Diversity and Distribution of Deep-Sea Fishes off the Emperor Seamounts, Northwestern Pacific Ocean, with DNA Barcodes, Phylogenetic, and Biogeographic Considerations
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographic Coverage
2.2. Sampling Conditions
2.3. Morphology
2.4. Faunistic Analysis
2.5. Molecular Analysis
3. Results
3.1. Family Etmopteridae
3.1.1. Centroscyllium excelsum Shirai et Nakaya, 1990 (Figure 2A)
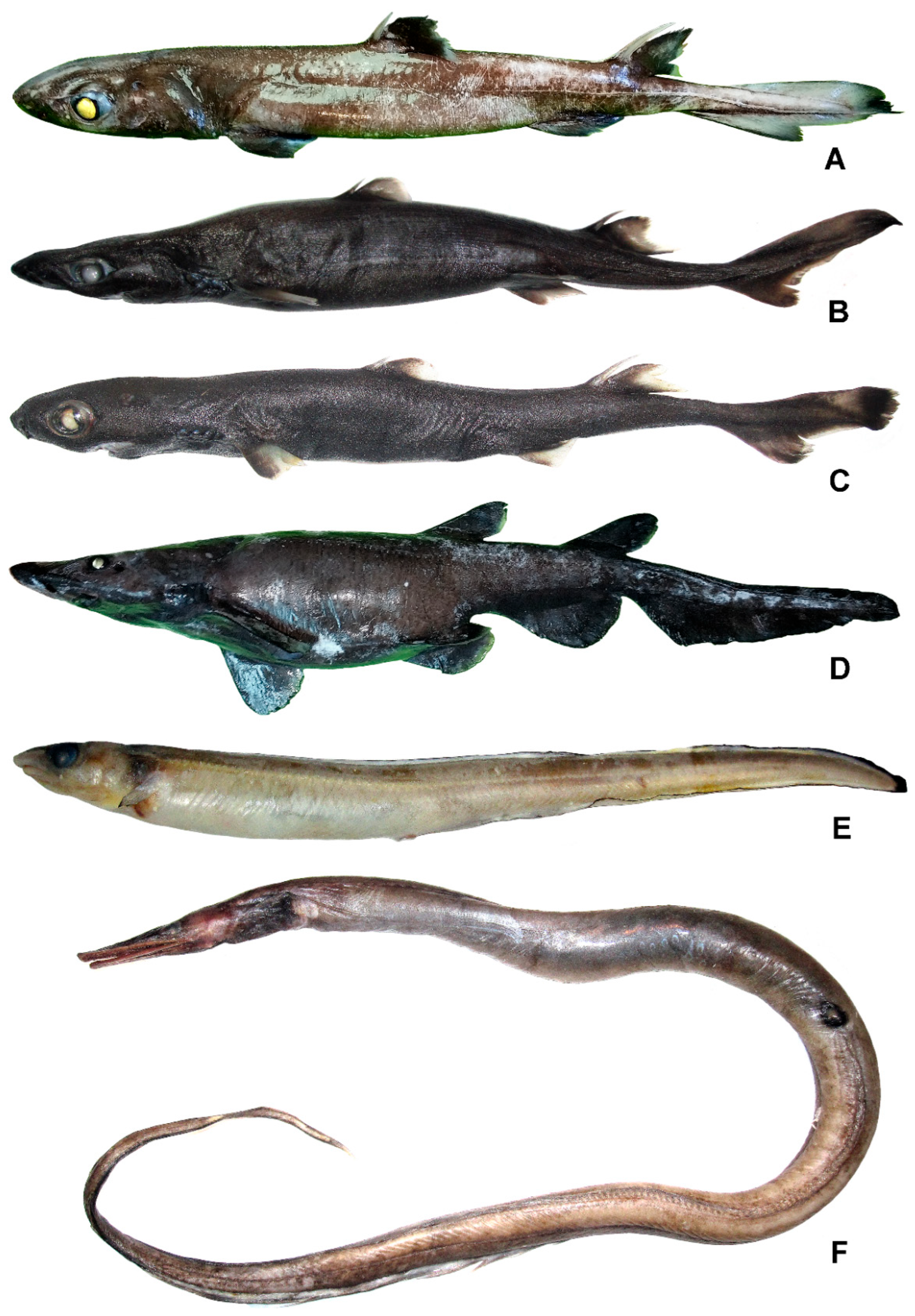
3.1.2. Etmopterus cf. lailae Ebert, Papastamatiou, Kajiura et Wetherbee, 2017 (Figure 2B)
3.1.3. Etmopterus pusillus (Lowe, 1839) (Figure 2C)
3.2. Family Pentanchidae
Apristurus fedorovi Dolganov, 1983 (Figure 2D)
3.3. Family Congridae
Gnathophis johnsoni Prokofiev, Frable, Emelianova, Orlova (Saveleva) et Orlov, 2025 (Figure 2E)
3.4. Family Nettastomatidae
Nettastoma parviceps Günther, 1877 (Figure 2F)
3.5. Family Synaphobranchidae
3.5.1. Meadia abyssalis (Kamohara, 1938) (Figure 3A)
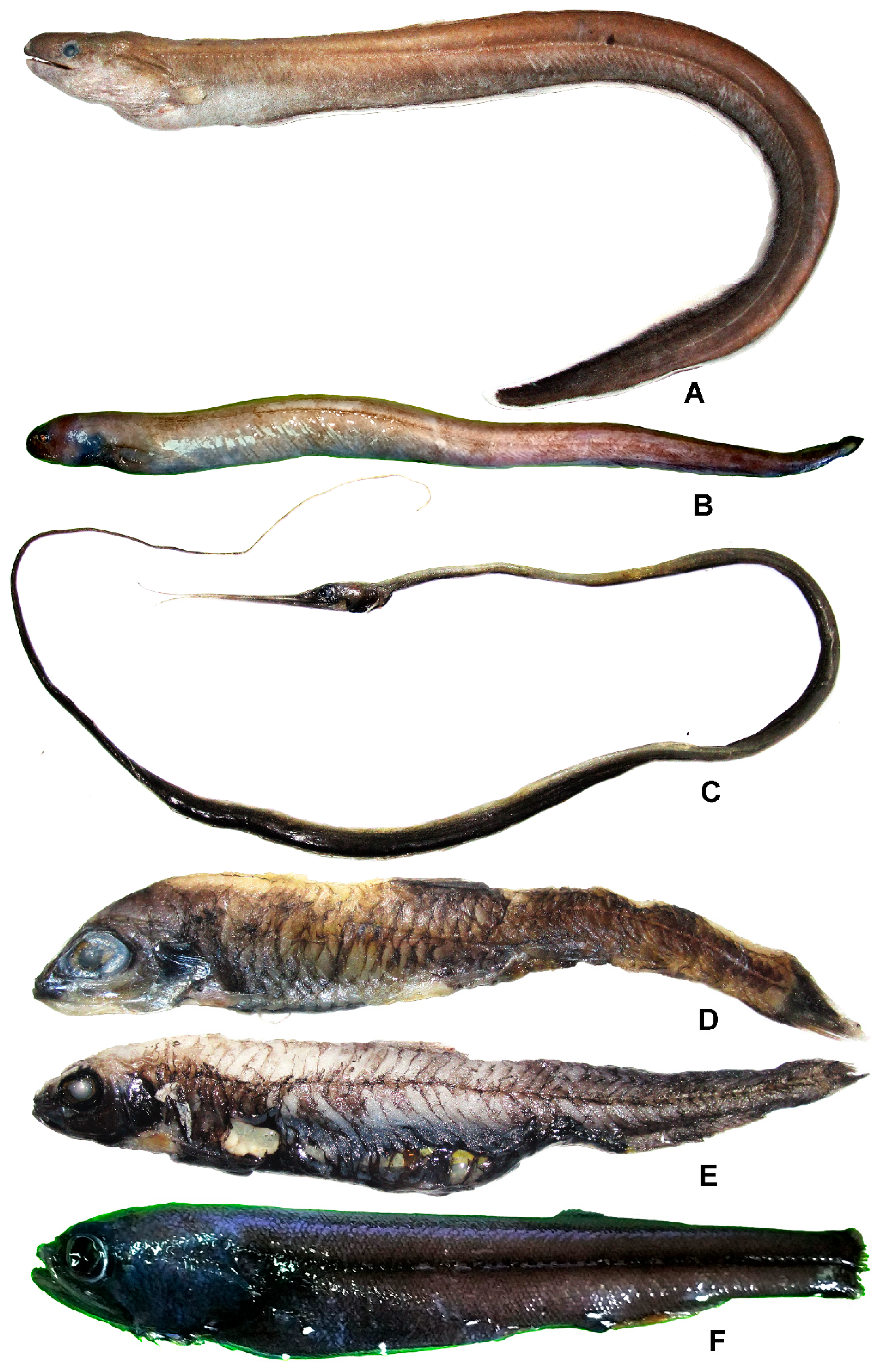
3.5.2. Simenchelys parasitica Gill, 1879 (Figure 3B)
3.6. Family Nemichthyidae
Nemichthys scolopaceus Richardson, 1848 (Figure 3C)
3.7. Family Bathylagidae
3.7.1. Lipolagus ochotensis (Schmidt, 1938) (Figure 3D)
3.7.2. Melanolagus bericoides (Borodin, 1929) (Figure 3E)
3.8. Family Platytroctidae
Sagamichthys abei Parr, 1953 (Figure 3F)
3.9. Family Gonostomatidae
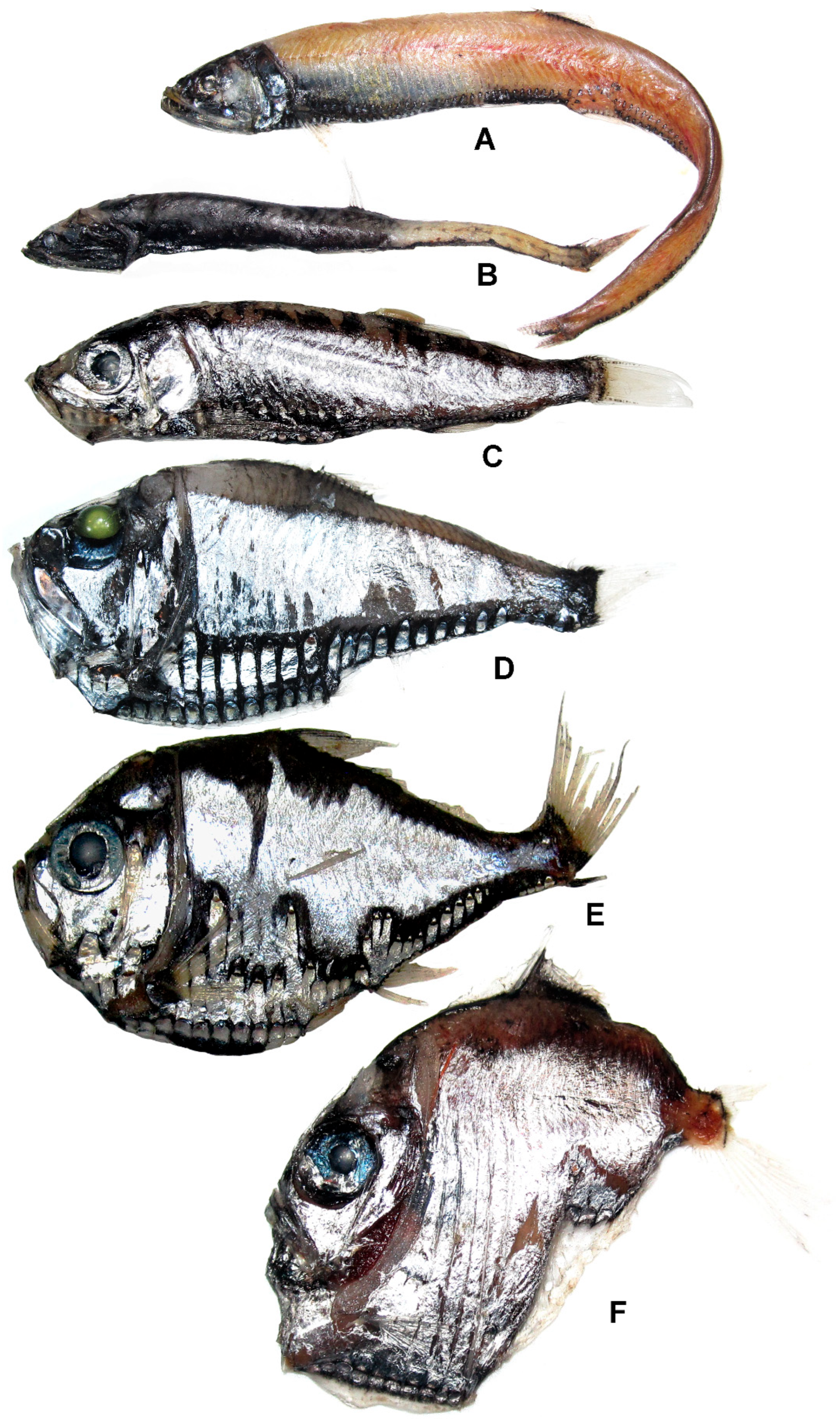
3.9.1. Diplophos orientalis Matsubara, 1940 (Figure 4A)
