Matching Forces Applied in Underwater Hull Cleaning with Adhesion Strength of Marine Organisms
Abstract
:1. Introduction
2. Underwater Hull Cleaning and Hull Grooming
3. Adhesion Strength of Marine Organisms
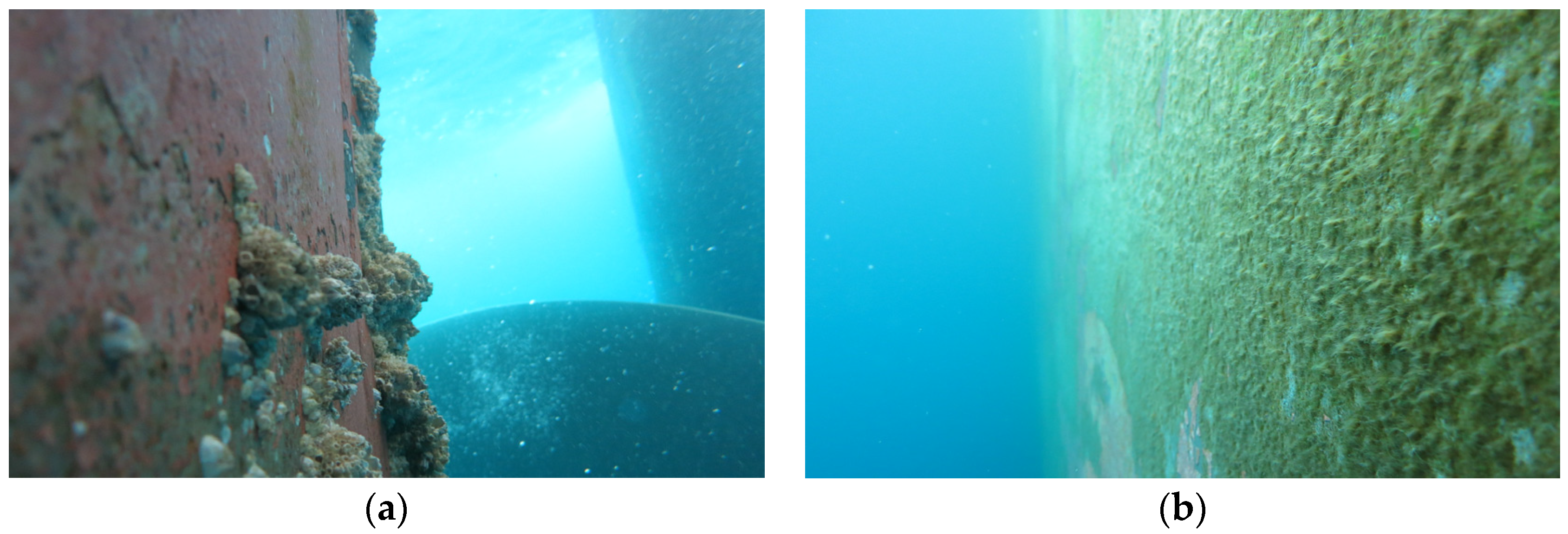
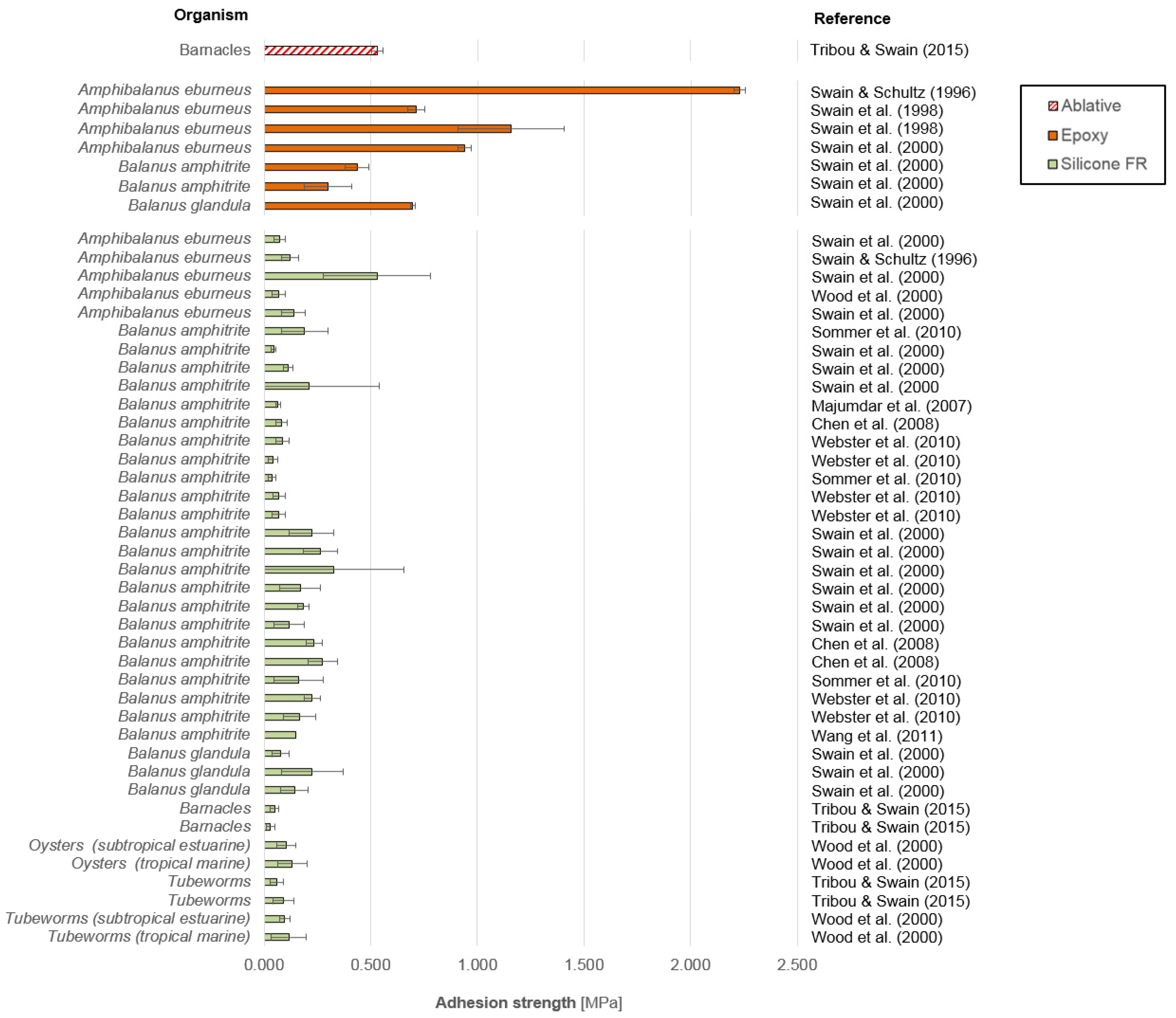
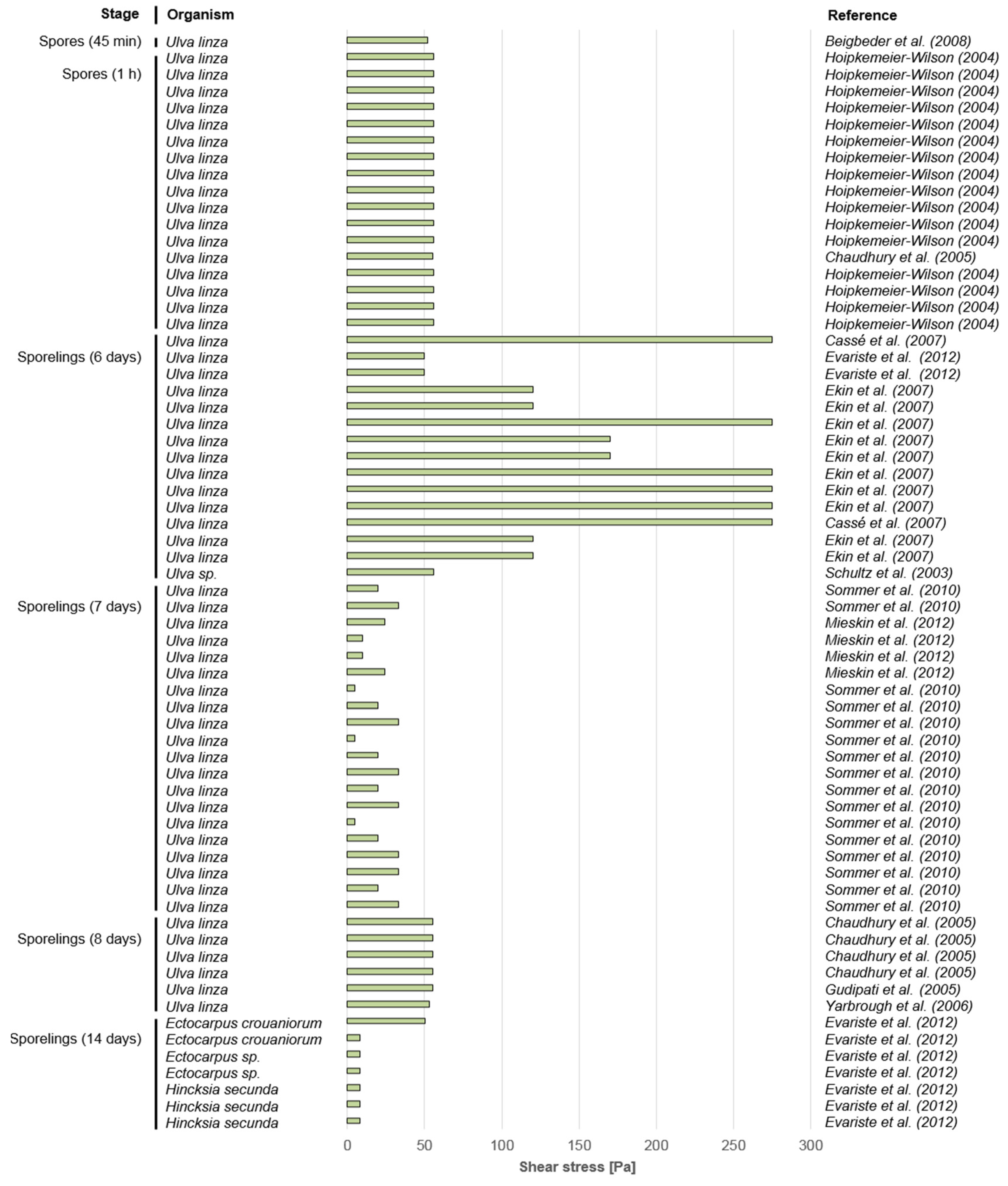
3.1. Macrofouling
