Methodological Studies on Estimates of Abundance and Diversity of Heterotrophic Flagellates from the Deep-Sea Floor
Abstract
:1. Introduction
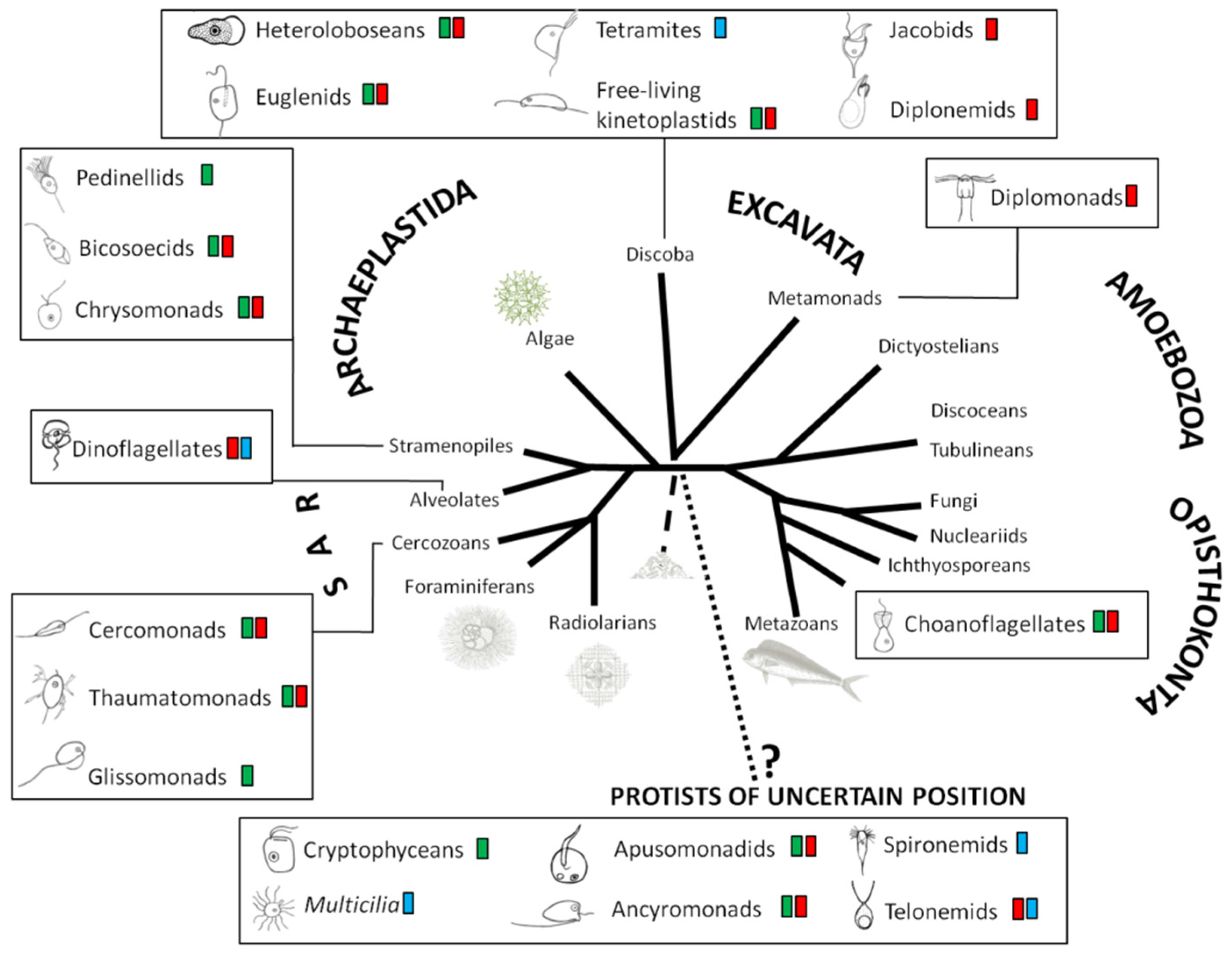
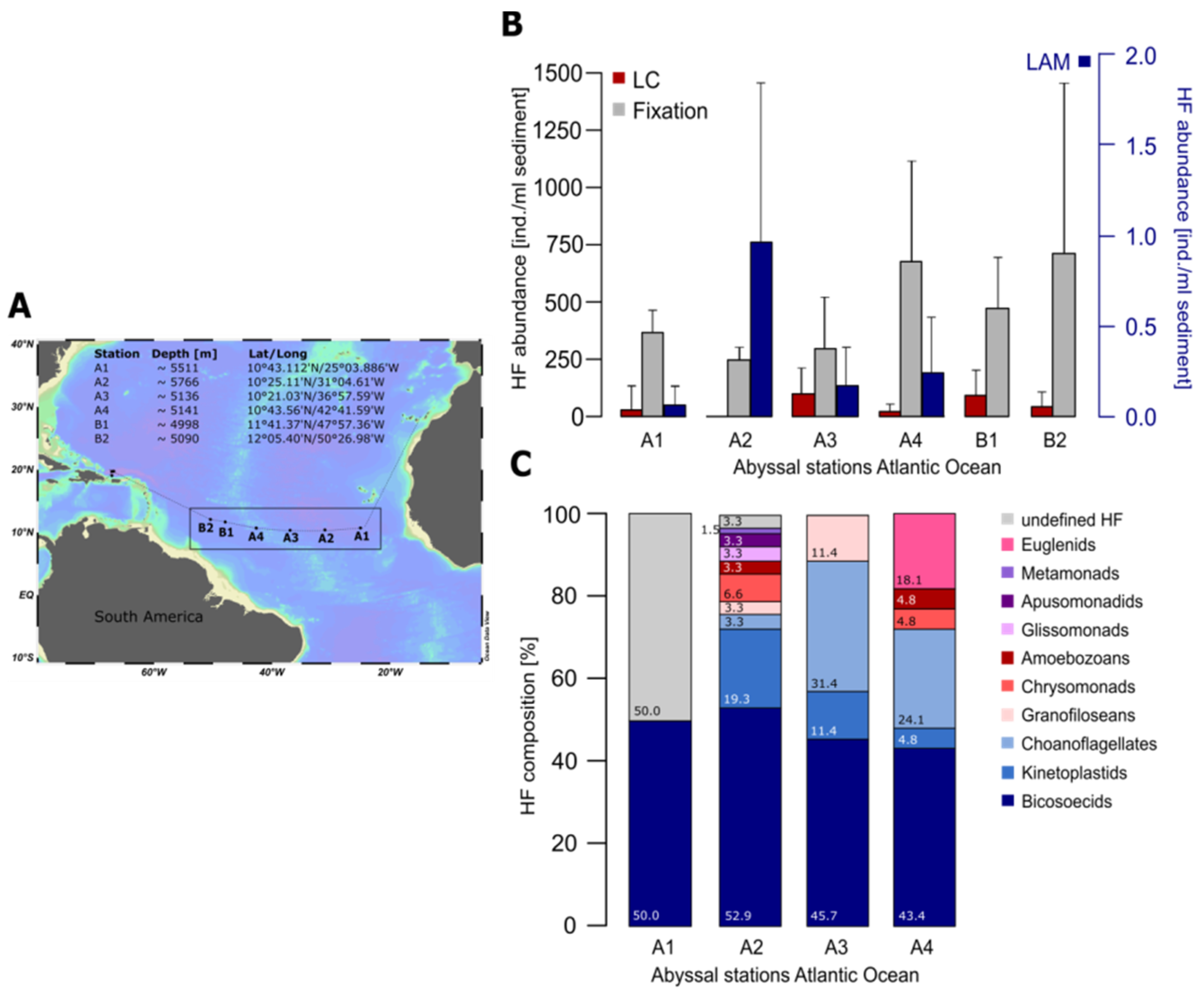
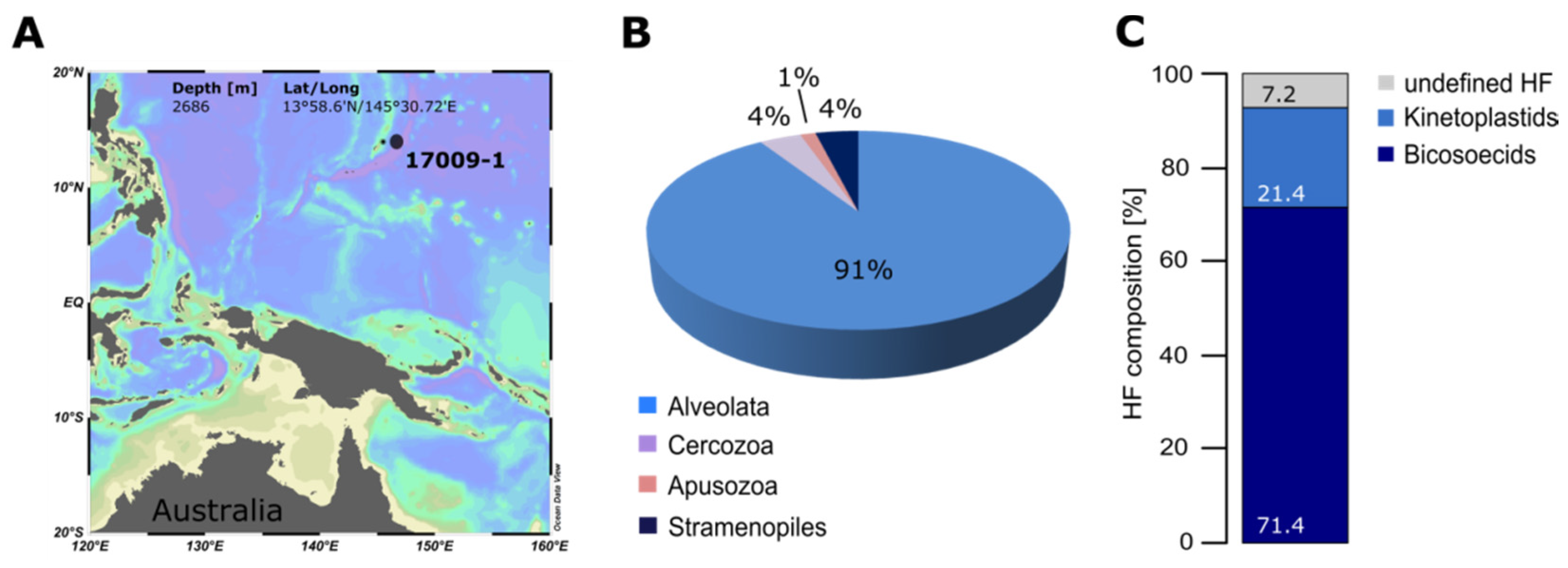
2. Quantification and Qualification of Deep-Sea Protists
2.1. How to Sample Deep-Sea Protists
2.2. Live-Counting
2.3. Fixation and Staining
2.4. Cultivation
2.5. Next Generation Sequencing (NGS)
3. Protocol for Detecting Nanofaunal Abundance and Diversity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gage, J.; Tyler, P.A. Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Danovaro, R.; Corinaldesi, C.; Rastelli, E.; Dell’Anno, A. Towards a better quantitative assessment of the relevance of deep-sea viruses, Bacteria and Archaea in the functioning of the ocean seafloor. Aquat. Microb. Ecol. 2015, 75, 81–90. [Google Scholar] [CrossRef]
- Bik, H.M.; Sung, W.; De Ley, P.; Baldwin, J.G.; Sharma, J.; Rocha-Olivares, A.; Thomas, W.K. Metagenetic community analysis of microbial eukaryotes illuminates biogeographic patterns in deep-sea and shallow water sediments. Mol. Ecol. 2012, 21, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Caron, D.A.; Peele, E.R.; Lim, E.L.; Dennett, M.R. Picoplankton and nanoplankton and their trophic coupling in surface waters of the Sargasso Sea south of Bermuda. Limnol. Oceanogr. 1999, 44, 259–272. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Bacterivory and herbivory: Key roles of phagotrophic protists in pelagic food webs. Microb. Ecol. 1994, 28, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.B.; Boetius, A. Feast and famine-microbial life in the deep-sea bed. Nat. Rev. Micro. 