3.9.2. Sigmops elongatus (Günther, 1878)
3.9.3. Sigmops gracilis (Günther, 1878)
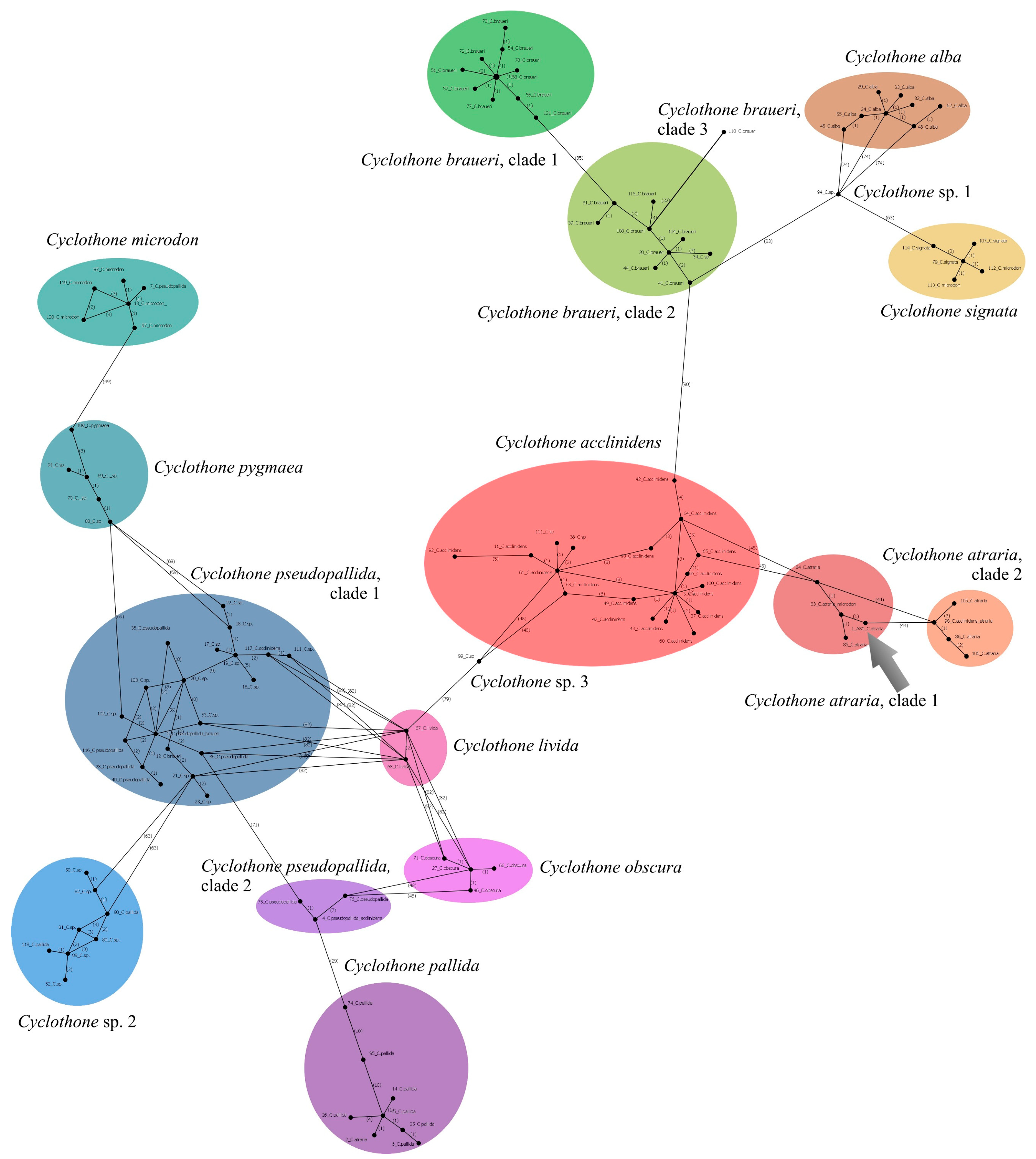
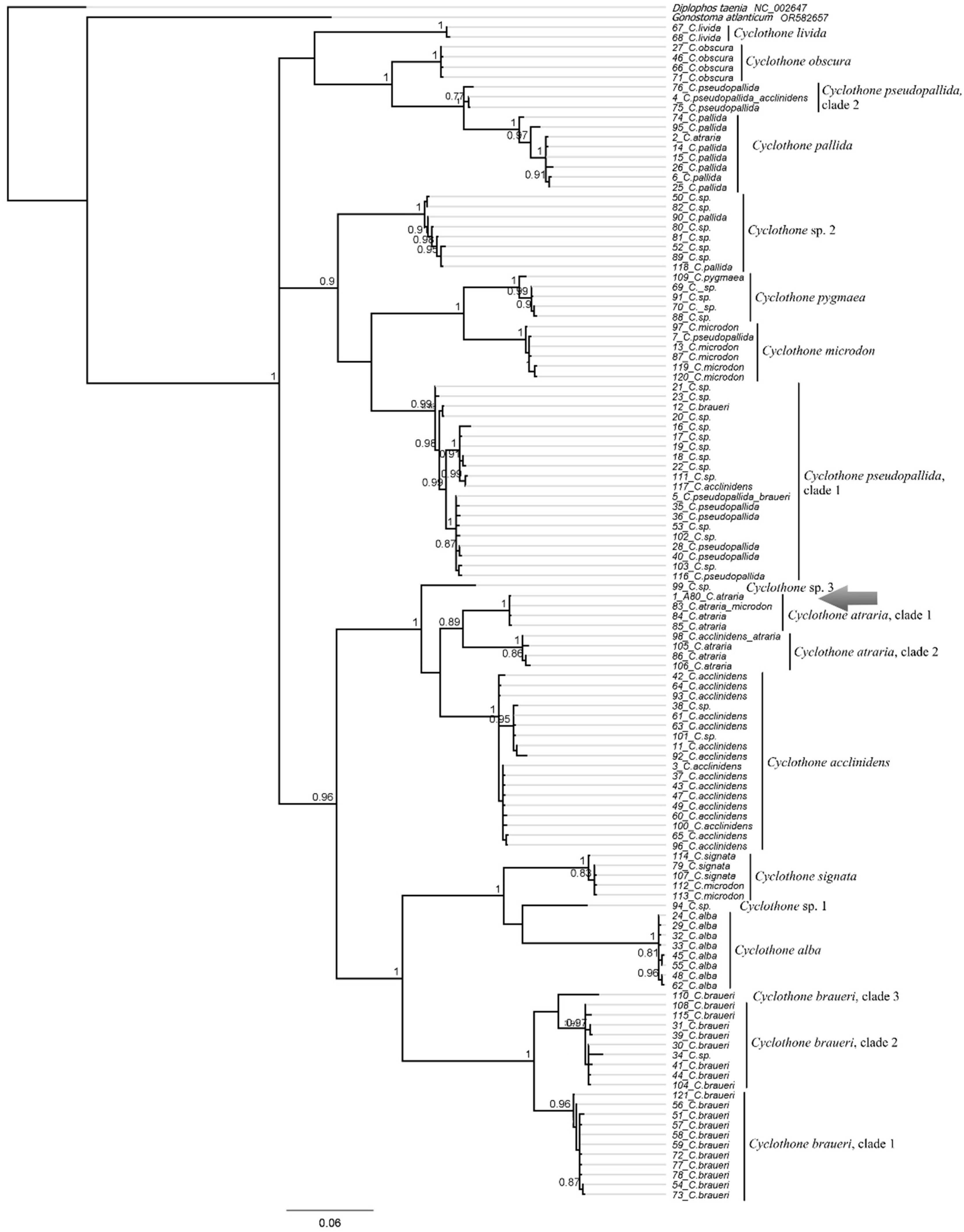
3.9.4. Cyclothone atraria Gilbert, 1905 (Figure 4B)
3.10. Family Sternoptychidae
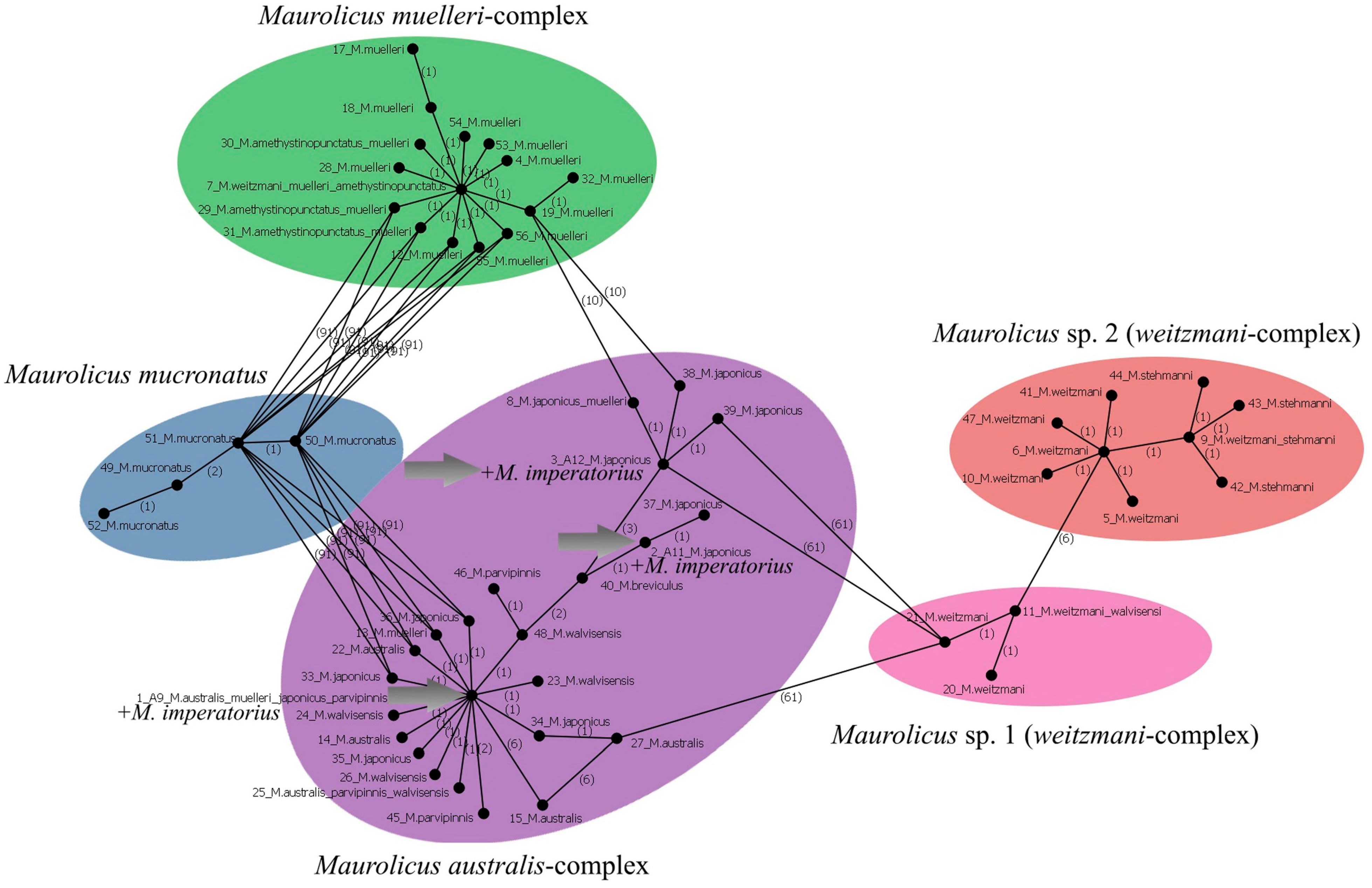
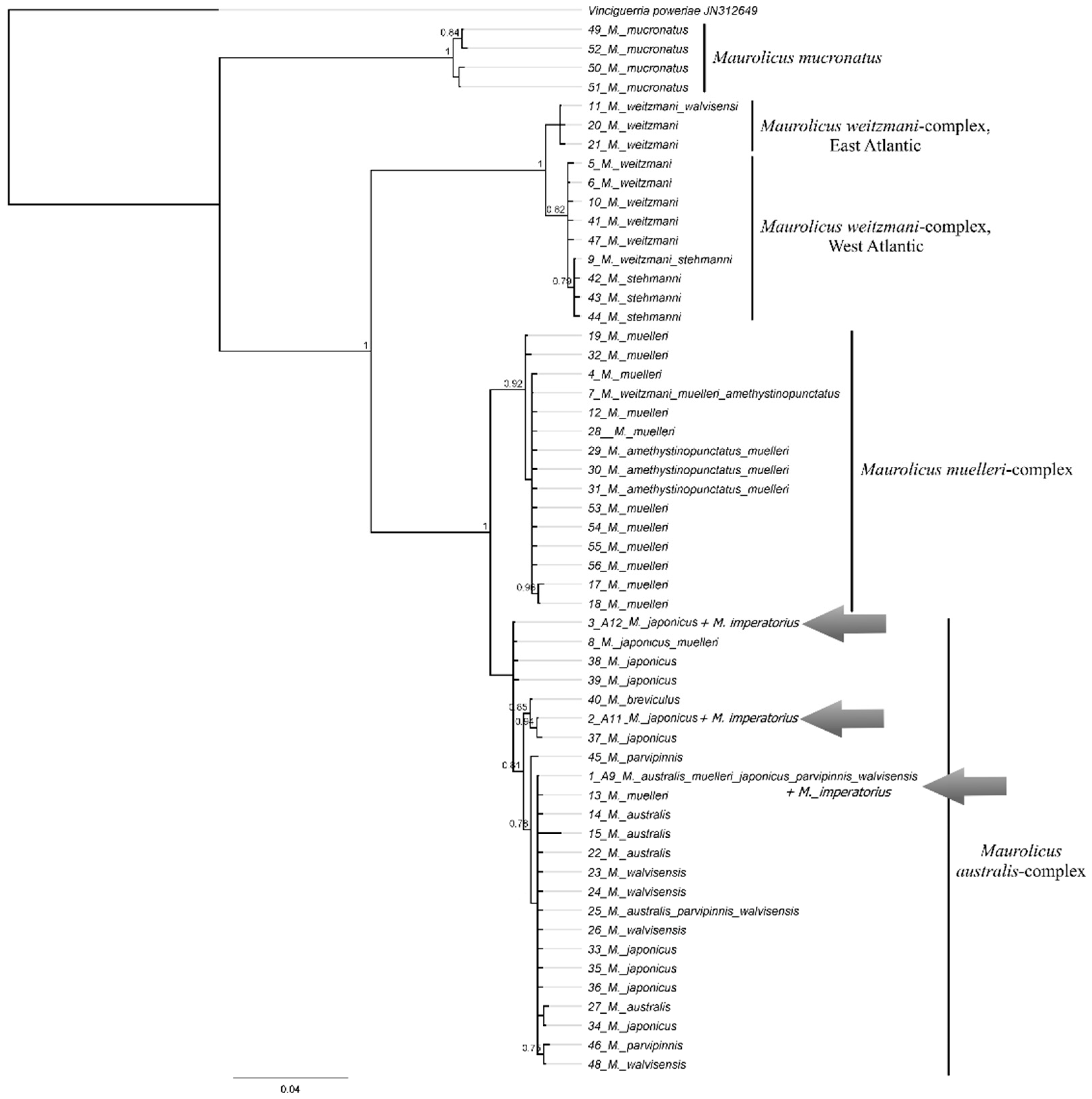
3.10.1. Maurolicus imperatorius Parin et Kobyliansky, 1993 (Figure 4C)
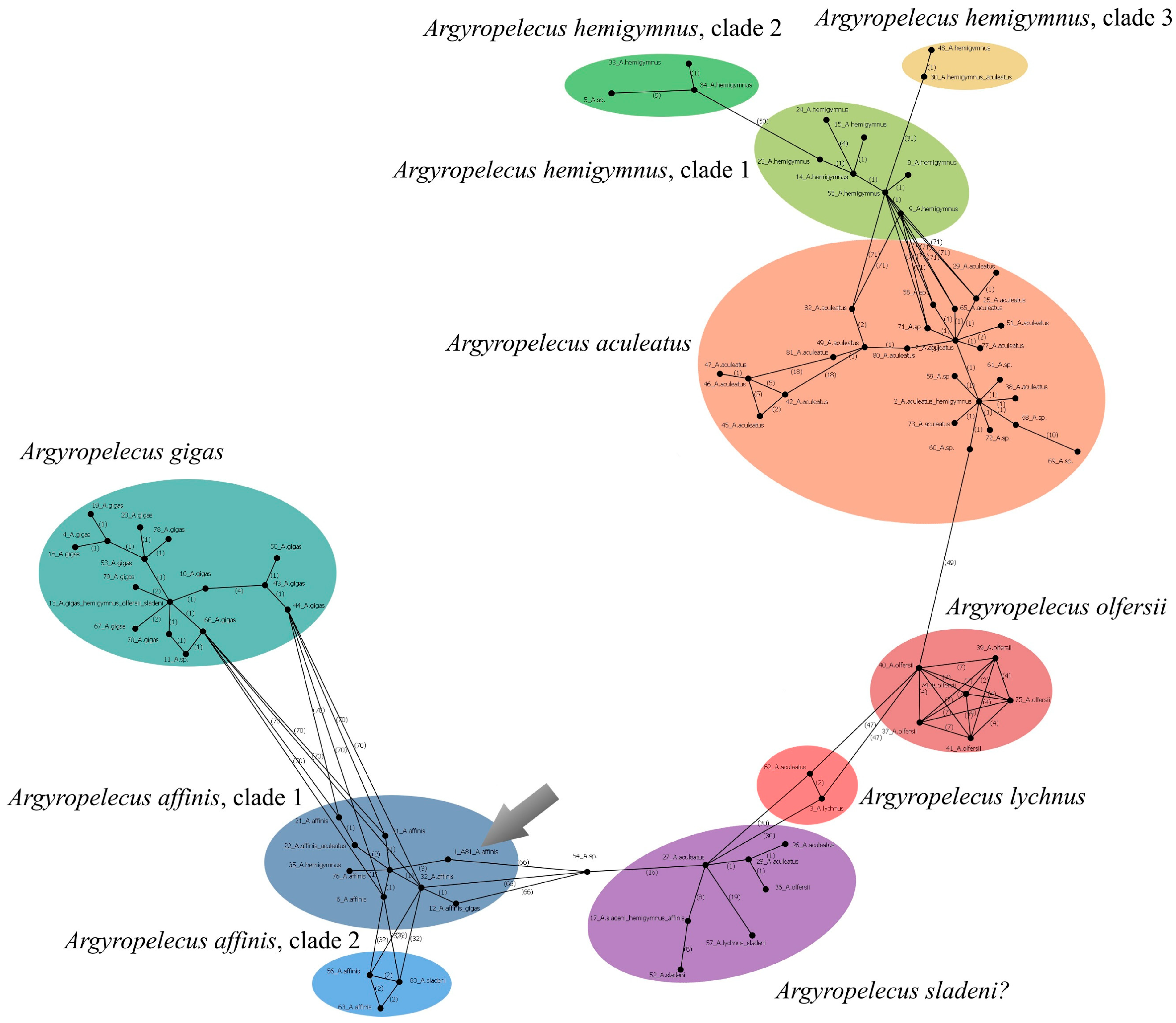
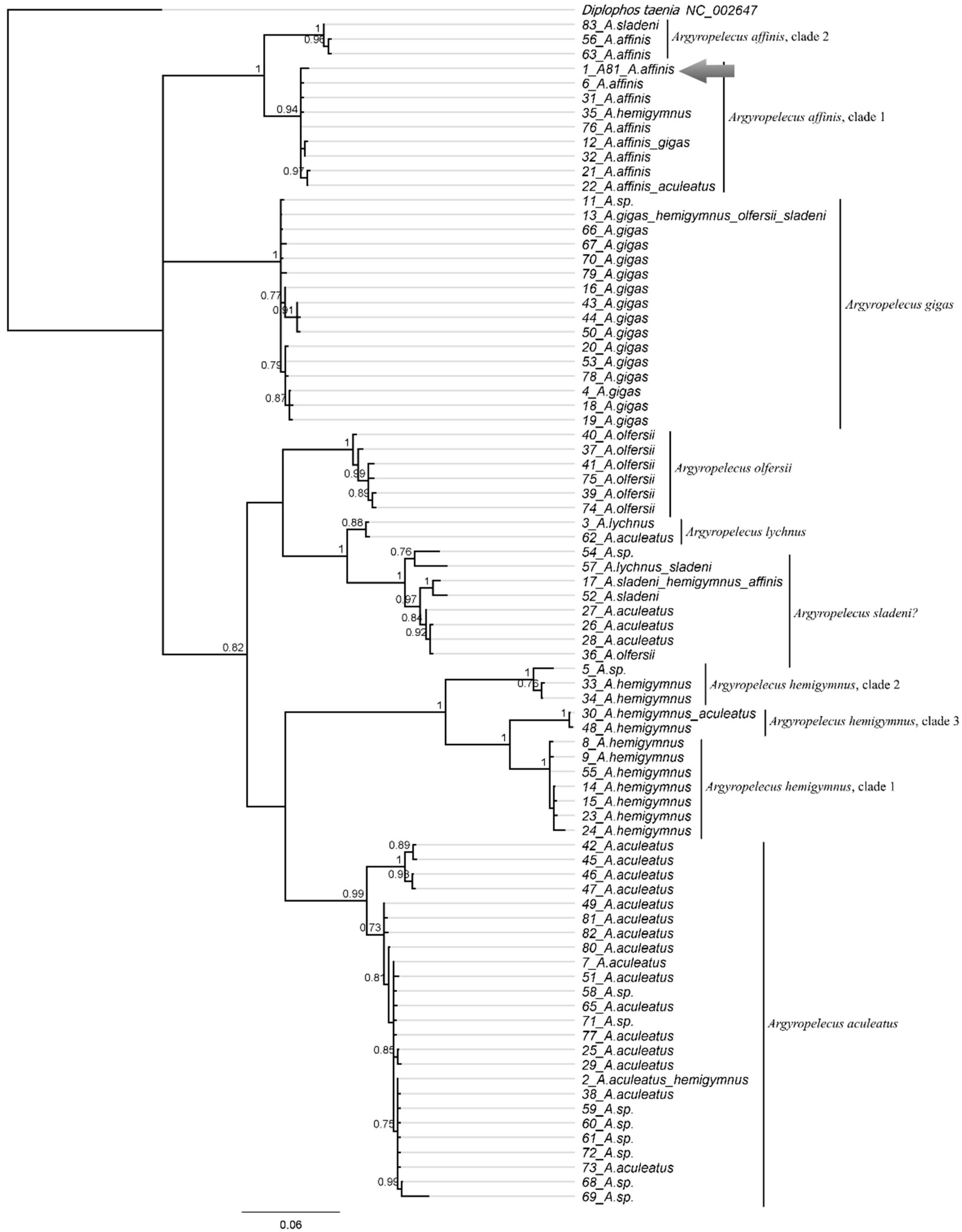
3.10.2. Argyropelecus affinis Garman, 1899 (Figure 4D)
3.10.3. Argyropelecus sladeni Regan, 1908
3.10.4. Polyipnus matsubarai Schultz, 1961 (Figure 4E)
3.10.5. Sternoptyx diaphana Hermann, 1781 (Figure 4F)
3.11. Family Phosichthyidae
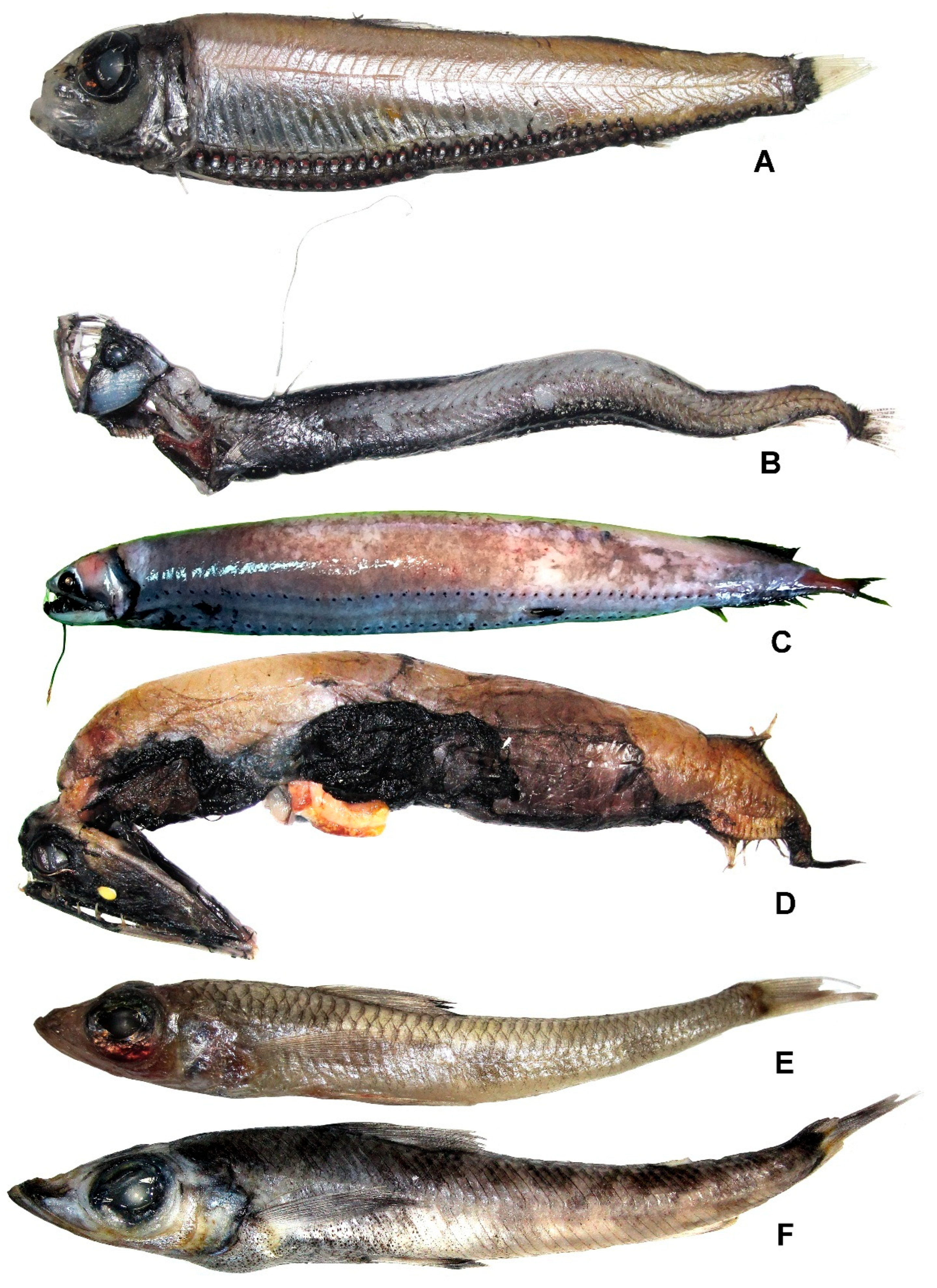
Ichthyococcus elongatus Imai, 1941 (Figure 11A)
3.12. Family Stomiidae
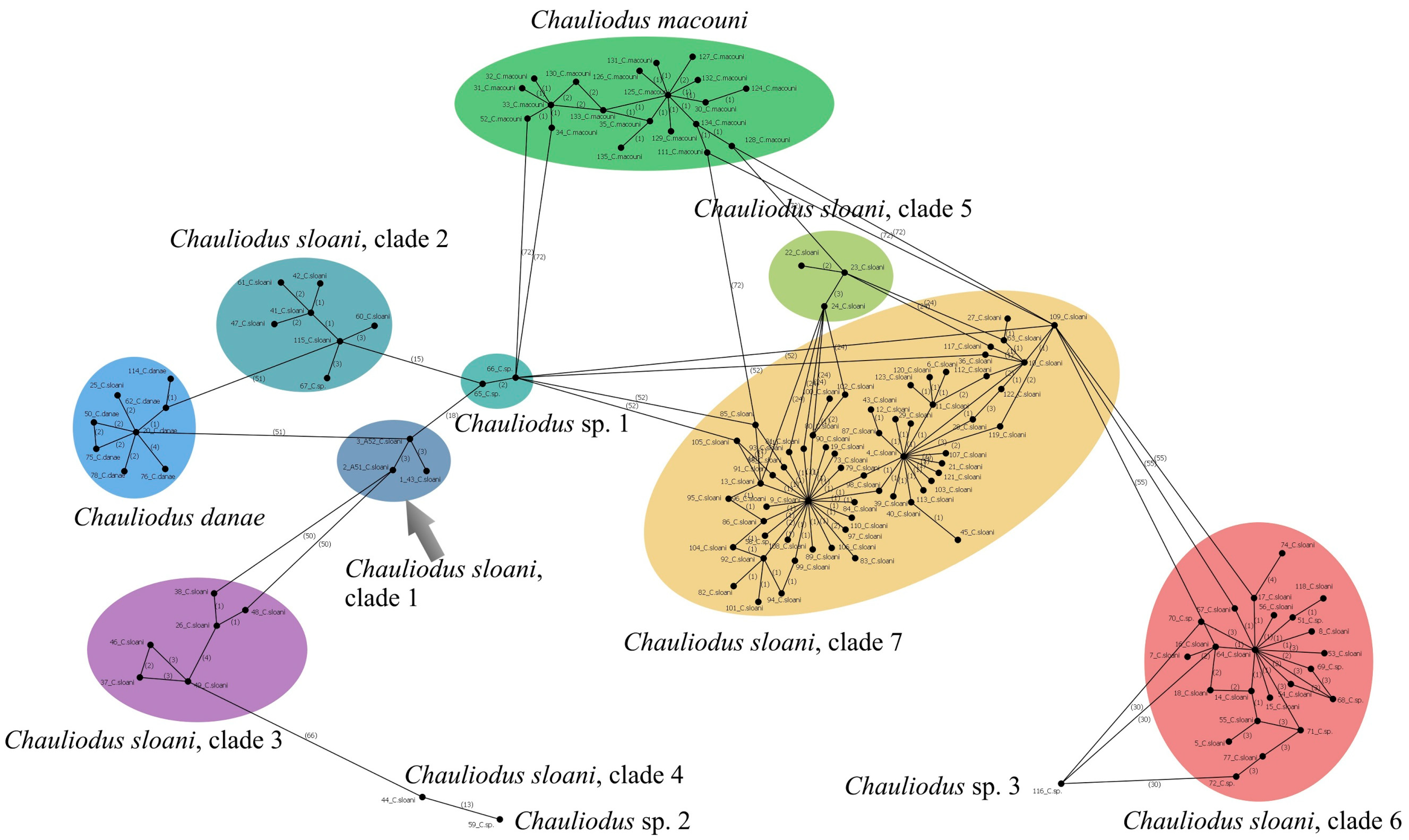
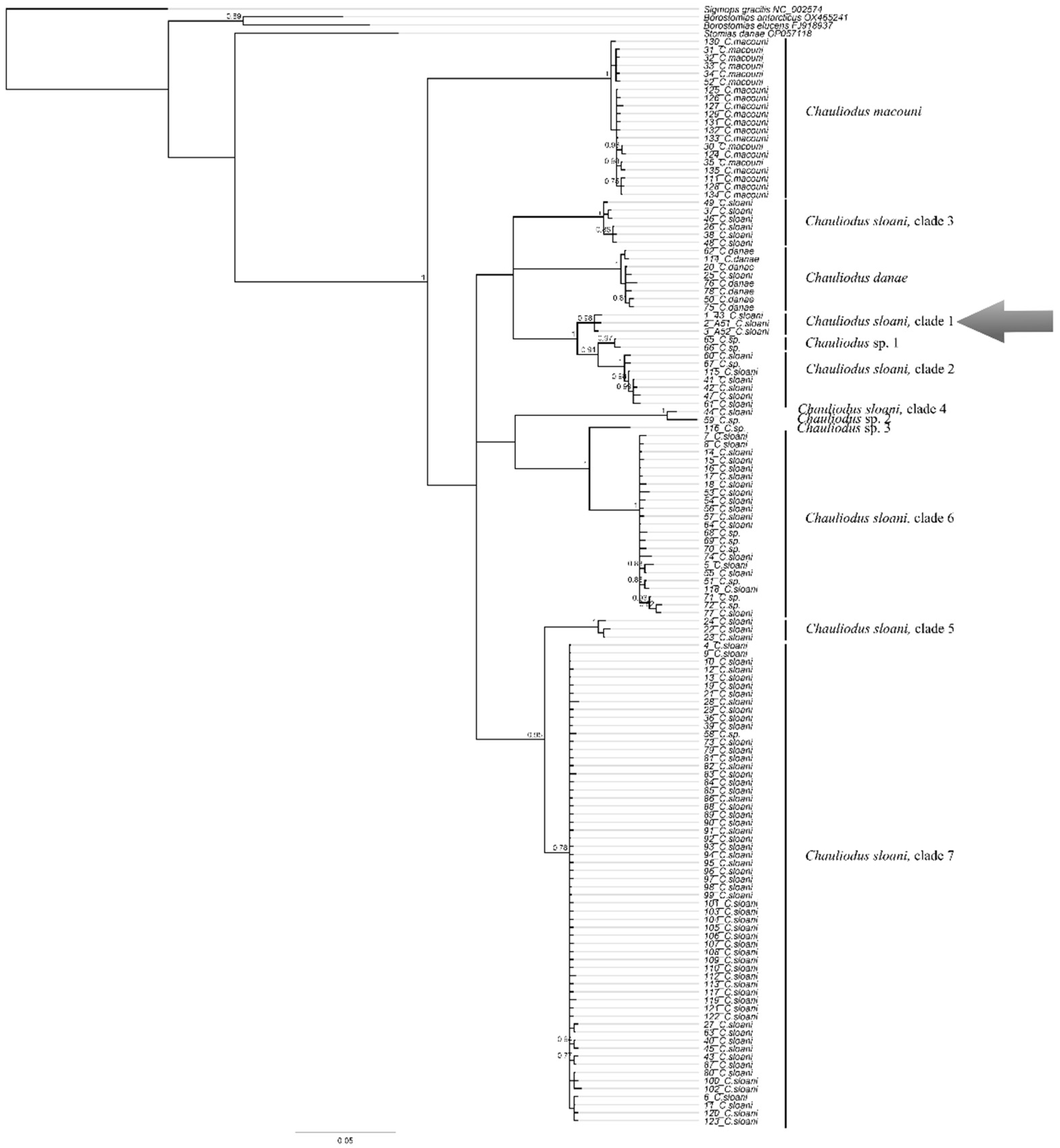
3.12.1. Chauliodus gr. sloani Bloch et Schneider, 1801 (Figure 11B)
3.12.2. Eustomias (Biradiostomias) securicula Prokofiev et Orlov, 2022
3.12.3. Melanostomias valdiviae Brauer, 1902
3.12.4. Opostomias mitsuii Imai, 1941 (Figure 11C)
3.12.5. Idiacanthus antrostomus Gilbert, 1890
3.12.6. Malacosteus niger Ayres, 1848 (Figure 11D)
3.13. Family Paraulopidae
Paraulopus filamentosus (Okamura, 1982) (Figure 11E)
3.14. Family Chlorophthalmidae
Chlorophthalmus imperator Fujiwara, Wada et Motomura, 2019 (Figure 11F)
3.15. Family Scopelarchidae
