Fish Hybridization Leads to Uncertainty Regarding Ciguatera Fish Poisoning Risk; Confirmation of Hybridization and Ciguatoxin Accumulation with Implications for Stakeholders
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Toxin Extraction
2.2. In Vitro N2a Cytotoxicity Assay
2.3. Analysis of C-CTX-1 by LC-MS/MS
2.4. Species Identification
3. Results
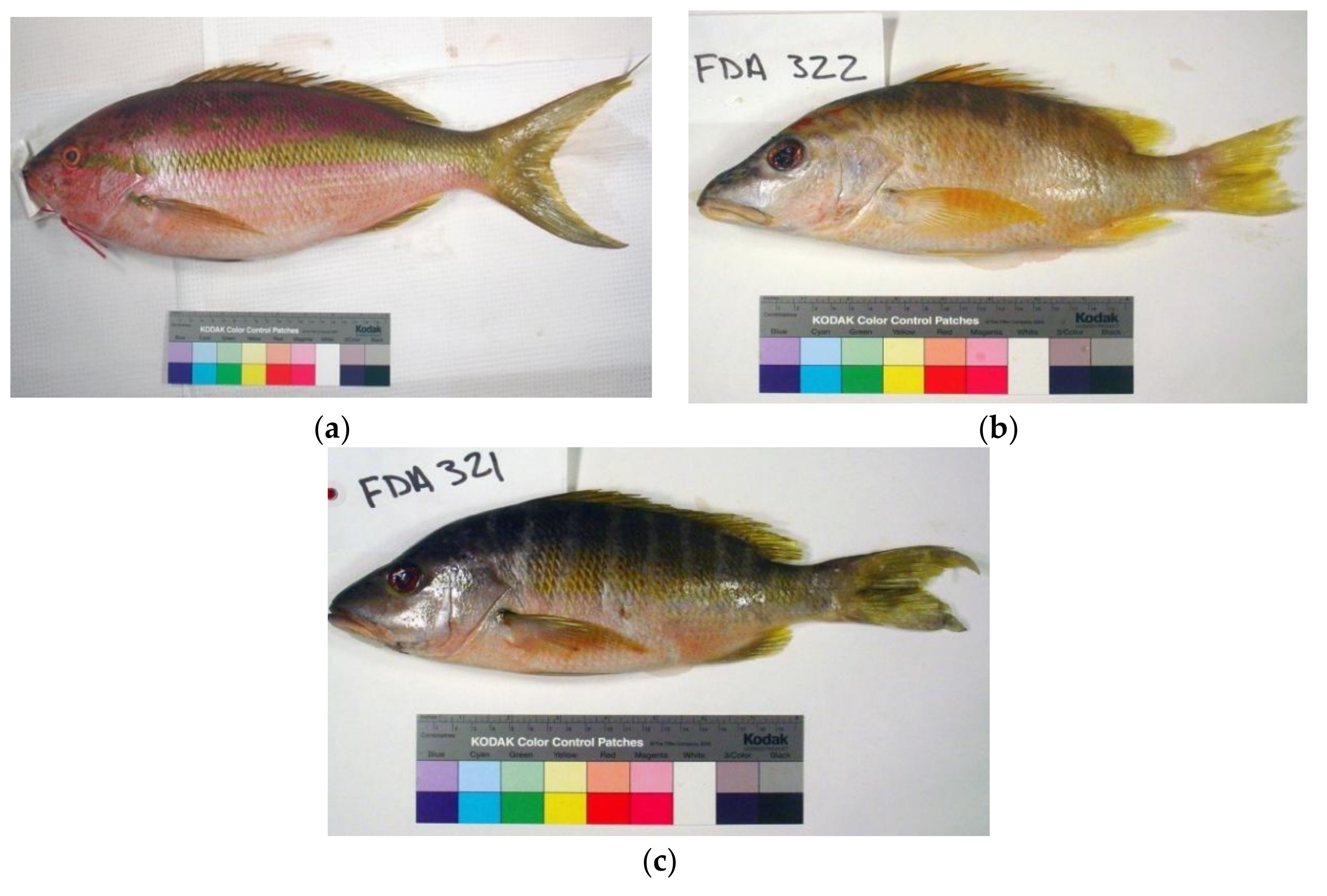
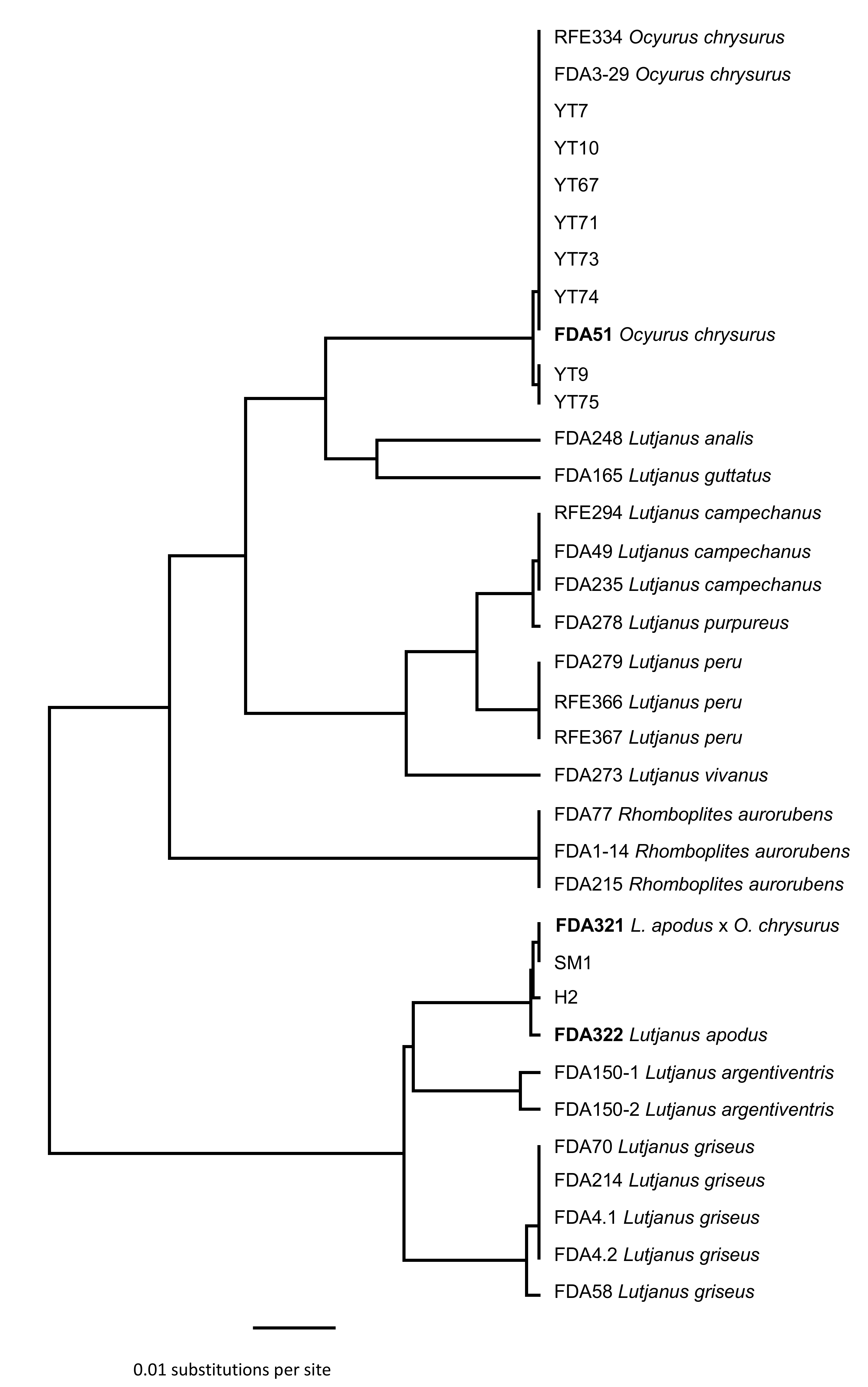
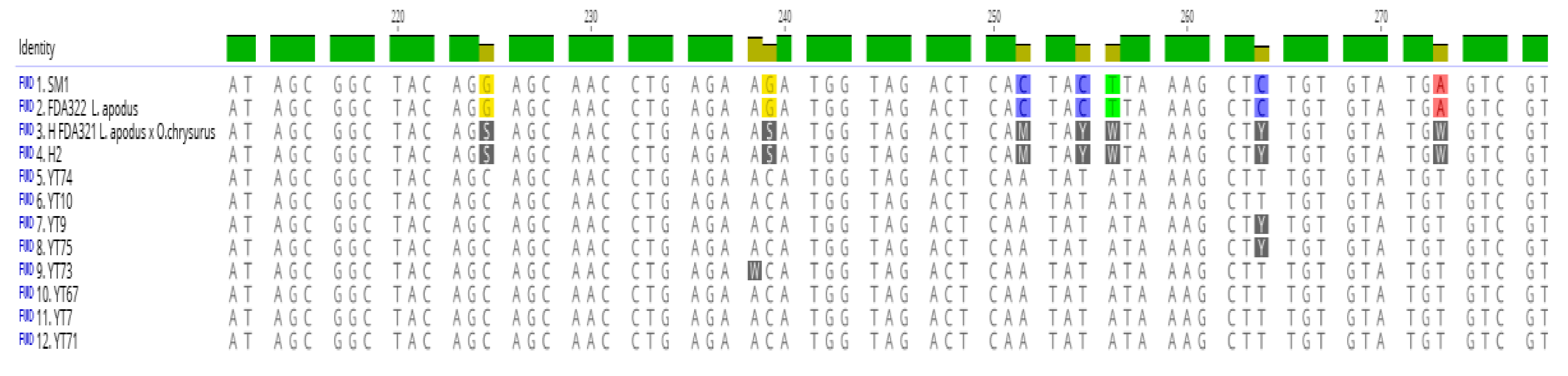
3.1. Identification of Maternal and Paternal Lineages in Suspected Hybrids
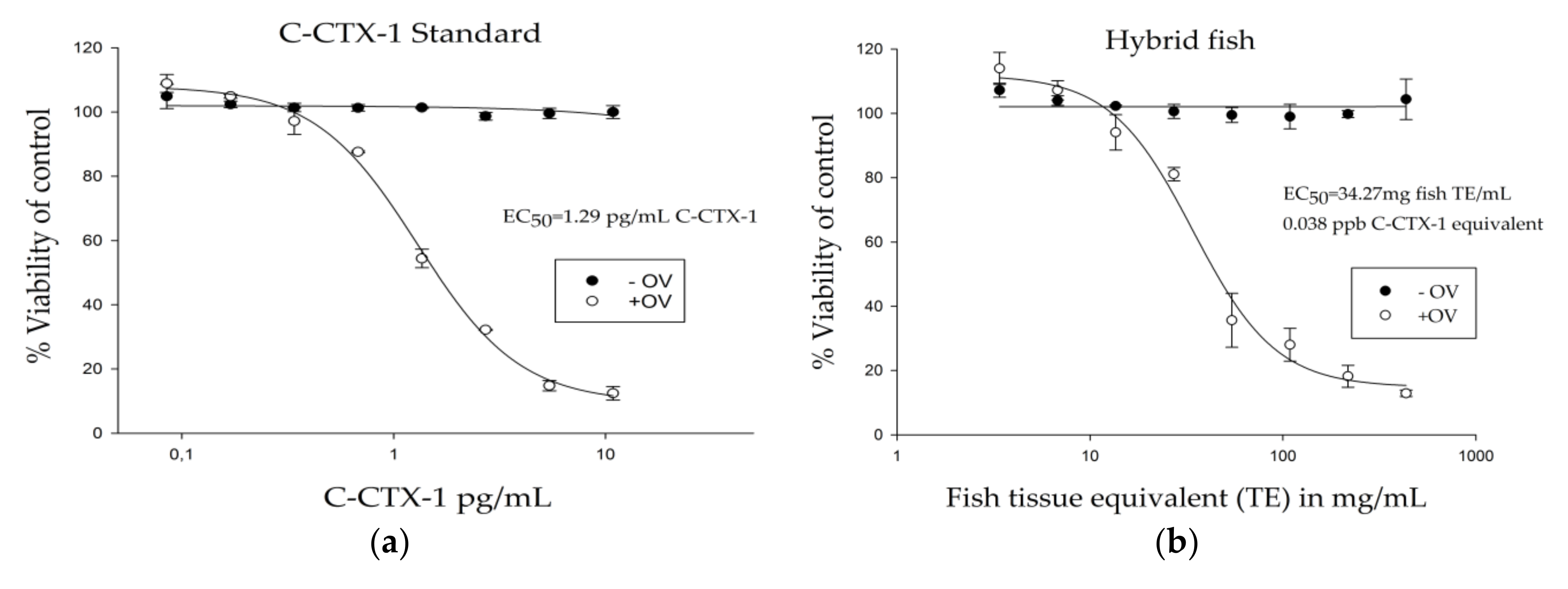
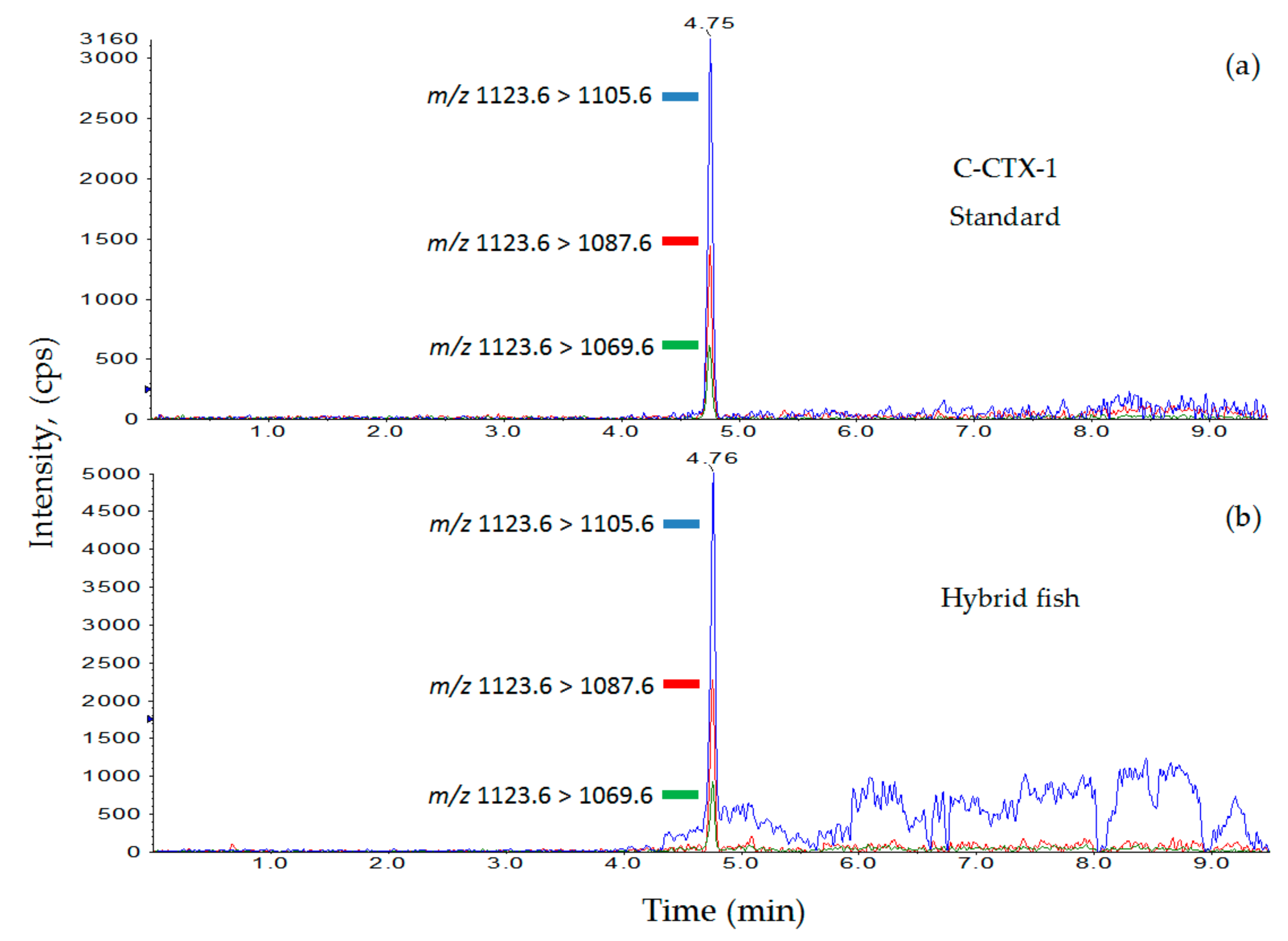
