Morphological and Immunohistochemical Study of Ventral Photophores of Ichthyococcus ovatus (Cocco, 1838) (Fam: Stomiidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Tissue Treatment
2.2. Immunohistochemistry
3. Results
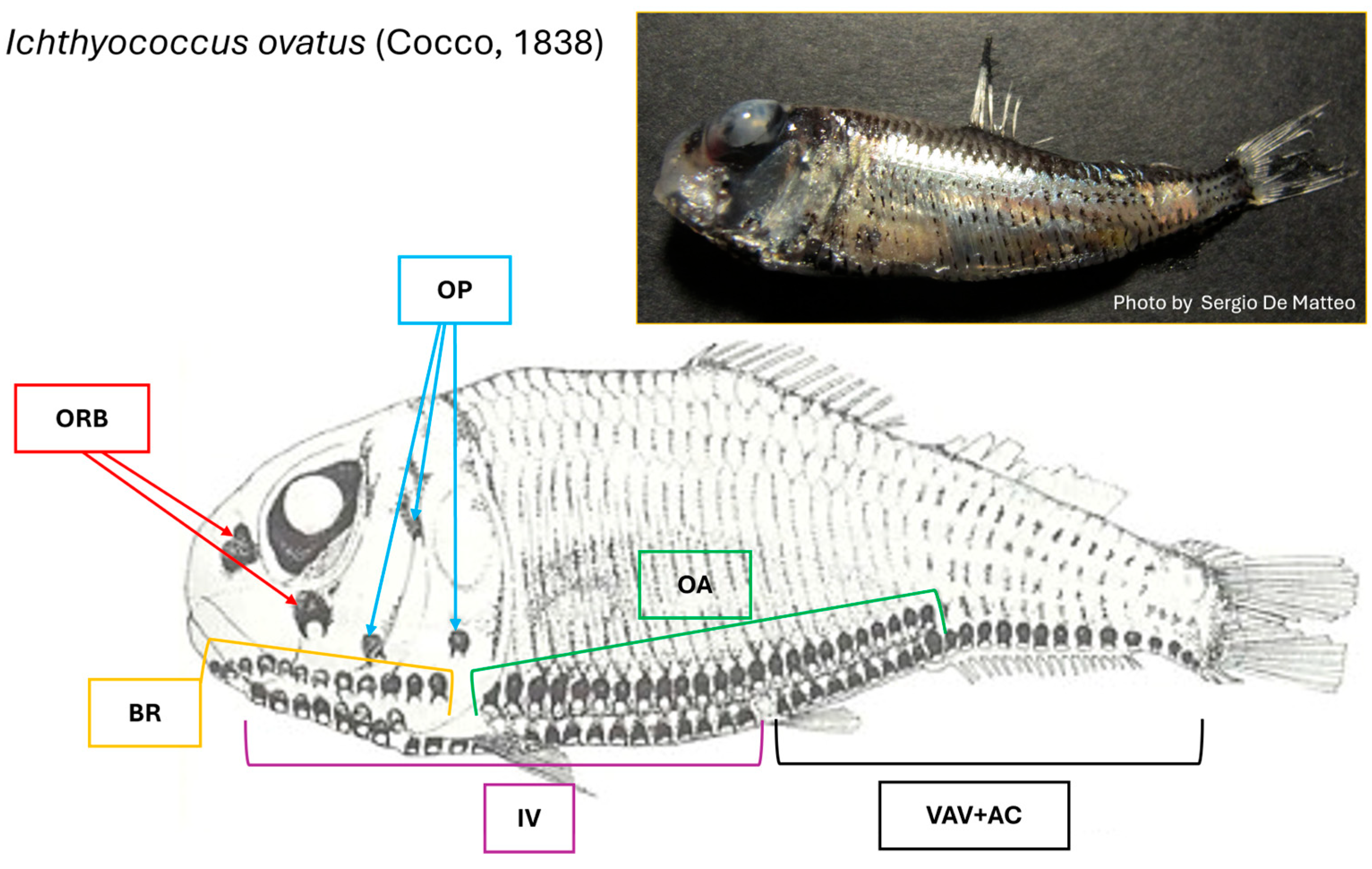
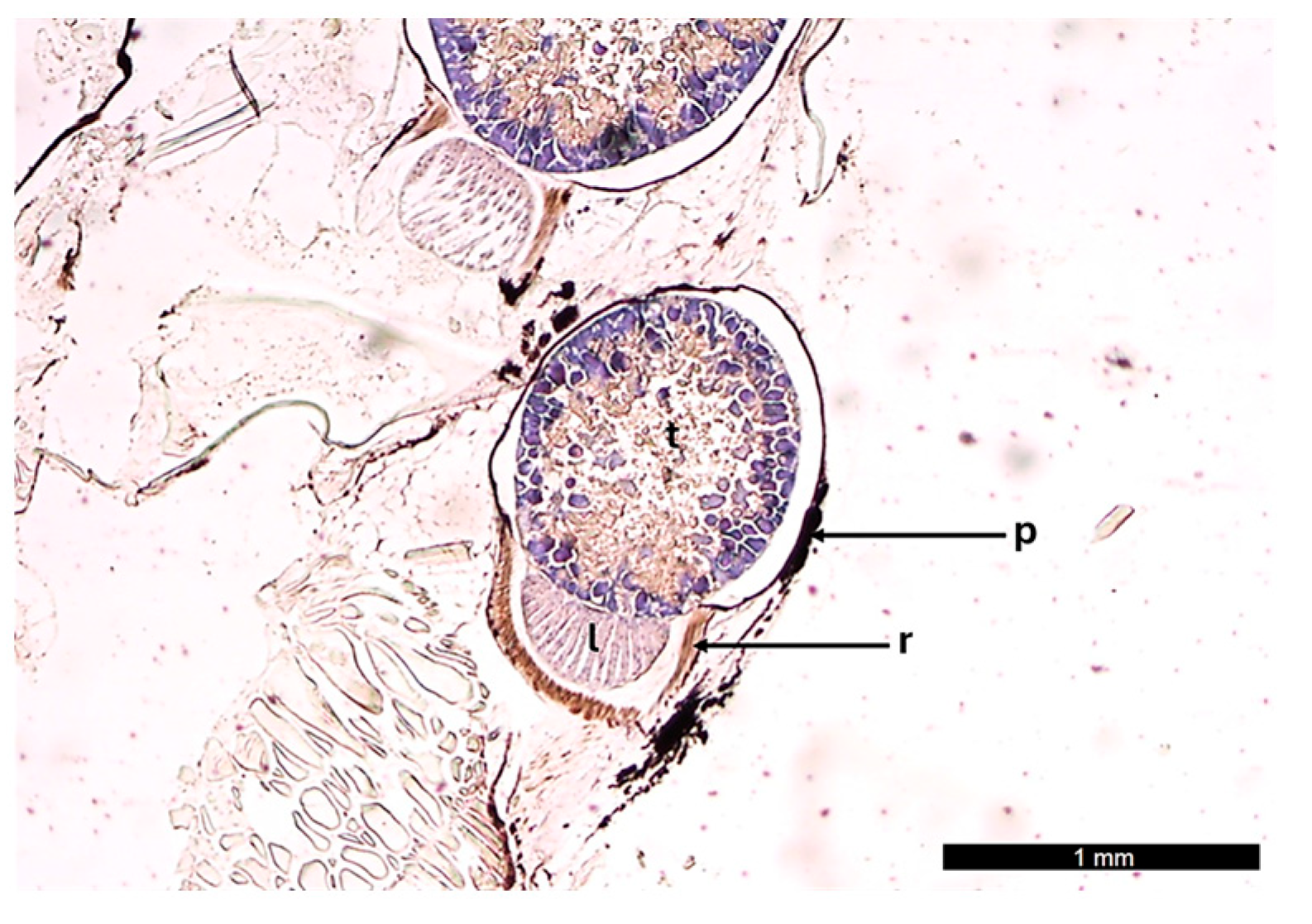
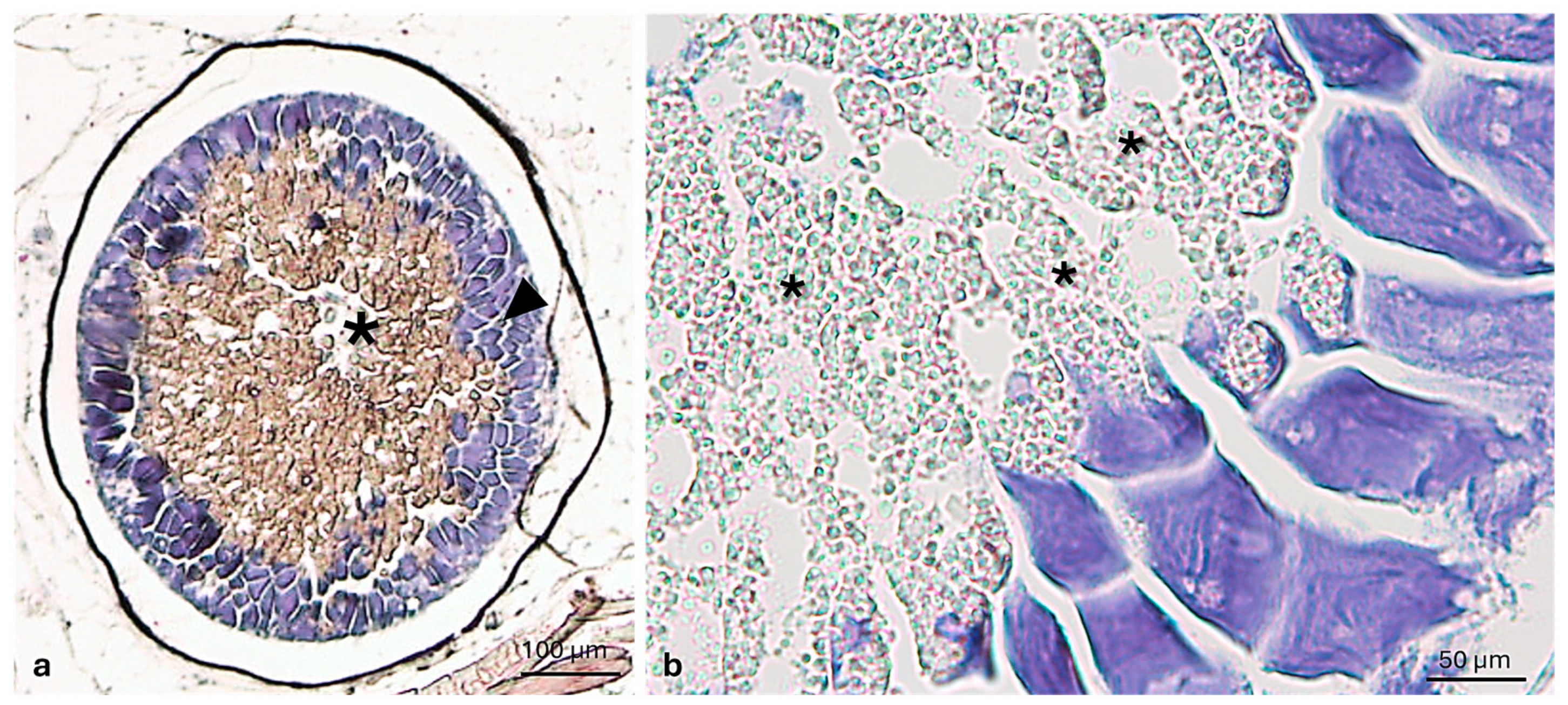
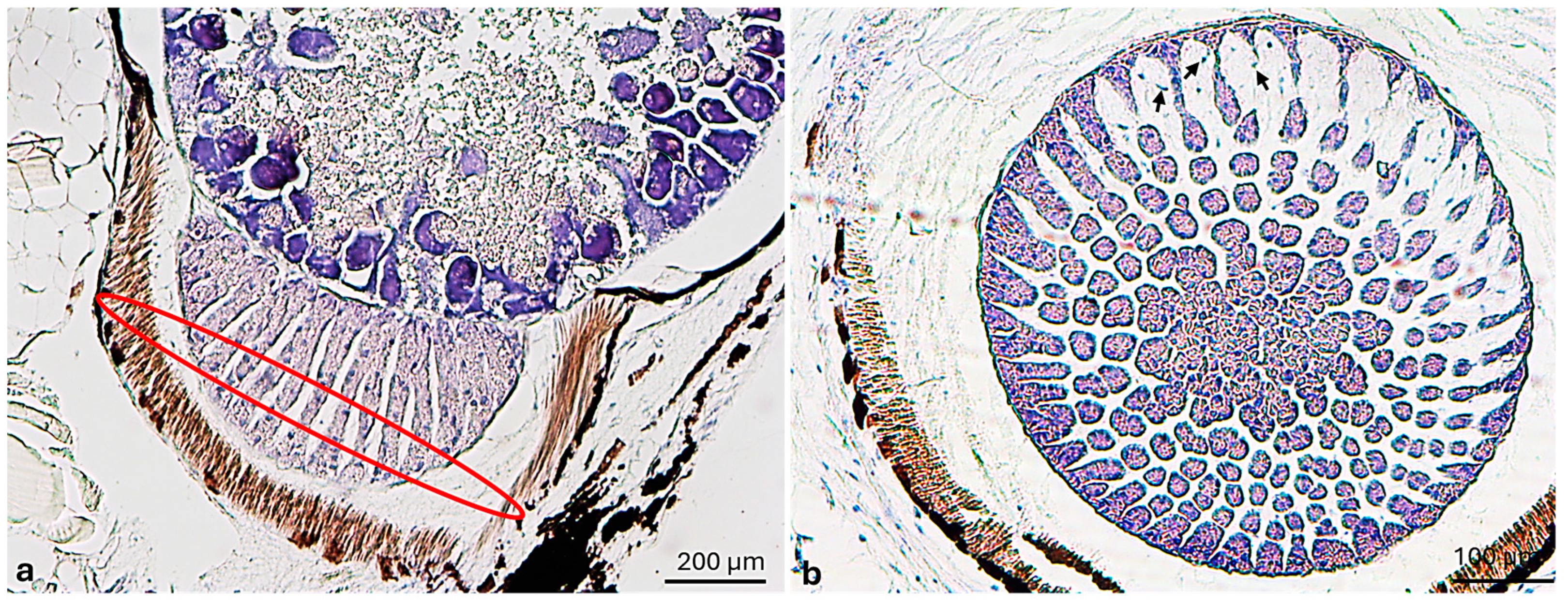
3.1. Morphological Analysis
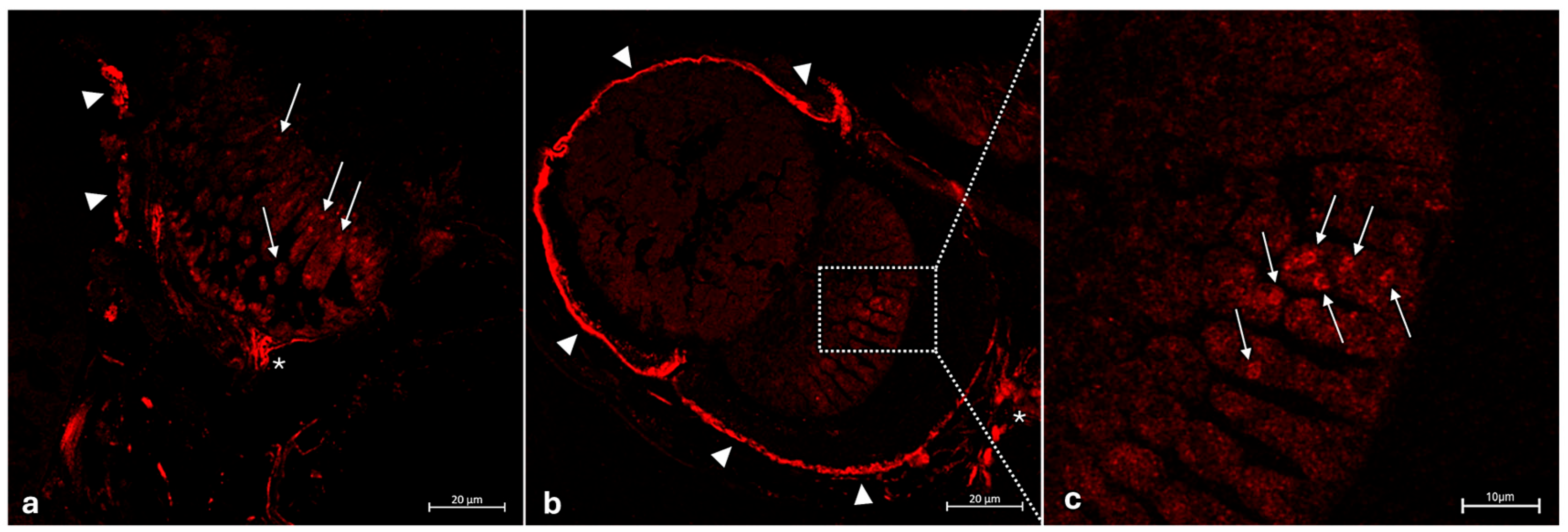
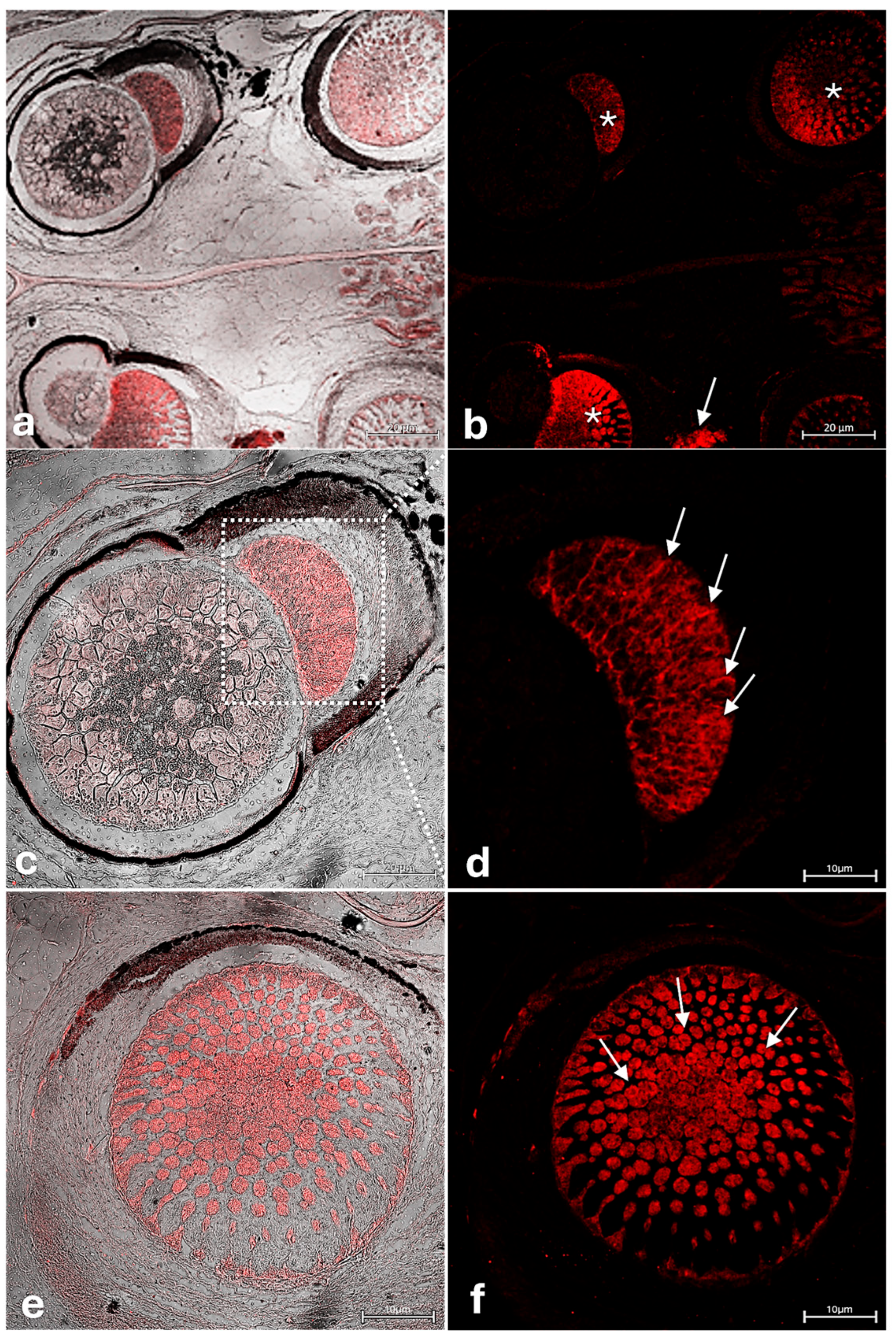
3.2. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavallaro, M.; Pansera, L.; Mhalhel, K.; Abbate, F.; Levanti, M.; Guerrera, M.C.; Montalbano, G.; Briglia, M.; Aragona, M.; Laurà, R. The Skin Photophores of Chauliodus sloani Bloch & Schneider, 1801 (Pisces: Stomiidae): A Morphological, Ultrastructural and Immunohistochemical Study. Animals 2025, 15, 1738. [Google Scholar] [CrossRef]
- Herring, P.J. Systematic distribution of bioluminescence in living organisms. J. Biolumin. Chemilumin. 1987, 1, 147–163. [Google Scholar] [CrossRef]
- Schramm, S.; Weiß, D. Bioluminescence—The Vibrant Glow of Nature and its Chemical Mechanisms. ChemBioChem 2024, 25, e202400106. [Google Scholar] [CrossRef]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Krönström, J.; Holmgren, S.; Baguet, F.; Salpietro, L.; Mallefet, J. Nitric oxide in control of luminescence from hatchetfish (Argyropelecus hemigymnus) photophores. J. Exp. Biol. 2005, 208, 2951–2961. [Google Scholar] [CrossRef][Green Version]
