Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile
Abstract
1. Introduction
2. Genus Cystoseira sensu lato
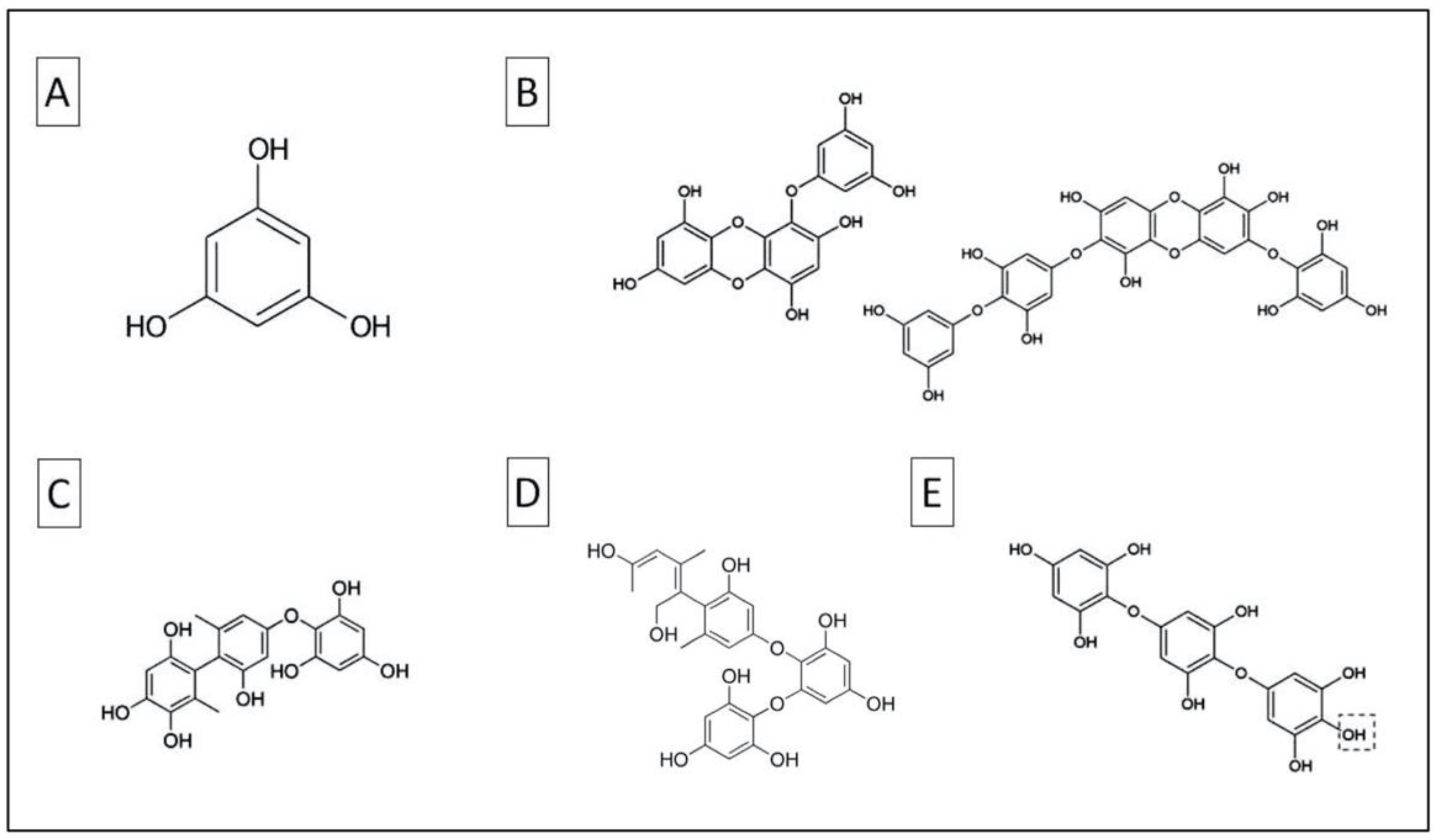
2.1. Phlorotannins
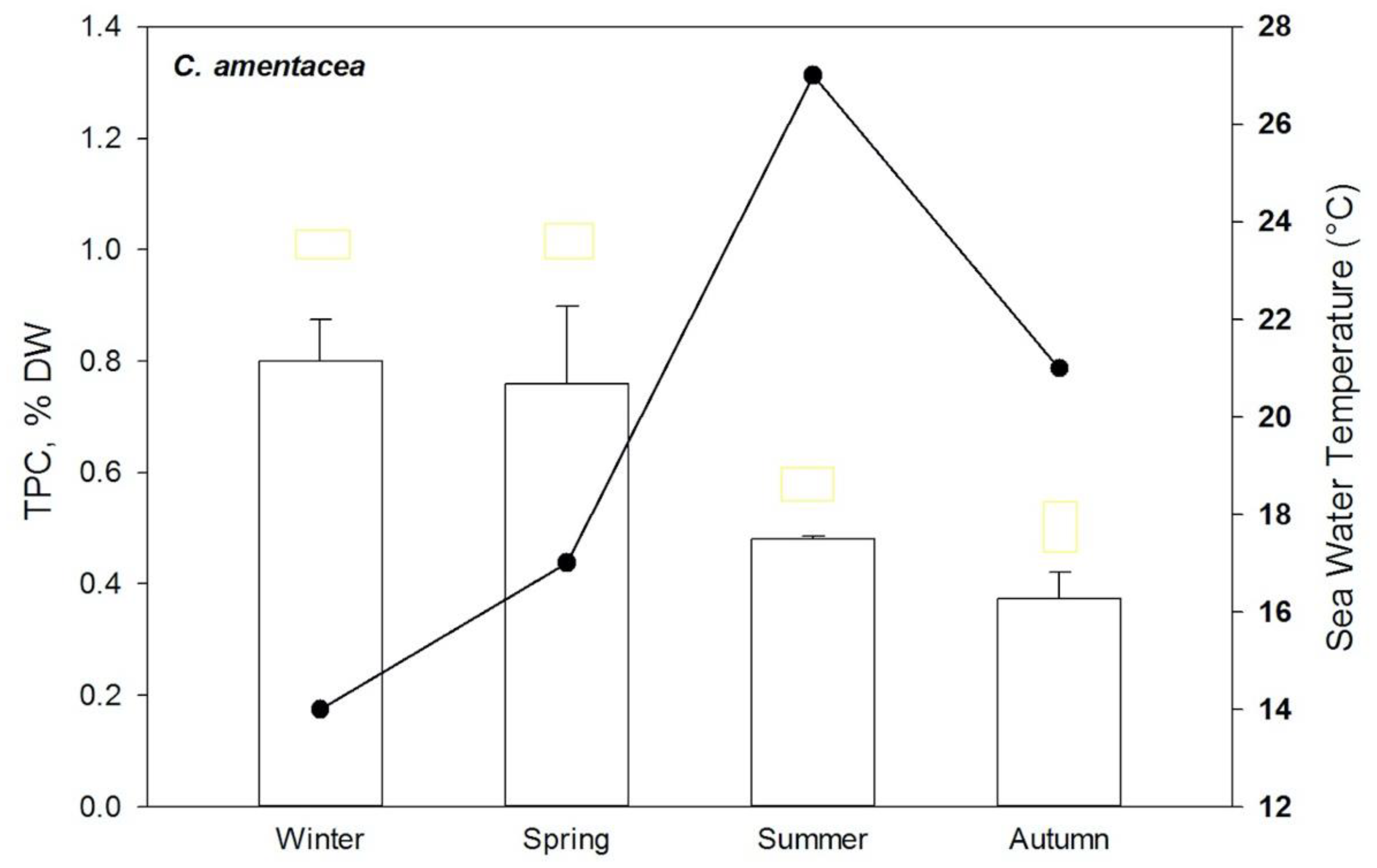
2.2. Ecological Role of Phlorotannins
3. Posidonia oceanica
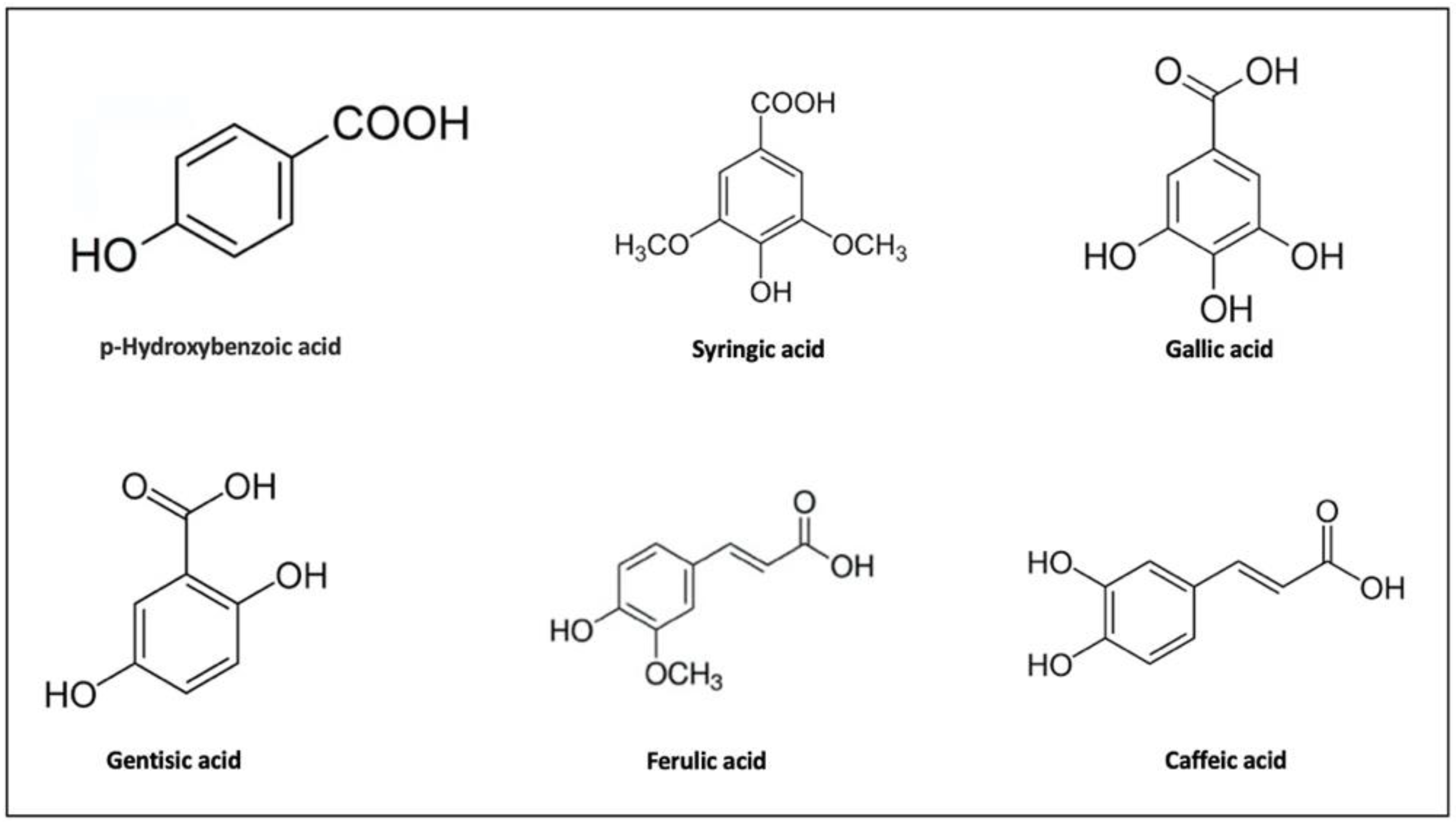
3.1. Phenolic Compounds
3.2. Ecological Role of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zapata, O.; McMillan, C. Phenolic acids in seagrasses. Aquat. Bot. 1979, 7, 307–317. [Google Scholar] [CrossRef]
- Agostini, S.; Desjobert, J.M.; Pergent, G. Distribution of phenolic compounds in the seagrass Posidonia oceanica. Phytochemistry 1998, 48, 611–617. [Google Scholar] [CrossRef]
- Heglmeier, A.; Zidorn, C. Secondary metabolites of Posidonia oceanica (Posidoniaceae). Biochem. Syst. Ecol. 2010, 38, 964–970. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Jégou, C.; Cérantola, S.; Guérard, F.; Le Lann, K. Phlorotannins from Sargassaceae species: Interesting molecules for ecophysiological and valorisation purposes. Adv. Bot. Res. 2014, 71, 379–412. [Google Scholar]
- Grignon-Dubois, M.; Rezzonico, B. Phenolic fingerprint of the seagrass Posidonia oceanica from four locations in the Mediterranean Sea: First evidence for the large predominance of chicoric acid. Bot. Mar. 2015, 58, 379–391. [Google Scholar] [CrossRef]
- Zidorn, C. Secondary metabolities of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic Content of Brown Algae (Phaeophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Conde Álvarez, R.M.; Figueroa, F.L. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.; Cabello-Pasini, A.; Márquez-Garrido, E.; Figueroa, F.L. Intrathallus variation of phenolic compounds, antioxidant activity and phenolsulfatase activity in Cystoseira tamariscifolia (Phaeophyceae) from southern Spain. Cienc. Mar. 2014, 40, 1–10. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2006, 43, 1–91. [Google Scholar]
- Celis-Plá, P.S.M.; Martínez, B.; Quintano, E.; García-Sánchez, M.; Pedersen, A.; Navarro, N.P.; Copertino, M.S.; Mangaiyarkarasi, N.; Mariath, R.; Figueroa, F.L.; et al. Short-term ecophysiological and biochemical responses of Cystoseira tamariscifolia and Ellisolandia elongata to changes in solar irradiance and nutrient levels. Aquat. Biol. 2014, 22, 227–243. [Google Scholar] [CrossRef]
- Constabel, C.P. A survey of herbivore-inducible defensive proteins and phytochemicals. In Induced Plant Defenses against Pathogens and Herbivores; Agrawal, A.A., Tuzun, S., Bent, E., Eds.; APS Press: St Paul, MN, USA, 1999; pp. 137–166. [Google Scholar]
- Targett, N.M.; Arnold, T.M. Effects of secondary metabolites on digestion in marine herbivores. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 391–411. [Google Scholar]
- Haznedaroglu, M.Z.; Zeybek, U. HPLC determination of chicoric acid in leaves of Posidonia oceanica. Pharm. Biol. 2007, 45, 745–748. [Google Scholar] [CrossRef]
- Pergent, G.; Boudouresque, C.F.; Dumay, D.; Pergent-Martini, C.; Wyllie-Echeverria, S. Competition between the invasive macrophyte Caulerpa taxifolia and the seagrass Posidonia oceanica: Contrasting strategies. BMC Ecol. 2008, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Micheli, C.; Cupido, R.; Lombardi, C.; Belmonte, A.; Peirano, A. Changes in genetic structure of Posidonia oceanica at Monterosso al Mare (Ligurian Sea) and its resilience over a decade (1998–2009). Environ. Manag. 2012, 50, 598–606. [Google Scholar] [CrossRef]
- Rotini, A.; Belmonte, A.; Barrote, I.; Micheli, C.; Peirano, A.; Santos, R.O.; Silva, J.; Migliore, L. Effectiveness and consistency of a suite of descriptors for assessing the ecological status of seagrass meadows (Posidonia oceanica L. Delile). Estuar. Coast. Shelf Sci. 2013, 130, 252–259. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From Ecology to Biotechnology, Study of the Defense Strategies of Algae and Halophytes (from Trapani Saltworks, NW Sicily) with a Focus on Antioxidants and Antimicrobial Properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; Ben Rais Lasram, F.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under siege: Spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Lejeusne, C.; Chevaldonne, P.; Pergent-Martini, C.; Boudouresque, C.F.; Perez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef]
