Underway Measurement of Dissolved Inorganic Carbon (DIC) in Estuarine Waters
Abstract
1. Introduction
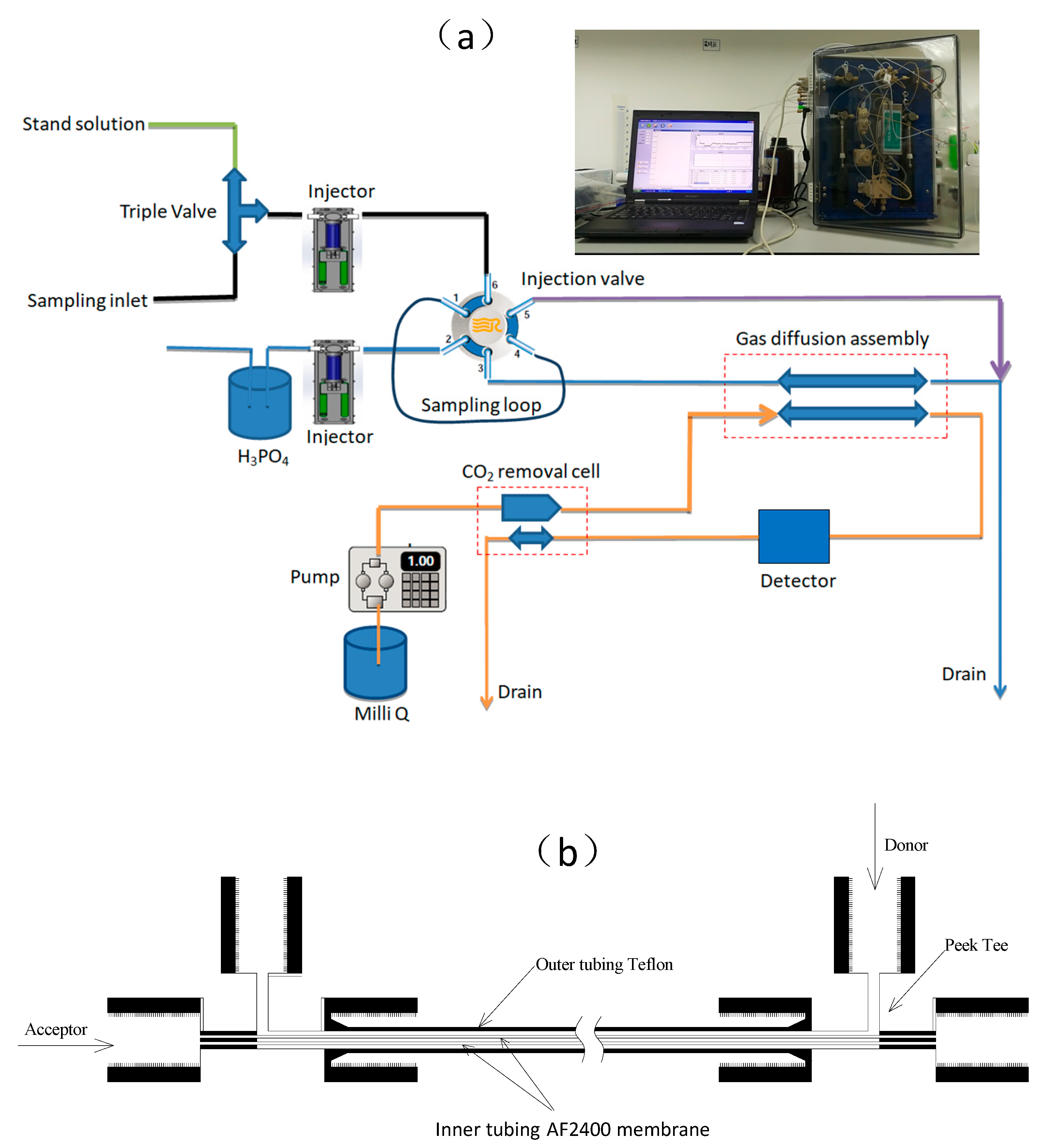
2. Measurement System
2.1. DIC Analysis System
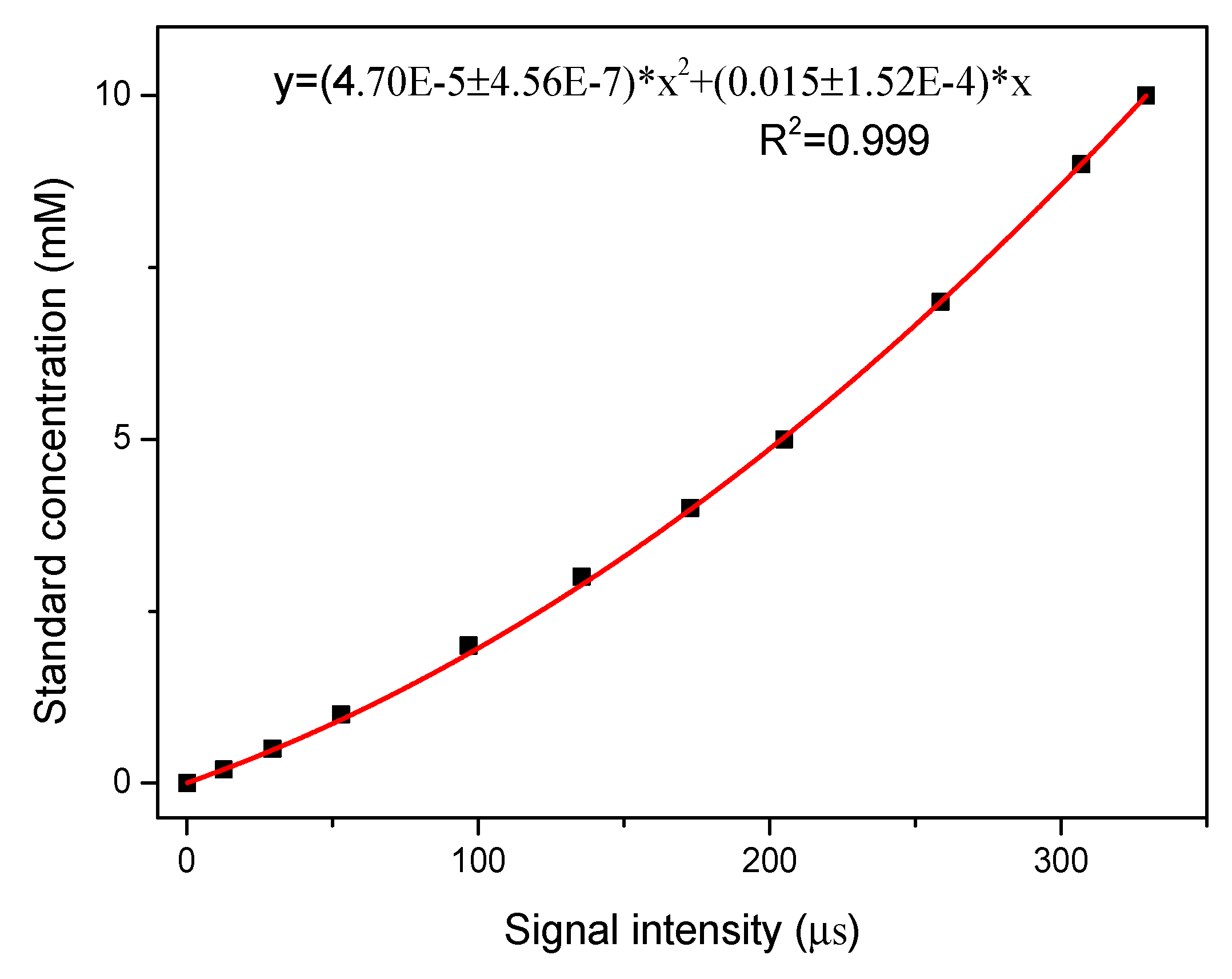
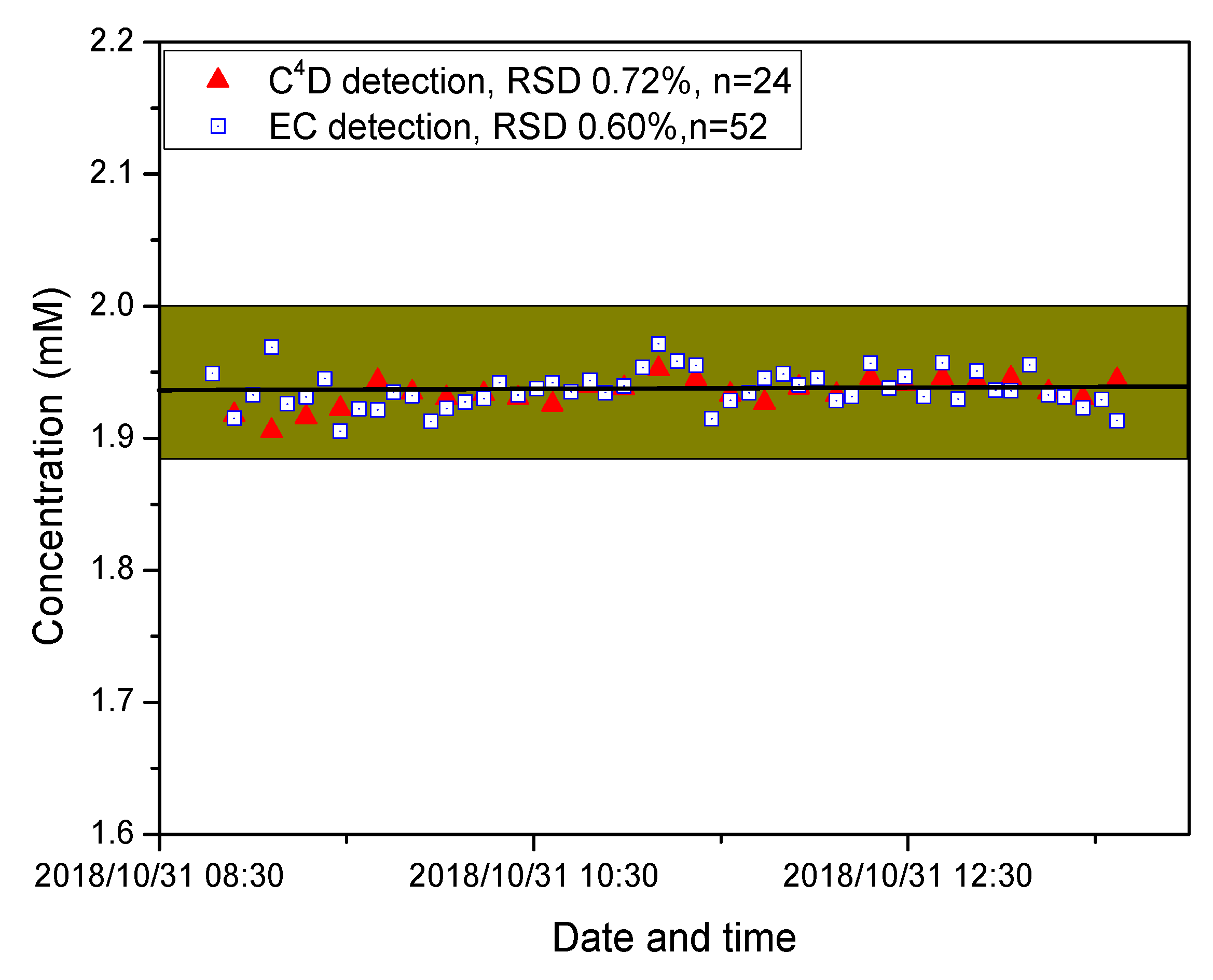
2.2. Performance of the DIC System with Standard Solutions
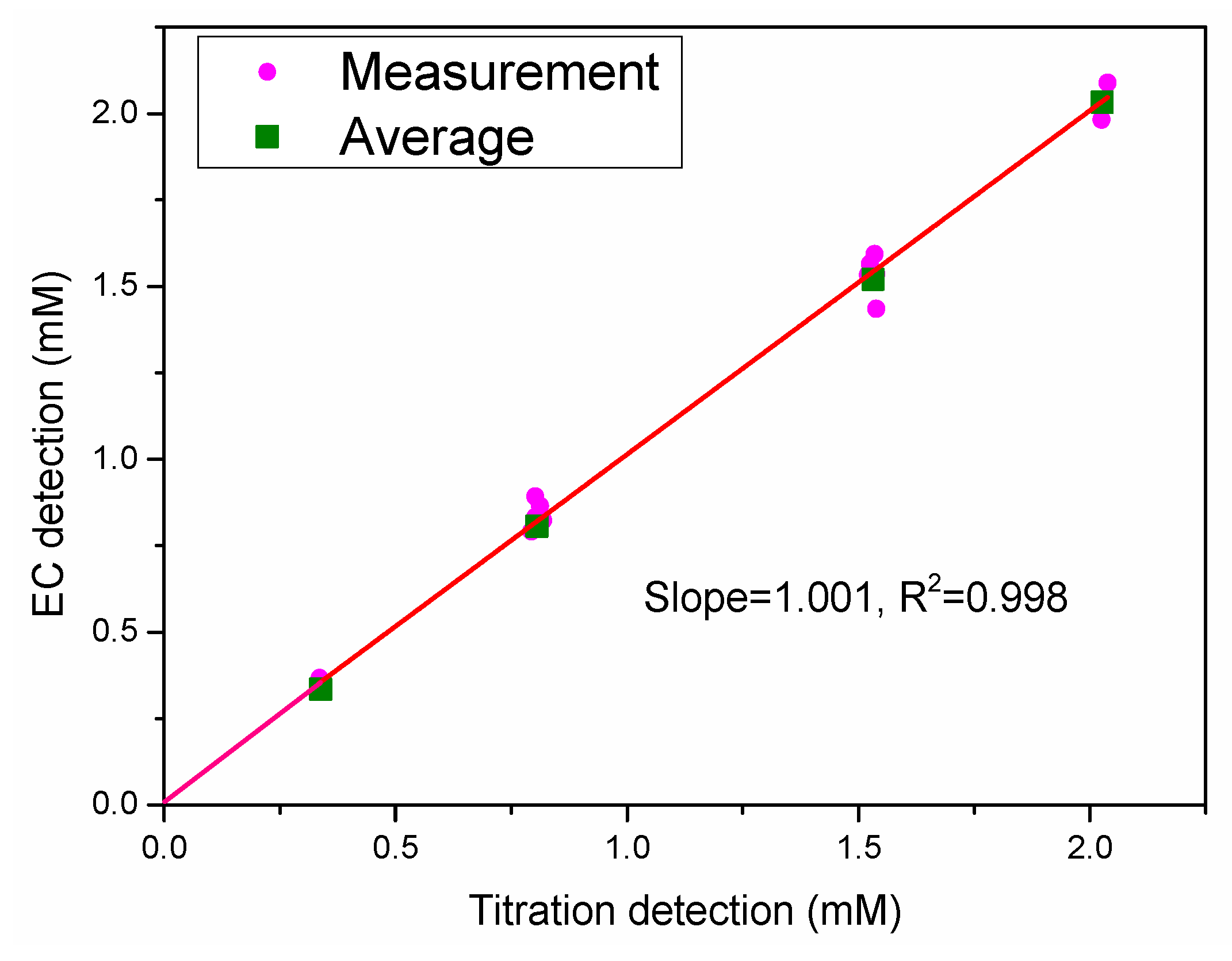
2.3. Comparisons with other DIC Determination Systems
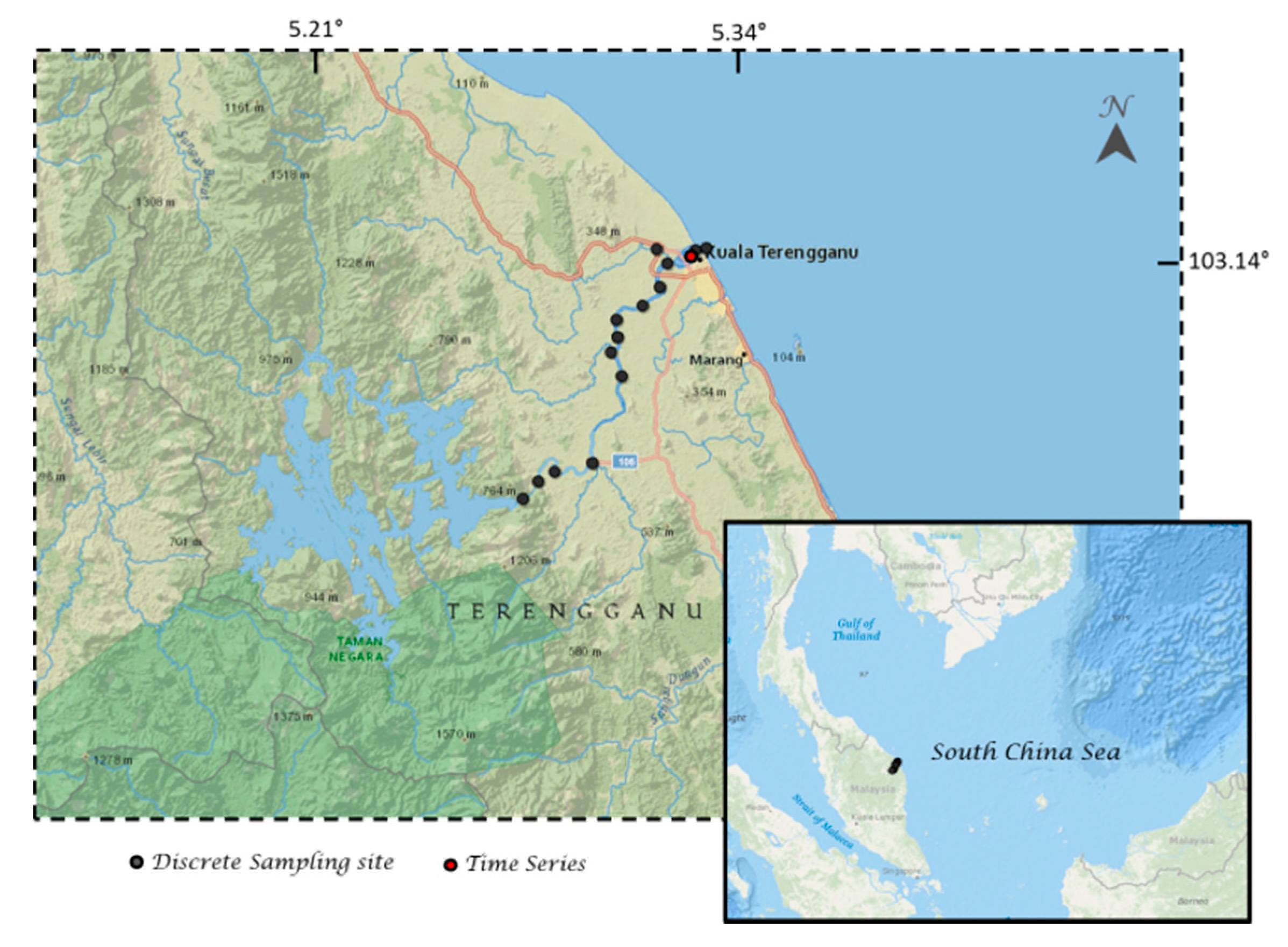
2.4. Shipboard Measurments
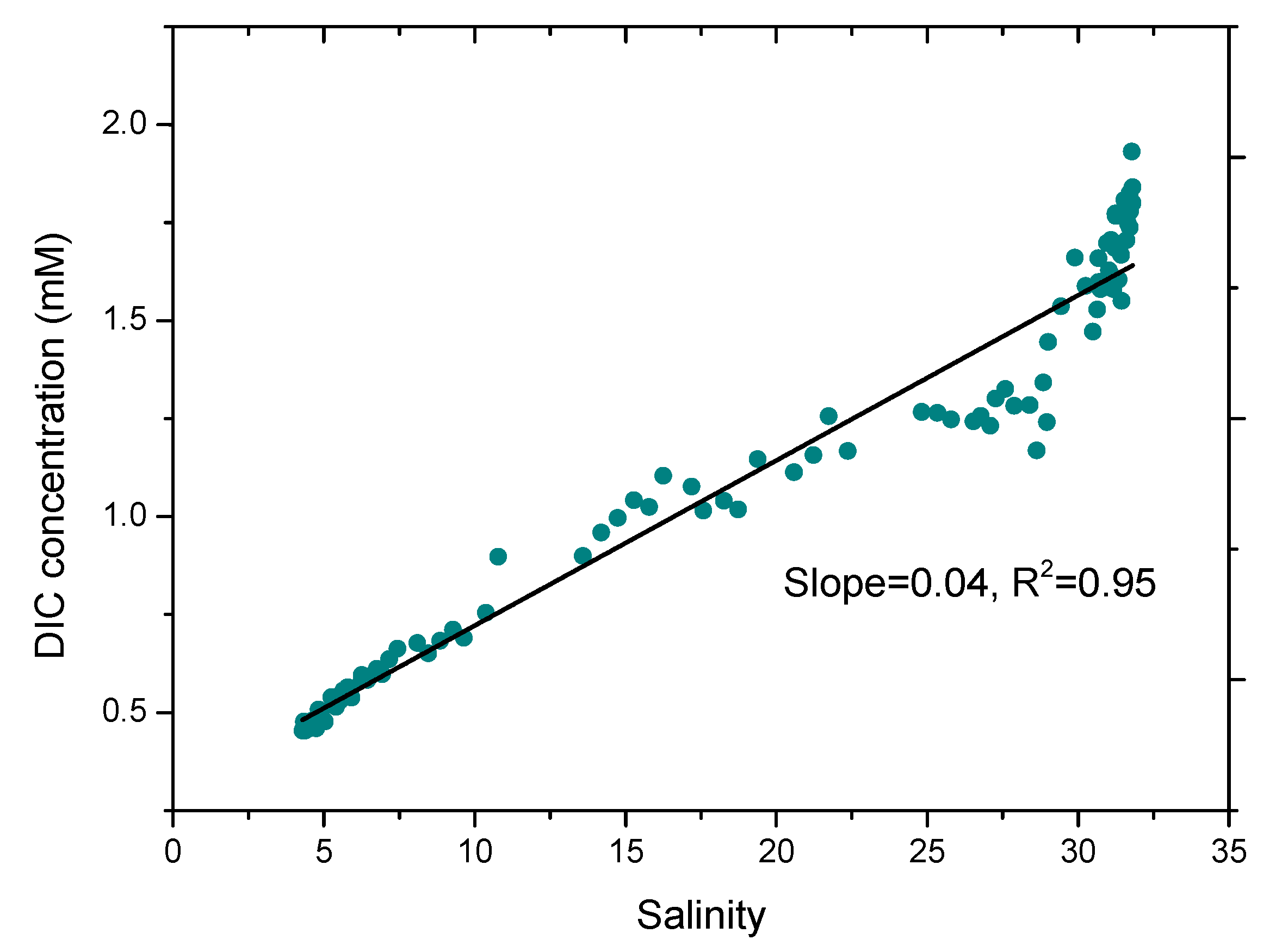
3. Results and Discussion
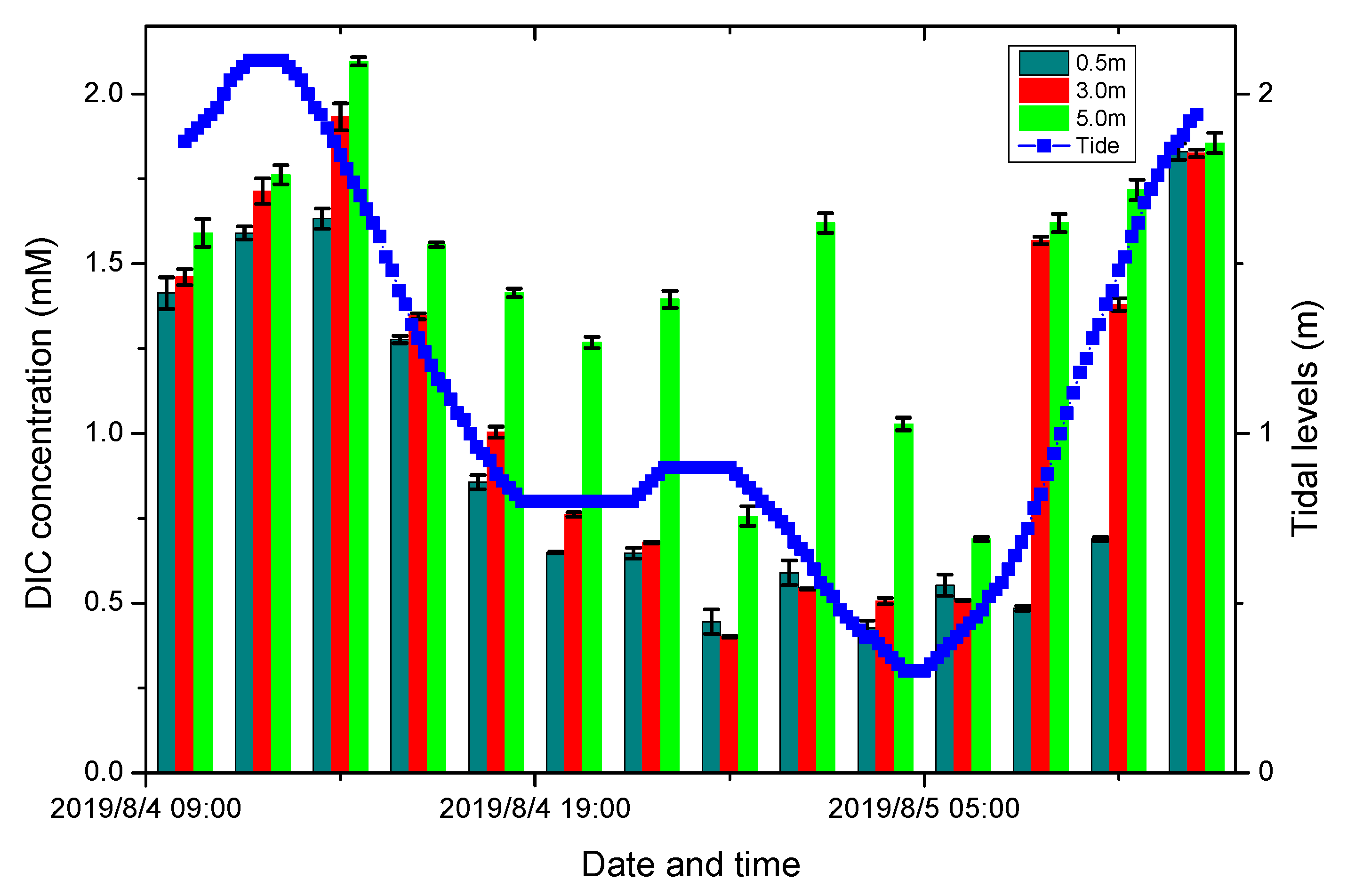
Application of On Board Sea Water DIC Observation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeebe, R.; Wolf-Gladrow, D. CO2 in Seawater: Equilibrium, Kinetics, Isotopes; Elsevier Science, Elsevier: Amsterdam, The Neitherland, 2001. [Google Scholar]