3.2. Ciguatoxin Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicholson, G.M.; Lewis, R.J. Ciguatoxins: Cyclic polyether modulators of voltage-gated Iion channel function. Mar. Drugs 2006, 4, 82–118. [Google Scholar] [CrossRef]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, C.R.; Richlen, M.L.; Brandt, M.E.; Smith, T.B. Effects of grazing, nutrients, and depth on the ciguatera-causing dinoflagellate Gambierdiscus in the US Virgin Islands. Mar. Ecol. Prog. Ser. 2015, 531, 91–104. [Google Scholar] [CrossRef]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrin. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Dickey, R.W.; Plakas, S.M. Ciguatera: A public health perspective. Toxicon 2010, 56, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Olsen, D.A.; Nellis, D.W.; Wood, R.S. Ciguatera in the Eastern Caribbean. Mar. Fish. Rev. 1984, 46, 13–18. [Google Scholar]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of Ciguatera Fish Poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Feldman, R.L.; Nau, A.W.; Kibler, S.R.; Litaker, R.W. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon 2010, 56, 698–710. [Google Scholar] [CrossRef]
- Tosteson, T.R. The diversity and origins of toxins in ciguatera fish poisoning. P. R. Health Sci. J. 1995, 14, 117–129. [Google Scholar] [PubMed]
