Abstract
In recent years, the massive influx of pelagic Sargassum spp. has generated great interest in the scientific community, highlighting the urgency of addressing the physiology and biochemical composition of these species. Until now, the presence of lignified cells in the tissue of Sargassum natans and Sargassum fluitans has not been reported. Although ‘‘lignin-like’’ compounds have been identified in green algae, the presence of true lignin in the Sargassum genus has not been confirmed. Our work is the first report of lignified cells forming the secondary cell wall in these Sargassum. This study used histological techniques applied to thick sections for identifying lignin-like tissues in Sargassum spp. The dyes as Safranin O and Toluidine have been used to differentiate lignin and cellulose in conducting tissue and to indicate the presence, absence, and distribution of these compounds in tissues. This work is the initial study of the cell wall heteropolymers structure and arrangement in Sargassum spp., providing insights into the unique cell wall architecture of these seaweeds.
1. Introduction
The Caribbean and western Atlantic equatorial surface waters have been historically considered oligotrophic [1]; however, large phytoplankton blooms in the eastern Caribbean during 2009 and 2010 were observed and attributed to unusually large Amazon river plumes; these events, together with satellite data, indicated that such events had not happened in the past 30 years [2,3]. Likewise, during Amazon non-flood years before 2011, Sargassum spp. (Phaeophyceae, Fucales) blooms and subsequent impacts to coastal beaches were small or non-existent [4,5]. Since 2011, large quantities of Sargassum have been observed recurrently in what was recently called the great Atlantic Sargassum belt [4], and several studies have recognized an area that comprises a stretch of open ocean between West Africa and South America as the new source of the pelagic Sargassum spp. that arrive in the tropical Atlantic and the Caribbean.
Accumulations of Sargassum occur naturally and regularly in coastal areas, although in smaller quantities than the atypical arrival of what is called “golden tides” occurring since 2011, with a maximal accumulation of more than 20 million metric tons observed in June 2018 on the Caribbean area [4]. Sargassum arrivals play an ecological role in beach nourishment and shoreline stability and provide nutrients for dune plants and sea birds that feed on fauna carried by the macroalgae. However, the massive influx of Sargassum and subsequent decaying on the beach may not be aesthetically acceptable to beach users, resulting in the demise of local tourism industries; in addition, there are negative ecological consequences in affected areas [6,7,8].
During the displacement of pelagic Sargassum through well-described transport pathways between the Equatorial Atlantic and the Caribbean Sea [9], the abiotic stresses such as UV-B (ultraviolet B) radiation can result in severe damage to nucleic acids, proteins, pigmentation, rubisco, photosynthesis, growth, and reproduction [10]; additionally, toxic heavy metals have been reported in pelagic Sargassum [11]. Exposition to stress can stimulate the production of several secondary compounds such as flavonoids, tannins, and phenolic compounds, all of which are thought to have played an important role in plant transition from water to land [12,13,14,15,16,17,18].
In the framework of circular economy, invasive species of seaweed are good candidates for use as a resource, and biorefinery is a strategy that allows the full use of biomass (e.g., biogas, biofertilizer, alginate, and fine chemicals), however, knowledge of the biomass adequate composition is required in order to reach an adequate valorization of them [7,19].
Although the presence of polyphenolic and lignin-like compounds has been reported in various algae in response to stress conditions [20,21,22], the presence of true lignin in Sargassum genus has not been confirmed.
Many studies have focused on the processes of lignin detected in cell walls by use of histological techniques [23,24]. Over the years, several dyes and different staining techniques have been used to differentiate lignin and cellulose in xylem, which can be used to demonstrate the presence/absence and distribution of these compounds in various tissues [23,24,25].
The aim of this preliminary study was to characterize complex polyphenol compounds in pelagic Sargassum spp. using a histological staining approach. This work provides a foundational baseline to further understand the cell wall heteropolymer architecture and arrangement of these macroalgae. This will allow for more efficient pretreatment strategies to access the valuable compounds offered by Sargassum spp. under the concept of biorefinery.
