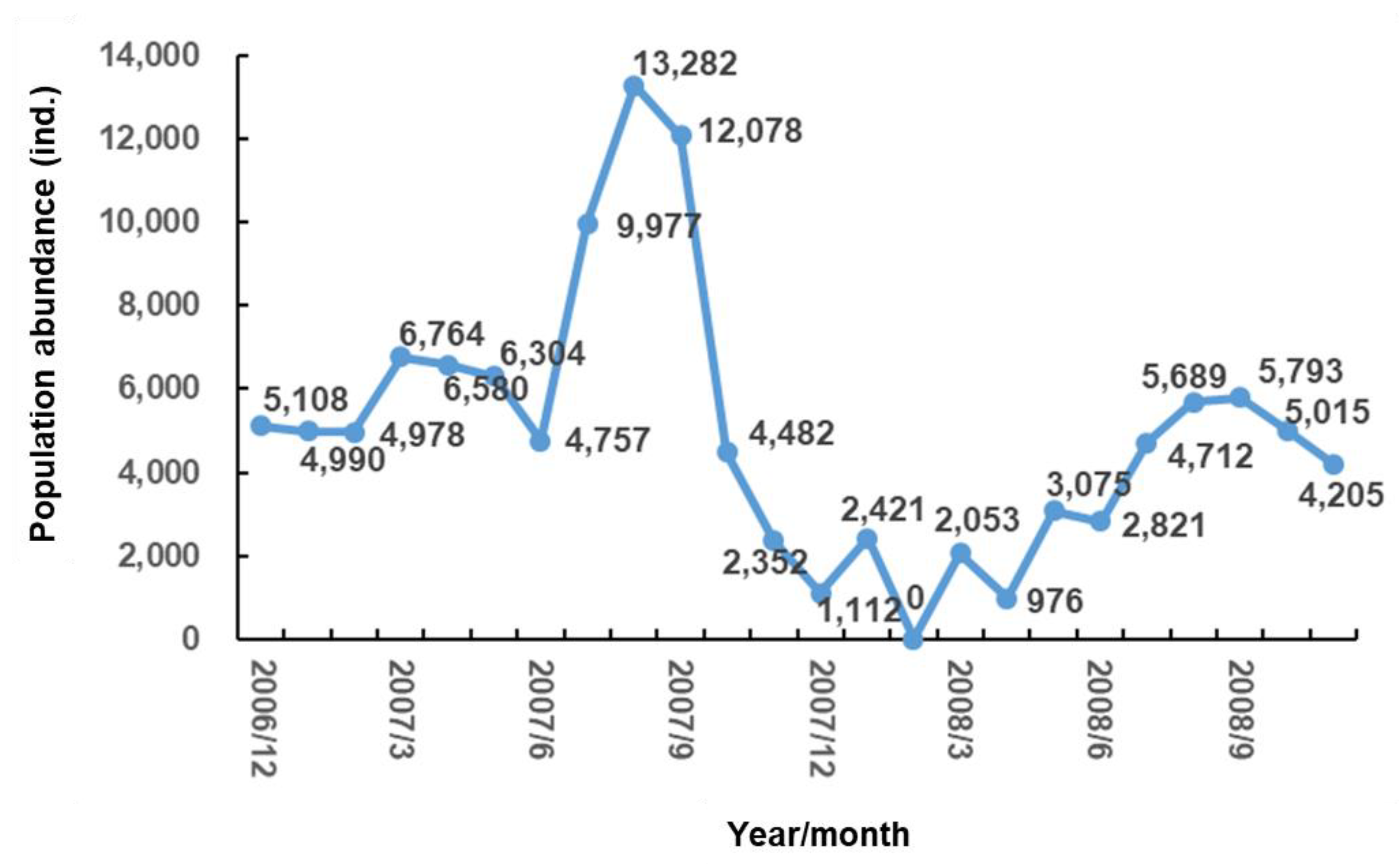
3.1. Biological Survey and Community Structure
3.2. Identifying Indicator Species
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.Y.; Shih, S.S. Impacts of vegetation changes on the hydraulic and sediment transport characteristics in Guandu mangrove wetland. Ecol. Eng. 2004, 23, 85–94. [Google Scholar] [CrossRef]
- Lee, T.M.; Yeh, H.C. Applying remote sensing techniques to monitor shifting wetland vegetation: A case study of Danshui River estuary mangrove communities, Taiwan. Ecol. Eng. 2009, 35, 487–496. [Google Scholar] [CrossRef]
- Shih, S.S.; Yang, S.C.; Lee, H.Y.; Hwang, G.W.; Hsu, Y.M. Development of a salinity-secondary flow-approach model to predict mangrove spreading. Ecol. Eng. 2011, 37, 1174–1183. [Google Scholar] [CrossRef]
- Yang, S.C.; Shih, S.S.; Hwang, G.W.; Adams, J.B.; Lee, H.Y.; Chen, C.P. The salinity gradient influences on the inundation tolerance thresholds of mangrove forests. Ecol. Eng. 2013, 51, 59–65. [Google Scholar] [CrossRef]
- Silva Lima, J.S.; Atalah, J.; Sanchez-Jerez, P.; Zalmon, I.R. Evaluating the performance and management of artificial reefs using artificial reef multimetric index (ARMI). Ocean Coast. Manag. 2020, 198, 105350. [Google Scholar] [CrossRef]
- Kuo, Y.Y.; Shih, C.H.; Lee, Y.C.; Chang, W.T.; Chu, T.J. Identifying Indicator Species in Habitats Created by Coastal Structures. China Ocean Eng. 2010, 24, 117–134. [Google Scholar]
- Siddig, A.A.H.; Ellison, A.M.; Ochs, A.; Leeman, C.V.; Lau, M.K. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D. Flagships, umbrellas, and keystones: Is single-species management passé in the landscape era? Conserv. Biol. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Caro, T.M. Umbrella species: Critique and lessons from East Africa. Anim. Conserv. 2003, 6, 171–181. [Google Scholar] [CrossRef]
- Morrison, M.L. Restoring Wildlife: Ecological Concepts and Practical Applications; Society for Ecological Restoration International: Island, WA, USA, 2009. [Google Scholar]
- Caro, T. Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship, and Other Surrogate Species; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 9, 479–486. [Google Scholar] [CrossRef]
- Landres, P.B.; Verner, J.; Thomas, J.W. Ecological uses of vertebrate indicator species: A critique. Conserv. Biol. 1988, 2, 316–328. [Google Scholar] [CrossRef]