- Radke, E.G.; Grattan, L.M.; Cook, R.L.; Smith, T.B.; Anderson, D.M.; Morris, J.G. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. Am. J. Trop. Med. Hyg. 2013, 88, 908–913. [Google Scholar] [CrossRef]
- Loeffler Christopher, R.; Robertson, A.; Flores Quintana Harold, A.; Silander Miguel, C.; Smith Tyler, B.; Olsen, D. Ciguatoxin prevalence in 4 commercial fish species along an oceanic exposure gradient in the US Virgin Islands. Environ. Toxicol. Chem. 2018, 37, 1852–1863. [Google Scholar] [CrossRef]
- Clark, R.D.; Pittman, S.; Battista, T.A.; Caldow, C. Survey and Impact Assessment of Derelict Fish Traps in St. Thomas and St. John, US Virgin Islands; US Department of Commerce, National Oceanic and Atmospheric Administration, National Ocean Service: Silver Spring, MD, USA, 2012. [Google Scholar]
- Randall, J.E. Food Habits of Reef Fishes of the West Indies; Institute of Marine Sciences, University of Miami: Coral Gables, FL, USA, 1967. [Google Scholar]
- U.S. Food Drug Administration. Fish and Fishery Products Hazards and Controls Guidance; US Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2011. Available online: https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/seafood/ucm2018426.htm (accessed on 9 August 2018).