2. Materials and Methods
2.1. Biomass Collection and Preparation
Samples of pelagic Sargassum spp. were collected in May 2019 at Puerto Morelos, Quinta Roo, Mexico. The harvested macroalgae were first washed with distilled water to remove salts and sand, and the samples were identified (S. natans and S. fluitans) using morphological keys [26] and then divided into two batches: one portion was used for histochemical analysis, and the other portion was dried at ambient temperature. Dried samples were pulverized and sieved using an 80-mesh screen to obtain particles less than 0.177 mm in size, which were further used for the chemical composition analysis.
2.2. Physical-Chemical Characterization
2.2.1. Chemical Composition
The dried material was used for the determination of the extractives in organic solvents in hot water, and Klason lignin was determined in triplicate according to the TAPPI T222 om-02 [27] modified technique. In the first step, extractives were obtained with benzene-ethanol (2:1 v/v) and ethanol (95% v/v) by Soxhlet extraction, and over a 4 to 5 h period the heaters were adjusted to provide a boiling rate with no less than 24 cycles to assure the extractions of the specimens. Rotatory evaporation at 40 °C (IKA HB10 digital, Staufen, Germany) was used to concentrate the extraction, according to TAPPI 204 CM [28]. Second, the content of extractives soluble in hot water was determined according to TAPPI standard method T 207 cm-99 [29]; then, 1 g of free extractives material was subjected to acid hydrolysis with 15 mL of 72% (v/v) H2SO4. By using a laboratory water bath (Novatech, Tlaquepaque, Mexico), the sample was kept at a constant temperature of 15 °C and agitation for 2 h. Third, the suspension was diluted into deionized water to reach a 4% (v/v) concentration and boiled for 4 h. The diluted suspension was filtered by an F-type glass filter previously conditioned to constant weight, and the insoluble material was dried in a convection oven. The lignin content was determined using the following equation,
where mf is the recovered weight (g), mi is the weight of sample-free extractives (g), and Exttot is the percentage of total extractives (%).
2.2.2. Thermogravimetric Analysis (TGA)
TGA analysis of Sargassum samples (S. natans and S. fluitans) was carried out using a Perkin Elmer TGA 8000 (PerkinElmer, Walthman, MA, USA). A TG curve was obtained under a nitrogen atmosphere with a flow rate of 10 mL/min and a heating rate of 10 °C/min from 50 to 700 °C with a sample mass of 10 mg in a platinum pan. The mass loss rate in the derivative form (derivative thermogravimetry, DTG) was also calculated in triplicate.
2.2.3. Scanning Electron Microscopy (SEM)
To study the microscopic structure of the raw material and carbon residue from the TGA test of macroalgae, a scanning electron microscope (SEM, model JSM-6360LV, JEOL, Tokyo, Japan) was used. Macroalgae samples were mounted on a metallic stub using double-sided adhesive tape coated with a 15 nm gold layer and observed at 20 kV.
2.2.4. Fourier Transforms Infrared (FT-IR) Analysis
The functional groups of Sargassum samples (S. natans and S. fluitans) were determined using a Bruker spectrophotometer FT-IR Tensor II model (Milton, ON, Canada). Samples were analyzed in Platinum ATR (Attenuated Total Reflection), and spectra were obtained in the region of 4000–400 cm−1 at 4 cm−1 resolution with 32 scans.
2.2.5. Histological Analysis
To analyze the internal structures of pelagic Sargassum, a modification to a previously reported [30,31] process was applied to stem segments of approximately 0.5 mm long. All samples were fixed in FAA solution (40% formaldehyde, glacial acetic acid, 95% ethanol, and distilled water, 10:5:50:35 parts, respectively) for 96 h and kept at 4 °C in the darkness during the whole histological process. Samples were rinsed with distilled water and dehydrated with ethanol (30, 50, 70, 85, 96, and 100% v/v). At the beginning of each treatment, vacuum was applied for 15 min, and the samples continued in the solution for another 45 min. Before the ethanol concentration was increased, every treatment was repeated twice, time with a new solution [32].