- Orellana, S.; Hernández, M.; Sansón, M. Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycol. 2019, 54, 447–465. [Google Scholar] [CrossRef]
- Pergent, G.; Bazairi, H.; Bianchi, C.; Boudouresque, C.F.; Buia, M.C.; Calvo, S.; Clabaut, P.; Harmelin-Vivien, M.; Mateo, M.; Montefalcone, M.; et al. Climate change and Mediterranean seagrass meadows: A synopsis for environmental managers. Mediterr. Mar. Sci. 2014, 15, 462–473. [Google Scholar] [CrossRef]
- Mineur, F.; Arenas, F.; Assis, J.; Davies, A.J.; Engelen, A.H.; Fernandes, F.; Malta, E.-J.; Thibaut, T.; Nguyen, T.V.; Vaz-Pinto, F.; et al. European seaweeds under pressure: Consequences for communities and ecosystem functioning. J. Sea Res. 2015, 98, 91–108. [Google Scholar] [CrossRef]
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira, Sargassum) in the Albères coast (northwestern Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Ceccherelli, G.; Oliva, S.; Pinna, S.; Piazzi, L.; Procaccini, G.; Marin-Guirao, L.; Dattolo, E.; Gallia, R.; La Manna, G.; Gennaro, P.; et al. Seagrass collapse due to synergistic stressors is not anticipated by phenological changes. Oecologia 2018, 186, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Stiger, V.; Deslandes, E.; Payri, C.E. Phenolic contents of two brown algae, Turbinaria ornata and Sargassum mangarevense on Tahiti (French Polynesia): Interspecific, ontogenic and spatio-temporal variations. Bot. Mar. 2004, 47, 402–409. [Google Scholar] [CrossRef]
- Migliore, L.; Rotini, A.; Randazzo, D.; Albanese, N.N.; Giallongo, A. Phenol contents and 2D electrophoresis protein pattern: A promising tool to monitor Posidonia meadow health state. BMC Ecol. 2007, 7, 6. [Google Scholar] [CrossRef]
- Arnold, T.; Mealey, C.; Leahey, H.; Miller, A.W.; Hall Spencer, J.M.; Milazzo, M.; Maers, K. Ocean acidification and the loss of phenolic substances in marine plants. PLoS ONE 2012, 7, e35107. [Google Scholar] [CrossRef]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10 (Suppl. 2), S2608–S2614. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Comparison of chemical profile and antioxidant properties of the brown algae. Int. J. Food Sci. Technol. 2018, 53, 174–181. [Google Scholar] [CrossRef]
- Lemesheva, V.; Tarakhovskaya, E. Physiological functions of phlorotannins. Biol. Commun. 2018, 63, 70–76. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernando, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.; Jouini, M.; Amor, H.B.H.; Mzoughi, Z.; Dridi, M.; Said, R.F.; Bouraoui, A. Phytochemical analysis and evaluation of the antioxidant, anti-Inflammatory, and antinociceptive potential of phlorotannin-rich fractions from three Mediterranean brown seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.H.S.; Surendraraj, A.; Al-Ghunaim, A.; Al-Yamani, F. Chemical profile and antioxidant activities of 26 selected species of seaweeds from Kuwait coast. J. Appl. Phycol. 2019, 31, 2653–2668. [Google Scholar] [CrossRef]
- Ragan, M.A.; Glombitza, K.W. Phlorotannins, brown alga polyphenols. Prog. Phycol. Res. 1986, 4, 129–241. [Google Scholar]
- Pavia, H.; Cervin, G.; Lindgren, A.; Aberg, P. Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 1997, 157, 139–146. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive phenolic compounds from algae. In Bioactive Compounds from Marine Foods: Plants and Animal Sources; Hernandez-Ledesma, B., Herrero, M., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2013; pp. 113–129. [Google Scholar]
- Arnold, T.M.; Targett, N.M. Marine tannins: The importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002, 28, 1919–1934. [Google Scholar] [CrossRef]
- Dumay, O.; Costa, J.; Desjobert, J.M.; Pergent, G. Variations in the concentration of phenolic compounds in the seagrass Posidonia oceanica under conditions of competition. Phytochemistry 2004, 65, 3211–3220. [Google Scholar] [CrossRef]