2007, 5, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Pernice, M.C.; Forn, I.; Gomes, A.; Lara, E.; Alonso-Sáez, L.; Arrieta, J.M.; del Carmen Garcia, F.; Hernando-Morales, V.; MacKenzie, R.; Mestre, M.; et al. Global abundance of planktonic heterotrophic protists in the deep ocean. ISME J. 2015, 9, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Pernice, M.C.; Giner, C.R.; Logares, R.; Perera-Bel, J.; Acinas, S.G.; Duarte, C.M.; Gasol, J.M.; Massana, R. Large variability of bathypelagic microbial eukaryotic communities across the world’s oceans. ISME J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gooday, A.J.; Rathburn, A.E. Temporal variability in living deep-sea benthic foraminifera: A review. Earth Sci. Rev. 1999, 46, 187–212. [Google Scholar] [CrossRef]
- Burnett, B.R. Quantitative sampling of nanobiota (microbiota) of the deep-sea benthos-III. The bathyal San Diego Trough. Deep Sea Res. 1981, 28, 649–663. [Google Scholar] [CrossRef]
- Lighthart, B. Planktonic and benthic bacterivorous protozoa at eleven stations in Puget Bay, Australia. J. Fish. Res. Can. 1969, 26, 299–304. [Google Scholar] [CrossRef]
- Alongi, D.M. Bacterial growth rates, production and estimates of detrital carbon utilization in deep-sea sediments of the Solomon and Coral Seas. Deep Sea Res. 1990, 37, 731–746. [Google Scholar] [CrossRef]
- Burnett, B.R. Quantitative sampling of microbiota of the deep-sea benthos-I. Sampling techniques and some data from the abyssal central North Pacific. Deep Sea Res. 1977, 24, 781–789. [Google Scholar] [CrossRef]
- Bak, R.P.M.; Nieuwland, G. Seasonal variation in bacterial and flagellate communities of deep-sea sediments in a monsoonal upwelling system. Deep Sea Res. II 1997, 44, 1281–1292. [Google Scholar] [CrossRef]