Benthalbella infans Zugmayer, 1911 (Figure 14A)
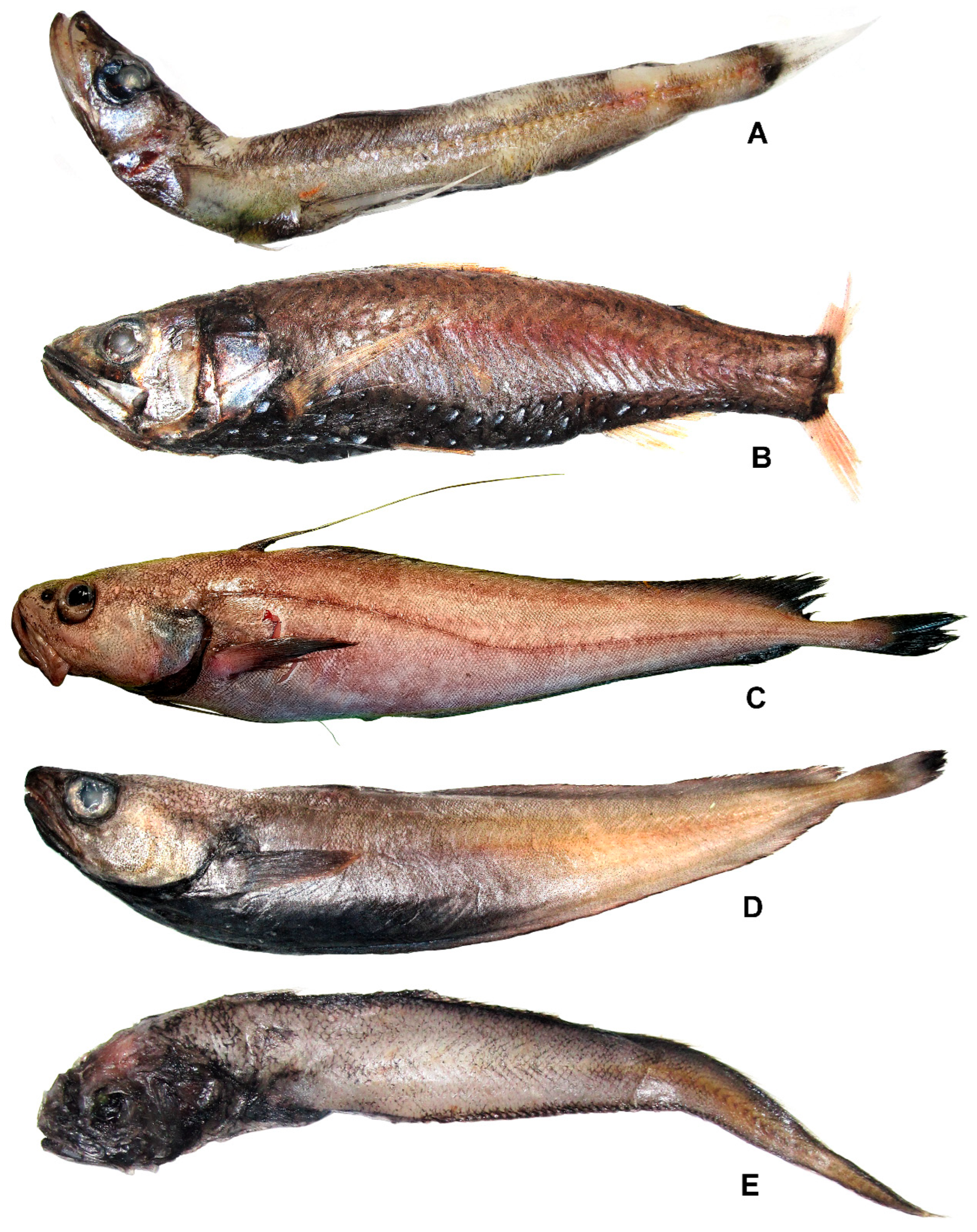
3.16. Family Neoscopelidae
Neoscopelus cf. macrolepidotus Johnson, 1863 (Figure 14B)
3.17. Family Myctophidae
3.18. Family Moridae
3.18.1. Lepidion inosimae (Günther, 1887) (Figure 14C)
3.18.2. Physiculus cynodon Sazonov, 1986 (Figure 14D)
3.19. Family Melanonidae
Melanonus zugmayeri Norman, 1930 (Figure 14E)
3.20. Family Macrouridae
3.20.1. Bathygadus antrodes (Jordan et Gilbert, 1904)
3.20.2. Coelorinchus matsubarai Okamura, 1982 (Figure 17A)
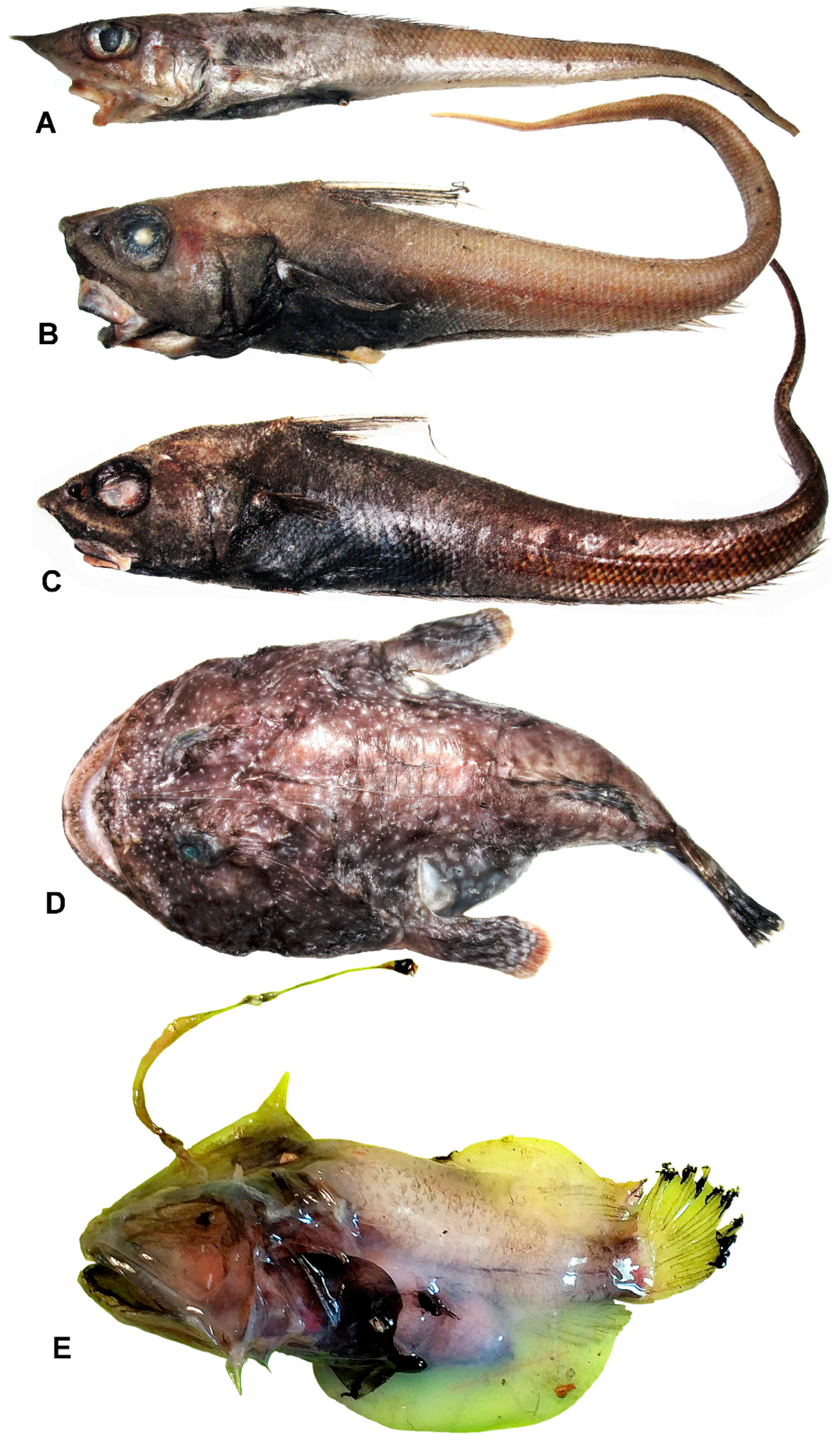
3.20.3. Nezumia obliquata (Gilbert, 1905) (Figure 17B)
3.20.4. Nezumia cf. proxima (Smith et Radcliffe, 1912) (Figure 17C)
3.20.5. Squalogadus modificatus Gilbert et Hubbs, 1916
3.21. Family Lophiidae
Lophiodes bruchius Caruso, 1981 (Figure 17D)
3.22. Family Oneirodidae
Bertella idiomorpha Pietsch, 1973 (Figure 17E)
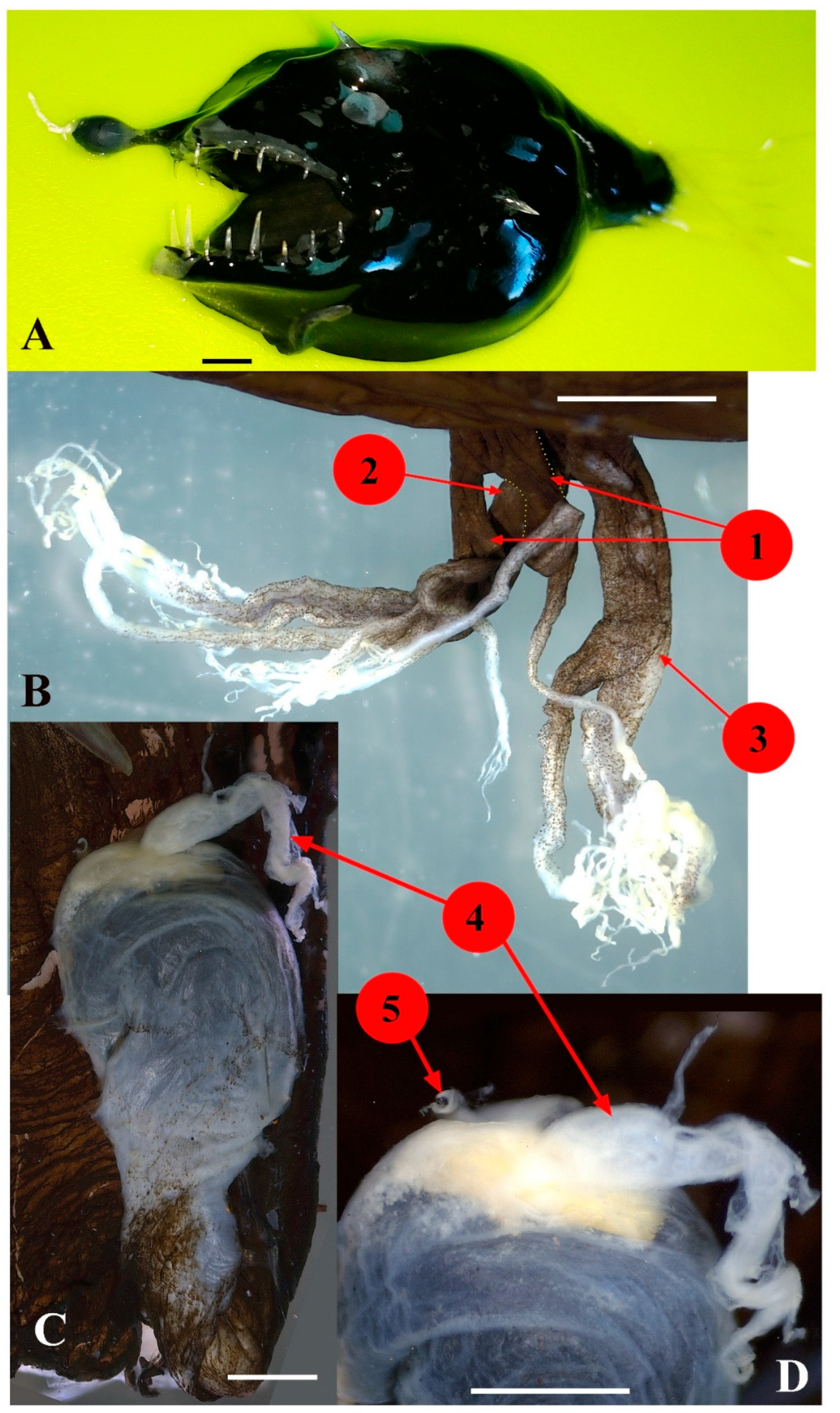
3.23. Family Linophrynidae
Linophryne arborifera Regan, 1925 (Figure 18)
3.24. Family Centriscidae
Macroramphosus gracilis (Lowe, 1839) (Figure 19A)
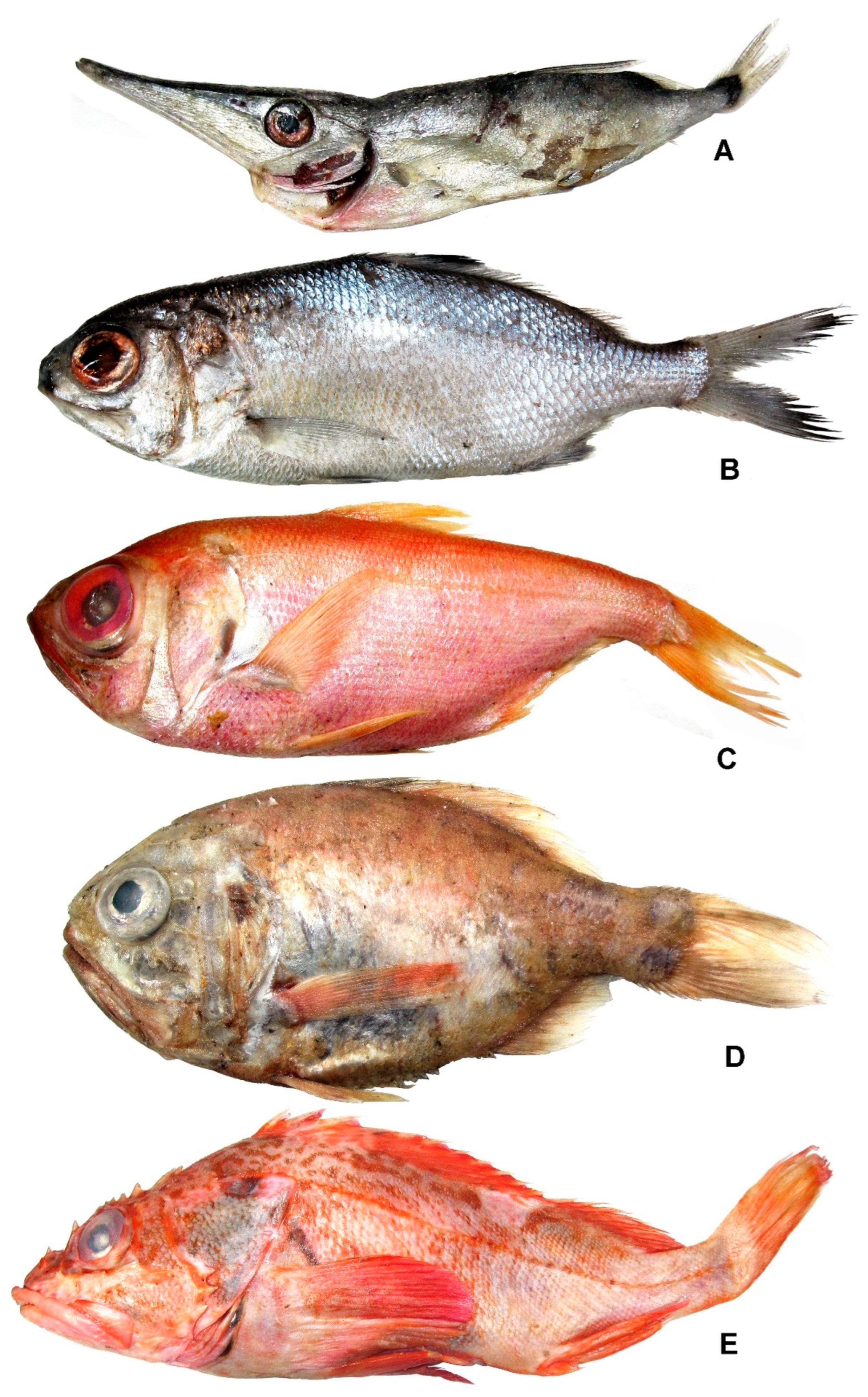
3.25. Family Polymixiidae
Polymixia sp. (Figure 19B)
3.26. Family Melamphaidae
Scopeloberyx malayanus (Weber, 1913)
3.27. Family Berycidae
Beryx splendens Lowe, 1834 (Figure 19C)
3.28. Family Trachichthyidae
Hoplostethus crassispinus Kotlyar, 1980 (Figure 19D)
3.29. Family Sebastidae
3.29.1. Adelosebastes latens Eschmeyer, Abe et Nakano, 1979 (Figure 19E)