- Sieg, R.D.; Kubanek, J. Chemical Ecology of Marine Angiosperms: Opportunities at the Interface of Marine and Terrestrial Systems. J. Chem. Ecol. 2013, 39, 687–711. [Google Scholar] [CrossRef]
- Giaccone, G.; Bruni, A. Le Cistoseire e la vegetazione sommersa del Mediterraneo. Atti Ist. Veneto Sci. Lett. Arti 1973, 131, 59–103. [Google Scholar]
- Ballesteros, E. Structure and dynamics of the community of Cystoseira zosteroides (Turner) C. Agardh (Fucales, Phaeophyceae) in the Northwestern Mediterranean. Sci. Mar. 1990, 54, 217–229. [Google Scholar]
- Hereu, B.; Mangialajo, L.; Ballesteros, E.; Thibaut, T. On the occurrence, structure and distribution of deep-water Cystoseira (Phaeophyceae) populations in the Port-Cros National Park (Northwestern Mediterranean). Eur. J. Phycol. 2008, 43, 263–273. [Google Scholar] [CrossRef]
- Sales, M.; Ballesteros, E. Shallow Cystoseira (Fucales: Ochrophyta) assemblages thriving in sheltered areas from Menorca (NW Mediterranean): Relationships with environmental factors and anthropogenic pressures. Estuar. Coast. Shelf Sci. 2009, 84, 476–482. [Google Scholar] [CrossRef]
- Nikolić, V.; Žuljević, A.; Mangialajo, L.; Antolić, B.; Kušpilić, G.; Ballesteros, E. Cartography of littoral rocky-shore communities (CARLIT) as a tool for ecological quality assessment of coastal waters in the Eastern Adriatic Sea. Ecol. Indic. 2013, 34, 87–93. [Google Scholar] [CrossRef]
- Graham, M.H. Effects of local deforestation on the diversity and structure of southern California giant kelp forest food webs. Ecosystems 2004, 7, 341–357. [Google Scholar] [CrossRef]
- Sales, M.; Ballesteros, E. Long-term comparison of algal assemblages dominated by Cystoseira crinita (Fucales, Heterokontophyta) from Cap Corse (Corsica, North Western Mediterranean). Eur. J. Phycol. 2010, 45, 404–412. [Google Scholar] [CrossRef]
- Martínez, B.; Arenas, F.; Trilla, A.; Viejo, R.M.; Carreño, F. Combining physiological threshold knowledge to species distribution models is key to improving forecasts of the future niche for macroalgae. Glob. Chang. Biol. 2015, 21, 1422–1433. [Google Scholar] [CrossRef]
- EEC, 1992. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- Council of Europe. Convention Relative à La Conservation De La Vie Sauvage Et Du Milieu Naturel de L’Europe; Council of Europe: Strasbourg, France, 1979. [Google Scholar]
- UNEP/MAP. Report of the 16th Ordinary Meeting of the Contracting Parties to the Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean and its Protocols, Marrakesh, Morocco, 3–5 November 2009; Mediterranean Action Plan: Athens, Greece, 2009; p. 321. [Google Scholar]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Verlaque, M. Decline and local extinction of Fucales in French Riviera: The harbinger of future extinctions? Medit. Mar. Sci. 2015, 16, 206–224. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The fate of Cystoseira crinita, a forest-forming Fucale (Phaeophyceae, Stramenopiles), in France (North Western Mediterranean Sea). Estuar. Coast. Shelf Sci. 2016, 181, 196–208. [Google Scholar] [CrossRef]
- EC, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community actions in the field of water policy. Off. J. Eur. Communities 2000, 22, 1–73. [Google Scholar]
- European Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for Community actions in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Communities 2008, 164, 19–40. [Google Scholar]
- Orlando-Bonaca, M.; Lipej, L.; Malej, A.; Francé, J.; Cermelj, B.; Bajt, O.; Kovac, N.; Mavric, B.; Turk, V.; Mozetic, P.; et al. Izbor Elementov Za Vzpostavitev Programa Spremljanja Stanja Morskega Okolja (po členu 11 ODMS) = Selection of Elements to Establish the Monitoring Program of the Marine Environment (Article 11 MSFD). In National Report in Slovenian; Porocila MBP, 144; Marine Biology Station Piran, National Institute of Biology: Piran, Slovenia, 2013; p. 29. [Google Scholar]