- Danovaro, R.; Marrale, D.; Della Croce, N.; Dell’Anno, A.; Fabiano, M. Heterotrophic nanoflagellates, bacteria, and labile organic compounds in continental shelf and deep-sea sediments of the Eastern Mediterranean. Microb. Ecol. 1998, 35, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.; Balagué, V.; Forn, I.; Lekunberri, I.; Massana, R. Culturing bias in marine heterotrophic flagellates analyzed through seawater enrichment incubations. Microb. Ecol. 2013, 66, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, J.; Audic, S.; Adl, S.M.; Bass, D.; Belbahri, L.; Berney, C.; Bowser, S.S.; Cepicka, I.; Decelle, J.; Dunthorn, M.; et al. CBOL Protist working group: Barcoding eukaryotic richness beyond the animal, plant and fungal kingdoms. PLoS Biol. 2012, 10, e1001419. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, K.; Massana, R. Protistan grazing on marine bacterioplankton. In Microbial Ecology of the Oceans, 2nd ed.; Wiley: New York, NY, USA, 2008; pp. 383–441. [Google Scholar]
- Massana, R.; Guillou, L.; Diez, B.; Pedrós-Alió, C. Unveiling the organisms behind novel eukaryotic ribosomal DNA sequences from the ocean. Appl. Environ. Microbiol. 2002, 68, 4554–4558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arndt, H.; Dietrich, D.; Auer, B.; Cleven, E.-J.; Gräfenhan, T.; Weitere, M.; Mylnikov, A.P. Functional diversity of heterotrophic flagellates in aquatic ecosystems. In The Flagellates—Unity, Diversity and Evolution; Leadbeater, B.S.C., Green, J.C., Eds.; Taylor & Francis Ltd: London, UK, 2000; pp. 240–268. [Google Scholar]