3.29.2. Helicolenus avius Abe et Eschmeyer, 1972 (Figure 20A)
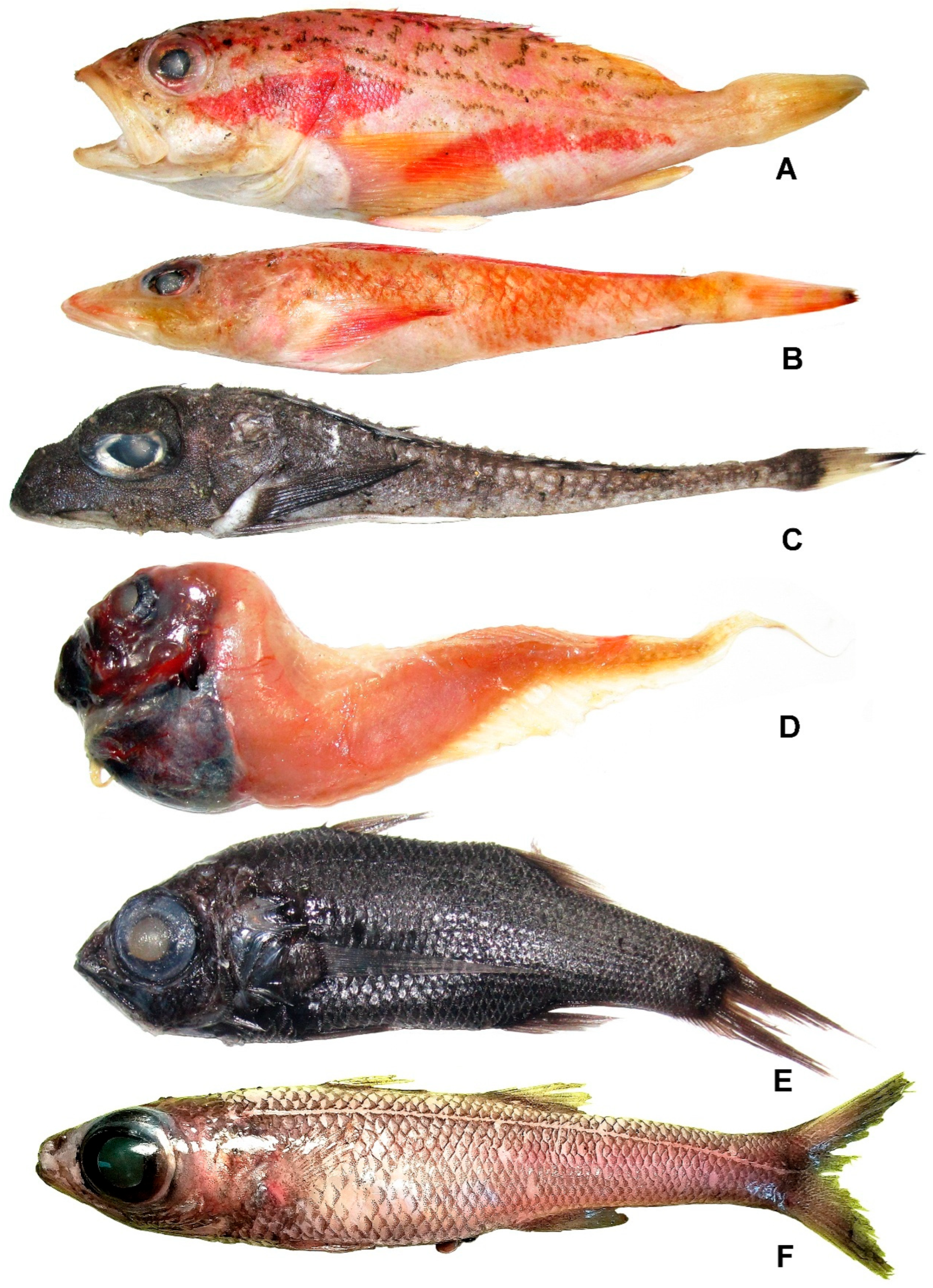
3.30. Family Plectrogeniidae
Bembradium roseum Gilbert, 1905 (Figure 20B)
3.31. Family Rhamphocottidae
Marukawichthys pacificus Yabe, 1983 (Figure 20C)
3.32. Family Liparidae
Psednos kaganovskii Prokofiev et Orlov, 2025 (Figure 20D)
3.33. Family Howellidae
Howella parini Fedoryako, 1976 (Figure 20E)
3.34. Family Epigonidae
Epigonus denticulatus Dieuzeide, 1950 (Figure 20F)
3.35. Family Pentacerotidae
Pentaceros wheeleri (Hardy, 1983) (Figure 23A)
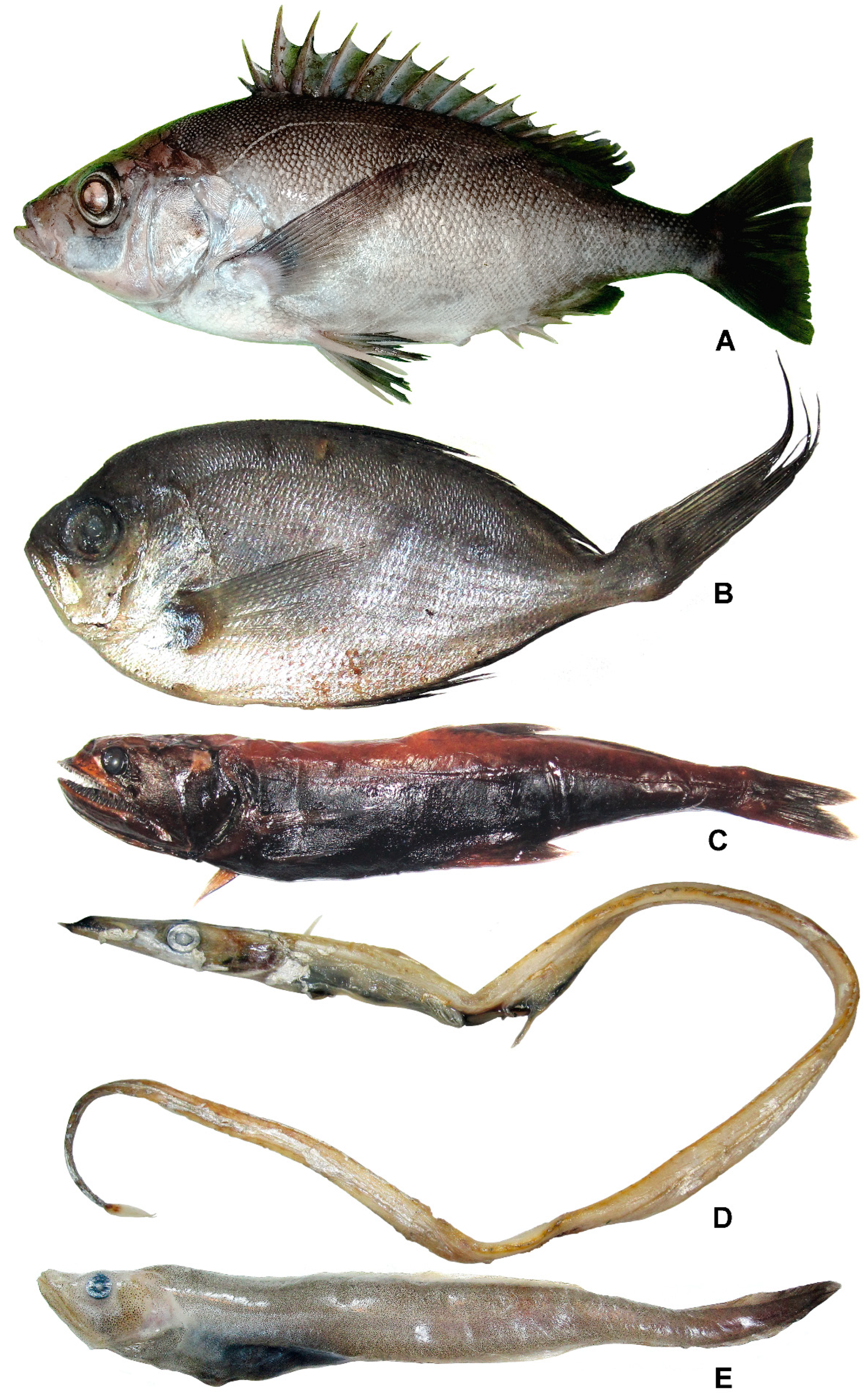
3.36. Family Bramidae
Brama japonica Hilgendorf, 1878 (Figure 23B)
3.37. Family Chiasmodontidae
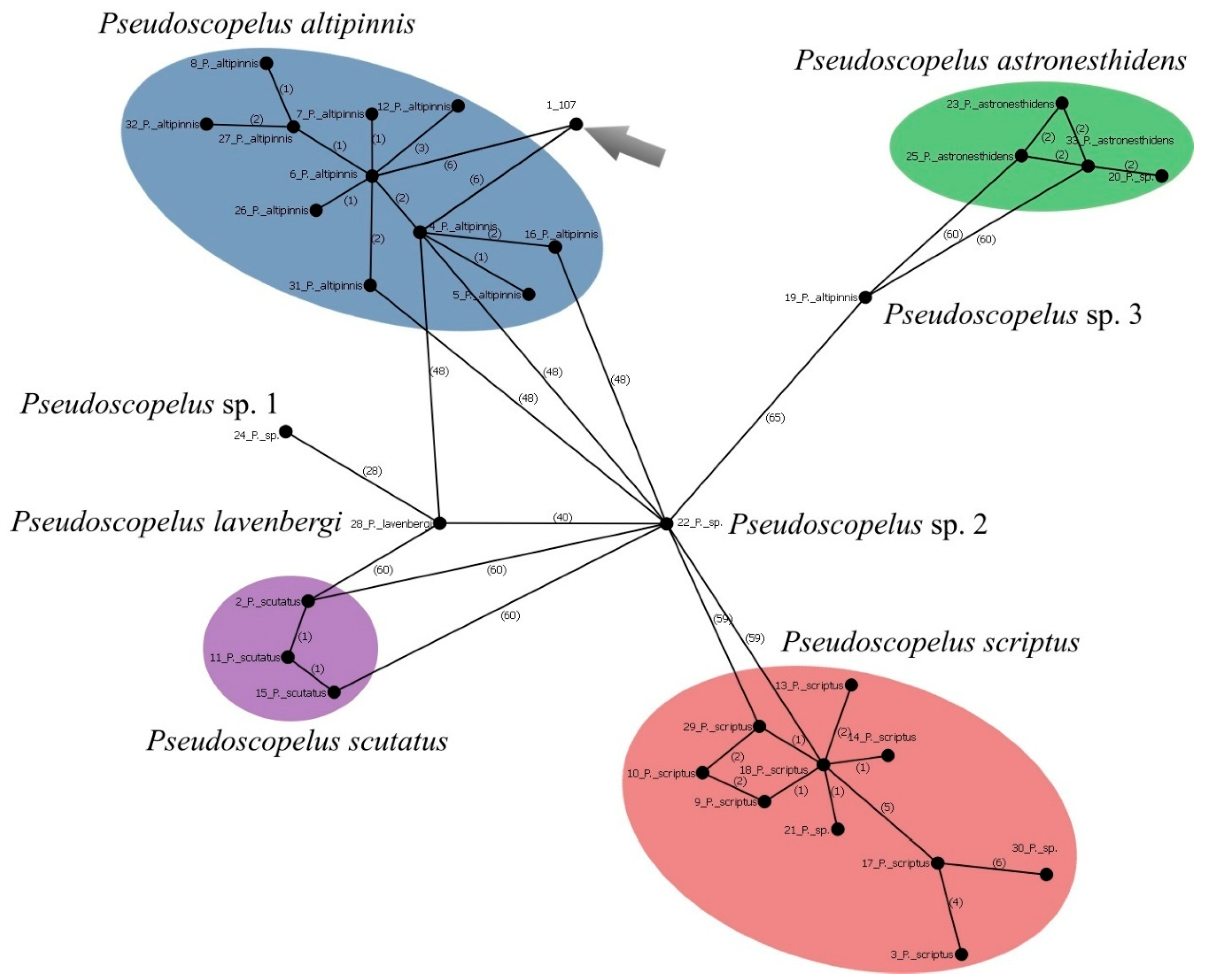
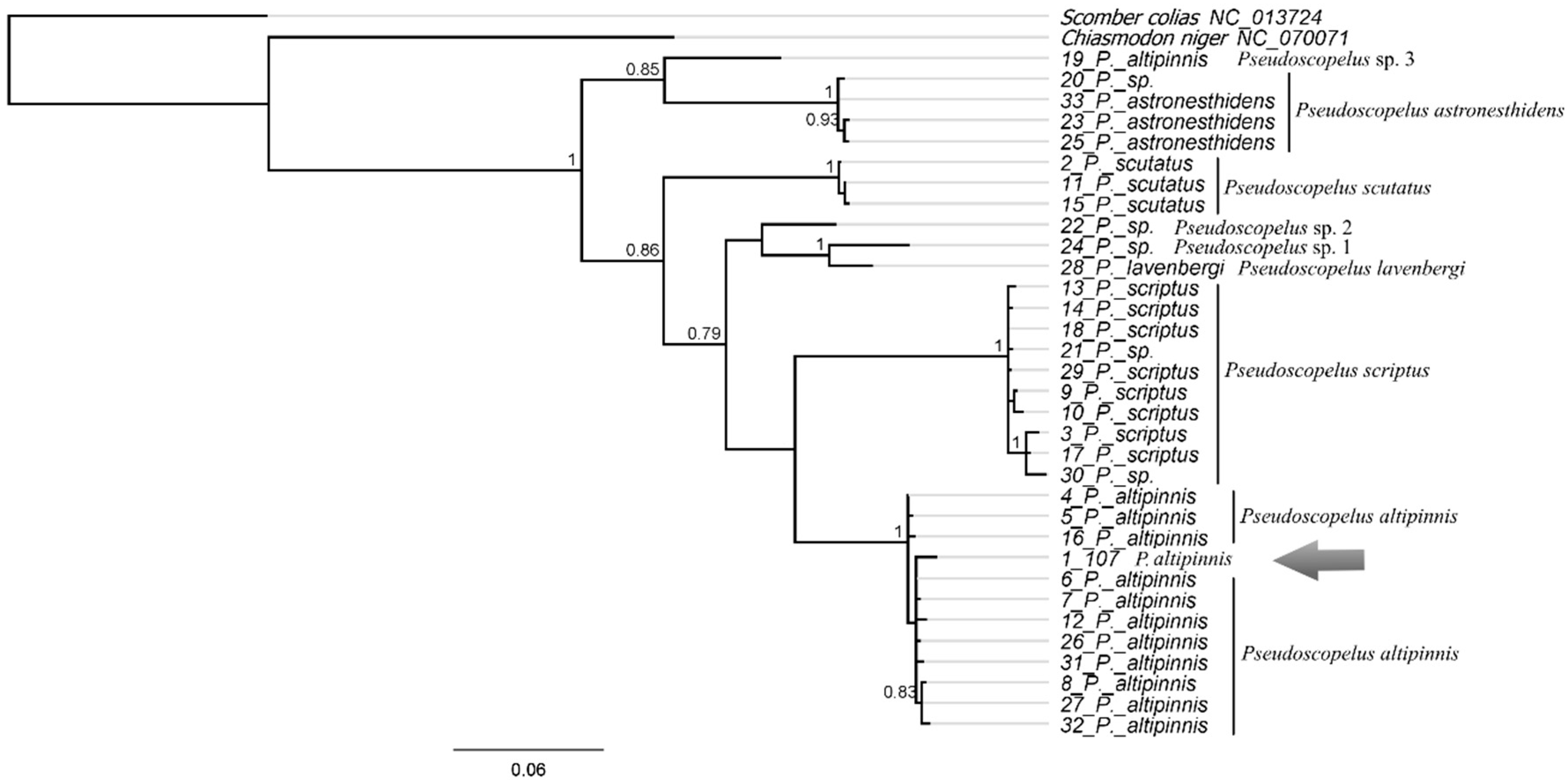
Pseudoscopelus altipinnis Parr, 1933 (Figure 23C)
3.38. Family Trichiuridae
Benthodesmus pacificus Parin et Becker, 1970 (Figure 23D)
3.39. Family Zoarcidae
Lycodapus imperatorius Prokofiev, Balanov, Emelianova, Orlov et Orlova, 2022 (Figure 23E)
3.40. Family Pleuronectidae
3.40.1. Microstomus bathybius (Gilbert, 1890) (Figure 26A)
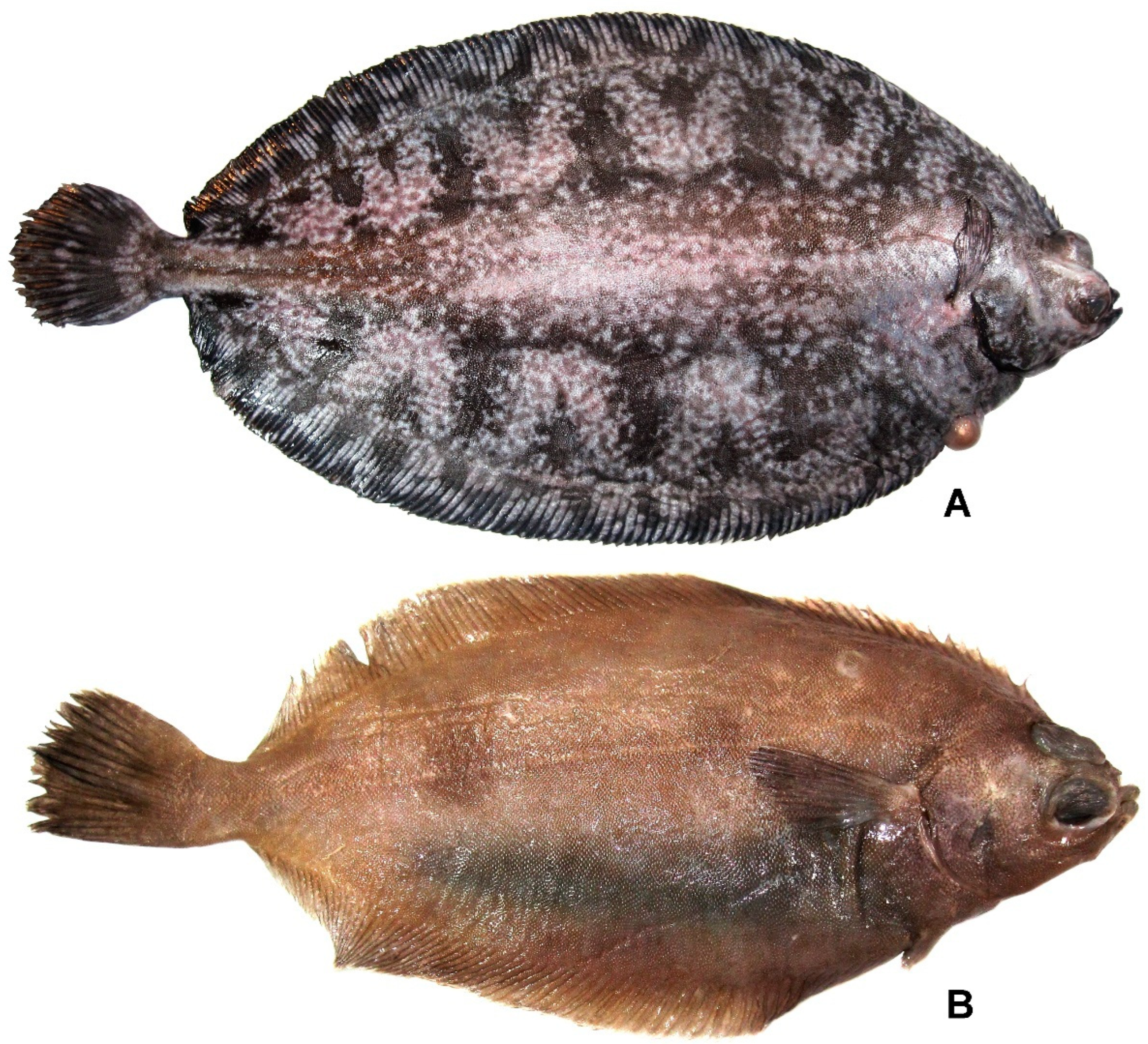
3.40.2. Microstomus shuntovi Borets, 1983 (Figure 26B)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, Y.; Sasaki, T. Trawl Fishery in the Central North Pacific Seamounts; Division of Northern Waters Groundfish Resources, Far Seas Fisheries Research Laboratory: Shizuoka, Japan, 1977; pp. 1–45.