- Claes, J.M.; Aksnes, D.L.; Mallefet, J. Phantom hunter of the fjords: Camouflage by counterillumination in a shark (Etmopterus spinax). J. Exp. Mar. Biol. Ecol. 2010, 388, 28–32. [Google Scholar] [CrossRef]
- Zaccone, G.; Abelli, L.; Salpietro, L.; Zaccone, D.; Macrì, B.; Marino, F. Nervous control of photophores in luminescent fishes. Acta Histochem. 2011, 113, 387–394. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Chapter 38—Activation of NOS by Ca2+-Dependent and Ca2+-Independent Mechanisms. In Nitric Oxide; Ignarro, L.J., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 621–632. [Google Scholar] [CrossRef]
- Wright, N.T.; Prosser, B.L.; Varney, K.M.; Zimmer, D.B.; Schneider, M.F.; Weber, D.J. S100A1 and Calmodulin Compete for the Same Binding Site on Ryanodine Receptor*. J. Biol. Chem. 2008, 283, 26676–26683. [Google Scholar] [CrossRef]
- Rebbeck, R.T.; Nitu, F.R.; Rohde, D.; Most, P.; Bers, D.M.; Thomas, D.D.; Cornea, R.L. S100A1 Protein Does Not Compete with Calmodulin for Ryanodine Receptor Binding but Structurally Alters the Ryanodine Receptor Calmodulin Complex. J. Biol. Chem. 2016, 291, 15896–15907. [Google Scholar] [CrossRef]
- Aragona, M.; Mhalhel, K.; Cometa, M.; Franco, G.A.; Montalbano, G.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Abbate, F.; Vega, J.A.; et al. Piezo 1 and Piezo 2 in the Chemosensory Organs of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2024, 25, 7404. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; Calapai, F.; et al. Localization of BDNF and Calretinin in Olfactory Epithelium and Taste Buds of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 4696. [Google Scholar] [CrossRef]