- Nitsche, F.; Arndt, H. A new choanoflagellate species from Taiwan: Morphological and molecular biological studies of Diplotheca elongata nov. spec. and D. costata. Eur. J. Protistol. 2008, 44, 220–226. [Google Scholar] [CrossRef] [PubMed]
- López-García, P.; Rodríguez-Valera, F.; Pedrós-Alió, C.; Moreira, D. Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 2001, 409, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Arístegui, J.; Gasol, J.M.; Duarte, C.M.; Herndl, G.J. Microbial oceanography of the dark ocean’s pelagic realm. Limnol. Oceanogr. 2009, 54, 1501–1529. [Google Scholar] [CrossRef]
- López-García, P.; Philippe, H.; Gail, F.; Moreira, D. Autochthonous eukaryotic diversity in hydrothermal sediment and experimental microcolonizers at the Mid-Atlantic Ridge. Proc. Natl. Acad. Sci. USA 2003, 100, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Simpson, A.G.B.; Lane, C.E.; Lukeš, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–493. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, V.; Orsi, W.; Leslin, C.; Epstein, S.S.; Bunge, J.; Jeon, S.; Yakimov, M.M.; Behnke, A.; Stoeck, T. Protistan community patterns within the brine and halocline of deep hypersaline anoxic basins in the eastern Mediterranean Sea. Extremophiles 2009, 13, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, T.; Epstein, S. Novel eukaryotic lineages inferred from small-subunit rRNA analyses of oxygen-depleted marine environments. Appl. Environ. Microbiol. 2003, 69, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, V.P.; Kysela, D.T.; Teske, A.; de Vera Gomez, A.; Sogin, M.L. Benthic eukaryotic diversity in the Guaymas Basin hydrothermal vent environment. Proc. Natl. Acad. Sci. USA 2002, 99, 7658–7662. [Google Scholar] [CrossRef] [PubMed]