- Humphreys, R.L., Jr.; Tagami, D.T.; Seki, M.P. Seamount fishery resources within the southern Emperor-Northern Hawaiian Ridge. In Proceedings of Second Symposium on Resources Investigations in the Northwestern Hawaiian Islands; Honolulu, HI, USA, 25–27 May 1983, University of Hawaii Seagrant: Honolulu, HI, USA, 1984; Volume 1, pp. 283–327. [Google Scholar]
- Snytko, V.A.; Tuponogov, V.N.; Kolpakov, N.V. The contribution of the scientists of TINRO-Center to study of bottom and near-bottom fishes. Izv. TINRO 2005, 141, 173–208. [Google Scholar]
- Bensch, A.; Gianni, M.; Gréboval, D.; Sanders, J.; Hjort, A. Worldwide Review of Bottom Fisheries in the High Seas; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2009; Volume 522, pp. 1–145. [Google Scholar]
- Darnitsky, V.B. Oceanological Processes Near Seamounts and Ridges of the Open Ocean; TINRO-Center: Vladivostok, Russia, 2010; pp. 1–119. [Google Scholar]
- Baitalyuk, A.A.; Karyakin, K.A.; Orlov, A.M. Thalassobathyal resources of the Emperor Seamounts: Fishery, stock status and possibility for distant fishery. Probl. Fish. 2010, 11, 801–816. [Google Scholar]
- Victorero, L.; Watling, L.; Deng Palomares, M.L.; Nouvian, C. Out of sight, but within reach: A global history of bottom-trawled deep-sea fisheries from >400 m depth. Front. Mar. Sci. 2018, 5, 98. [Google Scholar] [CrossRef]
- Orlov, A.M.; Tuponogov, V.N. Past and present ichthyological and fisheries research at the Emperor Seamounts: Lessons from the Soviet/Russian experience in the Central North Pacific. Rev. Fish. Sci. Aquac. 2025. [Google Scholar] [CrossRef]
- Delgado, J.P.; Brennan, M.L.; Stokes, E.; Wagner, D. A forgotten maritime highway: Maritime cultural heritage of the Emperor Seamounts with implications for high seas conservation. J. Marit. Archaeol. 2024, 19, 41–80. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kiyota, M.; Hayashibara, T.; Nonaka, M.; Imahara, Y.; Tachikawa, H. Megafaunal composition of cold-water corals and other deep-sea benthos in the southern Emperor Seamounts area, North Pacific Ocean. Galaxea J. Coral Reef Stud. 2017, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Kiyota, M. Application of association analysis for identifing indicator taxa of vulnerable marine ecosystems in the Emperor Seamounts area, North Pacific Ocean. Ecol. Indic. 2017, 78, 301–310. [Google Scholar] [CrossRef]
- Baco, A.R.; Morgan, N.B.; Roark, E.B.; Biede, V. Bottom-contact fisheries disturbance and signs of recovery of precious corals in the Northwestern Hawaiian Islands and Emperor Seamount Chain. Ecol. Indic. 2023, 148, 110010. [Google Scholar] [CrossRef]
- Borets, L.A.; Sokolovsky, A.S. Species composition of ichthyoplankton of the Hawaiian submarine ridge and Emperor Seamounts. Izv. TINRO 1978, 102, 43–50. [Google Scholar]
- Kodolov, L.S.; Gavrilov, G.M.; Kokareva, L.N. Species composition of the Melanostomiatidae (Osteichthyes) family in the area of the Emperor Seamounts. Izv. TINRO 1980, 104, 163–167. [Google Scholar]
- Novikov, N.P.; Kodolov, L.S.; Gavrilov, G.M. Preliminary list of fishes of the Emperor Underwater Ridge. In Fishes of the Open Ocean; Institute of Oceanology of the USSR Academy of Sciences: Moscow, Russia, 1981; pp. 32–35. [Google Scholar]
- Borets, L.A. Ichthyofauna of the Northwestern and Hawaiian underwater ridges. J. Ichthyol. 1986, 26, 1–13. [Google Scholar]
- Mundy, B.C. Checklist of the Fishes of the Hawaiian Archipelago (Bishop Museum Bulletin in Zoology); Bishop Museum: Honolulu, HI, USA, 2005; Volume 6, pp. 1–704. [Google Scholar]
- Belyaev, V.A.; Darniskiy, V.B. Features of oceanography and ichthyofauna composition on the Emperor Ridge. In Deep Sea 2003: Conference of the Governance and Management of Deep-Sea Fisheries. Part 1: Conference Reports; Shotton, R., Ed.; FAO: Rome, Italy, 2005; pp. 107–124. [Google Scholar]
- Sawada, K.; Okamoto, M.; Hoshino, K.; Yonezaki, S. Bycatch Species by Japanese Bottom Fisheries in the Emperor Seamounts; NPFC-2019-SSC BF02-WP03; North Pacific Fisheries Commission: Tokyo, Japan, 2019; pp. 1–23.
- Hoshino, K.; Sawada, K. Comments on Compiled Bycatch Species List for the Emperor Seamounts (ESM); NPFC-2020-SSC BFME01-IP01; North Pacific Fisheries Commission: Tokyo, Japan, 2020; pp. 1–25.
- Hoshino, K.; Okamoto, M.; Sawada, K. The Field Guide for Identifications of Fishes of the Emperor Seamount Chain Captured by Bottom Fisheries; North Pacific Fisheries Commission: Tokyo, Japan, 2024; pp. 1–92.
- Prokofiev, A.M.; Balanov, A.A.; Emelianova, O.R.; Orlov, A.M.; Orlova, S.Y. A new species of Lycodapus from the Emperor Seamount Chain, northwestern Pacific Ocean (Teleostei: Zoarcidae). Diversity 2022, 14, 972. [Google Scholar] [CrossRef]
- Prokofiev, A.M.; Emelyanova, O.R.; Orlov, A.M.; Orlova, S.Y. A new species of Diaphus associated with seamounts of the Emperor Chain, north-western Pacific Ocean (Teleostei: Myctophiformes: Myctophidae). J. Mar. Sci. Eng. 2022, 10, 65. [Google Scholar] [CrossRef]
- Prokofiev, A.M.; Frable, B.W.; Emelianova, O.R.; Saveleva, S.Y.; Orlov, A.M. A new species of the eel genus Gnathophis (Congridae, Anguilliformes) from the seamounts of the Emperor–Hawaiian Chain, Western and Central North Pacific. J. Mar. Sci. Eng. 2025, 13, 772. [Google Scholar] [CrossRef]
- Prokofiev, A.M.; Orlov, A.M. Eustomias securicula sp. nov.—The second representative of the subgenus Biradiostomias (Melanostomiidae) in the Pacific Ocean. J. Ichthyol. 2022, 62, 316–319. [Google Scholar] [CrossRef]
- Prokofiev, A.M.; Orlov, A.M. A new species of the genus Psednos (Teleostei: Liparidae) from the Emperor Seamount Chain and the first record of this genus from the northwestern Pacific Ocean. Zoosyst. Ross. 2025, 34, 272–278. [Google Scholar] [CrossRef]
- Lomakin, I.E.; Kochelab, V.V. Geology of the Emperor mountains and some questions of tectonics of the north-west Pacific. Geol. Miner. World Ocean. 2019, 15, 57–72. [Google Scholar] [CrossRef]
- Bystrov, Y.N.; Mikhailovsky, A.P. (Eds.) Description of Seamounts and Underwater Elevations of the World Oceans Fishing Grounds (High Seas); Ministry of Defense of the USSR: Moscow, Russia; Ministry of Fisheries of the USSR: Moscow, Russia, 1989; Volume 2, pp. 1–386. [Google Scholar]
- Somov, A.A.; Kanzeparova, A.N.; Vazhova, A.S.; Khleborodov, A.S.; Zuev, M.A.; Slabinsky, A.M.; Orlova, S.Y.; Kurnosov, D.S.; Belyaev, V.A.; Orlov, A.M. Some preliminary results of research on Emperor Seamounts in April, 2019. Tr. VNIRO 2019, 175, 208–2019. [Google Scholar] [CrossRef]
- Kurnosova, A.S.; Somov, A.A.; Kanzeparova, A.N.; Zuev, M.A.; Orlova, S.Y.; Kurnosov, D.S.; Orlov, A.M. Mesopelagic micronekton and macroplankton and the conditions of its habitat in the northeastern Pacific Ocean. Oceanology 2022, 62, 68–79. [Google Scholar] [CrossRef]
- Masuda, H.; Amaoka, K.; Araga, C.; Uyeno, T.; Yoshino, T. (Eds.) The Fishes of the Japanese Archipelago: Two Volumes; Tokai University Press: Tokyo, Japan, 1984; pp. 1–838. [Google Scholar]
- Amaoka, K.; Nakaya, K.; Yabe, M. The Fishes of Northern Japan; Kita-Nihon Kayo Center Co. Ltd.: Sapporo, Japan, 1995; pp. 1–390. [Google Scholar]
- Nakabo, T. (Ed.) Fishes of Japan with Pictorial Keys to the Species; Tokai University Press: Tokyo, Japan, 2002; pp. 1–1749. [Google Scholar]
- Hubbs, C.L.; Lagler, K.F. Fishes of the Great Lakes Region; University of Michigan Press: Ann Arbor, MI, USA, 1958; pp. xvii + 276. [Google Scholar]
- Compagno, L.J.V. Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date; FAO Fisheries Synopses; FAO: Rome, Italy, 1984; Volume 125, pp. 251–655. [Google Scholar]
- Eschmeyer, W.N. A systematic review of the scorpionfishes of the Atlantic Ocean (Pisces: Scorpaenidae). Occas. Pap. Calif. Acad. Sci. 1969, 79, 1–143. [Google Scholar]
- Bertelsen, E. Notes on Linophrynidae V: A revision of the deepsea anglerfishes of the Linophryne arborifera-group (Pisces, Ceratioidei). Steenstrupia 1980, 6, 29–70. [Google Scholar]
- Böhlke, E.B. Methods and terminology. In Fishes of the Western North Atlantic; Memoir of the Sears Foundation for Marine Research; Sears Foundation: New Haven, CT, USA, 1989; Volume 1, pp. 1–7. [Google Scholar]
- Paulin, C.D. Review of the morid genera Gadella, Physiculus, and Salilota (Teleostei: Gadiformes) with descriptions of seven new species. N. Z. J. Zool 1989, 16, 93–133. [Google Scholar] [CrossRef]
- Morrow, J.E.; Gibbs, R.H., Jr. Melanostomiatidae. In Fishes of the Western North Atlantic Part 4; Bigelow, H.B., Cohen, D.M., Dick, M.M., Gibbs, R.H., Jr., Grey, M., Morrow, J.E., Jr., Schultz, L.P., Walters, V., Eds.; Sears Foundation: New Haven, CT, USA, 1964; Volume 1, pp. 351–522. [Google Scholar]
- Prokofiev, A.M.; Kukuev, E.I. Systematics and Distribution of the Swallowerfishes of the Genus Pseudoscopelus (Chiasmodontidae); KMK Scientific Press Ltd.: Moscow, Russia, 2007; pp. 1–162. [Google Scholar]
- Odum, E. Ecology. 2 Vols; Mir: Moscow, Russia, 1986; pp. 1–328–1–376. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006; pp. 1–192. [Google Scholar]
- Barsukov, V.V. The species composition of the genus Helicolenus (Sebastinae, Scorpaenidae, Pisces) and a description of a new species. J. Ichthyol. 1973, 13, 161–167. [Google Scholar]
- Barsukov, V.V.; Borets, L.A. New data on the kinmei rosefish, Helicolenus fedorovi (Sebastinae, Scorpaenidae). Vop. Ikhtiol 1983, 23, 179–185. [Google Scholar]
- Barsukov, V.V.; Fedorov, V.V. Species of the genus Hozukius (Scorpaenidae, Sebastinae) from the guyots of the Hawaiian Submarine Ridge. J. Ichthyol. 1975, 15, 869–876. [Google Scholar]
- Barsukov, V.V.; Borets, L.A.; Kodolov, L.S.; Snytko, V.A. New data on Adelosebastes latens Eschmeyer, Abe et Nakano, 1979 (Scorpaenidae). Vopr. Ikhtiol 1983, 23, 538–543. [Google Scholar]
- Borets, L.A. Anthias rubromaculatus sp. n., a new species of fishes (Serranidae) from submarine mountains of the Hawaii Ridge. Biol. Morya 1982, 8, 68–70. [Google Scholar]
- Dolganov, V.N. A new species of shark from the north-west Pacific Ocean. Biol. Morya 1985, 3, 64–65. [Google Scholar]
- Dolganov, V.N. Squalus boretzi sp. n. (Squalidae), a new squalid shark species from the Emperor Seamount Chain, Pacific Ocean. Biol. Morya 2019, 45, 279–285. [Google Scholar] [CrossRef]
- Dolganov, V.N. On the little-known sharks Etmopterus villosus (Etmopteridae) and Scymnodalatias sherwoodi (Somniosidae) from the Pacific Ocean. J. Ichthyol. 2019, 59, 275–279. [Google Scholar] [CrossRef]
- Dolganov, V.N. Formation of the fauna of cartilaginous fishes of the Emperor Seamount Chain. Biol. Morya 2023, 49, 361–366. [Google Scholar] [CrossRef]
- Dolganov, V.N. On finding a rare species of chimera Chimaera owstoni Tanaka (Holocephali, Chimaeridae) in the area of the Northwestern underwater ridge. Vopr. Ikhtiol. 1982, 22, 1039. [Google Scholar]
- Dolganov, V.N. On catch of deep-sea shark Chlamydoselachus anguineus (Chlamydoselachidae) on Emperor Seamounts. Izv. TINRO 2018, 194, 68–69. [Google Scholar] [CrossRef]
- Fedorov, V.V.; Snytko, V.A.; Matiushin, V.M. Morphological characteristics and some data on the biology of Helicolenus avius Abe et Eschmeyer, 1972 (Pisces, Scorpaenidae) from the area of the southern part of the Emperor Seamounts. Investig. Biol. Fish Fish. Oceanogr. 1975, 6, 56–63. [Google Scholar]
- Korostelev, N.B.; Baytalyuk, A.A.; Maltsev, I.V.; Orlov, A.M. First data on the age and growth in Pacific flatnose Antimora microlepis (Moridae) from the waters of the underwater Emperor Mountain Range (Northwestern Pacific). J. Ichthyol. 2020, 60, 891–899. [Google Scholar] [CrossRef]
- Korostelev, N.B.; Maltsev, I.V.; Orlov, A.M. First data on the age and growth of Schmidt’s cod Lepidion schmidti (Moridae) from waters of the Emperor Seamounts (Northwestern Pacific). J. Mar. Sci. Eng. 2023, 11, 1212. [Google Scholar] [CrossRef]
- Korostelev, N.B.; Volvenko, I.V.; Belyakov, V.V.; Baytaliuk, A.A.; Bush, A.G.; Kanzeparova, A.N.; Orlov, A.M. “Firefly” of the submarine mountains: New data on Physiculus cynodon (Moridae, Teleostei) from Emperor Seamounts and Northwestern Hawaiian Ridge. J. Mar. Sci. Eng. 2023, 11, 2355. [Google Scholar] [CrossRef]
- Korostelev, N.B.; Volvenko, N.B.; Maltsev, I.V.; Orlov, A.M. Brought to the surface from obscurity: The first data on the distribution and biology of Coelorhinchus gilberti (Macrouridae, Gadiformes, Teleostei) off the Emperor Seamounts (Northwestern Pacific). Deep-Sea Res. Pt. II 2025, 220, 105461. [Google Scholar] [CrossRef]
- Kurbanov, Y.K.; Michalutin, E.A. Data on Pacific grenadier Coryphaenoides acrolepis (Macrouridae) from the area of underwater uplifts of Emperor Seamount Chain (northern part of the Pacific Ocean). In Proceedings of the National (All-Russian) Scientific and Practical Conference “Natural Resources, Their Current State, Protection, Commercial and Technical Use” Vol. 1. No. XII; Kamchatka State Technical University: Petropavlovsk-Kamchatsky, Russia, 2021; pp. 44–48. [Google Scholar]
- Kurbanov, Y.K.; Michalutin, E.A. New finds of chimaeras (Holocephali: Chimaeriformes) in the region of the Emperor Seamount Chain (Northern Pacific Ocean). J. Ichthyol. 2022, 62, 1307–1311. [Google Scholar] [CrossRef]
- Kurbanov, Y.K.; Michalutin, E.A. New data on Adelosebastes latens (Sebastidae) from the region of the underwater elevations of the Hawaiian-Emperor Seamount Chain (Northern Pacific Ocean). J. Ichthyol. 2022, 62, 79–88. [Google Scholar] [CrossRef]
- Kurbanov, Y.K.; Michalutin, E.A. Distribution and some aspects of biology of Hozukius guyotensis (Sebastidae) in the area of the Emperor Seamount Chain. J. Ichthyol. 2023, 63, 280–287. [Google Scholar] [CrossRef]
- Pakhorukov, N.P. Behavior and distribution of bottom and near-bottom fish on the Emperor Seamount Chain (the Pacific Ocean). J. Ichthyol. 2005, 45, 103–110. [Google Scholar]
- Sazonov, Y.I. Two new macrourid species (Gadiformes) from northern Pacific seamounts. J. Ichthyol. 1985, 25, 13–21. [Google Scholar]
- Sazonov, Y.I. Physiculus cynodon sp. n. (Gadiformes, Moridae) from submarine mountains in the northern part of Pacific Ocean. Zool. Zhur 1986, 65, 305–308. [Google Scholar]
- Sazonov, Y.I. Additions to the list of macrourids (Gadiformes, Bathygadidae and Macrouridae) from the Northwest Pacific Ridge. J. Ichthyol. 1994, 34, 98–115. [Google Scholar]
- Snytko, V.A. Rockfishes of the Northern Part of the Pacific Ocean; TINRO: Vladivostok, Russia, 2001; pp. 1–468.
- Zolotov, O.G.; Spirin, I.Y.; Zudina, S.M. New data on the range, biology, and abundance of skilfish Erilepis zonifer (Anoplopomatidae). J. Ichthyol. 2014, 54, 251–265. [Google Scholar] [CrossRef]
- Ebert, D.A.; Papastamatiou, Y.P.; Kajiura, S.M.; Wetherbee, B.M. Etmopterus lailae sp. nov., a new lanternshark (Squaliformes: Etmopteridae) from the Northwestern Hawaiian Islands. Zootaxa 2017, 4237, 371–382. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Phil. Trans. Roy. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Emelianova, O.R.; Bulatov, O.A.; Grigorov, I.V.; Orlov, A.M.; Orlova, S.Y. Polymorphism of mtDNA gene Cyt b of walleye pollock, Gadus chalcogrammus (Gadidae), in the Chukchi Sea, western Bering Sea, and Sea of Okhotsk. Deep Sea Res. II 2022, 206, 105216. [Google Scholar] [CrossRef]
- Emelianova, O.R.; Grigorov, I.V.; Orlov, A.M.; Orlova, S.Y. Polymorphism of mtDNA gene Cyt b of the Chukchi Sea polar cod, Boreogadus saida (Gadidae, Gadiformes). Deep Sea Res. II 2022, 206, 105212. [Google Scholar] [CrossRef]
- Silva, W.A., Jr.; Costa, M.C.; Valente, V.; Sousa, J.F.; Paçó-Larson, M.L.; Espreafico, E.M.; Camargo, S.S.; Monteiro, E.; Holanda, A.J.; Zago, M.A.; et al. PCR template preparation for capillary DNA sequencing. Biotechniques 2001, 30, 537–542. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ashton, B.; Buxton, S.; Cheung, M.; Cooper, A.; Duran, C.; Field, M.; Heled, J.; Kearse, M.; Markowitz, S.; et al. Geneious v5.4. 2011. Available online: http://www.geneious.com (accessed on 24 October 2025).
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.; Huelsenbeck, J. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Shirai, S.; Nakaya, K. A new squalid species of the genus Centroscyllium from the Emperor Seamount Chain. Jpn. J. Ichthyol. 1990, 36, 391–398. [Google Scholar] [CrossRef]
- Shirai, S.; Tachikawa, H. Taxonomic resolution of the Etmopterus pusillus species group (Elasmobranchii, Etmopteridae), with description of E. bigelowi, n. sp. Copeia 1993, 2, 483–495. [Google Scholar] [CrossRef]
- Ebert, D.A. Deep-Sea Cartilaginous Fishes of the Indian Ocean. Vol. 1. Sharks; FAO Species Catalogue for Fishery Purposes; FAO: Rome, Italy, 2013; Volume 8, pp. 1–256. [Google Scholar]
- Dolganov, V.N. Manual for Identification of Cartilaginous Fishes of Far East Seas of USSR and Adjacent Waters; TINRO: Vladivostok, Russia, 1983; pp. 1–92.