- Celis-Plá, P.S.M.; Bouzon, Z.L.; Hall-Spencer, J.M.; Schmidt, E.C.; Korbee, N.; Figueroa, F.L. Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Mar. Environ. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef]
- Strain, E.M.A.; Thomson, R.J.; Micheli, F.; Mancuso, F.P.; Airoldi, L. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob. Chang. Biol. 2014, 20, 3300–3312. [Google Scholar] [CrossRef]
- Iveša, L.; Djakovac, T.; Devescovi, M. Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 2016, 106, 162–173. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Targett, N.M.; Arnold, T.M. Minireview-predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 1998, 34, 195–205. [Google Scholar] [CrossRef]
- Barre, S.; Potin, P.; Leblanc, C.; Delage, L. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar. Drugs 2010, 8, 988–1010. [Google Scholar] [CrossRef]
- Martínez, J.H.; Castañeda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; Le Lann, K.; Connan, S.; Jechoux, G.; Deslandes, E.; Stiger-Pouvreau, V. Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquat. Bot. 2006, 85, 339–346. [Google Scholar] [CrossRef]
- Le Lann, K.; Connan, S.; Stiger-Pouvreau, V. Phenology, TPC and size-fractioning phenolics variability in temperate Sargassaceae (Phaeophyceae, Fucales) from Western Brittany: Native vs. introduced species. Mar. Environ. Res. 2012, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Le Lann, K.; Ferret, C.; VanMee, E.; Spagnol, C.; Lhuillery, M.; Payri, C.; Stiger-Pouvreau, V. Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: Spatial and specific variability. Phycol. Res. 2012, 60, 37–50. [Google Scholar] [CrossRef]
- Bruno de Sousa, C.; Gangadhar, K.N.; Macridachis, J.; Pavão, M.; Morais, T.R.; Campino, L.; Varela, J.; Lago, J.H.G. Cystoseira algae (Fucaceae): Update on their chemical entities and biological activities. Tetrahedron 2017, 28, 1486–1505. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E.A.; Wiencke, C. Phenolic compounds in the embryo development of several northern hemisphere fucoids. Plant Biol. 2000, 2, 24–33. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Custódio, L.; Acosta, G.; Lago, J.H.G.; Morais, T.R.; Bruno de Sousa, C.; Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Lima, R.T.; et al. Can macroalgae provide promising anti-tumoral compounds? A closer look at Cystoseira tamariscifolia as a source for antioxidant and anti-hepatocarcinoma compounds. PeerJ 2016, 4, e1704. [Google Scholar] [CrossRef]
- Sellimi, S.; Benslima, A.; Barragan-Montero, V.; Hajji, M.; Nasri, M. Polyphenolic-protein-polysaccharide ternary conjugates from Cystoseira barbata Tunisian seaweed as potential biopreservatives: Chemical, antioxidant and antimicrobial properties. Int. J. Biol. Macromol. 2017, 105, 1375–1383. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Whitman, S.L.; Ehlig, J.M. Differences in herbivore preferences, phlorotannin production, and nutritional quality between juvenile and adult tissues from marine brown algae. Mar. Biol. 2001, 139, 201–210. [Google Scholar] [CrossRef]
- Mannino, A.M.; Vaglica, V.; Oddo, E. Interspecific variation in total phenol content in temperate brown algae. J. Biol. Res. 2017, 90. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Ar Gall, E. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Mannino, A.M.; Vaglica, V.; Oddo, E. Seasonal variation in total phenolic content of Dyctiopteris polypodioides (Dictyotaceae) from the Sicilian coast. Flora Mediterr. 2014, 24, 39–50. [Google Scholar] [CrossRef]
- Mannino, A.M.; Vaglica, V.; Cammarata, M.; Oddo, E. Effects of temperature on total phenolic compounds in Cystoseira amentacea (C. Agardh) Bory (Fucales, Phaeophyceae) from southern Mediterranean. Plant Biosyst. 2016, 150, 152–160. [Google Scholar] [CrossRef]
- Steinberg, P.D.; Paul, V.J. Fish feeding and chemical defenses of tropical brown algae in Western Australia. Mar. Ecol. Prog. Ser. 1990, 58, 253–259. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Paul, V.J. The Biogeography of polyphelonic compounds in marine macroalgae: Temperate brown algal defenses deter feeding by tropical herbivorous fishes. Oceanologia 1990, 84, 158–163. [Google Scholar] [CrossRef]