- Cairns, J., Jr.; Pratt, J.R. A history of biological monitoring using benthic macroinvertebrates. In Freshwater Biomonitoring and Benthic Macroinvertebrates; Rosenberg, D.M., Resh, V.H., Eds.; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Bartell, S.M. Biomarkers, bioindicators, and ecological risk assessment—A brief review and evaluation. Environ. Bioindic. 2006, 1, 39–52. [Google Scholar] [CrossRef]
- Burger, J. Bioindicators: Types, development, and use in ecological assessment and research. Environ. Bioindic. 2006, 1, 22–39. [Google Scholar] [CrossRef]
- Shih, C.H.; Kuo, Y.Y.; Chu, T.J.; Chou, W.C.; Chang, W.T.; Lee, Y.C. Eco-environmental Impact Assessment of Artificial Beach Nourishment on Benthic Invertebrate Community at Anping Coast, Taiwan. China Ocean Eng. 2011, 25, 215–236. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, P.H.; Shih, C.H.; Shiao, J.C.; Tzeng, T.D.; Chang, W.C. The impact of religious release fish on conservation. Glob. Ecol. Biogeogr. 2021, 27, e01556. [Google Scholar]
- Primack, R.B. Essentials of Conservation Biology, 5th ed.; Sinauer Associates, Inc. Press: Palgrave Macmillan, MA, USA, 2010. [Google Scholar]
- Moraitis, M.L.; Tsikopoulou, I.; Geropoulos, A.; Dimitriou, P.D.; Papageorgiou, N.; Giannoulaki, M.; Valavanis, V.D.; Karakassis, I. Molluscan indicator species and their potential use in ecological status assessment using species distribution modeling. Mar. Environ. Res. 2018, 140, 10–17. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Chozas, S.; Correia, O.; Santos, A.M.C.; Hortal, J. Using niche models of indicator species to predict the distribution of xerophytic shrub dune communities. Web Ecol. 2016, 16, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Fleming, J.E.; Sutherland, C.; Sterrett, S.C.; Grant, E.H.C. A latent process model approach to improve the utility of indicator species. Oikos 2020, 129, 1753–1762. [Google Scholar] [CrossRef]
- Ünal, S.; Şahin, B.; Urla, Ö.; Efe, B. The Use of Indicator Species and Ecological Degradation Model for Rangeland Condition Assessment in Turkey. Tarla Bitk. Merk. Araşt. Enst. Derg. 2017, 26, 190–202. [Google Scholar]
- De Jonge, M.M.; Benítez-López, A.; Hennekens, S.; Santini, L.; Huijbregts, M.A.; Schipper, A.M. Conditional love? Co-occurrence patterns of drought-sensitive species in European grasslands are consistent with the stress-gradient hypothesis. Glob. Ecol. Biogeogr. 2021, 30, 1609–1620. [Google Scholar] [CrossRef]