- Cole, J.J. Chapter 6 The carbon cycle: With a brief introduction to global biogeochemistry. Fundam. Ecosyst. Sci. 2013, 109–135. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements, PICES Special Publication 3; North Pacific Marine Science Organization: Sydney, NS, Canada, 2007. [Google Scholar]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of Anthropogenic CO2 on the CaCO3 System in the Oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Giesbrecht, K.E.; Miller, L.A.; Davelaar, M.; Zimmermann, S.; Carmack, E.; Johnson, W.K.; Macdonald, R.W.; McLaughlin, F.; Mucci, A.; Williams, W.J.; et al. Measurements of the dissolved inorganic carbon system and associated biogeochemical parameters in the Canadian Arctic, 1974–2009. Earth Syst. Sci. Data 2014, 6, 91–104. [Google Scholar] [CrossRef]
- Peng, T.H.; Wanninkhof, R.; Bullister, J.L.; Feely, R.A.; Takahashi, T. Quantification of decadal anthropogenic CO2 uptake in the ocean based on dissolved inorganic carbon measurements. Nature 1998, 396, 560–563. [Google Scholar] [CrossRef]
- Noguchi, T.; Hatta, M.; Yamanaka, T.; Okamura, K. Fast measurement of dissolved inorganic carbon concentration for small-volume interstitial water by acid extraction and nondispersive infrared gas analysis. Anal. Sci. 2013, 29, 9–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pearson, P.N.; Palmer, M.R. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 2000, 406, 695–699. [Google Scholar] [CrossRef]
- King, D.W.; Kester, D.R. Determination of seawater pH from 1.5 to 8.5 using colorimetric indicators. Mar. Chem. 1989, 26, 5–20. [Google Scholar] [CrossRef]
- Bellerby, R.G.J.; Olsen, A.; Johannessen, T.; Croot, P. A high precision spectrophotometric method for on-line shipboard seawater pH measurements: The automated marine pH sensor (AMpS). Talanta 2002, 56, 61–69. [Google Scholar] [CrossRef]