- Germanà, A.; Guerrera, M.C.; Laurà, R.; Levanti, M.; Aragona, M.; Mhalhel, K.; Germanà, G.; Montalbano, G.; Abbate, F. Expression and Localization of BDNF/TrkB System in the Zebrafish Inner Ear. Int. J. Mol. Sci. 2020, 21, 5787. [Google Scholar] [CrossRef]
- Germanà, A.; Marino, F.; Guerrera, M.C.; Campo, S.; de Girolamo, P.; Montalbano, G.; Germanà, G.P.; Ochoa-Erena, F.J.; Ciriaco, E.; Vega, J.A. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 2008, 71, 248–255. [Google Scholar] [CrossRef]
- Kraemer, A.M.; Saraiva, L.R.; Korsching, S.I. Structural and functional diversification in the teleost S100 family of calcium-binding proteins. BMC Evol. Biol. 2008, 8, 48. [Google Scholar] [CrossRef]
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the Sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef]
- Paitio, J.; Oba, Y. Luminous fishes: Endocrine and neuronal regulation of bioluminescence. Aquac. Fish. 2024, 9, 486–500. [Google Scholar] [CrossRef]
- Smith, W.L.; Girard, M.G.; Walker, H.J., Jr.; Davis, M.P. The phylogeny of bristlemouths, lightfishes, and portholefishes with a revised family-level classification of the dragonfishes (Teleostei: Stomiiformes). Prof. Pap. NMFS 2024, 24, 167–184. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References; California Academy of Sciences: San Francisco, CA, USA, 2024. [Google Scholar]