- Ashton, E.C.; Macintosh, D.J. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For. Ecol. Manag. 2002, 166, 111–129. [Google Scholar] [CrossRef]
- Macintosh, D.J.; Ashton, E.C.; Havanon, S. Mangrove rehabilitation and intertidal biodiversity: A study in the Ranong Mangrove Ecosystem, Thailand. Estuar. Coast. Shelf Sci. 2002, 55, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Duke, N.C.; Meynecke, J.O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diop, S. Vulnerability assessment of mangroves to environmental change. Estuar. Coast. Shelf Sci. 2003, 58, 1–2. [Google Scholar] [CrossRef]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Chang. Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, A.C. Effects of mangrove removal on benthic communities and sedi-ment characteristics at Mangawhai Harbour, northern New Zealand. ICES J. Mar. Sci. 2010, 67, 1087–1104. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Shih, C.H. Sustainable management of coastal wetlands in Taiwan: A review for invasion, conservation, and removal of mangroves. Sustainability 2019, 11, 4305. [Google Scholar] [CrossRef] [Green Version]
- Opdam, P.; Pouwels, R.; Van Rooij, S.; Steingröver, E.; Vos, C. Setting Biodiversity Targets in Participatory Regional Planning: Introducing Ecoprofiles. Ecol. Soc. 2008, 13, 2021. Available online: http://www.jstor.org/stable/26267947 (accessed on 12 October 2020). [CrossRef] [Green Version]
- Shannon, E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois: Urbana, IL, USA; Chicago, IL, USA, 1963. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Cazelles, K.; Araújo, M.B.; Mouquet, N.; Gravel, D. A theory for species co-occurrence in interaction networks. Theor. Ecol. 2016, 9, 39–48. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Branton, M.; Richardson, J.S. Assessing the value of the umbrella-species concept for conservation planning with meta-analysis. Conserv. Biol. 2011, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Roberge, J.M.; Angelstam, P. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Chu, T.J. Ecological restoration in Kaya Water Recycling Center, Hsinchu City—Habitat Improvement Restoration (Sub-Project 2-1: Research on U. formosensis and Its Habitat), 2006–2008. Available online: https://ecologicallabs.com/ecosystem-restoration/ (accessed on 15 October 2020). (In Chinese).