- ICZN. International Code of Zoological Nomenclature; International Trust for Zoological Nomenclature: London, UK; The Natural History Museum: London, UK, 1999; pp. 1–106. [Google Scholar]
- Smith, D.G.; Böhlke, J.E.; Castle, P.H.J. A revision of the nettastomatid eel genera Nettastoma and Nettenchelys (Pisces: Anguilliformes), with descriptions of six new species. Proc. Biol. Soc. Wash 1981, 94, 535–560. [Google Scholar]
- Quero, J.C.; Saldanha, L. Poissons anguilliformes de l’Ile de la Réunion (Océan Indien): Description d’une nouvelle espèce. Cybium 1995, 19, 61–88. [Google Scholar]
- Vo, V.Q.; Ho, H.C.; Dao, H.V.; Tran, H.H.T. A new arrowtooth eel of genus Meadia (Synaphobranchidae: Ilyophinae) from Vietnam, South China Sea. Zootaxa 2021, 4952, 181–191. [Google Scholar] [CrossRef]
- Sulak, K.J.; Shcherbachev, Y.N. Zoogeography and systematics of six deep-living genera of synaphobranchid eels, with a key to taxa and description of two new species of Ilyophis. Bull. Mar. Sci. 1997, 60, 1158–1194. [Google Scholar]
- Nielsen, J.G.; Smith, D.G. The Eel Family Nemichthyidae (Pisces, Anguilliformes); Dana-report; Scandinavian Science Press: Copenhagen, Denmark, 1978; Volume 88, pp. 1–71. [Google Scholar]
- Smith, D.G.; Nielsen, J.G. Family Nemichthyidae. In Fishes of the Western North Atlantic; Böhlke, E.B., Ed.; Sears Foundation of Marine Research: New Haven, CT, USA, 1989; pp. 441–459. [Google Scholar]
- Robertson, D.R.; Angulo, A.; Baldwin, C.C.; Pitassy, D.E.; Driskell, A.; Weigt, L.A.; Navarro, I.J. Deep-water bony fishes collected by the B/O Miguel Oliver on the shelf edge of Pacific Central America: An annotated, illustrated and DNA-barcoded checklist. Zootaxa 2017, 4348, 1–125. [Google Scholar] [CrossRef]
- Orlov, A.M.; Tokranov, A.M. Checklist of deep-sea fishes of the Russian northwestern Pacific Ocean found at depths below 1000 m. Prog. Oceanogr. 2019, 176, 102143. [Google Scholar] [CrossRef]
- Rass, T.S.; Kashkina, A.A. Bathylagid fishes (Pisces, Bathylagidae) of the northern Pacific. Tr. Inst. Okeanol. AN SSSR 1967, 84, 209–221. [Google Scholar]
- Kobyliansky, S.G. Materials for the revision of the genus Bathylagus Günther (Bathylagidae): The group of “light” deep-sea smelts. J. Ichthyol. 1985, 25, 1–17. [Google Scholar]
- Kobyliansky, S.G. Materials for a revision of the family Bathylagidae (Teleostei, Salmoniformes). Tr. Inst. Okeanol. AN SSSR 1986, 121, 6–50. [Google Scholar]
- Cohen, D.M. Suborder Argentinoidea. In Fishes of the Western North Atlantic. Pt. 4; Memoir of the Sears Foundation for Marine Research; Sears Foundation: New Haven, CT, USA, 1964; Volume 1, pp. 1–70. [Google Scholar]
- Sazonov, Y.I. Materials on the systematics and distribution of fishes of the family Searsiidae (Salmoniformes, Alepocephaloidei). Tr. Inst. Okeanol. Akad. Nauk SSSR 1976, 104, 26–72. [Google Scholar]
- Matsui, T.; Rosenblatt, R.H. Review of the deep-sea fish family Platytroctidae (Pisces: Salmoniformes). Bull. Scripps Inst. Oceanogr. 1987, 26, 1–159. [Google Scholar]
- Sazonov, Y.I.; Balanov, A.A.; Fedorov, V.V. Alepocephaloid fishes (Alepocephaloidei) from the western North Pacific Ocean. Tr. Inst. Okeanol. RAS 1993, 128, 40–68. [Google Scholar]
- Ozawa, T.; Oda, K.; Ida, T. Systematics and distribution of the Diplophos taenia species complex (Gonostomatidae), with a description of a new species. Jpn. J. Ichthyol. 1990, 37, 98–115. [Google Scholar] [CrossRef]
- Mukhacheva, V.A. On systematics, distribution and biology of the Gonostoma species (Pisces, Gonostomatidae). Tr. Inst. Okeanol. Akad. Nauk SSSR 1972, 93, 203–249. [Google Scholar]
- Mukhacheva, V.A. The composition of species of the genus Cyclothone (Pisces, Gonostomatidae) in the Pacific Ocean. Tr. Inst. Okeanol. Akad. Nauk SSSR 1964, 73, 93–138. [Google Scholar]
- Mukhacheva, V.A. Cyclothones (gen. Cyclothone, fam. Gonostomatidae) of the world and their distributions. Tr. Inst. Okeanol. Akad. Nauk SSSR 1974, 96, 205–249. [Google Scholar]
- Parin, N.V.; Kobyliansky, S.G. Review of the genus Maurolicus (Sternoptychidae, Stomiiformes), with re-establishing validity of five species considered junior synonyms of M. muelleri and descriptions of nine new species. Tr. Inst. Okeanol. RAS 1993, 128, 69–107. [Google Scholar]
- Rees, D.J.; Poulsen, J.Y.; Sutton, T.T.; Costa, P.A.; Landaeta, M.F. Global phylogeography suggests extensive eucosmopolitanism in Mesopelagic Fishes (Maurolicus: Sternoptychidae). Sci. Rep. 2020, 10, 20544. [Google Scholar] [CrossRef]
- Savinykh, V.F.; Baytalyuk, A.A. New data on biology of the pearlfish Maurolicus imperatorius (Sternopthychidae) from the Emperor Seamount Chain. J. Ichthyol. 2010, 50, 148–158. [Google Scholar] [CrossRef]
- Baird, R.C. The systematics, distribution, and zoogeography of the marine hatchetfishes (family Sternoptychidae). Bull. Mus. Comp. Zool. Harvard Coll. 1971, 142, 1–128. [Google Scholar]
- Borodulina, O.D. Materials on the systematics and distribution of the oceanic hatchetfishes, genera Argyropelecus and Sternoptyx (Sternoptychidae, Osteichthyes). Tr. Inst. Okeanol. Akad. Nauk SSSR 1978, 111, 27–59. [Google Scholar]
- Harold, A.S. A taxonomic revision of the sternoptychid genus Polyipnus (Teleostei: Stomiiformes) with an analysis of phylogenetic relationships. Bull. Mar. Sci. 1994, 54, 428–534. [Google Scholar]
- Huang, D.; Zhang, X.; Jiang, Z.; Zhang, J.; Arbi, I.; Jiang, X.; Huang, X.; Zhang, W. Seasonal fluctuations of ichthyoplankton assemblage in the northeastern South China sea influenced by the Kuroshio intrusion. J. Geophys. Res. Oceans 2017, 122, 7253–7266. [Google Scholar] [CrossRef]
- Teramura, A.; Koeda, K.; Matsuo, A.; Sato, M.P.; Senou, H.; Ho, H.-C.; Suyama, Y.; Kikuchi, K.; Hirase, S. Assessing the effectiveness of DNA barcoding for exploring hidden genetic diversity in deep-sea fishes. Mar. Ecol. Prog. Ser. 2022, 701, 83–98. [Google Scholar] [CrossRef]
- Badcock, J.; Baird, R.C. Remarks on systematics, development, and distribution of the hatchetfish genus Sternoptyx (Pisces, Stomiatoidei). Fish. Bull. 1980, 77, 803–820. [Google Scholar]
- Mukhacheva, V.A. Review of the genus Ichthyococcus Bonaparte (Photichthyidae). Vopr. Ikhtiol 1980, 20, 771–786. [Google Scholar]
- Morrow, J.E. Taxonomy of the deep sea fishes of the genus Chauliodus. Bull. Mus. Comp. Zool 1961, 125, 247–294. [Google Scholar]
- Parin, N.V.; Novikova, N.S. Systematics of viperfishes (Chauliodontidae, Osteichthyes) and their distribution in the World Ocean. Tr. Inst. Okeanol. Akad. Nauk SSSR 1974, 96, 255–315. [Google Scholar]
- Parin, N.V.; Pokhilskaya, G.N. On the taxonomy and distribution of the mesopelagic fish genus Melanostomias Brauer (Melanostomiatidae, Osteichthyes). Tr. Inst. Okeanol. Akad. Nauk SSSR 1978, 111, 61–86. [Google Scholar]
- Novikova, N.S. Idiacanthids of the Indian and Pacific Oceans. Tr. Inst. Okeanol. Akad. Nauk SSSR 1967, 84, 159–208. [Google Scholar]
- Kenaley, C.P. Revision of the stoplight loosejaw genus Malacosteus (Teleostei: Stomiidae: Malacosteinae), with description of a new species from the temperate southern hemisphere and Indian Ocean. Copeia 2007, 4, 886–900. [Google Scholar] [CrossRef]
- Clarke, T.A. Some aspects of the ecology of stomiatoid fishes in the Pacific Ocean near Hawaii. Fish. Bull. 1974, 72, 337–351. [Google Scholar]
- Sutton, T.T.; Hopkins, T.L. Trophic ecology of the stomiid (Pisces: Stomiidae) fish assemblage of the eastern Gulf of Mexico: Strategies, selectivity and impact of a top mesopelagic predator group. Mar. Biol. 1996, 127, 179–192. [Google Scholar] [CrossRef]
- Sato, T.; Nakabo, T. A revision of the Paraulopus oblongus group (Aulopiformes: Paraulopidae) with description of a new species. Ichthyol. Res. 2003, 50, 164–177. [Google Scholar] [CrossRef]
- Fujiwara, K.; Wada, H.; Motomura, H. A new species of the greeneye genus Chlorophthalmus (Teleostei: Chlorophthalmidae) from the central North Pacific. Zootaxa 2019, 4555, 396–406. [Google Scholar] [CrossRef]
- Johnson, R.K. A revision of the alepisauroid family Scopelarchidae (Pisces: Myctophiformes). Field. Zool 1974, 66, 1–249. [Google Scholar]
- Nafpaktitis, B.G. Family Neoscopelidae. In Fishes of the Western North Atlantic. Part 7; Sears Foundation for Marine Research: New Haven, CT, USA, 1977; pp. 1–11. [Google Scholar]
- Bañón, R.; Barros-García, D.; Arronte, J.C.; Rábade, S.; Del Rio, J.L.; Baldó, F.; De Carlos, A. Diving deeper into the taxonomy of the Neoscopelus species complex (Myctophiformes: Neoscopelidae) with the description of Neoscopelus serranoi sp. nov. Zootaxa 2024, 5529, 487–510. [Google Scholar] [CrossRef]
- Savinykh, V.F.; Balanov, A.A. New findings of bathypelagic fishes in Pacific Ocean. J. Ichthyol. 1999, 39, 415–418. [Google Scholar]
- Badcock, J.; Araujo, T.M. On the significance of variation in a warm water cosmopolitan species, nominally Ceratoscopelus warmingii (Pisces, Myctophidae). Bull. Mar. Sci. 1988, 42, 16–43. [Google Scholar]
- Becker, V.E. Myctophid Fishes of the World Ocean; Nauka: Moscow, Russia, 1983; pp. 1–248. [Google Scholar]
- Nakaya, K.; Amaoka, K.; Abe, K. A review of the genus Lepidion (Gadiformes, Moridae) from the Northwestern Pacific. Jpn. J. Ichthyol. 1980, 27, 41–47. [Google Scholar]
- Paulin, C.D. First record of Lepidion inosimae (Günther) and L. schmidti Svetovidov (Pisces: Moridae) from New Zealand. N. Z. J. Zool 1984, 11, 59–62. [Google Scholar] [CrossRef]
- Vinu, J.; Rajeesh Kumar, M.P.; Sumod, K.S.; Deepa, K.P.; Hashim, M.; Sanjeevan, V.N.; Sudhakar, M. Occurrence of a rare gigantic sized deep-sea cod Lepidion inosimae (Günther, 1887) in the Northwestern Indian Ocean. Mar. Biodivers. 2017, 47, 575–578. [Google Scholar] [CrossRef]
- Howes, G.J.; Crimmen, O.A. A review of the Bathygadidae (Teleostei: Gadiformes). Bull. Brit. Mus. Nat. Hist. (Zool.) 1990, 56, 155–203. [Google Scholar]
- Gilbert, C.H. The deep-sea fishes of the Hawaiian Islands. Bull. U.S. Fish. Comm. 1905, 23, 577–713. [Google Scholar]
- Gilbert, C.H.; Hubbs, C.L. Contributions to the biology of the Philippine Archipelago and adjacent regions, Vol. 1. Papers on collections gathered by the Albatross, Philippine Expedition, 1907–1910. Pt. 7: The macrouroid fishes of the Philippine Islands and the East Indies. Bull. United States Natl. Mus. 1920, 100, 369–588. [Google Scholar]
- Okamura, O. Macrouridae. In Fishes of the Kyushu-Palau Ridge and Tosa Bay; Okamura, O., Amaoka, K., Mitani, F., Eds.; Japan Fisheries Resource Conservation Association: Tokyo, Japan, 1982; pp. 140–181, 345–354. [Google Scholar]
- Iwamoto, T. Family Macrouridae. In Gadiform Fishes of the World (Order Gadiformes). An Annotated and Illustrated Catalogue of Cods, Hakes, Grenadiers and Other Gadiform Fishes Known to Date; Cohen, D.M., Inada, T., Iwamoto, T., Scialabba, N., Eds.; FAO Species Catalogue; Vol. 10. FAO Fisheries Synopsis no. 125; FAO: Rome, Italy, 1990; pp. 90–317. [Google Scholar]
- Nakayama, N. Grenadiers (Teleostei: Gadiformes: Macrouridae) of Japan and adjacent waters, a taxonomic monograph. Megataxa 2020, 3, 1–383. [Google Scholar] [CrossRef]
- Prokofiev, A.M. New grenadier Coelorinchus ganymedes sp. nova from the waters of Polynesia (Macrouridae). J. Ichthyol. 2021, 61, 175–181. [Google Scholar] [CrossRef]
- Nakayama, N.; Endo, H. Redescription of Nezumia infranudis (Gilbert & Hubbs, 1920), with the first record of the species from the Eastern Indian Ocean (Actinopterygii: Gadiformes: Macrouridae). Mar. Biol. Res. 2015, 11, 1108–1115. [Google Scholar] [CrossRef]
- Iwamoto, T.; Merrett, N.R. Pisces Gadiformes: Taxonomy of grenadiers of the New Caledonian region, southwest Pacific. Mem. Mus. Nat. Hist. Natur. Ser. A Zool 1997, 176, 473–570. [Google Scholar]
- Caruso, J.H. The systematics and distribution of the lophiid anglerfishes: I. A revision of the genus Lophiodes with the description of two new species. Copeia 1981, 3, 522–549. [Google Scholar] [CrossRef]
- Pietsch, T.W. A new genus and species of deep-sea anglerfish (Pisces: Oneirodidae) from the northern Pacific Ocean. Copeia 1973, 2, 193–199. [Google Scholar] [CrossRef]
- Pietsch, T.W. Oceanic Anglerfishes: Extraordinary Diversity in the Deep Sea; University of California Press: Oakland, CA, USA, 2009; pp. 1–576. [Google Scholar] [CrossRef]
- Prokofiev, A.M. New species and new records of deepsea anglerfish of the family Oneirodidae. J. Ichthyol. 2014, 54, 602–607. [Google Scholar] [CrossRef]
- Bilecenoglu, M. Status of the genus Macroramphosus (Syngnathiformes: Centriscidae) in the eastern Mediterranean Sea. Zootaxa 2006, 1273, 55–64. [Google Scholar] [CrossRef]
- Kotlyar, A.N. Systematics and the distribution of fishes of the family Polymixidae (Polymixioidei, Beryciformes). J. Ichthyol. 1984, 24, 1–20. [Google Scholar]
- Kotlyar, A.N. A new species of the genus Polymixia from the submarine Kyushu-Palau Ridge, and notes on other representatives of the genus (Polymixiidae, Beryciformes). Vopr. Ikhtiol 1992, 32, 11–26. [Google Scholar]
- Kotlyar, A.N. Beryciform Fishes of the World Ocean; VNIRO Publishing: Moscow, Russia, 1996; pp. 1–368. [Google Scholar]
- Kotlyar, A.N. Revision of the genus Scopeloberyx (Melamphaidae). Part 2. Oligorakered species of the group S. robustus. J. Ichthyol. 2004, 44, 677–689. [Google Scholar]
- Kotlyar, A.N. Systematics and distribution of trachichthyid fishes (Trachichthyidae, Beryciformes) of the Indian Ocean. Tr. Inst. Okeanol. Akad. Nauk SSSR 1980, 110, 177–224. [Google Scholar]
- Eschmeyer, W.N.; Abe, T.; Nakano, S. Adelosebastes latens, a new genus and species of scorpionfish from the North Pacific Ocean (Pisces, Scorpaenidae). Uo 1979, 30, 77–84. [Google Scholar]
- Orr, J.W.; Baker, D.C. New North American records of the northeast Pacific scorpaenids Adelosebastes latens and Sebastes glaucus. Alaska Fish. Res. Bull. 1996, 3, 94–102. [Google Scholar]
- Orr, J.W.; Brown, M.A.; Baker, D.C. Guide to Rockfishes (Scorpaenidae) of the Genera Sebastes, Sebastolobus, and Adelosebastes of the Northeast Pacific Ocean; NOAA Tech. Memo. NMFS-SWFC.; U.S. Department of Commerce: Seattle, WA, USA; National Oceanic and Atmospheric Administration: Seattle, WA, USA; National Marine Fisheries Service: Seattle, WA, USA; Alaska Fisheries Science Center: Seattle, WA, USA, 2000; Volume 117, pp. 1–47.