- Cavallaro, M.; Guerrera, M.C.; Abbate, F.; Levanti, M.B.; Laurà, R.; Ammendolia, G.; Malara, D.; Stipa, M.G.; Battaglia, P. Morphological, ultrastructural and immunohistochemical study on the skin ventral photophores of Diaphus holti Tåning, 1918 (Family: Myctophidae). Acta Zool. 2020, 102, 405–411. [Google Scholar] [CrossRef]
- Gjøsæter, J.; Kawaguchi, K. A Review of the World Resources of Mesopelagic Fish; Food and Agriculture Organization of the United Nations: Rome, Italy, 1980. [Google Scholar]
- Stukel, M.R.; Ducklow, H.W. Stirring Up the Biological Pump: Vertical Mixing and Carbon Export in the Southern Ocean. Glob. Biogeochem. Cycles 2017, 31, 1420–1434. [Google Scholar] [CrossRef]
- Clardy, T.R.; Gopalan, J.; Rabaoui, L.J. Morphology of photophores in juvenile Vinciguerria mabahiss (Stomiiformes: Phosichthyidae). Ichthyol. Res. 2025. [Google Scholar] [CrossRef]
- Martin, R.P.; Carr, E.M.; Sparks, J.S. Variation in lanternfish (Myctophidae) photophore structure: A comprehensive comparative analysis. PLoS ONE 2024, 19, e0310976. [Google Scholar] [CrossRef]
- Harvey, E.N. The luminous organs of fishes. In The Physiology of Fishes; Brown, M.E., Ed.; Elsevier: New York, NY, USA, 1957; Volume 3, pp. 345–366. [Google Scholar]
- Haygood, M.G.; Edwards, D.B.; Mowlds, G.; Rosenblatt, R.H. Bioluminescence of myctophid and stomiiform fishes is not due to bacterial luciferase. J. Exp. Zool. 1994, 270, 225–231. [Google Scholar] [CrossRef]