- Dammann, A.E.; Swingle, W.E.; Yntema, J.A. Study of the Fisheries Potential of the Virgin Islands; Special Report; Virgin Island Ecological Research Station: St. John, US Virgin Islands, 1969; pp. 1–197. [Google Scholar]
- Halstead, B.W. Poisonous fishes. Public Health Rep. 1958, 73, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Pottier, I.; Vernoux, J.-P.; Lewis, R. Ciguatera Fish Poisoning in the Caribbean Islands and Western Atlantic. In Reviews of Environmental Contamination and Toxicology; Ware, G., Ed.; Springer: New York, NY, USA, 2001; Volume 168, pp. 99–141. [Google Scholar]
- Boucaud-Maitre, D.; Vernoux, J.P.; Pelczar, S.; Daudens-Vaysse, E.; Aubert, L.; Boa, S.; Ferracci, S.; Garnier, R. Incidence and clinical characteristics of ciguatera fish poisoning in Guadeloupe (French West Indies) between 2013 and 2016: A retrospective cases-series. Sci. Rep. 2018, 8, 3095. [Google Scholar] [CrossRef]
- Hammerschlag-Peyer, C.M.; Layman, C.A. Intrapopulation variation in habitat use by two abundant coastal fish species. Mar. Ecol. Prog. Ser. 2010, 415, 211–220. [Google Scholar] [CrossRef][Green Version]
- Cummings, N.J. The Biology of Yellowtail Snapper, Ocyurus Chrysurus, with Emphasis on Populations in the Caribbean; Sustain. Fish. Division Contrib. (SFD) No, 045; U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Sustainable Fisheries Division: Miami, FL, USA, 2004. [Google Scholar]
- Pittman, S.J.; Monaco, M.E.; Friedlander, A.M.; Legare, B.; Nemeth, R.S.; Kendall, M.S.; Poti, M.; Clark, R.D.; Wedding, L.M.; Caldow, C. Fish with chips: Tracking reef fish movements to evaluate size and connectivity of Caribbean marine protected areas. PLoS ONE 2014, 9, e96028. [Google Scholar] [CrossRef]
- Rooker, J.R. Feeding ecology of the schoolmaster snapper, Lutjanus apodus (Walbaum), from southwestern Puerto Rico. Bull. Mar. Sci. 1995, 56, 881–894. [Google Scholar]
- De la Moriniere, C.; Pollux, B.; Nagelkerken, I.; Hemminga, M.; Huiskes, A.; Van Der Velde, G. Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: Stable isotopes and gut-content analysis. Mar. Ecol. Prog. Ser. 2003, 246, 279–289. [Google Scholar] [CrossRef]
- Cocheret de la Morinière, E.; Pollux, B.J.A.; Nagelkerken, I.; van der Velde, G. Diet shifts of Caribbean grunts (Haemulidae) and snappers (Lutjanidae) and the relation with nursery-to-coral reef migrations. Estuar. Coast. Shelf Sci. 2003, 57, 1079–1089. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Fishbase, Version (02/2019), 2016. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 17 April 2019).
- Handy, S.M.; Deeds, J.R.; Ivanova, N.V.; Hebert, P.D.N.; Hanner, R.H.; Ormos, A.; Weigt, L.A.; Moore, M.M.; Yancy, H.F. A single-laboratory validated method for the generation of DNA barcodes for the identification of fish for regulatory compliance. J. AOAC Int. 2011, 94, 201–210. [Google Scholar] [PubMed]
- Eischeid, A.C.; Stadig, S.R.; Handy, S.M.; Fry, F.S.; Deeds, J. Optimization and evaluation of a method for the generation of DNA barcodes for the identification of crustaceans. LWT 2016, 73, 357–367. [Google Scholar] [CrossRef]
- Chow, S.; Hazama, K. Universal PCR primers for S7 ribosomal protein gene introns in fish. Mol. Ecol. 1998, 7, 1255–1256. [Google Scholar]
- Yaakub, S.M.; Bellwood, D.R.; Herwerden, L.v.; Walsh, F.M. Hybridization in coral reef fishes: Introgression and bi-directional gene exchange in Thalassoma (family Labridae). Mol. Phylogenet. Evol. 2006, 40, 84–100. [Google Scholar] [CrossRef]
- Dickey, R. Ciguatera toxins: Chemistry, toxicology, and detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection; Botana, L.M., Ed.; CRC Press: New York, NY, USA, 2008; Volume 173, pp. 479–500. [Google Scholar]