- Doi, T.; Takano, M.; Okamura, K.; Ura, T.; Gamo, T. In-situ survey of nanomolar manganese in seawater using an autonomous underwater vehicle around a volcanic crater at Teishi Knoll, Sagami Bay. Jpn. J. Oceanogr. 2008, 64, 471–477. [Google Scholar] [CrossRef]
- Johnson, K.M.; Arthur, E.K.; Sieburth, J.M. Coulometric TCO2 analysis for marine studies: An introduction. Mar. Chem. 1985, 16, 61–82. [Google Scholar] [CrossRef]
- Cai, W.-J. Air-sea exchange of carbon dioxide in ocean margins: A province-based synthesis. Geophys. Res. Lett. 2006, 33, 12603. [Google Scholar] [CrossRef]
- Millero, F.J.; Dickson, A.G.; Eischeid, G. Assessment of the quality of the shipboard measurements of total alkalinity on the WOCE Hydrographic Program Indian Ocean CO2 survey cruises 1994–1996. Mar. Chem. 1998, 63, 9–20. [Google Scholar] [CrossRef]
- Kaltin, S.; Haraldsson, C.; Anderson, L.G. A rapid method for determination of total dissolved inorganic carbon in seawater with high accuracy and precision. Mar. Chem. 2005, 96, 53–60. [Google Scholar] [CrossRef]
- Pencharee, S.; Faber, P.A.; Ellis, P.S.; Cook, P.; Intaraprasert, J.; Grudpan, K.; Mckelyie, I.D. Underway determination of dissolved inorganic carbon in estuarine waters by gas-diffusion flow analysis with C4D detection. Anal. Methods 2012, 4, 1278. [Google Scholar] [CrossRef]
- Hall, P.O.J.; Aller, R.C. Rapid, small-volume, flow injection analysis for ΣCO2 and NH4+ in marine and freshwaters. Limnol. Oceanogr. 1992, 37, 1113–1119. [Google Scholar] [CrossRef]
- Monser, L.; Adhoum, N.; Sadok, S. Gas diffusion-flow injection determination of total inorganic carbon in water using tungsten oxide electrode. Talanta 2004, 62, 389–394. [Google Scholar] [CrossRef]