Tissues were embedded in Glycol methacrylate plastic resin according to manufacturer’s instructions (JB-4 Embedding Kit, Polysciences, Inc. Parrington, PA, USA). Cross and tangential sections 2–4 µm thick were prepared from tissues using an HM 325 rotary microtome (Themo Fisher Scientific, Waltham, MA, USA).
Histological sections were stained independently with Safranin O dye (0.5%, w/v; in 95% ethanol) for 30 s and with 0.5% toluidine blue in 200 mM acetate buffer [31]. Then, the sections were washed with distilled water for 30 s and allowed to dry at room temperature. In all cases, permanent preparations were made using Polymount, Inc. mounting medium. All histological sections were analyzed and photographed under a light field microscope model Eclipse LV100DA-U (Nikon Co., Tokyo, Japan).
2.2.6. Elemental Analysis
Sargassum samples (S. natans and S. fluitans) and the collected leachate (generated during the natural decomposition of the collected samples) were digested in triplicate according to Fernandez et al. [33]: briefly, the digestion of samples was carried out with 0.5 g of each species in triplicate, which were poured into a 75 mL teflon vial, to which 7.0 mL of 69% HNO3, 1.5 mL of H2O2, 0.5 mL of HF (40–45%) and 2.0 mL of deionized water were added. For the quantification of the elements present in the samples under study, an inductively coupled plasma–optical emission spectrometry (ICP-OES) was used (Perkin Elmer Optima 8000, Waltham, MA, USA). A calibration curve was established for each analyte in triplicate.
3. Results and Discussion
3.1. Biomass Composition and Characterization
The composition of macroalgae biomass (S. natans and S. fluitans) is summarized in Table 1. In both species of Sargassum, a high content of aromatic polyphenol complex or lignin-like materials were present (25.4 and 29.5% respectively). The unusual values of lignin-like materials reported here were obtained in samples collected in the summer at the Mexican Caribbean and coincide with the values reported by Ardalan et al. [34] in brown macroalga Sargassum angustifolium, which were found at rates of 25.8% in summer and 11.8% in winter. Likewise, Yazdani et al. [35] reported that the brown macroalga Nizimuddinia zanardini contains 12.3% of lignin-like materials. These variations in the contents of lignin-like compounds can be the results of different types of abiotic stresses such as UV radiation and high temperature, which are adverse conditions generated by climate change [10,12,13,14,15,16,17,18].

Table 1.
Composition of macroalgal biomass (%) harvested in May 2019. Data shown are the mean ± SD, n = 3.
In order to carry out the thermogravimetric analysis (TGA) on Sargassum samples, three stages were selected (Figure 1). The first stage indicated a loss of 12% in mass, in the range of 50 to 150 °C, which is attributed to the loss of water as was reported by Davis et al. Al et al. [19,36] also found a loss of mass in the range of 8–15% in samples of these same species collected in the Caribbean Sea. The second stage is comprised between 180 to 360 °C with a loss of mass of 35% and is seen in the DTGA curves as two strong peaks at 250 and 320 °C. The overlapping of the peaks indicates the complexity of degradation reaction in this zone that is attributed to the decomposition of hemicellulose and cellulose, respectively.
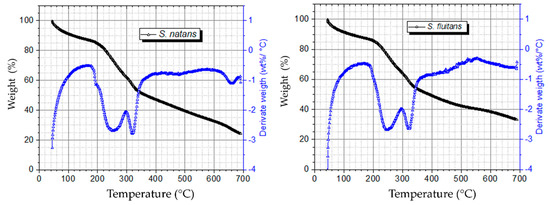
Figure 1.
TGA and DTG curves of Sargassum spp. (a) S. natans; (b) S. fluitans.