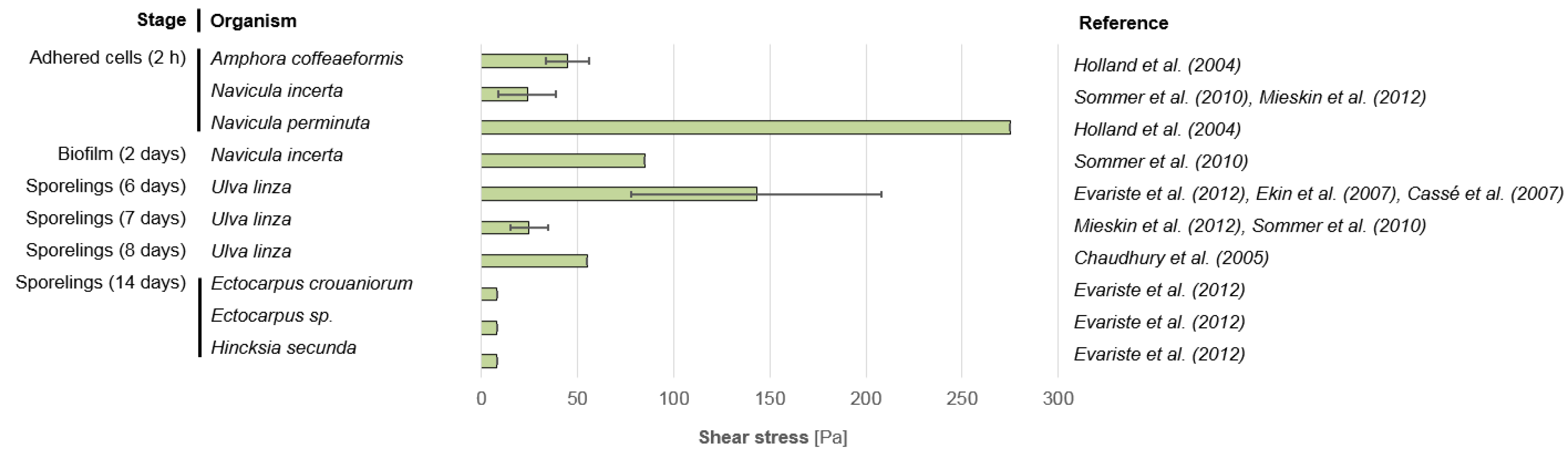
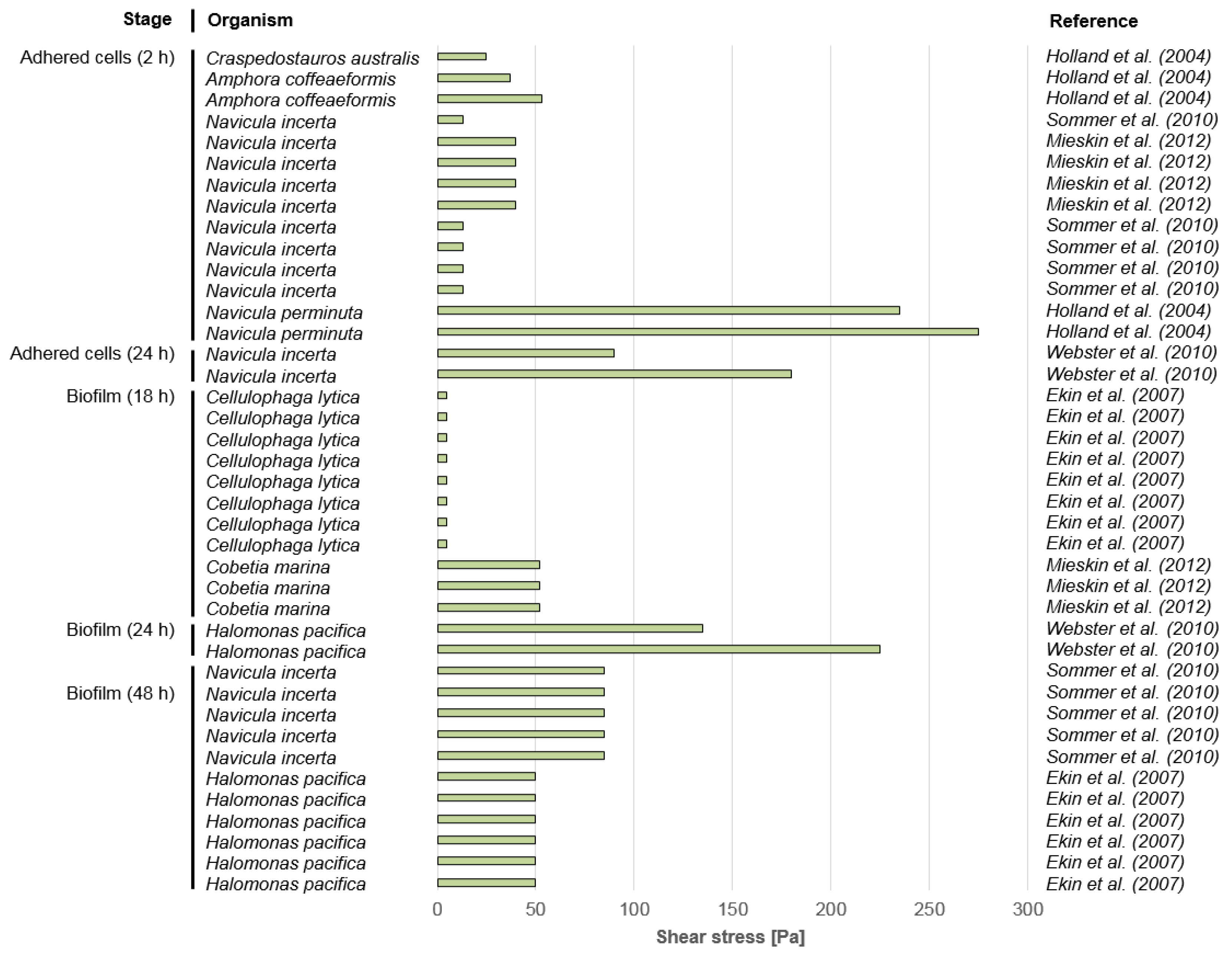
3.2. Microfouling
4. Matching Cleaning/Grooming Forces with Adhesion Strength
- for developers of cleaning technology: (1) shear forces should target microfouling or early stages of macrofouling; (2) forces should be easy to control by the user, with as few adjustable parameters as possible; (3) variability in shear force for each level of cleaning strength should be minimized; and (4) information should be compiled on effects on different types of coatings, microfouled samples and surface roughness;
- for users (e.g., diving companies), ship owners and as tool for various decision makers: (1) underwater cleaning of hulls covered with macrofouling should be avoided, as a rule; (2) the cleaning strength level should be adjusted by taking into account information available from the manufacturer of the cleaning system, as to get a conservatively low first estimate of strength needed for the task, taking into consideration the type and age of coating.