- Ank, G.; da Gama, B.A.P.; Pereira, R.C. Latitudinal variation in phlorotannin contents from Southwestern Atlantic brown seaweeds. PeerJ 2019, 7, e7379. [Google Scholar] [CrossRef]
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses; CRC Press: Boca Raton, FL, USA, 2004; p. 320. [Google Scholar]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Mccarthy, J.J., III; Hustead, C.L.; Kearns, L.J. Phlorotannin allocation among tissues of northeastern pacific kelps and rockweeds. J. Phycol. 1999, 35, 482–492. [Google Scholar] [CrossRef]
- Cérantola, S.; Breton, F.; Gall, E.A.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Breton, F.; Cerantola, S.; Ar Gall, E. Distribution and radical scavenging activity of phenols in Ascophyllum nodosum (Phaeophyceae). J. Exp. Mar. Biol. Ecol. 2011, 399, 167–172. [Google Scholar] [CrossRef]
- Cruces, E.; Huovinen, P.; Gómez, I. Phlorotannin and antioxidant responses upon short-term exposure to UV radiation and elevated temperature in three South Pacific kelps. Photochem. Photobiol. 2012, 88, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Schoenwaelder, M.E.A. The biology of phenolic containing vesicles. Algae 2008, 23, 163–175. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-Gonzalez, B.; Korbee, N. Vulnerability and acclimation to increased UVB in the three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef]
- Jormalainen, V.; Honkanen, T.; Koivikko, R.; Eränen, J. Induction of phlorotannin production in a brown alga: Defense or resource dynamics? Oikos 2003, 103, 640–650. [Google Scholar] [CrossRef]
- Karez, C.S.; Pereira, R.C. Metal contents in polyphenolic fractions extracted from the brown alga Padina gymnospora. Bot. Mar. 1995, 38, 151–155. [Google Scholar] [CrossRef]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Martínez, B.; Korbee, N.; Hall-Spencer, J.M.; Figueroa, F.L. Ecophysiological responses to elevated CO2 and temperature in Cystoseira tamariscifolia (Phaeophyceae). Clim. Chang. 2017, 142, 67–81. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Hall-Spencer, J.M.; Horta, P.A.; Milazzo, M.; Korbee, N.; Cornwall, C.E.; Figueroa, F.L. Macroalgal responses to ocean acidification depend on nutrient and light levels. Front. Mar. Sci. 2015, 2, 26. [Google Scholar] [CrossRef]
- Witman, J.D.; Dayton, P.K. Rocky subtidal communities. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Press: Sunderland, MA, USA, 2001; pp. 339–366. [Google Scholar]
- Silchenko, A.S.; Imbs, T.I.; Zvyagintseva, T.N.; Fedoreyev, S.; Ermakova, S. Brown alga metabolites–inhibitors of marine organism fucoidan hydrolases. Chem. Nat. Compd. 2017, 53, 345–350. [Google Scholar] [CrossRef]
- Van Alstyne, K.L. Herbivore grazing increases polyphenolic defenses in the intertidal brown alga Fucus distichus. Ecology 1988, 69, 655–663. [Google Scholar] [CrossRef]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- Shibata, T.; Hama, Y.; Miyasaki, T.; Ito, M.; Nakamura, T. Extracellular secretion of phenolic substances from living brown algae. J. Appl. Phycol. 2006, 18, 787–794. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. The evolution of thallus form and survival strategies in benthic marine macroalgae: Field and laboratory tests of a functional form model. Am. Nat. 1980, 116, 25–44. [Google Scholar] [CrossRef]
- Steneck, R.S.; Dethier, M.N. A functional group approach to the structure of algal-dominated communities. Oikos 1994, 69, 476–498. [Google Scholar] [CrossRef]
- Pavia, H.; Brock, E. Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 2000, 193, 285–294. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Orth, R.J.; Duarte, C.M. (Eds.) Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 1–691. [Google Scholar]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarra, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3. [Google Scholar] [CrossRef]
- den Hartog, C.; Kuo, J.J. Taxonomy and Biogeography in Seagrasses. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–23. [Google Scholar]