- Levinton, J.S.; Judge, M.L.; Kurdziel, J.P. Functional differences between the major and minor claws of fiddler crabs (Uca, family Ocypodidae, order Decapoda, Subphylum Crustacea): A result of selection or developmental constraint? J. Exp. Mar. Biol. Ecol. 1995, 193, 147–160. [Google Scholar] [CrossRef]
- Kuo, Y.Y.; Lee, L.C. Construct the Coastal Landscape and Ecology Environment; Chansbook: Hsinchu, Taiwan, 2006. (In Chinese) [Google Scholar]
- Pander, J.; Geist, J. Ecological indicators for measuring stream restoration success. Ecol. Indic. 2013, 30, 106–118. [Google Scholar] [CrossRef]



| Species | Time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | ||||||||||||
| Oct | Nov | Dec | Jan | Feb | Mar | Arp | May | June | July | Aug | Sep | ||
| Helicana doerjesi | 0.002 | 0.004 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.002 | 0.001 | 0.001 | 0.002 | 9.2 × 10−4 |
| Helice formosensis | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.002 | 0.001 | 0.007 | 0.007 | 0.074 | 0.075 | 0.074 | 2.7 × 10−3 |
| Mictyris brevidactylus | 0.079 | 0.077 | 0.072 | 0.069 | 0.074 | 0.071 | 0.074 | 0.075 | 0.076 | 0.071 | 0.076 | 0.075 | 7.4 × 10−2 |
| Macrophthalmus banzai | 0.139 | 0.077 | 0.072 | 0.039 | 0.074 | 0.040 | 0.074 | 0.041 | 0.076 | 0.071 | 0.076 | 0.075 | 6.7 × 10−2 |
| Macrophthalmusab reviatus | 0.001 | 0.007 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 7.1 × 10−4 |
| Ocypode ceratophthalmus | 0.002 | 0.007 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.002 | 0.001 | 0.000 | 0.000 | 0.001 | 7.1 × 10−4 |
| Ocypode stimpsoni | 0.000 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 8.5 × 10−4 |
| Ocypode sinensis | 0.007 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 2.0 × 10−4 |
| Uca arcuata | 0.135 | 0.023 | 0.012 | 0.007 | 0.039 | 0.004 | 0.039 | 0.039 | 0.246 | 0.074 | 0.250 | 0.074 | 4.1 × 10−2 |
| Uca formosensis | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.004 | 0.001 | 0.012 | 0.013 | 0.013 | 1.1 × 10−3 |
| Uca lactea | 0.013 | 0.013 | 0.002 | 0.004 | 0.012 | 0.004 | 0.012 | 0.069 | 0.246 | 0.074 | 0.250 | 0.074 | 2.2 × 10−2 |
| Uca perplexa | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.5 × 10−4 |
| Uca borealis | 0.012 | 0.013 | 0.002 | 0.001 | 0.001 | 0.002 | 0.004 | 0.004 | 0.041 | 0.004 | 0.076 | 0.013 | 5.2 × 10−3 |
| Scopimera longidactyla | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.4 × 10−4 |
| Scopimera globosa | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.5 × 10−4 |
| Scopimera bitympana | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 3.3 × 10−4 |
| Scylla serrata | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.5 × 10−4 |
| Pagurus dubius | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.4 × 10−4 |
| Diogenes penicillatus | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.5 × 10−4 |
| Parapagurus diogenes | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 1.9 × 10−4 |
| Parapagurus obtusifrons | 0.004 | 0.004 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 | 0.001 | 5.0 × 10−4 |
| Total species(S) | 18 | 19 | 14 | 11 | 14 | 13 | 12 | 14 | 14 | 12 | 14 | 14 | |
| Species richness index(D) | 0.19 | 0.19 | 0.23 | 0.20 | 0.17 | 0.22 | 0.20 | 0.21 | 0.17 | 0.22 | 0.17 | 0.16 | |
| Pielu’s evenness index(J’) | 2.16 | 2.12 | 1.83 | 1.86 | 2.02 | 1.95 | 1.96 | 1.97 | 2.11 | 1.89 | 2.06 | 2.13 | |
| Shannon index(H’) | 0.75 | 0.72 | 0.69 | 0.77 | 0.77 | 0.76 | 0.79 | 0.75 | 0.80 | 0.76 | 0.78 | 0.81 | |