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dürr, S.; Thomason, J.C. Biofouling; Dürr, S., Thomason, J.C., Eds.; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Drake, J.M.; Lodge, D.M. Hull fouling is a risk factor for intercontinental species exchange in aquatic ecosystems. Aquat. Invasions 2007, 2, 121–131. [Google Scholar] [CrossRef]
- Townsin, R.L. The Ship Hull Fouling Penalty. Biofouling 2003, 19, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lewthwaite, J.C.; Molland, A.F.; Thomas, K.W. An Investigation into the Variation of Ship Skin Frictional Resistance with Fouling. In RINA Transactions; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 1984; pp. 269–284. [Google Scholar]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic Impact of Biofouling on a Naval Surface Ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- International Maritime Organization (IMO). A Transparent and Reliable Hull and Propeller Performance Standard; MEPC 63/4/8; IMO: London, UK, 2011; pp. 1–6. [Google Scholar]
- Blanco-Davis, E.; del Castillo, F.; Zhou, P. Fouling Release Coating Application as an Environmentally Efficient Retrofit: A Case Study of a Ferry-Type Ship. Int. J. Life Cycle Assess. 2014, 19, 1705–1715. [Google Scholar] [CrossRef]
- Champ, M.A. A Review of Organotin Regulatory Strategies, Pending Actions, Related Costs and Benefits. Sci. Total Environ. 2000, 258, 21–71. [Google Scholar] [CrossRef]
- Schulz, S.; Pastuch, J. How One Shipyard Is Making Paint Removal Cleaner and Greener. JPCL 2003, 20, 50–54. [Google Scholar]
- Song, Y.C.; Woo, J.H.; Park, S.H.; Kim, I.S. A Study on the Treatment of Antifouling Paint Waste from Shipyard. Mar. Pollut. Bull. 2005, 51, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Earley, P.J.; Swope, B.L.; Barbeau, K.; Bundy, R.; McDonald, J.; Rivera-Duarte, I. Life Cycle Contributions of Copper from Vessel Painting and Maintenance Activities. Biofouling 2014, 30, 51–68. [Google Scholar] [CrossRef] [PubMed]
- International Maritime Organization (IMO). 2011 Guidelines for the Control and Management of Ships’ Biofouling to Minimize the Transfer of Invasive Aquatic Species; MEPC 62/24/Add.1; IMO: London, UK, 2011; pp. 1–25. [Google Scholar]
- Takata, L.; Falkner, M.; Gilmore, S. Commercial Vessel Fouling in California: Analysis, Evaluation, and Recommendations to Reduce Nonidegenous Species Release from the Non-Ballast Water Vector; California State Lands Commission, Marine Facilities Division: Sacramento, CA, USA, 2006.
- Tribou, M.; Swain, G.W. Grooming Using Rotating Brushes as a Proactive Method to Control Ship Hull Fouling. Biofouling 2015, 31, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Schottle, R.; Brown, P. Copper Loading Assessment from in-Water Hull Cleaning Following Natural Fouling, Shelter Island Yacht Basin, San Diego Bay, San Diego, California. In Proceedings of the 11th Triennial International Conference on Ports, San Diego, CA, USA, 25–28 March 2007; pp. 1–10.
- Floerl, O.; Norton, N.; Inglis, G.J.; Hayden, B.; Middleton, C.; Smith, M.; Alcock, N.; Fitridge, I. Efficacy of Hull Cleaning Operations in Containing Biological Material—I. Risk Assessment; MAF Biosecurity: Wellington, New Zealand, 2005. [Google Scholar]
- Tribou, M.; Swain, G.W. The Use of Proactive in-Water Grooming to Improve the Performance of Ship Hull Antifouling Coatings. Biofouling 2010, 26, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, B. Is the Much Sought-After, Better Alternative Underwater Hull Coating System Already Here? In Proceedings of the RINA, Royal Institution of Naval Architects—International Conference on Marine Coatings, London, UK, 18 April 2013; pp. 38–43.
- NSTM. Chapter 081—Water-Borne Underwater Hull Cleaning of Navy Ships; Direction Of Commander, Naval Sea Systems Command: Washington, DC, USA, 2006. [Google Scholar]
- Floerl, O.; Peacock, L.; Seaward, K.; Inglis, G. Review of Biosecurity and Contaminant Risks Associated with in-Water Cleaning; Commonwealth of Australia: Canberra, Australia, 2010.
- Bohlander, J. Review of Options for in-Water Cleaning of Ships; MAF Biosecurity: Wellington, New Zealand, 2009. [Google Scholar]
- Roche, R.C.; Monnington, J.M.; Newstead, R.G.; Sambrook, K.; Griffith, K.; Holt, R.H.F.; Jenkins, S.R. Recreational Vessels as a Vector for Marine Non-Natives: Developing Biosecurity Measures and Managing Risk through an in-Water Encapsulation System. Hydrobiologia 2015, 750, 187–199. [Google Scholar] [CrossRef]
- Atalah, J.; Brook, R.; Cahill, P.; Fletcher, L.M.; Hopkins, G.A. It’s a Wrap: Encapsulation as a Management Tool for Marine Biofouling. Biofouling 2016, 32, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Holm, E.R.; Haslbeck, E.G.; Horinek, A. Evaluation of Brushes for Removal of Fouling from Fouling-Release Surfaces, Using a Hydraulic Cleaning Device. Biofouling 2003, 19, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Hearin, J.; Hunsucker, K.Z.; Swain, G.; Gardner, H.; Stephens, A.; Lieberman, K. Analysis of Mechanical Grooming at Various Frequencies on a Large Scale Test Panel Coated with a Fouling-Release Coating. Biofouling 2016, 32, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P.; Walker, J.M.; Steppe, C.N.; Flack, K.A. Impact of Diatomaceous Biofilms on the Frictional Drag of Fouling-Release Coatings. Biofouling 2015, 31, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Hearin, J.; Hunsucker, K.Z.; Swain, G.; Stephens, A.; Gardner, H.; Lieberman, K.; Harper, M. Analysis of Long-Term Mechanical Grooming on Large-Scale Test Panels Coated with an Antifouling and a Fouling-Release Coating. Biofouling 2015, 31, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Useche, L.V.V.; Wahab, M.M.A.; Parker, G.A. Brush Dynamics: Models and Characteristics. In Proceedings of the ASME 8th Biennial Conference on Engineering Systems Design and Analysis, Volume 3: Dynamic Systems and Controls, Symposium on Design and Analysis of Advanced Structures, and Tribology, Torino, Italy, 4–7 July 2006; pp. 441–450.