- Bremer, B.; Bremer, K.; Chase, M.W.; Fay, M.F.; Reveal, J.L.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F.; Anderberg, A.A.; Moore, M.J.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Protection and Conservation of Posidonia Oceanica Meadows; RAMOGE and RAC/SPA Publish: Tunis, Tunisia, 2012; pp. 1–202. [Google Scholar]
- Procaccini, G.; Buia, M.C.; Gambi, M.C.; Perez, M.; Pergent, G.; Pergent-Martini, C.; Romer, J. The Seagrasses of Western Mediterranean. In World Atlas of Seagrasses; Green, E.P., Short, F.T., Eds.; University of California Press: Berkeley, CA, USA, 2003; pp. 48–58. [Google Scholar]
- Gnisci, V.; Cognetti De Martiis, S.; Belmonte, A.; Micheli, C.; Piermattei, V.; Bonamano, S.; Marcelli, M. Assessment of the ecological structure of Posidonia oceanica (L.) Delile on the northern coast of Lazio, Italy (central Tyrrhenian, Mediterranean). Ital. Bot. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Trav. Sci. Parc Natl. Port-Cros 2004, 20, 97–146. [Google Scholar]
- Pergent-Martini, C.; Leoni, V.; Pasqualini, V.; Ardizzone, G.D.; Balestri, E.; Bedini, R.; Belluscio, A.; Belsher, T.; Borg, J.; Boudouresque, C.F.; et al. Descriptors of Posidonia oceanica meadows: Use and application. Ecol. Indic. 2005, 5, 213–230. [Google Scholar] [CrossRef]
- Micheli, C.; Paganin, P.; Peirano, A.; Caye, C.; Meinesz, A.; Bianchi, C.N. Genetic variability of Posidonia oceanica (L.) Delile in relation to local factors and biogeographic patterns. Aquat. Bot. 2005, 82, 210–221. [Google Scholar] [CrossRef]
- Bonacorsi, M.; Pergent-Martini, C.; Breand, N.; Pergent, G. Is Posidonia oceanica regression a general feature in the Mediterranean Sea? Mediterr. Mar. Sci. 2013, 14, 193–203. [Google Scholar] [CrossRef]
- Cariello, L.; Zanetti, L. Distribution of chicoric acid during leaf development of Posidonia oceanica. Bot. Mar. 1979, 22, 359–360. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant phenolics-Secondary metabolites with diverse functions. In Recent Advances in Polyphenol Research; Daayf, F., Lattanzio, V., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2008; Volume 1, pp. 1–35. [Google Scholar] [CrossRef]
- Boumaza, S.; Boudefoua, N.; Boumaza, R.; Semroud, R. Effects of urban effluents on spatial structure, morphology and total phenols of Posidonia oceanica: Comparison with a reference site. J. Exp. Mar. Biol. Ecol. 2014, 457, 113–119. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, C.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent ad advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Frazier, A.R. Characterization of protein–polyphenol interactions. Trends Food Sci. Technol. 2004, 15, 186–190. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–Phenolic Interactions as a Factor Affecting the Physicochemical Properties of White Bean Proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef]
- Serve, L.; Piovetti, L.; Combout, G. Analyse des substances phenoliques des restes de Posidonia oceanica. (L.) Delile provenanton sediments holocenes et de deposits actuels. In GIS Posidonia International Workshop; Boudouresque, C.F., Jeudy de Grissac, A., Olivier, J., Eds.; Gis Posidonie Publish: Marseille, France, 1984; pp. 137–144. [Google Scholar]
- Cuny, P.; Serve, L.; Jupin, H.; Boudouresque, C.F. Water soluble phenolic compounds of the marine phanerogam Posidonia oceanica in a Mediterranean area colonised by the introduced chlorophyte Caulerpa taxifolia. Aquat. Bot. 1995, 52, 237–242. [Google Scholar] [CrossRef]
- Kaal, J.; Serrano, O.; Nierop, K.G.J.; Schellekens, J.; Martínez Cortizas, A.; Mateo, M.-Á. Molecular composition of plant parts and sediment organic matter in a Mediterranean seagrass (Posidonia oceanica) mat. Aquat. Bot. 2016, 133, 50–61. [Google Scholar] [CrossRef]
- Cornara, L.; Pastorino, G.; Borghesi, B.; Salis, A.; Clericuzio, M.; Marchetti, C.; Damonte, G.; Burlando, B. Posidonia oceanica (L.) Delile Ethanolic exstract modulates cell activities with skin health applications. Mar. Drugs 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Bitam, F.; Ciavatta, M.L.; Manzo, E.; Villani, G.; Gavagnin, M. The first record of neolignans from the marine phanerogam Posidonia oceanica. Phytochem. Lett. 2012, 5, 696–699. [Google Scholar] [CrossRef]