| Simpson index(SI) | 2.49 | 2.67 | 2.17 | 1.78 | 2.33 | 1.86 | 1.73 | 1.93 | 1.88 | 1.53 | 1.77 | 1.74 | |
| Species | Time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | ||||||||||||
| Oct | Nov | Dec | Jan | Feb | Mar | Arp | May | June | July | Aug | Sep | Rank | |
| Helicana doerjesi | 12 | 8 | 10 | 11 | 5 | 7 | 9 | 9 | 7 | 9 | 9 | 8 | 7 |
| Helice formosensis | 18 | 11 | 10 | 9 | 10 | 6 | 7 | 5 | 6 | 1 | 6 | 3 | 6 |
| Mictyris brevidactylus | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 4 | 4 | 1 | 1 |
| Macrophthalmus banzai | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 3 | 3 | 4 | 4 | 1 | 2 |
| Macrophthalmusab reviatus | 17 | 6 | 7 | 8 | 8 | 8 | 12 | 11 | 11 | 8 | 11 | 12 | 9 |
| Ocypode ceratophthalmus | 13 | 7 | 9 | 10 | 7 | 12 | 9 | 8 | 9 | 11 | 13 | 11 | 9 |
| Ocypode stimpsoni | 19 | 10 | 8 | 5 | 6 | 10 | 6 | 10 | 12 | 10 | 10 | 9 | 8 |
| Ocypode sinensis | 9 | 11 | 12 | 12 | 13 | 14 | 13 | 14 | 15 | 13 | 15 | 15 | 14 |
| Uca arcuata | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 1 | 1 | 1 | 3 | 3 |
| Uca formosensis | 19 | 19 | 14 | 6 | 10 | 9 | 8 | 6 | 10 | 6 | 7 | 7 | 11 |
| Uca lactea | 6 | 4 | 6 | 4 | 4 | 3 | 4 | 2 | 1 | 1 | 1 | 3 | 4 |
| Uca perplexa | 15 | 13 | 12 | 12 | 14 | 14 | 13 | 15 | 15 | 13 | 15 | 15 | 16 |
| Uca borealis | 8 | 4 | 4 | 6 | 9 | 5 | 5 | 7 | 5 | 7 | 3 | 6 | 5 |
| Scopimera longidactyla | 15 | 13 | 15 | 12 | 15 | 14 | 13 | 15 | 15 | 13 | 15 | 15 | 20 |
| Scopimera globosa | 4 | 20 | 15 | 12 | 15 | 14 | 13 | 15 | 15 | 13 | 15 | 15 | 16 |
| Scopimera bitympana | 7 | 16 | 15 | 12 | 12 | 12 | 9 | 13 | 14 | 11 | 13 | 13 | 13 |
| Scylla serrata | 11 | 13 | 15 | 12 | 15 | 14 | 13 | 15 | 15 | 13 | 15 | 15 | 16 |
| Pagurus dubius | 14 | 16 | 15 | 12 | 15 | 14 | 13 | 15 | 15 | 13 | 15 | 15 | 21 |
| Diogenes penicillatus | 4 | 20 | 15 | 12 | 15 | 14 | 13 | 15 | 15 | 13 | 15 | 15 | 16 |
| Parapagurus diogenes | 19 | 16 | 15 | 12 | 15 | 11 | 13 | 15 | 8 | 13 | 12 | 13 | 15 |
| Parapagurus obtusifrons | 10 | 8 | 5 | 12 | 15 | 14 | 13 | 12 | 13 | 13 | 8 | 10 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, T.-J.; Shih, C.-H.; Lu, Y.-M.; Shih, Y.-J.; Wang, J.-Q.; Huang, L.-M. Incorporating Species-Conditional Co-Occurrence When Selecting Indicator Species to Monitor Restoration after Mangrove Removal from the Siangshan Wetland, Taiwan. J. Mar. Sci. Eng. 2021, 9, 1044. https://doi.org/10.3390/jmse9101044
Chu T-J, Shih C-H, Lu Y-M, Shih Y-J, Wang J-Q, Huang L-M. Incorporating Species-Conditional Co-Occurrence When Selecting Indicator Species to Monitor Restoration after Mangrove Removal from the Siangshan Wetland, Taiwan. Journal of Marine Science and Engineering. 2021; 9(10):1044. https://doi.org/10.3390/jmse9101044
Chicago/Turabian StyleChu, Ta-Jen, Chun-Han Shih, Yu-Ming Lu, Yi-Jia Shih, Jia-Qiao Wang, and Liang-Ming Huang. 2021. "Incorporating Species-Conditional Co-Occurrence When Selecting Indicator Species to Monitor Restoration after Mangrove Removal from the Siangshan Wetland, Taiwan" Journal of Marine Science and Engineering 9, no. 10: 1044. https://doi.org/10.3390/jmse9101044
APA StyleChu, T.-J., Shih, C.-H., Lu, Y.-M., Shih, Y.-J., Wang, J.-Q., & Huang, L.-M. (2021). Incorporating Species-Conditional Co-Occurrence When Selecting Indicator Species to Monitor Restoration after Mangrove Removal from the Siangshan Wetland, Taiwan. Journal of Marine Science and Engineering, 9(10), 1044. https://doi.org/10.3390/jmse9101044