- Baguet, F. Les Photophores des Poissons Lumineux. Rev. Quest. Sci. 1977, 148, 21–41. [Google Scholar]
- Herring, P.J. Bioluminescence of marine organisms. Nature 1977, 267, 788–793. [Google Scholar] [CrossRef]
- Anctil, M. Stimulation of bioluminescence in lanternfishes (Myctophidae). II. Can. J. Zool. 1972, 50, 233–237. [Google Scholar] [CrossRef]
- Anctil, M.; Case, J.F. The caudal luminous organs of lanternfishes: General innervation and ultrastructure. Am. J. Anat. 1977, 149, 1–21. [Google Scholar] [CrossRef]
- Edwards, A.; Herring, P. Observations on the comparative morphology and operation of the photogenic tissues of myctophid fishes. Mar. Biol. 1977, 41, 59–70. [Google Scholar] [CrossRef]
- Herring, P.J. How to survive in the dark: Bioluminescence in the deep sea. Symp. Soc. Exp. Biol. 1985, 39, 323–350. [Google Scholar]
- Davis, M.P.; Sparks, J.S.; Smith, W.L. Repeated and widespread evolution of bioluminescence in marine fishes. PLoS ONE 2016, 11, e0155154. [Google Scholar] [CrossRef]
- Paitio, J.; Oba, Y.; Meyer-Rochow, V.B. Bioluminescent fishes and their eyes. In Luminescence—An Outlook on the Phenomena and Their Applications; Intech Open: London, UK, 2016. [Google Scholar]
- Paitio, J.; Yano, D.; Muneyama, E.; Takei, S.; Asada, H.; Iwasaka, M.; Oba, Y. Reflector of the body photophore in lanternfish is mechanistically tuned to project the biochemical emission in photocytes for counterillumination. Biochem. Biophys. Res. Commun. 2020, 521, 821–826. [Google Scholar] [CrossRef]