- Papenbrock, J. Highlights in Seagrasses’ phylogeny, physiology and metabolism: What makes them Special? ISRN Bot. 2012. [Google Scholar] [CrossRef]
- Klap, V.A.; Hemminga, M.A.; Boon, J.J. Retention of lignin in seagrasses: Angiosperms that returned to the sea. Mar. Ecol. Prog. Ser. 2000, 194, 1–11. [Google Scholar] [CrossRef]
- Ferrat, L.; Pergent-Martini, C.; Romeo, M.; Pergent, G. Hydrosoluble phenolic compounds production in a Mediterranean seagrass according to mercury contamination. Gul. Mex. Sci. 2003, 21, 108. [Google Scholar]
- Rotini, A.; Micheli, C.; Valiante, L.M.; Migliore, L. Assessment of Posidonia oceanica (L.) Delile conservation status by standard and putative approaches: The case study of Santa Marinella meadow (Italy, W Mediterranean). Open J. Ecol. 2011, 1, 48–56. [Google Scholar] [CrossRef]
- Ferrat, L.; Wyllie-Echeverria, S.; Cates Rex, G.; Pergent-Martini, C.; Pergent, G.; Zou, J.; Romero, M.; Pasqualini, V.; Fernandez, C. Posidonia oceanica and Zostera marina as Potential Biomarkers of Heavy Metal Contamination in Coastal Systems. In Ecological Water Quality-Water Treatment and Reuse; Voudouris, K., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 123–140. [Google Scholar] [CrossRef]
- Cannac, M.; Ferrat, L.; Pergent-Martini, C.; Pergent, G.; Pasqualini, V. Effects of fish farming on flavonoids in Posidonia oceanica. Sci. Total Environ. 2006, 370, 91–98. [Google Scholar] [CrossRef]
- Leoni, V.; Pasqualini, V.; Pergent-Martini, C.; Vela, A.; Pergent, G. Morphological responses of Posidonia oceanica to experimental nutrient enrichment of the canopy water. J. Exp. Mar. Biol. Ecol. 2006, 339, 1–14. [Google Scholar] [CrossRef]
- Steele, L.T.; Valentine, J.F. Idiosyncratic response of seagrass phenolic production following sea urchin grazing. Mar. Ecol. Prog. Ser. 2012, 466, 81–92. [Google Scholar] [CrossRef]
- Cozza, R.; Chiappetta, A.; Petrarulo, M.; Salimonti, A.; Rende, F.; Bitonti, M.B.; Innocenti, A.M. Cytophisiological features of Posidonia oceanica as putative markers of environmental conditions. Chem. Ecol. 2004, 20, 215–223. [Google Scholar] [CrossRef]
- Micheli, C.; Paglialonga, A.; Soldati, P.; Cremisini, C.; Chiavarini, S. Photosynthetic performance and polychlorinated biphenyl (PCB) accumulation by the macroalgae Ulva laetevirens. Sci. Total Environ. 1995, 171, 137–142. [Google Scholar] [CrossRef]
- Micheli, C.; Spinosa, F.; Aliani, S.; Gasparini, G.P.; Molcard, A.; Peirano, A. Genetic input by Posidonia oceanica (L.) Delile fruits dispersed by currents in the Ligurian Sea. Plant Biosyst. 2010, 144, 333–339. [Google Scholar] [CrossRef]
- Micheli, C.; D’Esposito, D.; Belmonte, A.; Peirano, A.; Valiante, L.M.; Procaccini, G. Genetic diversity and structure in two protected Posidonia oceanica meadows. Mar. Environ. Res. 2015, 109, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Pasqualini, V.; Pergent-Martini, C.; Vela, A.; Pergent, G. Physiological responses of Posidonia oceanica to experimental nutrient enrichment of the canopy water J. Exp. Mar. Biol. Ecol. 2007, 349, 73–83. [Google Scholar] [CrossRef]
- Machaix, J.-J.; Fleuriet, A.; Jay-Allemand, C. Les Composés Phénoliques des Végétaux, un Exemple de Métabolites Secondaires D’importance Economique; Presses Polytechniques et Universitaires Romandes: Lausanne, France, 2005; pp. 1–216. [Google Scholar]
- Yates, J.C.; Peckol, P. Effects of nutrient availability and herbivory on polyphenolics in the seaweed Fucus vesiculosus. Ecology 1993, 74, 1757–1766. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef]
- Goecker, M.E.; Heeck, K.L.; Valentine, J.F. Effect of nitrogen concentration in turtlegrass Thalassia testudium on consumption by the bucktooth parrofish Sparisoma radians. Mar. Ecol. Prog. Ser. 2005, 286, 239–248. [Google Scholar] [CrossRef]