- Soliño, L.; Widgy, S.; Pautonnier, A.; Turquet, J.; Loeffler, C.R.; Quintana, H.A.F.; Diogène, J. Prevalence of ciguatoxins in lionfish (Pterois spp.) from Guadeloupe, Saint Martin, and Saint Barthélmy Islands (Caribbean). Toxicon 2015, 102, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Hokama, Y.; Dickey, R.W.; Granade, H.R.; Lewis, R.; Yasumoto, T.; Wekell, M.M. Detection of sodium channel toxins: Directed cytotoxicity assays of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. J. AOAC Int. 1995, 78, 521–527. [Google Scholar] [PubMed]
- Pottier, I.; Vernoux, J.-P.; Jones, A.; Lewis, R.J. Characterisation of multiple Caribbean ciguatoxins and congeners in individual specimens of horse-eye jack (Caranx latus) by high-performance liquid chromatography/mass spectrometry. Toxicon 2002, 40, 929–939. [Google Scholar] [CrossRef]
- Deeds, J.R.; Handy, S.M.; Fry, J.F.; Granade, H.; Williams, J.T.; Powers, M.; Shipp, R.; Weigt, L.A. Protocol for building a reference standard sequence library for DNA-based seafood identification. J. AOAC Int. 2014, 97, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Hudson, C.; Batista, O.; Gabriela, M.; Camargo, P.G.; Oliveira, D.P.V.; Patrícia, B.P.; Cezar, A.F. Occurrence of the hybrid snapper between yellowtail snapper Ocyurus chrysurus (Bloch 1791) and lane snapper Lutjanus synagris (Linnaeus 1758) (Perciformes: Lutjanidae) in the Southwest Atlantic, Northeast Brazil. Pan Am. J. Aquat. Sci. 2012, 7, 45–49. [Google Scholar]
- Pottier, I.; Vernoux, J.P.; Lewis, R.J. Ciguatera fish poisoning in the Caribbean islands and western Atlantic. Rev. Environ. Contam. Toxicol. 2001, 168, 99–141. [Google Scholar]
- Market, S.F. Schedule of Ciguatera High-Risk Areas and Species Size Limits. Available online: http://www.sydneyfishmarket.com.au/Portals/0/Ciguatera_Schedule.pdf (accessed on 16 April 2019).
- U.S. Food Drug Administration. Guidance for Industry: Purchasing Reef Fish Species Associated with the Hazard of Ciguatera Fish Poisoning. 2013. Available online: https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/seafood/ucm375214.htm (accessed on 16 April 2019).
- Diogène, J.; Reverté, L.; Rambla-Alegre, M.; del Río, V.; de la Iglesia, P.; Campàs, M.; Palacios, O.; Flores, C.; Caixach, J.; Ralijaona, C.; et al. Identification of ciguatoxins in a shark involved in a fatal food poisoning in the Indian Ocean. Sci. Rep. 2017, 7, 8240. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loeffler, C.R.; Handy, S.M.; Flores Quintana, H.A.; Deeds, J.R. Fish Hybridization Leads to Uncertainty Regarding Ciguatera Fish Poisoning Risk; Confirmation of Hybridization and Ciguatoxin Accumulation with Implications for Stakeholders. J. Mar. Sci. Eng. 2019, 7, 105. https://doi.org/10.3390/jmse7040105
Loeffler CR, Handy SM, Flores Quintana HA, Deeds JR. Fish Hybridization Leads to Uncertainty Regarding Ciguatera Fish Poisoning Risk; Confirmation of Hybridization and Ciguatoxin Accumulation with Implications for Stakeholders. Journal of Marine Science and Engineering. 2019; 7(4):105. https://doi.org/10.3390/jmse7040105
Chicago/Turabian StyleLoeffler, Christopher R., Sara M. Handy, Harold A. Flores Quintana, and Jonathan R. Deeds. 2019. "Fish Hybridization Leads to Uncertainty Regarding Ciguatera Fish Poisoning Risk; Confirmation of Hybridization and Ciguatoxin Accumulation with Implications for Stakeholders" Journal of Marine Science and Engineering 7, no. 4: 105. https://doi.org/10.3390/jmse7040105
APA StyleLoeffler, C. R., Handy, S. M., Flores Quintana, H. A., & Deeds, J. R. (2019). Fish Hybridization Leads to Uncertainty Regarding Ciguatera Fish Poisoning Risk; Confirmation of Hybridization and Ciguatoxin Accumulation with Implications for Stakeholders. Journal of Marine Science and Engineering, 7(4), 105. https://doi.org/10.3390/jmse7040105