- Scheckenbach, F.; Wylezich, C.; Weitere, M.; Hausmann, K.; Arndt, H. Molecular identity of strains of heterotrophic flagellates isolated from surface waters and deep-sea sediments of the South Atlantic based on SSU rDNA. Aquat. Microb. Ecol. 2005, 38, 239–247. [Google Scholar] [CrossRef]
- Arndt, H.; Hausmann, K.; Wolf, M. Deep-sea heterotrophic nanoflagellates of the Eastern Mediterranean Sea: Qualitative and quantitative aspects of their pelagic and benthic occurrence. Mar. Ecol. Prog. Ser. 2003, 256, 45–56. [Google Scholar] [CrossRef]
- Scheckenbach, F.; Hausmann, K.; Wylezich, C.; Weitere, M.; Arndt, H. Large-scale patterns in biodiversity of microbial eukaryotes from the abyssal sea floor. Proc. Natl. Acad. Sci. USA 2010, 107, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, K.; Selchow, P.; Scheckenbach, F.; Weitere, M.; Arndt, H. Cryptic species in a morphospecies complex of heterotrophic flagellates: The case study of Caecitellus spp. Acta Protozool. 2006, 45, 415–431. [Google Scholar]
- Salani, F.S.; Arndt, H.; Hausmann, K.; Nitsche, F.; Scheckenbach, F. Analysis of the community structure of abyssal kinetoplastids revealed similar communities at larger spatial scales. ISME J. 2012, 6, 713–723. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Smith, D.; Herndl, G.; van Aken, H.; Bochdansky, A. Abundance of eukaryotic microbes in the deep subtropical North Atlantic. Aquat. Microb. Ecol. 2011, 65, 103–115. [Google Scholar] [CrossRef]
- Jeuck, A.; Arndt, H. A short guide to common heterotrophic flagellates of freshwater habitats based on the morphology of living organisms. Protist 2013, 164, 842–860. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, R. Ocean Data View. 2012. Available online: http://odv.awi.de (accessed on 28 August 2015).