- Finlay, J.A.; Callow, M.; Schultz, M.P.; Swain, G.W.; Callow, J.A. Adhesion Strength of Settled Spores of the Green Alga Enteromorpha. Biofouling 2002, 18, 251–256. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Mazurek, K.A. Impingement of Circular Turbulent Jets on Rough Boundaries. J. Hydraul. Res. 2005, 43, 689–695. [Google Scholar] [CrossRef]
- Guo, S.; Khoo, B.C.; Teo, S.L.M.; Zhong, S.; Lim, C.T.; Lee, H.P. Effect of ultrasound on cyprid footprint and juvenile barnacle adhesion on a fouling release material. Colloid Surface B 2014, 115, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Crawford, S.A.; Higgins, M.J.; Mulvaney, P.; Wetherbee, R. The application of atomic force microscopy to topographical studies and force measurements on the secreted adhesive of the green alga Enteromorpha. Planta 2000, 211, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Crisp, D.J.; Walker, G.; Young, G.A.; Yule, A.B. Adhesion and substrate choice in mussels and barnacles. J. Colloid Interface Sci. 1985, 104, 40–50. [Google Scholar] [CrossRef]
- Kamino, K. Barnacle Underwater Attachment. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin, Germany, 2006; pp. 145–166. [Google Scholar]
- ASTM International. Measurement of Barnacle Adhesion Strength in Shear (Reapproved 2000); ASTM Standard D5618; ASTM International: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Berglin, M.; Larsson, A.; Jonsson, P.R.; Gatenholm, P. The adhesion of the barnacle Balanus improvisus, to poly(dimethylsiloxane) fouling-release coatings and poly(methyl methacrylate) panels: The effect of barnacle size on strength and failure mode. J. Adhes Sci. Technol. 2001, 15, 1485–1502. [Google Scholar] [CrossRef]
- Larsson, A.I.; Mattsson-Thorngren, L.; Granhag, L.M.; Berglin, M. Fouling-Release of Barnacles from a Boat Hull with Comparison to Laboratory Data of Attachment Strength. J. Exp. Mar. Biol. Ecol. 2010, 392, 107–114. [Google Scholar] [CrossRef]
- Ackerman, J.D.; Ethier, C.R.; Allen, D.G.; Spelt, J.K. Investigation of Zebra Mussel Adhesion Strength Using Rotating Disks. J. Environ. Eng. 1992, 118, 708–724. [Google Scholar] [CrossRef]
- Swain, G.W.; Nelson, W.G.; Preedeekanit, S. The Influence of Biofouling Adhesion and Biotic Disturbance on the Development of Fouling Communities on Non-toxic Surfaces. Biofouling 1998, 12, 257–269. [Google Scholar] [CrossRef]
- Zargiel, K.; Swain, G.W. Static vs. Dynamic Settlement and Adhesion of Diatoms to Ship Hull Coatings. Biofouling 2014, 30, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Cassé, F.; Swain, G.W. The Development of Microfouling on Four Commercial Antifouling Coatings under Static and Dynamic Immersion. Int. Biodeterior. Biodegrad. 2006, 57, 179–185. [Google Scholar] [CrossRef]
- Swain, G.W.; Schultz, M.P. The Testing and Evaluation of Non-Toxic Antifouling Coatings. Biofouling 1996, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.; Anil, A.C.; Baier, R.E.; Chia, F.; Conte, E.; Cook, A.; Hadfield, M.; Haslbeck, E.; Holm, E.; Kavanagh, C.; et al. Biofouling and Barnacle Adhesion Data for Fouling-release Coatings Subjected to Static Immersion at Seven Marine Sites. Biofouling 2000, 16, 331–344. [Google Scholar] [CrossRef]
- Wood, C.D.; Truby, K.; Stein, J.; Wiebe, D.; Holm, E.; Wendt, D.; Smith, C.; Kavanagh, C.; Montemarano, J.; Swain, G.; Meyer, A. Temporal and Spatial Variations in Macrofouling of Silicone Fouling-Release Coatings. Biofouling 2000, 16, 311–322. [Google Scholar] [CrossRef]