- Tomas, F.; Abbott, J.M.; Steinberg, C.; Balk, M.; Williams, S.L.; Stachowicz, J.J. Plant genotype and nitrogen loading influence seagrass productivity, biochemistry, and plant–herbivore interactions. Ecology 2011, 92, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.C.; Kohrs, D.G.; Steller, D.L.; Alberte, R.S. Impact of CO2 enrichment on productivity and light requirements on eelgrass. Plant Physiol. 1997, 115, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Invers, O.; Tomàs, F.; Pérez, M.; Romero, J. Potential effect of increased global CO2 availability on the depth distribution of the seagrass Posidonia oceanica (L.) Delile: A tentative assessment using a carbon balance model. Bull. Mar. Sci. 2002, 71, 1191–1198. [Google Scholar]
- Arnold, T.; Freundlich, G.; Weilnau, T.; Verdi, A.; Tibbets, I. Impacts of groundwater discharge at Myora Springs (North Stradbroke Island, Australia) on the phenolic metabolism of eelgrass, Zostera muelleri, and grazing by the juvenile rabbitfish, Siganus fuscescens. PLoS ONE 2014, 9, e104738. [Google Scholar] [CrossRef]
- Verges, A.; Becerro, M.A.; Alcoverro, T.; Romero, J. Experimental evidence of chemical deterrence against multiple herbivores in the seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2007, 343, 107–114. [Google Scholar] [CrossRef][Green Version]
- Hernán, G.; Ramajo, L.; Basso, L.; Delgado, A.; Terrados, J.; Duarte, C.M.; Tomas, F. Seagrass (Posidonia oceanica) seedlings in a high-CO2 world: From physiology to herbivory. Sci. Rep. 2016, 6, 38017. [Google Scholar] [CrossRef]
- Endara, M.J.; Coley, P.D. The re source availability hypothesis revisited: A meta analisis. Funct. Ecol. 2011, 25, 389–398. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Boudouresque, C.F.; Pasqualini, V.; Pergent, G. Impact of fish farming facilities on Posidonia oceanica meadows: A review. Mar. Ecol. 2006, 27, 310–319. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Chang. Biol. 2010, 16, 2366–2375. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; AlmogiLabin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Patterns in introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast. Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Mannino, A.M. Human activities trigger change in marine landscape. Flora Mediterr. 2018, 28, 295–311. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Pergent, G. Marine phanerogams as a tool in the evaluation of marine trace metal contamination: An example from the Mediterranean. Int. J. Environ. Pollut. 2000, 13, 126–147. [Google Scholar] [CrossRef]
- Calvo, S.; Tomasello, A.; Di Maida, G.; Pirrotta, M.; Buia, M.C.; Cinelli, F.; Cormaci, M.; Furnari, G.; Giaccone, G.; Luzzu, F.; et al. Seagrasses along the Sicilian coasts. Chem. Ecol. 2010, 26, 249–266. [Google Scholar] [CrossRef]
- Domina, G.; Campisi, P.; Mannino, A.M.; Sparacio, I.; Raimondo, F.M. Environmental quality assessment of the Sicilian coast using a multi-disciplinary approach. Acta Zool. Bulg. 2018, (Suppl. 11), 11–18. [Google Scholar]
- Cozza, R.; Rende, F.; Ferrari, M.; Bruno, L.; Pacenza, M.; Dattola, L.; Bitonti, M.B. Biomonitoring of Posidonia oceanica beds by a multiscale approach. Aquat. Bot. 2019, 156, 14–24. [Google Scholar] [CrossRef]
- Jahnke, M.; Olsen, J.L.; Procaccini, G. A meta-analysis reveals a positive correlation between genetic diversity metrics and environmental status in the long-lived seagrass Posidonia oceanica. Mol. Ecol. 2015, 24, 2336–2348. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. https://doi.org/10.3390/jmse8010019
Mannino AM, Micheli C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. Journal of Marine Science and Engineering. 2020; 8(1):19. https://doi.org/10.3390/jmse8010019
Chicago/Turabian StyleMannino, Anna Maria, and Carla Micheli. 2020. "Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile" Journal of Marine Science and Engineering 8, no. 1: 19. https://doi.org/10.3390/jmse8010019
APA StyleMannino, A. M., & Micheli, C. (2020). Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. Journal of Marine Science and Engineering, 8(1), 19. https://doi.org/10.3390/jmse8010019