These results are consistent with what was previously reported by Ali et al. [37] for Sargassum spp. collected in the Red Sea, where two strong peaks and a shoulder at 276, 336 and 495 °C were reported, respectively, and correspond to the degradation of different biopolymers. These results also concur with the degradation temperature ranges of different components in some lignocellulosic biomasses [37,38,39]. Results obtained in the third stage indicate that at a decomposition zone of 360 to 700 °C, lignin compounds begin degradation [40]; note that differences can be observed in the shape of the curve depending on the species of Sargassum spp. Samples of S. natans showed a loss of mass of 18%, with a char residue of 22% at 700 °C. Likewise, S. fluitans showed a monotonic loss of mass up to 640 °C, with a char residue of 33% at 700 °C. While in this stage, substances with high molecular weight like polysaccharides, proteins and lignin are degraded, the values of char residue (22–33%) obtained in this study are in the range of those reported by Fernandez et al. [33], where the analysis of thermal degradation of lignin showed a remarkable char residue (37–40%). Similar results were obtained by Escobar et al. [41] for these species of Sargassum, where the residue obtained at temperatures above 550 °C was coke and ash. Overall, results obtained in this work showed marked differences in the decomposition zone of lignin between S. natans and S. fluitans.
Figure 2 shows the Sargassum charcoal samples obtained from the TGA analysis under SEM. In both species, S. natans (Figure 2c) and S. fluitans (Figure 2d) showed cracks (yellow arrows) that can be attributed to the volumetric expansion of lignine up to four times when subjected to thermal stress by temperatures greater than 260 °C and its subsequent relaxation when the temperature decreases according to that reported by El Moustaqim et al. [42].
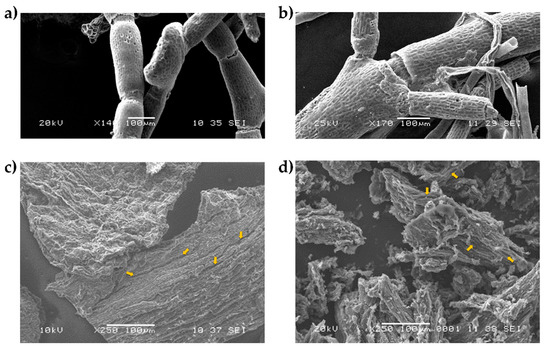
Figure 2.
Scanning electron microscopy (SEM) of Sargassum spp. raw material and char. (a) Raw material of S. natans; (b) raw material of S. fluitans; (c) char of S. natans and (d) char of S. fluitans.
Similarly, the higher content of lignin-like materials present in S. natans (Figure 2a) could be responsible for maintaining a greater integrity in the structure due to the persistence of residues of lignin-like materials at high temperatures of TGA analysis in comparison with the char surface from S. fluitans, which is more fragmented (Figure 2b).
3.2. FT-IR Analysis
FTIR spectra of S. natans and S. fluitans show a similar pattern of absorption bands (Figure 3). The strong absorption band at 3406 cm−1 corresponds to the higher exposure of hydroxyl groups (-OH) in compounds like cellulose, hemicellulose and lignin [8,43]. The strong intensity in the bands around 1600 and 1406 cm−1 represents the stretching of C=C that are associated with lignin and aromatic compounds, as reported by Thompson et al. and Silva et al. [8,43] for samples of Sargassum spp. and lignocellulosic biomass (sugarcane bagasse). The bands at 1055 and 897 cm−1 are associated with C-O-C vibrations and are indicatives of anomeric regions of the xylans of hemicellulose and stretch of B-glycosidic bonds of cellulose and hemicellulose core structures, as described in [8,43,44]. The weak peak observed at 833 cm−1 represents the C-H bond out-of-plane in p-hydroxyphenyl unit in lignin, which coincides with the report of Silva et al. [43] by lignocellulosic biomass.