- Satienperakul, S.; Cardwell, T.J.; Cattrall, R.W.; Mckelvie, I.D.; Taylor, D.M.; Kolev, S.D. Determination of carbon dioxide in gaseous samples by gas diffusion-flow injection. Talanta 2004, 62, 631–636. [Google Scholar] [CrossRef]
- Ymbern, O.; Sández, N.; Calvo-López, A.; Puyol, M.; Alonso-Chamarro, J. Gas diffusion as a new fluidic unit operation for centrifugal microfluidic platforms. Lab Chip 2014, 14, 1014. [Google Scholar] [CrossRef]
- Su, X.; Yu, B.; Tan, H.; Yang, X.; Nie, L.; Yao, S. Flow-injection determination of total ammonia and total carbon dioxide in blood based on gas-diffusion separation and with a bulk acoustic wave impedance sensor. J. Pharm. Biomed. Anal. 1998, 16, 759–769. [Google Scholar] [CrossRef]
- Ljunggren, E.; Karlberg, B. Determination of total carbon dioxide in beer and soft drinks by gas diffusion and flow injection analysis. J. Autom. Chem. 2008, 17, 105. [Google Scholar] [CrossRef]
- Kuban, V.; Dasgupta, P.K. Comparison of Photometry and Conductometry for the Determination of Total Carbonate by Gas Permeation Flow Injection Analysis. Talanta 1993, 40, 831–840. [Google Scholar] [CrossRef]
- Miller, J.C.; Miller, J.N. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson Education Limited, Harlow: London, UK, 2005. [Google Scholar]
- Ji, H.W.; Xu, H.; Xin, H.Z.; Ning, X. Analysis of dissolved inorganic carbon (DIC) in sea water. Trans. Oceanol. Limnol. 2002, 4, 16–24. [Google Scholar]
- Lee, H.L.; Tangang, F.; Gisen, J.I.; Suratman, S. Prediction of salinity intrusion in the sheltered estuary of Terengganu River in Malaysia using 1-D empirical intrusion model. Acta Oceanol. 