- Altun, T.; Celi, F.; Danabas, D. Bioluminescence in aquatic organisms. J. Anim. Vet. Adv. 2008, 7, 841–846. [Google Scholar]
- Battaglia, P.; Ammendolia, G.; Cavallaro, M.; Consoli, P.; Esposito, V.; Malara, D.; Rao, I.; Romeo, T.; Andaloro, F. Influence of lunar phases, winds and seasonality on the stranding of mesopelagic fish in the Strait of Messina (Central Mediterranean Sea). Mar. Ecol. 2017, 38, e12459. [Google Scholar] [CrossRef]
- Cavallaro, M. Studio Morfologico ed Ultrastrutturale dei Fotofori in Diverse Specie di Pesci Mesopelagici Dello Stretto di Messina. Ph.D. Thesis, Università degli Studi di Messina, Messina, Italy, 2017. [Google Scholar]
- Cavallaro, M.; Aragona, M.; Germanà, A. Morphological and immunohistochemical study on photophores of Gonostoma denudatum Rafinesque, 1810 (Fam: Gonostomatidae). Atti Accad. Peloritana Pericolanti Cl. Sci. Med. Biol. 2021, 109, 1–7. [Google Scholar] [CrossRef]
- Cavallaro, M.; Battaglia, P.; Guerrera, M.C.; Abbate, F.; Levanti, M.B.; Ammendolia, G.; Andaloro, F.; Germanà, A.; Laurà, R. Structure and ultrastructure study on photophores of the Madeira lanternfish, Ceratoscopelus maderensis (Lowe, 1839), Pisces: Myctophidae. Acta Zool. 2019, 100, 89–95. [Google Scholar] [CrossRef]