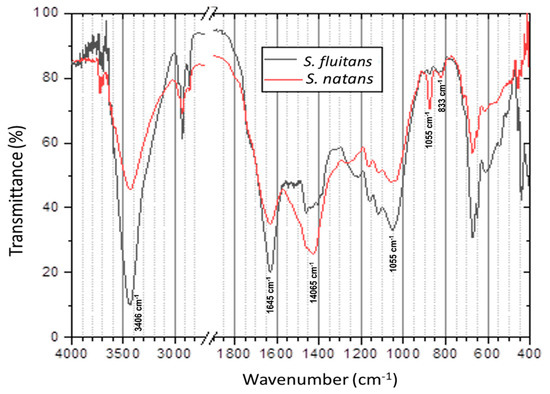
Figure 3.
FTIR spectra of pelagic Sargassum spp.
The results obtained by FTIR are consistent with the chemical composition values of the main polymeric compounds (cellulose, hemicellulose, and lignin) that are present in the two species of macroalgal biomass (Table 1), where the S. natans species shows the most intense bands (1406, 897 and 833 cm−1), which are associated with the presence of functional groups present in lignocellulosic material.
3.3. Histological Analysis
The histological analysis revealed the presence of a secondary cell wall and epidermis (secondary cell-wall layers) in the tissues of the S. natans and S. fluitans. The Safranin O staining showed a characteristic red coloration of lignified cells (Figure 4a,a’,c,c’), showing the presence of a secondary cell wall (yellow arrows) in both species (Figure 4a’,c’) [30,31].
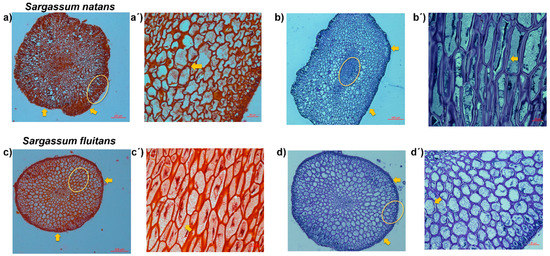
Figure 4.
Safranin O stained cross-sections of stem of Sargassum natans (a,a’), and Sargassum fluitans (c,c’). Toluidine blue-stained cross-sections of stem of Sargassum natans (b,b’), and Sargassum fluitans (d,d’). (a’,b’,c’,d’) represent the area inside the yellow circle.
Histological staining methods such as the Safranin O staining method observed by microscopy have been successfully used to visualize differences in lignification between and within cellular elements [23]. Until now, such developmentally specialized cell walls have been described in vascular plants and in the intertidal red alga Calliarthron cheilosporioides [13]. In the case of Sargassum, our hypothesis about the presence of the secondary cell wall is due to the exposition to different stresses, which may change lignin content and composition as defense responses to the stress e.g., high temperatures, radiation, high nutrient content, and the presence of metals [13,14].
In order to obtain a general overview of the cell wall Toluidine blue staining, a polychromatic stain was also used, allowing the visualization of several compounds (e.g., nucleic acids, polysaccharides, and lignin, which stain bright blue) [45,46]. Lignin is a complex organic polymer and is important for cell walls but can also be found between cells and in the cells of all vascular plants [46]. Toluidine blue O of stem cross-sections revealed the presence of lignin-like materials in the cell wall (Figure 4b,b’,d,d’), and cells of the epidermis and intercellular spaces (yellow arrow) are lignified since they show a blue coloration in both species (Figure 4b’,d’) [45,46].
3.4. Elemental Analysis
Both samples from Sargassum spp. (S. natans and S. fluitans) showed the presence of heavy metals, within different concentrations depending on the species (Table 2). As shown in Table 2 of the metals tested in this study, on average S. natans showed a higher concentration of the analyzed metals compared with S. fluitans. This is the case of B (228.83 mg/Kg) and As (115.66 mg/Kg) in S. natans compared with the same metals B (204.36 mg/Kg) and As (76.49 mg/Kg) of S. fluitans.

Table 2.
Element content (mg/kg) in Sargassum spp.
This variation is significant among both species of Sargassum and it can be attributed to the different concentrations of lignin-like materials found during characterization of the biomass of both species (Table 1), since these types of compounds play an important role in the bioabsorption process of heavy metals [21]. Only low concentrations of B and Al were found in consortium Sargassum leachate.