- Sommer, S.; Ekin, A.; Webster, D.C.; Stafslien, S.J.; Daniels, J.; VanderWal, L.J.; Thompson, S.E.M.; Callow, M.E.; Callow, J.A. A Preliminary Study on the Properties and Fouling-Release Performance of Siloxane-Polyurethane Coatings Prepared from Poly(dimethylsiloxane) (PDMS) Macromers. Biofouling 2010, 26, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Stafslien, S.; Daniels, J.; Webster, D.C. High Throughput Combinatorial Characterization of Thermosetting Siloxane–urethane Coatings Having Spontaneously Formed Microtopographical Surfaces. J. Coat. Technol. Res. 2007, 4, 131–138. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.; Kim, J.; Stafslien, S.; Wagner, R.; Patel, S.; Daniels, J.; Vander Wal, L.; Li, J.; Ward, K.; et al. UV-Curable, Oxetane-Toughened Epoxy-Siloxane Coatings for Marine Fouling-Release Coating Applications. Polym. Int. 2008, 57, 879–886. [Google Scholar] [CrossRef]
- Webster, D.C.; Pieper, R.J.; Nasrullah, M.J. Zwitterionic/Amphiphilic Pentablock Copolymers and Coatings Therefrom. U.S. Patent WO 2010/042804 A2, 15 April 2010. [Google Scholar]
- Wang, Y.; Betts, D.E.; Finlay, J.A.; Brewer, L.; Callow, M.E.; Callow, J.A.; Wendt, D.E.; Desimone, J.M. Photocurable Amphiphilic Perfluoropolyether/poly(ethylene Glycol) Networks for Fouling-Release Coatings. Macromolecules 2011, 44, 878–885. [Google Scholar] [CrossRef]
- Becka, A.; Loeb, G. Ease of Removal of Barnacles from Various Polymeric Materials. Biotechnol. Bioeng. 1984, 26, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Becker, K. Attachment Strength and Colonization Patterns of Two Macrofouling Species on Substrata with Different Surface Tension (in Situ Studies). Mar. Biol. 1993, 117, 301–309. [Google Scholar] [CrossRef]
- Kavanagh, C.J.; Schultz, M.P.; Swain, G.W.; Stein, J.; Truby, K.; Wood, C.D. Variation in Adhesion Strength of Balanus eburneus, Crassostrea virginica and Hydroides dianthus to Fouling-Release Coatings. Biofouling 2001, 17, 155–167. [Google Scholar] [CrossRef]
- Chiovitti, A.; Dugdale, T.M.; Wetherbee, R. Diatom Adhesives: Molecular and Mechanical Properties. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin, Germany, 2006; pp. 79–103. [Google Scholar]
- Pettitt, M.E.; Henry, S.L.; Callow, M.E.; Callow, J.A.; Clare, A.S. Activity of Commercial Enzymes on Settlement and Adhesion of Cypris Larvae of the Barnacle Balanus amphitrite, Spores of the Green Alga Ulva linza, and the Diatom Navicula perminuta. Biofouling 2004, 20, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Ekin, A.; Webster, D.C.; Daniels, J.W.; Stafslien, S.J.; Cassé, F.; Callow, J.A.; Callow, M.E. Synthesis, Formulation, and Characterization of Siloxane-Polyurethane Coatings for Underwater Marine Applications Using Combinatorial High-Throughput Experimentation. J. Coat. Technol. Res. 2007, 4, 435–451. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. The Ulva Spore Adhesive System. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin, Germany, 2006; pp. 63–78. [Google Scholar]
- Schultz, M.P.; Finlay, J.A.; Callow, M.E.; Callow, J.A. A Turbulent Channel Flow Apparatus for the Determination of the Adhesion Strength of Microfouling Organisms. Biofouling 2000, 15, 243–251. [Google Scholar] [CrossRef]
- Cassé, F.; Ribeiro, E.; Ekin, A.; Webster, D.C.; Callow, J.A.; Callow, M.E. Laboratory Screening of Coating Libraries for Algal Adhesion. Biofouling 2007, 23, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Dugdale, T.M.; Wetherbee, R.; Brennan, A.B.; Finlay, J.A.; Callow, J.A.; Callow, M.E. Adhesion and Motility of Fouling Diatoms on a Silicone Elastomer. Biofouling 2004, 20, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cassé, F.; Stafslien, S.J.; Bahr, J.A.; Daniels, J.; Finlay, J.A.; Callow, J.A.; Callow, M.E. Combinatorial materials research applied to the development of new surface coatings V. Application of a spinning water-jet for the semi-high throughput assessment of the attachment strength of marine fouling algae. Biofouling 2007, 23, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mieszkin, S.; Martin-Tanchereau, P.; Callow, M.E.; Callow, J.A. Effect