- Cavallaro, M.; Battaglia, P.; Guerrera, M.C.; Abbate, F.; Levanti, M.B.; Andaloro, F.; Germanà, A.; Laurà, R. New data on morphology and ultrastructure of skin photophores in the deep-sea squid Histioteuthis bonnellii (Férussac, 1834), Cephalopoda: Histioteuthidae. Acta Zool. 2017, 98, 271–277. [Google Scholar] [CrossRef]
- Cavallaro, M.; Battaglia, P.; Laurà, R.; Guerrera, M.C.; Abbate, F.; Germanà, A. The morphology of photophores in the garrick, Cyclothone braueri (Family: G onostomatidae): An ultrastructure study. Acta Zool. 2015, 96, 296–300. [Google Scholar] [CrossRef]
- Cavallaro, M.; Mammola, C.; Verdiglione, R. Structural and ultrastructural comparison of photophores of two species of deep-sea fishes: Argyropelecus hemigymnus and Maurolicus muelleri. J. Fish Biol. 2004, 64, 1552–1567. [Google Scholar] [CrossRef]
- Robison, B.H. Deep pelagic biology. J. Exp. Mar. Biol. Ecol. 2004, 300, 253–272. [Google Scholar] [CrossRef]
- Battaglia, P.; Pagano, L.; Consoli, P.; Esposito, V.; Granata, A.; Guglielmo, L.; Pedá, C.; Romeo, T.; Zagami, G.; Vicchio, T.M.; et al. Consumption of mesopelagic prey in the Strait of Messina, an upwelling area of the central Mediterranean Sea: Feeding behaviour of the blue jack mackerel Trachurus picturatus (Bowdich, 1825). Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 155, 103158. [Google Scholar] [CrossRef]
- Brauer, A. Die Tiefsee-Fische. II. Anatomischer Teil. Wiss. Ergebn. Dtsch. Tiefsee Exped. “Valdivia” 1908, 15, 1–266. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic PublicationFishBase. 2019. Available online: http://www.fishbase.org (accessed on 24 June 2025).