2017, 36, 57–66. [Google Scholar] [CrossRef][Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Lin, Q.; Poh, S.C.; Li, Y.; Zhan, L. Underway Measurement of Dissolved Inorganic Carbon (DIC) in Estuarine Waters. J. Mar. Sci. Eng. 2020, 8, 765. https://doi.org/10.3390/jmse8100765
Yan J, Lin Q, Poh SC, Li Y, Zhan L. Underway Measurement of Dissolved Inorganic Carbon (DIC) in Estuarine Waters. Journal of Marine Science and Engineering. 2020; 8(10):765. https://doi.org/10.3390/jmse8100765
Chicago/Turabian StyleYan, Jinpei, Qi Lin, Seng Chee Poh, Yuhong Li, and Liyang Zhan. 2020. "Underway Measurement of Dissolved Inorganic Carbon (DIC) in Estuarine Waters" Journal of Marine Science and Engineering 8, no. 10: 765. https://doi.org/10.3390/jmse8100765
APA StyleYan, J., Lin, Q., Poh, S. C., Li, Y., & Zhan, L. (2020). Underway Measurement of Dissolved Inorganic Carbon (DIC) in Estuarine Waters. Journal of Marine Science and Engineering, 8(10), 765. https://doi.org/10.3390/jmse8100765