Results observed here were similar to those previously reported by Rodriguez-Martinez et al. and Thompson et al. [11,47], where the concentration of metals such as As varied significantly depending on the Sargassum species under study. It is well known that abiotic stress and abnormally high concentrations of nutrients, high temperature and heavy metals can play an important role in phenolic compounds biosynthetic pathways and accumulation in plants and Sargassum fusiforme [14,17].
4. Conclusions
In summary, this study demonstrates the presence of high contents of lignin-like polyphenols in the species of S. natans and S. fluitans, where S. natans showed the highest concentrations of the analyzed elements and in some cases almost doubled the detected concentration in the other species. Histological results, by Safranin O and toluidine blue staining revealed the presence of lignified tissue forming a secondary cell wall in both species, which was consistent with the results obtained in the characterization by FTIR, where the presence of absorption bands characteristic of functional groups associated with the lignin molecule was observed. The elemental analysis revealed that at least two of the analyzed metals (Al and B) are solubilized in the leachates generated during the decomposition of Sargassum spp., which is a latent risk for the groundwater conditions (water quality) of the Yucatan peninsula. Additional work will focus on identifying complex aromatic polyphenols present in Sargassum spp., which will allow the design of more efficient pretreatment strategies to access the valuable compounds offered by Sargassum spp. under the concept of biorefinery, which could turn this problem into a source of sustainable raw material.
Author Contributions
All the authors contributed to this work: L.A.-G.: conceptualization, investigation, writing—original draft preparation. R.T.-T.: conceptualization, project administration, investigation. J.D.-M., R.C.-V., E.O.-M. and F.A.B.-P.: performed the experimental analysis, analyzed the data. R.M.L.-B. and G.C.-E.: Writing, Review and Editing of the paper. A.C.-V. and C.H.-Z.: resources, review and editing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for studies not involving human or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Santiago Durante-Aranda from Material Unit for technical assistance in SEM micrographs and Daniela Ortega-Camacho from Water Research Unit for technical assistance in elemental analysis by inductively coupled plasma–optical emission spectrometry (ICP-OES).
Conflicts of Interest
The authors declare no conflict of interest in this paper.
References
- Johns, E.M.; Lumpkin, R.; Putman, N.F.; Smith, R.H.; Muller-Karger, F.E.; Rueda, D.; Hu, C.; Wang, M.; Brooks, M.T.; Gramer, L.J. The establishment of a pelagic Sargassum population in the tropical Atlantic: Biological consequences of a basin-scale long distance dispersal event. Prog. Oceanogr. 2020, 182, 102269. [Google Scholar] [CrossRef]
- Johns, E.M.; Muhling, B.A.; Perez, R.C.; Müller-Karger, F.E.; Melo, N.; Smith, R.H.; Lamkin, J.T.; Gerard, T.L.; Malca, E. Amazon River water in the northeastern Caribbean Sea and its effect on larval reef fish assemblages during April 2009. Fish. Oceanogr. 2014, 23, 472–494. [Google Scholar] [CrossRef]