- Tanaka, T.; Rassoulzadegan, F. Full-depth profile (0–2000 m) of bacteria, heterotrophic nanoflagellates and ciliates in the NW Mediterranean Sea: Vertical partitioning of microbial trophic structures. Deep Sea Res. II 2002, 49, 2093–2107. [Google Scholar] [CrossRef]
- Fukuda, H.; Sohrin, R.; Nagata, T.; Koike, I. Size distribution and biomass of nanoflagellates in meso-and bathypelagic layers of the subarctic Pacific. Aquat. Microb. Ecol. 2007, 46, 203. [Google Scholar] [CrossRef]
- Paffenhöfer, G.-A. Abundance and distribution of nanoplankton in the epipelagic subtropical/tropical open Atlantic Ocean. J. Plankton Res. 2003, 25, 1535–1549. [Google Scholar] [CrossRef]
- Sherr, E.B.; Caron, D.A.; Sherr, B.F. Staining of heterotrophic protists for visualization via epifluorescence microscopy. In Handbook of Methods in Aquatic Microbial Ecology; Taylor & Francis: London, UK, 1993; pp. 213–228. [Google Scholar]
- Hondeveld, B.J.; Nieuwland, G.; Van Duyl, F.C.; Bak, R.P. Temporal and spatial variations in heterotrophic nanoflagellate abundance in North Sea sediments. Mar. Ecol. Prog. Ser. 1994, 109, 235–235. [Google Scholar] [CrossRef]
- Epstein, S.S. Simultaneous enumeration of protozoa and micrometazoa from marine sandy sediments. Aquat. Microb. Ecol. 1995, 9, 219–227. [Google Scholar] [CrossRef]
- Scherwass, A.; Wickham, S.A.; Arndt, H. Determination of the abundance of ciliates in highly turbid running waters—An improved method tested for the River Rhine. Arch. Hydrobiol. 2002, 156, 135–143. [Google Scholar] [CrossRef]
- Jeuck, A.; Nitsche, F.; Wylezich, C.; Wirth, O.; Hennemann, M.; Nopper, N.; Bergfeld, T.; Monir, S.; Scherwass, A.; Arndt, H. A comparison of some methods to quantify heterotrophic flagellates of different taxonomic groups. In Proceedings of the VII ECOP-ISOP joint meeting, Sevilla, Spain, 5–10 October 2015.
- Kubota, K. CARD-FISH for environmental microorganisms: Technical advancement and future applications. Microbes Environ. 2013, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pernthaler, A.; Pernthaler, J.; Amann, R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 2002, 68, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.I.; Ludwig, W.; Schleifer, K.-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microb. Rev. 1995, 59, 143–169. [Google Scholar]
- Beardsley, C.; Knittel, K.; Amann, R.I.; Pernthaler, J. Quantification and distinction of aplastidic and plastidic marine nanoplankton by fluorescence in situ hybridization. Aquat. Microb. Ecol. 2005, 41, 163–169. [Google Scholar] [CrossRef]
- Biegala, I.C.; Not, F.; Vaulot, D.; Simon, N. Quantitative assessment of picoeukaryotes in the natural environment by using taxon-specific oligonucleotide probes in association with tyramide signal amplification-fluorescence in situ hybridization and flow cytometry. Appl. Environ. Microbiol. 2003, 69, 5519–5529. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J.; De Long, E.F.; Olsen, G.J.; Pace, N.R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bact. 1988, 170, 720–726. [Google Scholar] [PubMed]
- Bochdansky, A.B.; Huang, L. Re-Evaluation of the EUK516 Probe for the Domain Eukarya Results in a Suitable Probe for the Detection of Kinetoplastids, an Important Group of Parasitic and Free-Living Flagellates. J. Eukaryot. Microbiol. 2010, 57, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.J.; Lee, W.J. Geographic distribution and diversity of free-living heterotrophic flagellates. In The Flagellates - Unity, Diversity and Evolution; Taylor & Francis Ltd: London, UK, 2000; pp. 269–287. [Google Scholar]
- Butler, H.; Rogerson, A. Temporal and spatial abundance of naked amoebae (Gymnamoebae) in marine benthic sediments of the Clyde Sea Area, Scotland. J. Eukaryot. Microbiol. 1995, 42, 724–730. [Google Scholar] [CrossRef]