of Bacterial Biofilms Formed on Fouling-Release Coatings from Natural Seawater and Cobetia marina, on the Adhesion of Two Marine Algae. Biofouling 2012, 28, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Evariste, E.; Gachon, C.M.M.; Callow, M.E.; Callow, J.A. Development and Characteristics of an Adhesion Bioassay for Ectocarpoid Algae. Biofouling 2012, 28, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, M.K.; Finlay, J.A.; Chung, J.Y.; Callow, M.E.; Callow, J.A. The Influence of Elastic Modulus and Thickness on the Release of the Soft-Fouling Green Alga Ulva linza (Syn. Enteromorpha linza) from Poly(dimethylsiloxane) (PDMS) Model Networks. Biofouling 2005, 21, 41–48. [Google Scholar] [PubMed]
- Beigbeder, A.; Degee, P.; Conlan, S.L.; Mutton, R.J.; Clare, A.S.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Dubois, P. Preparation and Characterisation of Silicone-Based Coatings Filled with Carbon Nanotubes and Natural Sepiolite and Their Application as Marine Fouling-Release Coatings. Biofouling 2008, 24, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Hoipkemeier-Wilson, L.; Schumacher, J.F.; Carman, M.L.; Gibson, A.L.; Feinberg, A.W.; Callow, M.E.; Finlay, J.A.; Callow, J.A.; Brennan, A.B. Antifouling Potential of Lubricious, Micro-Engineered, PDMS Elastomers against Zoospores of the Green Fouling Alga Ulva (Enteromorpha). Biofouling 2004, 20, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P.; Finlay, J.A.; Callow, M.E.; Callow, J.A. Three Models to Relate Detachment of Low Form Fouling at Laboratory and Ship Scale. Biofouling 2003, 19 (Suppl. S1), 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, C.S.; Finlay, J.A.; Callow, J.A.; Callow, M.E.; Wooley, K.L. The Antifouling and Fouling-Release Perfomance of Hyperbranched Fluoropolymer (HBFP)-Poly(ethylene Glycol) (PEG) Composite Coatings Evaluated by Adsorption of Biomacromolecules and the Green Fouling Alga Ulva. Langmuir 2005, 21, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, J.C.; Rolland, J.P.; DeSimone, J.M.; Callow, M.E.; Finlay, J.A.; Callow, J.A. Contact Angle Analysis, Surface Dynamics, and Biofouling Characteristics of Cross-Linkable, Random Perfluoropolyether-Based Graft Terpolymers. Macromolecules 2006, 39, 2521–2528. [Google Scholar] [CrossRef]
- Woods Hole Oceanografic Institute. Marine Fouling and Its Prevention; Chapter 18; George Banta Publishing Co.: Menasha, WI, USA, 1952; p. 313. [Google Scholar]
- Balashov, V.S.; Gromov, B.A.; Ermolov, I.L.; Roskilly, A.P. Cleaning by means of the HISMAR autonomous robot. Russ. Eng. Res. 2011, 31, 589–592. [Google Scholar] [CrossRef]
- Anil, A.C.; Khandeparker, L.; Desai, D.V.; Baragi, L.V.; Gaonkar, C.A. Larval development, sensory mechanisms and physiological adaptations in acorn barnacles with special reference to Balanus amphitrite. J. Exp. Mar. Bio. Ecol. 2010, 392, 89–98. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, D.; Granhag, L. Matching Forces Applied in Underwater Hull Cleaning with Adhesion Strength of Marine Organisms. J. Mar. Sci. Eng. 2016, 4, 66. https://doi.org/10.3390/jmse4040066
Oliveira D, Granhag L. Matching Forces Applied in Underwater Hull Cleaning with Adhesion Strength of Marine Organisms. Journal of Marine Science and Engineering. 2016; 4(4):66. https://doi.org/10.3390/jmse4040066
Chicago/Turabian StyleOliveira, Dinis, and Lena Granhag. 2016. "Matching Forces Applied in Underwater Hull Cleaning with Adhesion Strength of Marine Organisms" Journal of Marine Science and Engineering 4, no. 4: 66. https://doi.org/10.3390/jmse4040066
APA StyleOliveira, D., & Granhag, L. (2016). Matching Forces Applied in Underwater Hull Cleaning with Adhesion Strength of Marine Organisms. Journal of Marine Science and Engineering, 4(4), 66. https://doi.org/10.3390/jmse4040066