- Oviatt, C.A.; Huizenga, K.; Rogers, C.S.; Miller, W.J. What nutrient sources support anomalous growth and the recent Sargassum mass stranding on Caribbean beaches? A review. Mar. Pollut. Bull. 2019, 145, 517–525. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Arana, H.A.H.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Graham, C.; Vera, E.; Escalante-Mancera, E.; Álvarez-Filip, L.; van Tussenbroek, B.I. Temporal changes in the composition and biomass of beached pelagic Sargassum species in the Mexican Caribbean. Aquat. Bot. 2020, 167, 103275. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden Tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Putman, N.F.; Goni, G.J.; Gramer, L.J.; Hu, C.; Johns, E.M.; Trinanes, J.; Wang, M. Simulating transport pathways of pelagic Sargassum from the Equatorial Atlantic into the Caribbean Sea. Prog. Oceanogr. 2018, 165, 205–214. [Google Scholar] [CrossRef]
- Brown, M.T. UV-B radiation and the green tide-forming macroalga Ulva. In Aquatic Ecosystems in a Changing Climate; CRC Press: Boca Raton, FL, USA, 2018; ISBN 0429436130. [Google Scholar]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef]
- Holzinger, A.; Pichrtová, M. Abiotic stress tolerance of charophyte green algae: New challenges for omics techniques. Front. Plant Sci. 2016, 7, 678. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Nayaka, S.; Toppo, K.; Verma, S. Adaptation in Algae to Environmental Stress and Ecological Conditions. In Plant Adaptation Strategies in Changing Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 103–115. [Google Scholar]
- Sun, D.; Call, D.; Wang, A.; Cheng, S.; Logan, B.E. Geobacter sp. SD-1 with enhanced electrochemical activity in high-salt concentration solutions. Environ. Microbiol. Rep. 2014, 6, 723–729. [Google Scholar] [CrossRef]
- Liu, L.; Lin, L. Effect of Heat Stress on Sargassum fusiforme Leaf Metabolome. J. Plant Biol. 2020, 63, 229–241. [Google Scholar] [CrossRef]
- Rosado-Espinosa, L.A.; Freile-Pelegrín, Y.; Hernández-Nuñez, E.; Robledo, D. A comparative study of Sargassum species from the Yucatan peninsula coast: Morphological and chemical characterisation. Phycologia 2020, 59, 261–271. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2020, 143134. Available online: https://www.sciencedirect.com/science/article/abs/pii/S004896972036664X (accessed on 9 November 2020).
- Zagoskina, N.V.; Dubravina, G.A.; Alyavina, A.K.; Goncharuk, E.A. Effect of ultraviolet (UV-B) radiation on the formation and localization of phenolic compounds in tea plant callus cultures. Russ. J. Plant Physiol. 2003, 50, 270–275. [Google Scholar] [CrossRef]
- Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture system. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech. Histochem. 2007, 82, 209–216. [Google Scholar] [CrossRef]
- Estevez, J.M.; Fernández, P.V.; Kasulin, L.; Dupree, P.; Ciancia, M. Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 2009, 19, 212–228. [Google Scholar] [CrossRef]
- Ferreira, B.G.; Falcioni, R.; Guedes, L.M.; Avritzer, S.C.; Antunes, W.C.; Souza, L.A.; Isaias, R.M.S. Preventing false negatives for histochemical detection of phenolics and lignins in PEG-embedded plant tissues. J. Histochem. Cytochem. 2017, 65, 105–116. [Google Scholar] [CrossRef]
- Camacho, O.; Mattio, L.; Draisma, S.; Fredericq, S.; Diaz-Pulido, G. Morphological and molecular assessment of Sargassum (Fucales, Phaeophyceae). Syst. Biodivers. 2015, 13, 105–130. [Google Scholar] [CrossRef]
- TAPPI. 222 om-02: Acid-insoluble lignin in wood and pulp. In 2002–2003 TAPPI Test Methods; TAPPI: Atlanta, GA, USA, 2002. [Google Scholar]
- TAPPI. Solvent Extractives of Wood and Pulp (Proposed Revision of T 204 cm-97); TAPPI: Atlanta, GA, USA, 2007. [Google Scholar]
- TAPPI. Water Solubility of Wood and Pulp; TAPPI: Atlanta, GA, USA, 1999. [Google Scholar]