- Maslenikov, K.P.; Orr, J.W.; Stevenson, D.E. Range extensions and significant distributional records for eighty-two species of fishes in Alaskan marine waters. Northw. Natur. 2013, 94, 1–21. [Google Scholar] [CrossRef]
- Abe, T.; Eschmeyer, W.N. A new species of the scorpionfish genus Helicolenus from the north Pacific Ocean. Proc. Calif. Acad. Sci. (Ser. 4) 1972, 39, 47–53. [Google Scholar]
- Barsukov, V.V. Annotated and Illustrated Catalog of Rockfishes of the World Ocean. Proc. Zool. Inst. Russ. Acad. Sci. 2003, 295, 1–320. [Google Scholar]
- Kanayama, T. Scorpaenid fishes from the Emperor Seamount Chain. Res. Inst. Nor. Pac. Fish. Fac. Fish. Hokkaido Univ 1981, Spec. Vol., 119–129. [Google Scholar]
- Yabe, M. A new cottoid fish of the family Ereuniidae, Marukawichthys pacificus, from the central North Pacific. Jpn. J. Ichthyol. 1983, 30, 18–26. [Google Scholar]
- Prokofiev, A.M.; Iftime, A. A revision of the abandoned snailfish genus Menziesichthys with description of a new species (Teleostei, Scorpaeniformes, Liparidae). Trav. Mus. D’histoire Nat. Grigore Antipa 2020, 63, 93–115. [Google Scholar] [CrossRef]
- Chernova, N.V. A review of the genus Psednos (Pisces, Liparidae) with description of ten new species from the north Atlantic and southwestern Indian Ocean. Bull. Mus. Compar. Zool 2001, 155, 477–507. [Google Scholar]
- Chernova, N.V.; Stein, D.L. Ten new species of Psednos (Pisces, Scorpaeniformes: Liparidae) from the Pacific and North Atlantic Oceans. Copeia 2002, 3, 755–778. [Google Scholar] [CrossRef]
- Stein, D.L. A review of the snailfishes (Liparidae, Scorpaeniformes) of New Zealand, including descriptions of a new genus and sixteen new species. Zootaxa 2012, 3588, 1–54. [Google Scholar] [CrossRef]
- Duhamel, G. Description d’especes nouvelles de Careproctus et Paraliparis et donnees nouvelles sur ces genres et le genre Edentoliparis de l’ocean Austral (Cyclopteridae, Liparinae). Cybium 1992, 16, 183–207. [Google Scholar]
- Andriashev, A.P. Liparid fishes (Liparidae, Scorpaeniformes) of the Southern Ocean and adjacent waters. In Explorations of the Fauna of the Seas; Zoological Institute of Russian Academy of Science: St. Petersburg, Russia, 2003; Volume 53, p. 10021215645. [Google Scholar]
- Stein, D.L. Snailfishes (Family Liparidae) of the Ross Sea, Antarctica, and closely adjacent waters. Zootaxa 2012, 3285, 1–120. [Google Scholar] [CrossRef]
- Stein, D.L.; Chernova, N.V.; Andriashev, A.P. Snailfishes (Pisces: Liparidae) of Australia, including descriptions of thirty new species. Rec. Austral. Mus. 2001, 53, 341–406. [Google Scholar] [CrossRef]
- Orr, J.W.; Spies, I.; Stevenson, D.E.; Longo, G.C.; Kai, Y.; Ghods, S.; Hollowed, M. Molecular phylogenetics of snailfishes (Cottoidei: Liparidae) based on MtDNA and RADseq genomic analyses, with comments on selected morphological characters. Zootaxa 2019, 4642, 1–79. [Google Scholar] [CrossRef]
- Fedoryako, B.I. Materials on the systematics and distribution of the “oceanic Cheilodipteridae”. Tr. Inst. Okeanol. Akad. Nauk SSSR 1976, 104, 156–190. [Google Scholar]
- Amaoka, K.; Nakaya, K.; Abe, K. First record of the percichthyid fish Howella parini from Japan. Jpn. J. Ichthyol. 1978, 25, 149–152. [Google Scholar]
- Abramov, A.A. Species composition and distribution of Epigonus (Epigonidae) in the world ocean. J. Ichthyol. 1992, 32, 94–108. [Google Scholar]
- Kamysheva, T.P. Cmparative morphometric characters of Epigonus denticulatus Dieuzeide (Perciformes, Apogonidae) from the Gerakl and Hawaiian ranges. J. Ichthyol. 1985, 25, 36–43. [Google Scholar]
- Hardy, G.S. A revision of the fishes of the family Pentacerotidae (Perciformes). N. Z. J. Zool 1983, 10, 177–220. [Google Scholar] [CrossRef]
- Kim, S.Y. Phylogenetic systematics of the family Pentacerotidae (Actinopterygii: Order Perciformes). Zootaxa 2012, 3366, 1–111. [Google Scholar] [CrossRef]
- Orlov, A.M.; Rabazanov, N.I.; Nikiforov, A.I. Transoceanic Migrations of Fishlike Animals and Fish: Norm or Exclusion? J. Ichthyol. 2020, 60, 242–262. [Google Scholar] [CrossRef]
- Mead, G.W. Bramidae; Dana-report; AF Høst Publisher: Copenhagen, Denmark, 1972; Volume 81, pp. 1–166. [Google Scholar]
- Orlov, A.M.; Volvenko, I.V. Distribution and abundance of large pelagic predatory bony fishes in the northwestern Pacific over a half-century. Wat. Biol. Secur. 2025, 4, 100373. [Google Scholar] [CrossRef]
- Prokofiev, A.M. Swallowerfishes (Chiasmodontidae) of the East Pacific. J. Ichthyol. 2014, 54, 631–641. [Google Scholar] [CrossRef]
- Parin, N.V.; Becker, V.E. Materials for a revision of the trichiuroid fishes of the genus Benthodesmus, with the description of four new species and one new subspecies. Proc. Biol. Soc. Wash 1970, 83, 351–364. [Google Scholar]
- Parin, N.V.; Becker, V.E. Materials on taxonomy and distribution of some trichiuroid fishes (Pisces, Trichiuroidae: Scombrolabracidae, Gempylidae, Trichiuridae). Tr. Inst. Okeanol. Akad. Nauk SSSR 1972, 93, 110–204. [Google Scholar]
- Nakamura, I.; Parin, N.V. Snake Mackerels and Cutlassfishes of the World (Families Gempylidae and Trichiuridae); FAO Fisheries Synopis; FAO: Rome, Italy, 1993; Volume 125, pp. 1–136. [Google Scholar]
- Cooper, J.A.; Chapleau, F. Monophyly and intrarelationships of the family Pleuronectidae (Pleuronectiformes), with a revised classification. Fish. Bull. 1998, 96, 686–726. [Google Scholar]
- Orlov, A.M.; Tokranov, A.M. Distribution and some biological features of four poorly studied deep benthic flatfishes (Pleuronectiformes: Pleuronectidae) in the northwestern Pacific Ocean. Raffles Bull. Zool. Suppl. 2007, 14, 221–235. [Google Scholar]
- Borets, L.A. A new species of flounder, Microstomus shuntovi sp. n. (Pleuronectidae), and two rare flounder species (Bothidae) from seamounts of the northwestern and Hawaiian ridges. J. Ichthyol. 1983, 23, 10011024541. [Google Scholar]
- Parin, N.V.; Borodulina, O.D. Preliminary review of the bathypelagic fish genus Antigonia Lowe (Zeiformes, Caproidae). Tr. Inst. Okeanol. Akad. Nauk SSSR 1986, 121, 141–172. [Google Scholar]
- Parin, N.V. Three new species and new records of black scabbardfishes of genus Aphanopus (Trichiuridae). Vopr. Ikhtiol 1994, 34, 740–746. [Google Scholar]
- Orlov, A.M. New northwest Pacific record of the Pacific black scabbardfish Aphanopus arigato (Trichiuridae, Perciformes) in the vicinity of southeastern Kamchatka. Acta Ichthyol. Piscat. 1999, 29, 3–11. [Google Scholar] [CrossRef]
- Okamoto, M.; Motomura, H.; Hoshino, K.; Yanagimoto, T.; Hayashibara, T. New records of the no line scorpionfish, Phenacoscorpius megalops (Actinopterygii: Scorpaeniformes: Scorpaenidae), from the Emperor Seamounts, central North Pacific. Biogeography 2012, 14, 77–81. [Google Scholar]
- Iwamoto, T.; Okamoto, M. A new grenadier fish of the genus Lucigadus (Macrouridae, Gadiformes, Teleostei) from the Emperor Seamounts, northwestern Pacific. Proc. Calif. Acad. Sci. 2015, 62, 369–380. [Google Scholar]
- Prokofiev, A.M. Grammatonotus ambiortus sp. nova: A new species of callanthiids (Perciformes) from the western tropical Pacific. J. Ichthyol. 2006, 46, 13–17. [Google Scholar] [CrossRef]
- Prokofiev, A.M. A new species of the grenadier genus Coelorinchus from the Hawaiian-Emperor Seamount Chain (the Pacific Ocean) (Teleostei, Gadiformes, Macrouridae). Amur. Zool. J. 2020, 12, 299–310. [Google Scholar] [CrossRef]
- Møller, P.R.; Schwarzhans, W.W.; Lauridsen, H.; Nielsen, J.G. Bidenichthys okamotoi, a new species of the Bythitidae (Ophidiiformes, Teleostei) from the Koko Seamount, Central North Pacific. J. Mar. Sci. Eng. 2021, 9, 1399. [Google Scholar] [CrossRef]
- Orlov, A.M.; Tokranov, A.M. Some ecological and biological features of giant and popeye grenadiers in the Pacific waters off the northern Kuril Islands and southeastern Kamchatka. Am. Fish. Soc. Symp. 2008, 63, 225–260. [Google Scholar] [CrossRef]
- Tuponogov, V.N.; Orlov, A.M.; Kodolov, L.S. The most abundant grenadiers of the Russian Far East EEZ: Distribution and basic biological patterns. Am. Fish. Soc. Symp. 2008, 63, 285–316. [Google Scholar] [CrossRef]
- Alferof, A.I.; Kurnosov, D.S. Life Cycle Characteristics and Distribution of Giant Grenadier Coryphaenoides pectoralis (Macrouridae) in Northwest Bering Sea. J. Ichthyol. 2024, 64, 304–316. [Google Scholar] [CrossRef]
- Dautova, T.N.; Galkin, S.V.; Tabachnik, K.R.; Minin, K.V.; Kireev, P.A.; Moskovtseva, A.V.; Adrianov, A.V. First data on the structure of vulnerable marine ecosystems of the Emperor Chain seamounts: Indicator taxa, landscapes, biogeography. Russ. J. Mar. Biol. 2019, 45, 408–417. [Google Scholar] [CrossRef]
- Darnitskiy, V.B. On the history of seamounts studies in the Pacific Ocean (oceanographic processes). Izv. TINRO 2005, 141, 255–283. [Google Scholar]
- Fedosova, R.A. Distribution of mesoplankton biomass in the area of the Hawaiian underwater ridge. Izv. TINRO 1974, 92, 38–42. [Google Scholar]
- Fedosova, R.A. Some data on the feeding of the boarfish Pentaceros richardsoni Smith on the banks of the Hawaiian Ridge. Issled. Biol. Ryb. Prom. Okeanogr. 1976, 7, 29–36. [Google Scholar]
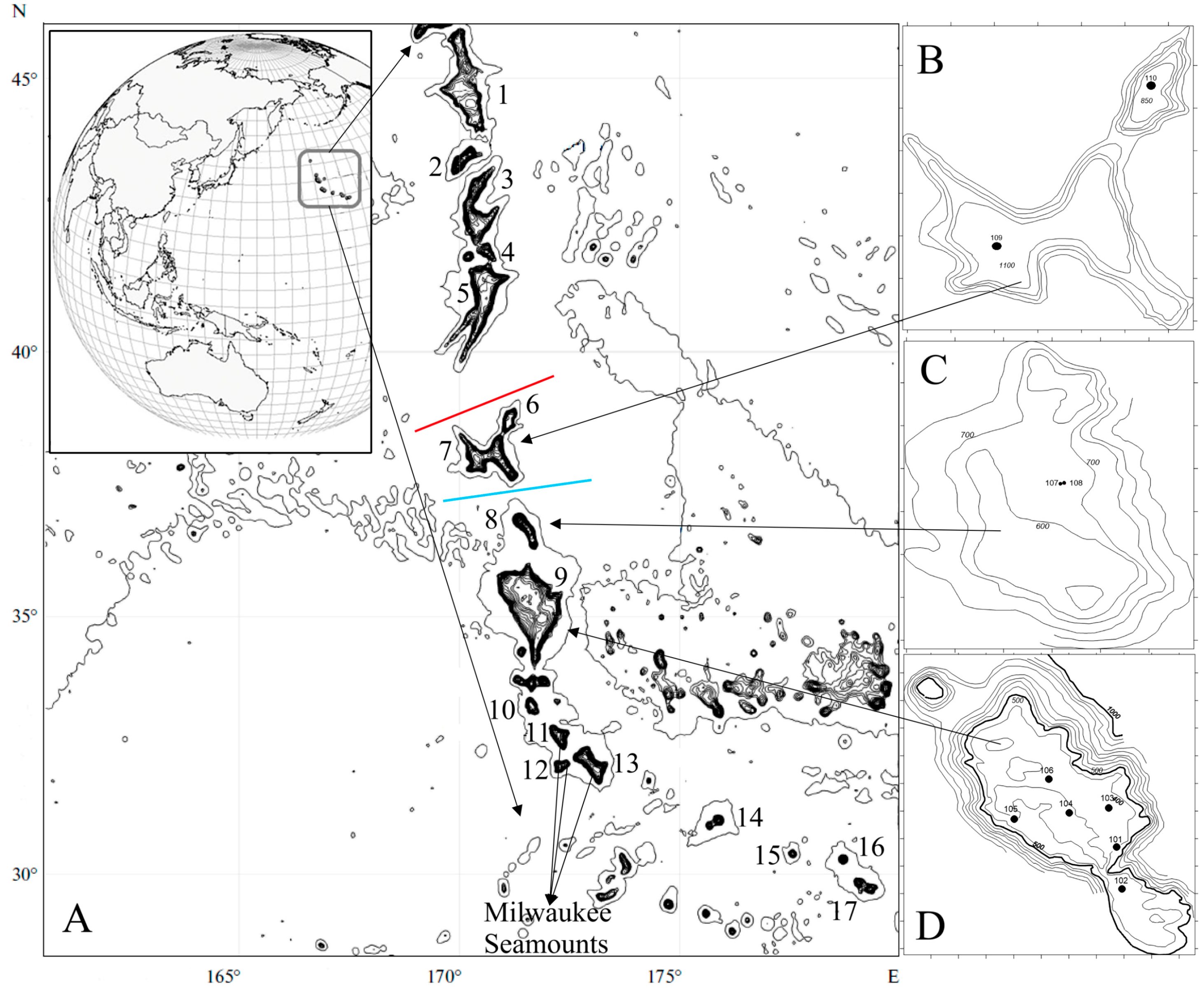
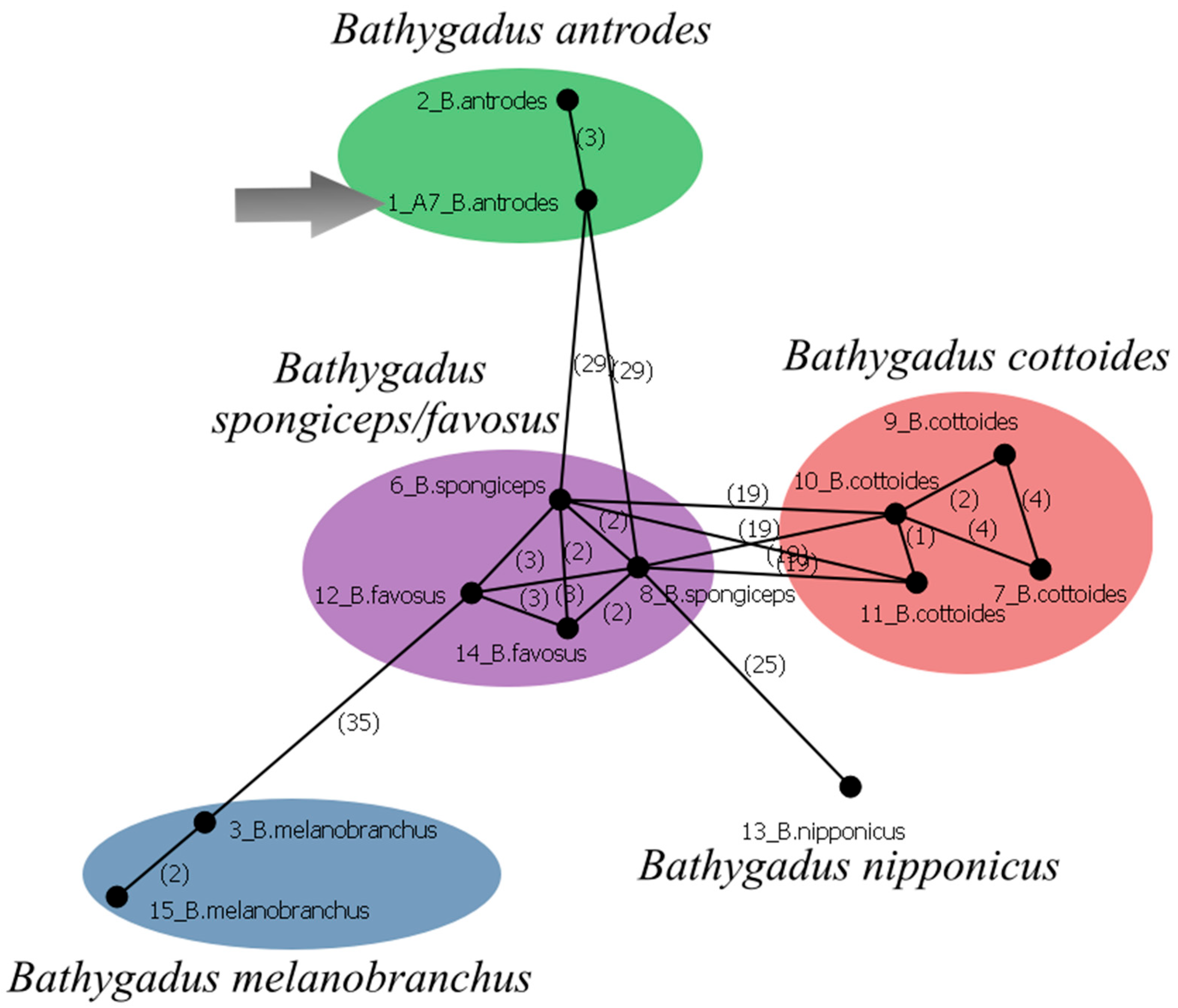
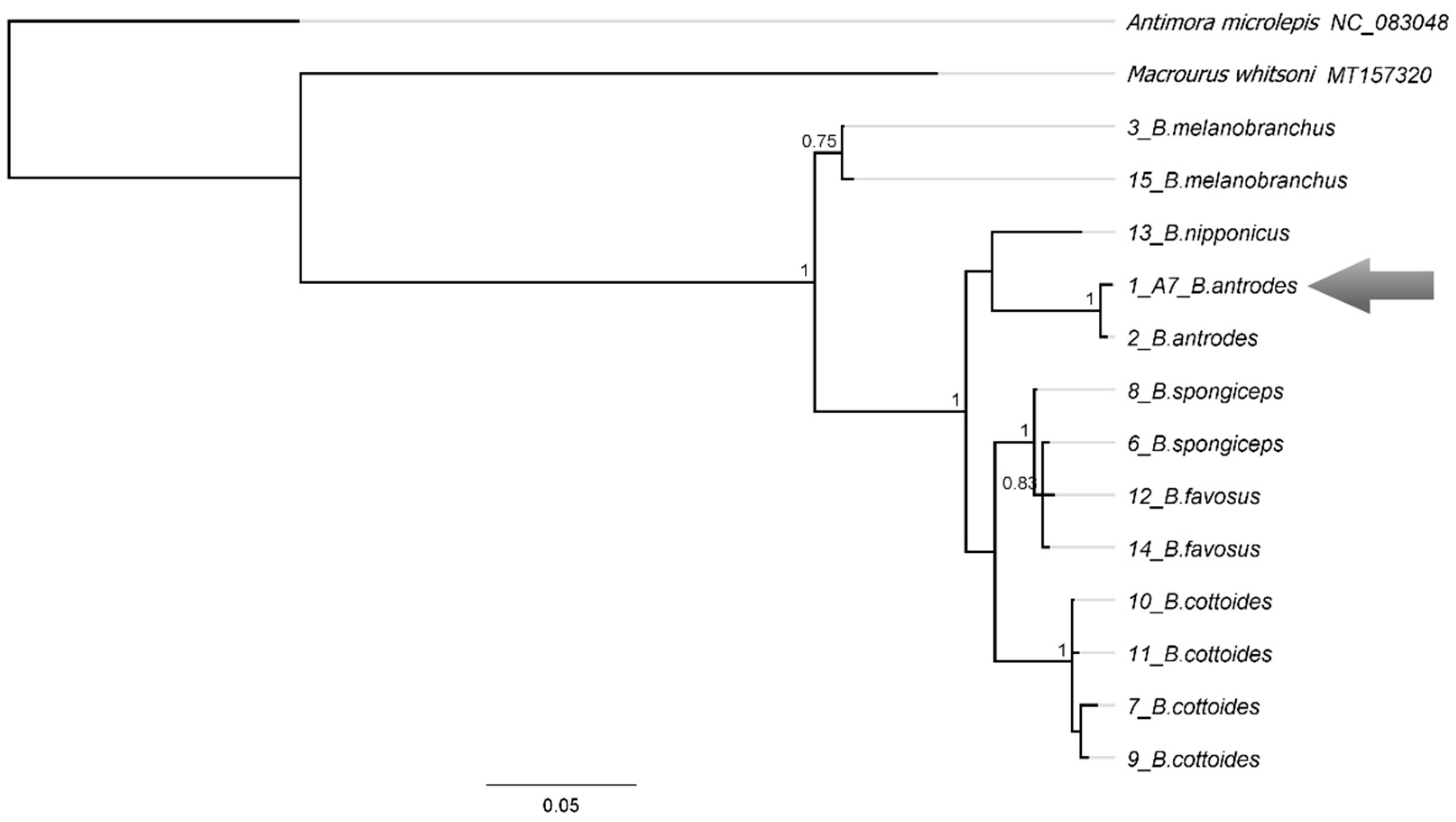
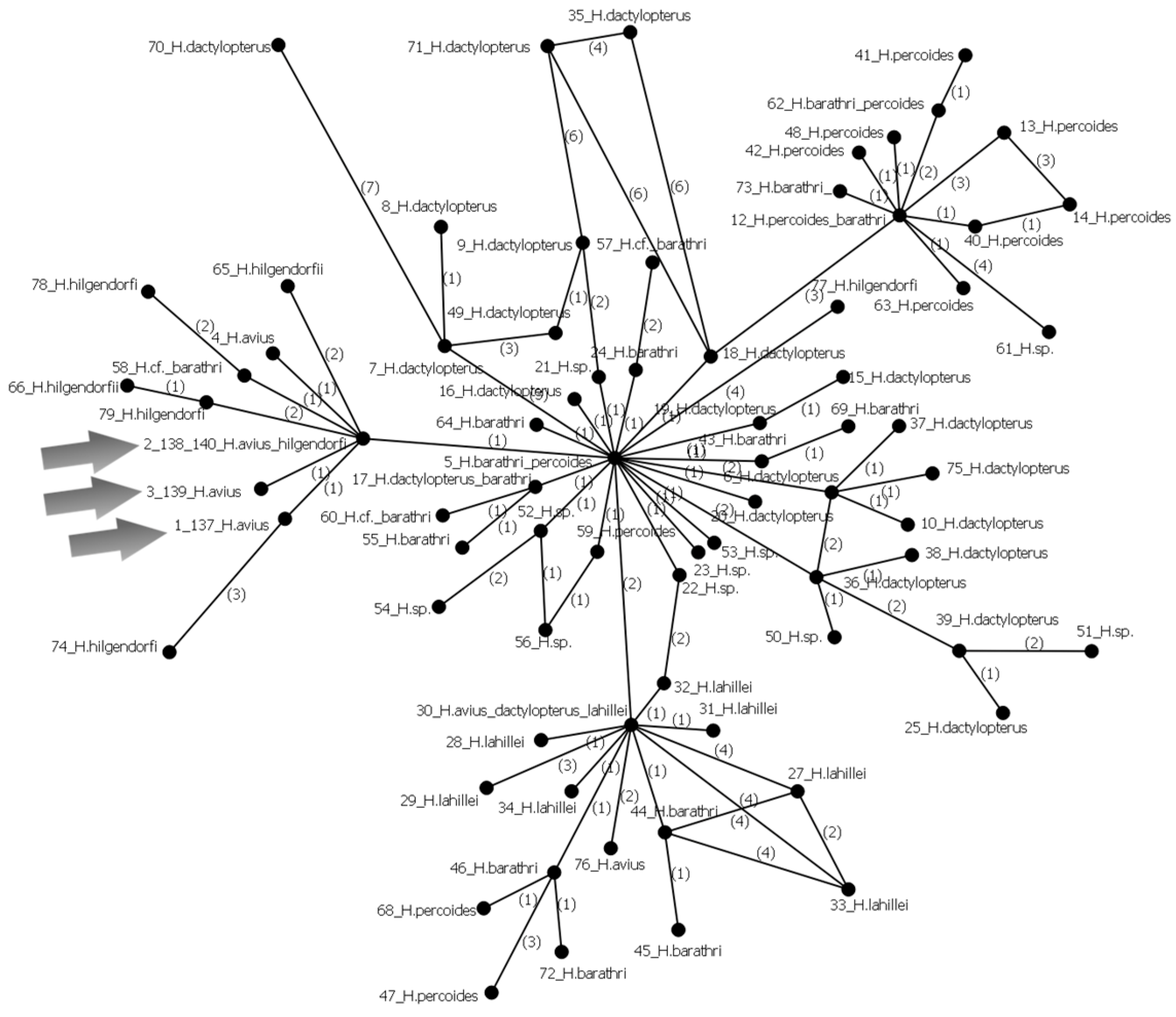
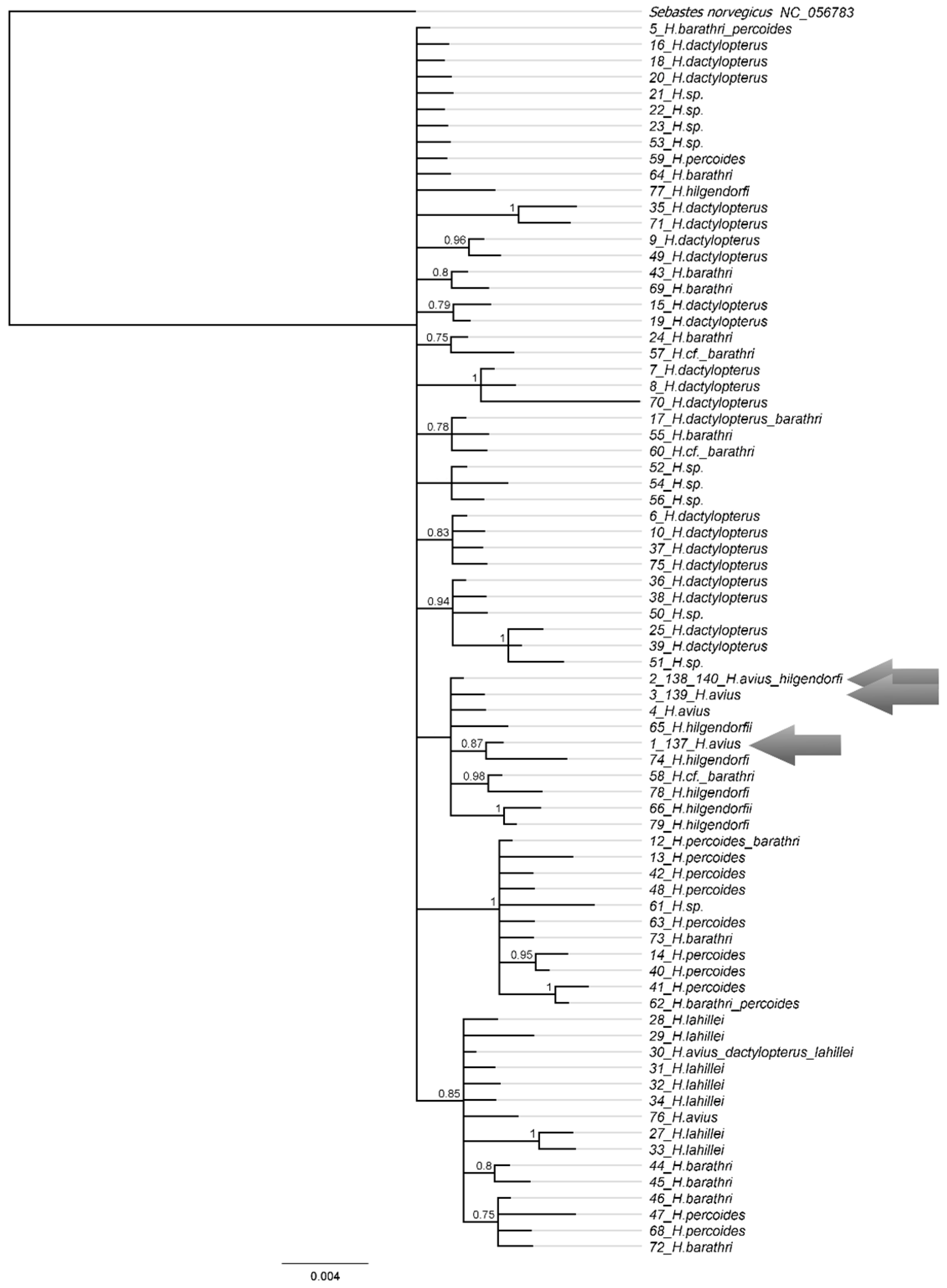
| Station No. | Seamount | Date | Latitude, N | Longitude, E | Depth, m | Bottom Temperature, °C |
|---|---|---|---|---|---|---|
| 101 | Koko | 9 April 2019 | 35°10′ | 171°46′ | 383–387 | 10.4 |