- Weber, A.A.-T.; Pawlowski, J. Can abundance of protists be inferred from sequence data: A case study of foraminifera. PLoS ONE 2013, 8, e56739. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimi, E.M.; Novati, S.; Bruno, R.; Bandi, C.; Mulatto, P.; Giannico, R.; Casiraghi, M.; Ferri, E. Errors in ribosomal sequence datasets generated using PCR-coupled “panbacterial” pyrosequencing, and the establishment of an improved approach. Mol. Cell. Probes 2013, 27, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Massana, R.; Not, F.; Marie, D.; Vaulot, D. Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol. 2005, 52, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Potvin, M.; Lovejoy, C. PCR-based diversity estimates of artificial and environmental 18S RNA gene libraries. J. Eukaryot. Microbiol. 2009, 56, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Kilias, E.S.; Metfies, K. Evaluating the potential of 18S rDNA clone libraries to complement pyrosequencing data of marine protists with near full-length sequence information. Mar. Biol. Res. 2014, 10, 771–780. [Google Scholar] [CrossRef]
- Dell’Anno, A. Extracellular DNA plays a key role in deep-sea ecosystem functioning. Science 2005, 309, 2179–2179. [Google Scholar] [CrossRef] [PubMed]
- Torti, A.; Lever, M.A.; Jørgensen, B.B. Origin, dynamics, and implications of extracellular DNA pools in marine sediments. Mar. Genom. 2015, 24, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, T.; Zuendorf, A.; Breiner, H.-W.; Behnke, A. A molecular approach to identify active microbes in environmental eukaryote clone libraries. Microb. Ecol. 2007, 53, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Not, F.; Gausling, R.; Azam, F.; Heidelberg, J.F.; Worden, A.Z. Vertical distribution of picoeukaryotic diversity in the Sargasso Sea. Environ. Microbiol. 2007, 9, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Stecher, A.; Neuhaus, S.; Lange, B.; Frickenhaus, S.; Beszteri, B.; Kroth, P.G.; Valentin, K. rRNA and rDNA based assessment of sea ice protist biodiversity from the central Arctic Ocean. Eur. J. Phycol. 2016, 51. [Google Scholar] [CrossRef]
- Logares, R.; Audic, S.; Bass, D.; Bittner, L.; Boutte, C.; Christen, R.; Claverie, J.-M.; Decelle, J.; Dolan, J.R.; Dunthorn, M.; et al. Patterns of rare and abundant marine microbial eukaryotes. Curr. Biol. 2014, 24, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Schoenle, A.; Werner, J.; Nitsche, F.; Arndt, H. Ciliates in the abyss: Occurrence and survival at high hydrostatic pressures. University of Cologne, Cologne, Germany, Unpublished work. 2015. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoenle, A.; Jeuck, A.; Nitsche, F.; Venter, P.; Prausse, D.; Arndt, H. Methodological Studies on Estimates of Abundance and Diversity of Heterotrophic Flagellates from the Deep-Sea Floor. J. Mar. Sci. Eng. 2016, 4, 22. https://doi.org/10.3390/jmse4010022
Schoenle A, Jeuck A, Nitsche F, Venter P, Prausse D, Arndt H. Methodological Studies on Estimates of Abundance and Diversity of Heterotrophic Flagellates from the Deep-Sea Floor. Journal of Marine Science and Engineering. 2016; 4(1):22. https://doi.org/10.3390/jmse4010022
Chicago/Turabian StyleSchoenle, Alexandra, Alexandra Jeuck, Frank Nitsche, Paul Venter, Dennis Prausse, and Hartmut Arndt. 2016. "Methodological Studies on Estimates of Abundance and Diversity of Heterotrophic Flagellates from the Deep-Sea Floor" Journal of Marine Science and Engineering 4, no. 1: 22. https://doi.org/10.3390/jmse4010022
APA StyleSchoenle, A., Jeuck, A., Nitsche, F., Venter, P., Prausse, D., & Arndt, H. (2016). Methodological Studies on Estimates of Abundance and Diversity of Heterotrophic Flagellates from the Deep-Sea Floor. Journal of Marine Science and Engineering, 4(1), 22. https://doi.org/10.3390/jmse4010022