- Catzín-Yupit, C.N.; Ramírez-Morillo, I.M.; Pool, F.A.B.; Loyola-Vargas, V.M. Ontogenic development and structure of the embryo, seed, and fruit of Jatropha curcas L.(Euphorbiaceae). South Afr. J. Bot. 2014, 93, 1–8. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Teyer, L.F.; Quiroz-Figueroa, F.; Loyola-Vargas, V.; Infante, D. Culture-induced variation in plants of Coffea arabica cv. Caturra rojo, regenerated by direct and indirect somatic embryogenesis. Mol. Biotechnol. 2003, 23, 107–115. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198. [Google Scholar]
- Fernández, F.; Boluda, C.J.; Olivera, J.; Gómez, L.A.G.B.; Gómez, E.E.A.M. Prospective elemental analysis of algal biomass accumulated at the Dominican Republic Shores during 2015. Cent. Azucar 2017, 44, 11–22. [Google Scholar]
- Ardalan, Y.; Jazini, M.; Karimi, K. Sargassum angustifolium brown macroalga as a high potential substrate for alginate and ethanol production with minimal nutrient requirement. Algal Res. 2018, 36, 29–36. [Google Scholar] [CrossRef]
- Yazdani, P.; Zamani, A.; Karimi, K.; Taherzadeh, M.J. Characterization of Nizimuddinia zanardini macroalgae biomass composition and its potential for biofuel production. Bioresour. Technol. 2015, 176, 196–202. [Google Scholar] [CrossRef]
- López-Sosa, L.B.; Alvarado-Flores, J.J.; Corral-Huacuz, J.C.; Aguilera-Mandujano, A.; Rodríguez-Martínez, R.E.; Guevara-Martínez, S.J.; Alcaraz-Vera, J.V.; Rutiaga-Quiñones, J.G.; Zárate-Medina, J.; Ávalos-Rodríguez, M.L.; et al. A Prospective Study of the Exploitation of Pelagic Sargassum spp. as a Solid Biofuel Energy Source. Appl. Sci 2020, 10, 8706. [Google Scholar] [CrossRef]
- Ali, I.; Bahadar, A. Red Sea seaweed (Sargassum spp.) pyrolysis and its devolatilization kinetics. Algal Res. 2017, 21, 89–97. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves. Thermochim. Acta 2013, 566, 36–43. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Escobar, B.; Pérez-Salcedo, K.Y.; Alonso-Lemus, I.L.; Pacheco, D.; Barbosa, R. N-doped porous carbon from Sargassum spp. as metal-free electrocatalysts for oxygen reduction reaction in alkaline media. Int. J. Hydrog. Energy 2017, 42, 30274–30283. [Google Scholar] [CrossRef]
- El Moustaqim, M.; El Kaihal, A.; El Marouani, M.; Men-La-Yakhaf, S.; Taibi, M.; Sebbahi, S.; El Hajjaji, S.; Kifani-Sahban, F. Thermal and thermomechanical analyses of lignin. Sustain. Chem. Pharm. 2018, 9, 63–68. [Google Scholar] [CrossRef]
- Silva, T.A.L.; Zamora, H.D.Z.; Varão, L.H.R. Effect of steam explosion pretreatment catalysed by organic acid and alkali on chemical and structural properties and enzymatic hydrolysis of sugarcane bagasse. Waste Biomass Valoriz. 2018, 9, 2191–2201. [Google Scholar] [CrossRef]
- Tapia-Tussell, R.; Avila-Arias, J.; Maldonado, J.D.; Valero, D.; Olguin-Maciel, E.; Pérez-Brito, D.; Alzate-Gaviria, L. Biological pretreatment of mexican caribbean macroalgae consortiums using Bm-2 strain (Trametes hirsuta) and its enzymatic broth to improve biomethane potential. Energies 2018, 11, 494. [Google Scholar] [CrossRef]
- Mitra, P.P.; Loqué, D. Histochemical staining of Arabidopsis thaliana secondary cell wall elements. JoVe (J. Vis. Exp.) 2014, 87, e51381. [Google Scholar]
- Herrera-Ubaldo, H.; de Folter, S. Exploring cell wall composition and modifications during the development of the gynoecium medial domain in Arabidopsis. Front. Plant Sci. 2018, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Efficiency of hydrothermal pretreatment on the anaerobic digestion of pelagic Sargassum for biogas and fertiliser recovery. Fuel 2020, 279, 118527. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).