| 102 | 35°01′ | 171°47′ | 353–358 | 10.6 | ||
| 103 | 35°19′ | 171°44′ | 347–359 | 10.8 | ||
| 104 | 35°17′30″ | 171°35′ | 291 | 11.7 | ||
| 105 | 10 April 2019 | 35°16′ | 171°23′30″ | 353–357 | 10.7 | |
| 106 | 35°24′30″ | 171°31′30″ | 344–336 | 10.6 | ||
| 107 | Annei (Lira) | 11 April 2019 | 36°47′30″ | 171°23′ | 643–649 | 4.9 |
| 108 | 36°47′30″ | 171°23′ | 642–653 | 4.7 | ||
| 109 | Ojin | 12 April 2019 | 37°57′ | 170°24′ | 1030 | 3.35 |
| 110 | Jingu | 14 April 2019 | 38°42′ | 171°07′ | 820 | 3.8 |
| Species | Laboratory DNA Sample Number (IORAS Voucher Specimen Number) | GenBank Accession Number | Sequence Length (bp) | Comments |
|---|---|---|---|---|
| Etmopteridae | ||||
| Etmopterus cf. lailae | 19 (P.05030) | PX647299 | 717 | First barcode-flagged and public sequences for this species |
| 63 (P.04998) | PX647313 | 717 | ||
| 64 (P.04998) | PX647314 | 717 | ||
| 65 (P.04998) | PX647315 | 717 | ||
| 66 (P.04998) | PX647316 | 717 | ||
| 67 (P.04998) | PX647317 | 717 | ||
| Etmopterus pusillus | 119 (P.05024) | PX647323 | 721 | |
| 120 (P.05024) | PX647324 | 721 | ||
| 121 (P.05024) | PX647325 | 721 | ||
| Congridae | ||||
| Gnatophis johnsoni | 70 (P.04999) | PV455848 | 618 | [24] |
| 71 (P.04999) | PV455851 | 618 | ||
| 72 (P.04999) | PV455850 | 618 | ||
| 73 (P.04999) | PV455849 | 618 | ||
| 74 (P.04999) | PV455847 | 618 | ||
| Bathylagidae | ||||
| Lipolagus ochotensis | 45 (P.05058) | PX647307 | 678 | |
| A74 (P.05032) | PX647344 | 468 | ||
| Melanolagus bericoides | 108 (P.05033) | PX660574 | 463 | We could not receive a good COI sequence from this sample, but we obtained the first public sequence of the Cyt b gene fragment |
| Gonostomatidae | ||||
| Sigmops elongatus | 44 (P.03668) | PX647306 | 677 | |
| Sigmops gracilis | A77 (P.03669) | PX647346 | 614 | |
| A79 (P.03669) | PX647347 | 430 | ||
| Cyclothone atraria | A80 (P.05035) | PX647348 | 622 | |
| Sternoptychidae | ||||
| Maurolicus imperatorius | A9 (P.05020) | PX647338 | 717 | First barcode-flagged and public sequence for this species |
| A11 (P.05020) | PX647339 | 717 | ||
| A12 (P.05020) | PX647340 | 717 | ||
| Argyropelecus affinis | A81 (P.05036) | PX647349 | 619 | |
| Polyipnus matsubarai | 16 (P.05015) | PX647298 | 608 | |
| Sternoptyx diaphana | A75 (P.05038) | PX647345 | 614 | |
| Stomiidae | ||||
| Chauliodus sloani | 43 (P.05060) | PX647305 | 539 | These sequences represent a separate lineage (possible cryptic species) within the C. sloani complex |
| A51 (P.05068) | PX647342 | 539 | ||
| A52 (P.05068) | PX647343 | 539 | ||
| Melanostomias valdiviae | 86 (P.05051) | PX647320 | 610 | |
| Opostomias mitsui | 13 (P.05069) | PX647297 | 539 | |
| Chlorophthalmidae | ||||
| Chlorophthalmus imperator | 48 (P.05011) | PX647308 | 611 | First barcode-flagged and public sequence for this species |
| 50 (P.05011) | PX647309 | 613 | ||
| Neoscopelidae | ||||
| Neoscopelus cf. macrolepidotus | 127 (S.0114) | PX647326 | 613 | |
| 128 (S.0114) | PX647327 | 613 | ||
| Moridae | ||||
| Lepidion inosimae | 53 (P.05042) | PX647311 | 659 | |
| Macrouridae | ||||
| Bathygadus antrodes | A7 (M.00109) | PX647337 | 726 | |
| Coelorinchus matsubarae | 7 (M.00109-001) | PX647291 | 722 | First barcode-flagged and public sequence for this species |
| 8 (M.00109-003) | PX647292 | 722 | ||
| 9 (M.00109-004) | PX647293 | 722 | ||
| 10 (M.00109-002) | PX647294 | 722 | ||
| 11 (M.00109-005) | PX647295 | 722 | ||
| Nezumia obliquata | 23 (M.00107-003) | PX647300 | 703 | First barcode-flagged and public sequence for this species |
| 24 (M.00107-002) | PX647301 | 703 | ||
| Nezumia cf. proxima | 25 (M.00107-001) | PX647302 | 474 | |
| Squalogadus modificatus | A18 (not catalogued) | PX647341 | 601 | |
| Lophiidae | ||||
| Lophioides bruchius | 56 (P.05002) | PX647312 | 679 | First barcode-flagged and public sequence for this species |
| Oneirodidae | ||||
| Bertella idiomorpha | 100 (P.05043) | PX647321 | 662 | |
| Macroramphosidae | ||||
| Macroramphosus gracilis | 5 (P.05021) | PX647289 | 680 | |
| Sebastidae | ||||
| Adelosebastes latens | 134 (P.05055) | PX647328 | 452 | First barcode-flagged and public sequence for this genus and species |
| 135 (P.05055) | PX647329 | 421 | ||
| Helicolenus avius | 137 (P.05005) | PX647330 | 675 | |
| 138 (P.05005) | PX647331 | 675 | ||
| 139 (P.05005) | PX647332 | 675 | ||
| 140 (P.05005) | PX647333 | 675 | ||
| Plectrogeniidae | ||||
| Bembradium roseum | 6 (P.05022) | PX647290 | 620 | |
| 26 (P.05013) | PX647303 | 620 | ||
| Howellidae | ||||
| Howella parini | 36 (P.05063) | PX647304 | 666 | Although it is the first barcode-flagged sequence for this species, it forms a single unique lineage with “Bathysphyraenops simplex” (likely misidentification; OR582658, no locality data) |
| 147 (P.05044) | PX647334 | 590 | ||
| 148 (P.05044) | PX647335 | 590 | ||
| 150 (P.05044) | PX647336 | 616 | ||
| Epigonidae | ||||
| Epigonus denticulatus | A100 (P.05008) | PX647350 | 664 | |
| Bramidae | ||||
| Brama japonica | 52 (P.05009) | PX647310 | 677 | |
| Chiasmodontidae | ||||
| Pseudoscopelus altipinnis | 107 (P.05045) | PX647322 | 684 | First barcode-flagged and public sequence for this species |
| Trichiuridae | ||||
| Benthodesmus pacificus | 12 (P.05018) | PX647296 | 614 | Although it is the first barcode-flagged sequence for this species, it is identical to the samples GU805059/DSFSF394-09 and KF489506/DSFSG511-11 from South Africa identified as B. simonyi |
| 68 (P.05010) | PX647318 | 640 | ||
| Zoarcidae | ||||
| Lycodapus imperatorius | 89 (P. 03641) | OP759467 | 582 | [26] |
| Pleuronectidae | ||||
| Microstomus shuntovi | 69 (P.05029) | PX647319 | 590 | |
| Species | Annei | Koko | Ojin | Jingu | Remarks |
|---|---|---|---|---|---|
| Centroscyllium excelsum (B) | + | ||||
| Etmopterus cf. lailae (B) | + | ||||
| Apristurus fedorovi (B) | + | ||||
| Gnathophis johnsoni (B) | + | Newly described species | |||
| Nettastoma parviceps (B) | + | ||||
| Meadia abyssalis (B) | + | ||||
| Simenchelys parasitica (B) | + | ||||
| Nemichthys scolopaceus (P) | + | ||||
| Lipolagus ochotensis (P) | + | + | |||
| Melanolagus bericoides (P) | + | + | First record for the area | ||
| Sagamichthys abei (B) | + | ||||
| Diplophos orientalis (P) | + | ||||
| Sigmops elogatus (P) | + | ||||
| Sigmops gracilis (P) | + | ||||
| Cyclothone atraria (P) | + | ||||
| Argyropelecus affinis (P) | + | First record for the area | |||
| Argyropelecus sladeni (P) | + | ||||
| Sternoptyx diaphana (P) | + | ||||
| Ichthyococcus elongatus (P) | + | ||||
| Eustomias securicula (P) | + | Newly described species | |||
| Melanostomias valdiviae (P) | + | ||||
| Opostomias mitsuii (P) | + | ||||
| Idiacanthus antrostomus (P) | + | ||||
| Malacosteus niger (P) | + | ||||
| Benthalbella infans (P) | + | ||||
| Diaphus balanovi (B) | + | + | Newly described species | ||
| Neoscopelus cf. macrolepidotus (B) | + | ||||
| Lepidion inosimae (B) | + | ||||
| Melanonus zugmayeri (P) | + | First record for the area | |||
| Bathygadus antrodes (B) | + | ||||
| Nezumia obliquata (B) | + | ||||
| Nezumia cf. proxima (B) | + | First record for the area | |||
| Squalogadus modificatus (B, P) | + | First record for the area | |||
| Bertella idiomorpha (P) | + | First record for the area | |||
| Linophryne arborifera (P) | + | First record for the Pacific Ocean | |||
| Macroramphosus gracilis (P) | + | ||||
| Scopeloberyx malayanus (P) | + | First record for the area | |||
| Bembradium roseum (B) | + | ||||
| Psednos kaganovskii (P) | + | Newly described species | |||
| Howella parini (B, P) | + | + | |||
| Pseudoscopelus altipinnis (P) | + | ||||
| Benthodesmus pacificus (B, P) | + | ||||
| Lycodapus imperatorius (P) | + | + | Newly described species | ||
| Microstomus bathybius (B) | + | + |
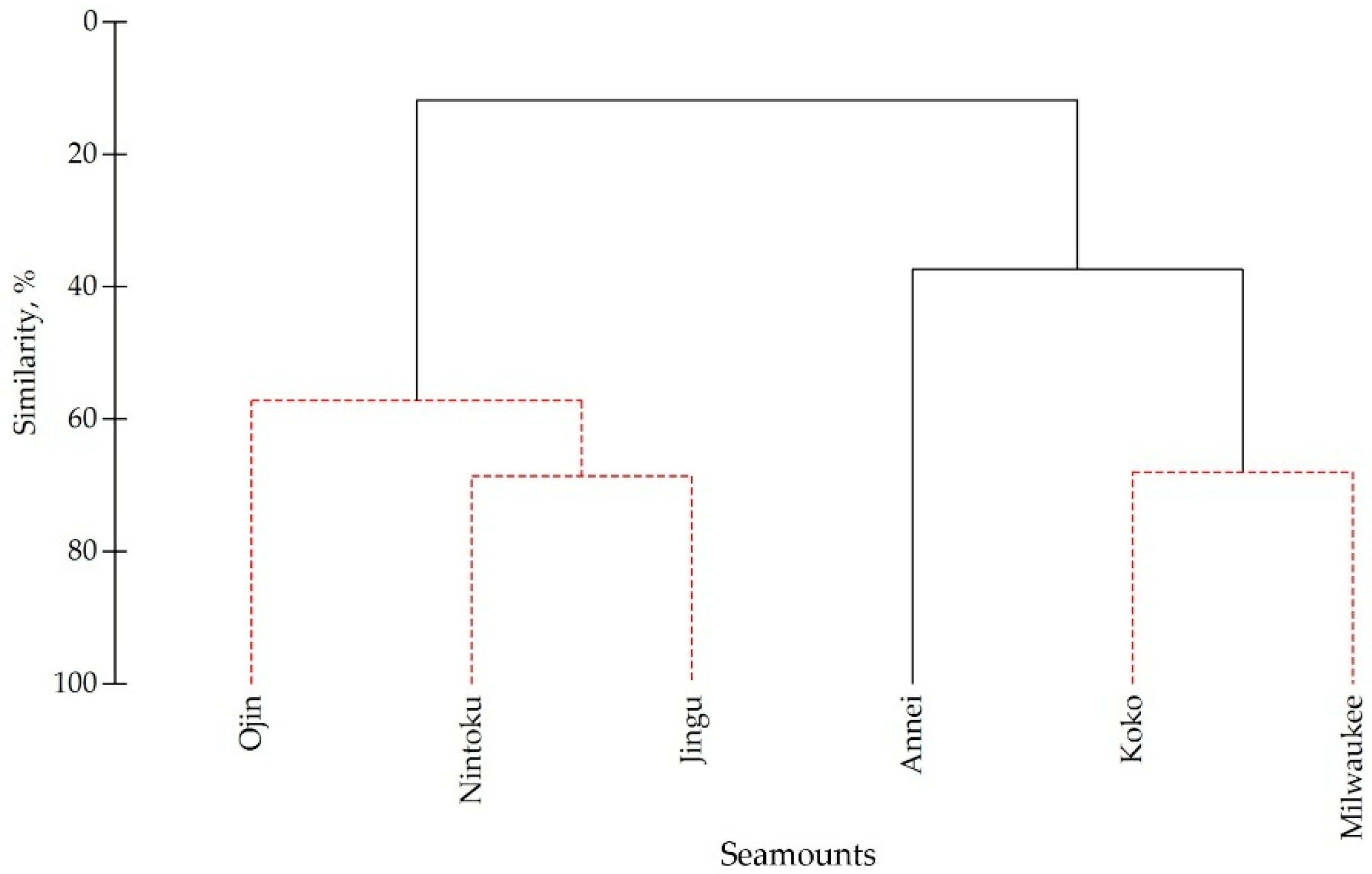
| Seamounts | Nintoku | Jingu | Ojin | Koko | Milwaukee |
|---|---|---|---|---|---|
| Nintoku | |||||
| Jingu | 68.57 | ||||
| Ojin | 57.14 | 63.16 | |||
| Koko | 17.91 | 25.71 | 28.57 | ||
| Milwaukee | 11.76 | 16.90 | 22.54 | 67.96 | |
| Annei | 35.90 | 42.86 | 52.38 | 40.54 | 37.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
M. Prokofiev, A.; R. Emelianova, O.; Y. Saveleva, S.; M. Orlov, A. Diversity and Distribution of Deep-Sea Fishes off the Emperor Seamounts, Northwestern Pacific Ocean, with DNA Barcodes, Phylogenetic, and Biogeographic Considerations. J. Mar. Sci. Eng. 2026, 14, 63. https://doi.org/10.3390/jmse14010063
M. Prokofiev A, R. Emelianova O, Y. Saveleva S, M. Orlov A. Diversity and Distribution of Deep-Sea Fishes off the Emperor Seamounts, Northwestern Pacific Ocean, with DNA Barcodes, Phylogenetic, and Biogeographic Considerations. Journal of Marine Science and Engineering. 2026; 14(1):63. https://doi.org/10.3390/jmse14010063
Chicago/Turabian StyleM. Prokofiev, Artem, Olga R. Emelianova, Svetlana Y. Saveleva, and Alexei M. Orlov. 2026. "Diversity and Distribution of Deep-Sea Fishes off the Emperor Seamounts, Northwestern Pacific Ocean, with DNA Barcodes, Phylogenetic, and Biogeographic Considerations" Journal of Marine Science and Engineering 14, no. 1: 63. https://doi.org/10.3390/jmse14010063
APA StyleM. Prokofiev, A., R. Emelianova, O., Y. Saveleva, S., & M. Orlov, A. (2026). Diversity and Distribution of Deep-Sea Fishes off the Emperor Seamounts, Northwestern Pacific Ocean, with DNA Barcodes, Phylogenetic, and Biogeographic Considerations. Journal of Marine Science and Engineering, 14(1), 63. https://doi.org/10.3390/jmse14010063