- Board, W.E. World Register of Marine Species. 2024. Available online: https://www.marinespecies.org (accessed on 24 June 2025).
- Van der Land, J.; Costello, M.; Zavodnik, D.; Santos, R.; Porteiro, F.; Bailly, N.; Eschmeyer, W.; Froese, R. Pisces. Collect. Patrim. Nat. 2001, 357–374. [Google Scholar]
- Abbate, F.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Montalbano, G.; Cavallaro, M.; Germanà, A. The tongue of Leopard Gecko (Eublepharis macularius): LM, SEM and confocal laser study. Anat. Histol. Embryol. 2020, 49, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Abbate, F.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Aragona, M.; Mhalhel, K.; Montalbano, G.; Germanà, A. Morphological characteristics of the blackspot seabream (Pagellus bogaraveo) tongue: A structural and immunohistochemical study. Anat. Histol. Embryol. 2022, 51, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Albano, M.; Savoca, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lo Cascio, P.; Hussein, M.M.; Capillo, G.; Pergolizzi, S.; et al. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology 2022, 11, 1653. [Google Scholar] [CrossRef]
- Zaccone, G.; Maina, J.; Germanà, A.; Montalbano, G.; Capillo, G.; Aragona, L.; Kuciel, M.J.; Lauriano, E.R.; Icardo, J.M. First demonstration of the neuroepithelial cells and their chemical code in the accessory respiratory organ and the gill of the sharptooth catfish, Clarias gariepinus: A preliminary study. Acta Zool. 2019, 100, 160–166. [Google Scholar] [CrossRef]
- Whitehead, P.J.P.; Bauchot, M.-L.; Hureau, J.-C.; Nielsen, J.; Tortonese, E. Fishes of the North-Eastern Atlantic and the Mediterranean; UNESCO: Paris, France, 1984; Volume 1. [Google Scholar]
- Cavallaro, M.; Ammendolia, G.; Andaloro, F.; Battaglia, P. First record of the mesopelagic fish Diaphus dumerilii (Bleeker, 1856) in the Mediterranean Sea. Mar. Biodivers. 2017, 47, 585–588. [Google Scholar] [CrossRef]
- Denton, E.; Gilpin-Brown, J.; Roberts, B. On the organization and function of the photophores of Argyropelecus. J. Physiol. 1969, 204, 38P–39P. [Google Scholar]
- Denton, E.; Gilpin-Brown, J.; Wright, P. On the ‘filters’ in the photophores of mesopelagic fish and on a fish emitting red light and especially sensitive to red light. J. Physiol. 1970, 208, 72P–73P. [Google Scholar]
- Denton, E.; Herring, P.J. On the filters in the ventral photophores of mesopelagic animals [proceedings]. J. Physiol. 1978, 284, 42P. [Google Scholar] [PubMed]
- Denton, E.J.; Herring, P.; Widder, E.; Latz, M.; Case, J. The roles of filters in the photophores of oceanic animals and their relation to vision in the oceanic environment. Proc. R. Soc. London. Ser. B. Biol. Sci. 1985, 225, 63–97. [Google Scholar] [CrossRef]
- Mallefet, J.; Duchatelet, L.; Hermans, C.; Baguet, F. Luminescence control of Stomiidae photophores. Acta Histochem. 2019, 121, 7–15. [Google Scholar] [CrossRef]
- Mallefet, J.; Shimomura, O. Presence of coelenterazine in mesopelagic fishes from the Strait of Messina. Mar. Biol. 1995, 124, 381–385. [Google Scholar] [CrossRef]
- Davis, A.L.; Sutton, T.T.; Kier, W.M.; Johnsen, S. Evidence that eye-facing photophores serve as a reference for counterillumination in an order of deep-sea fishes. Proc. Biol. Sci. 2020, 287, 20192918. [Google Scholar] [CrossRef]
- Krönström, J.; Dupont, S.; Mallefet, J.; Thorndyke, M.; Holmgren, S. Serotonin and nitric oxide interaction in the control of bioluminescence in northern krill, Meganyctiphanes norvegica (M. Sars). J. Exp. Biol. 2007, 210, 3179–3187. [Google Scholar] [CrossRef]
- Coccimiglio, M.L.; Jonz, M.G. Serotonergic neuroepithelial cells of the skin in developing zebrafish: Morphology, innervation and oxygen-sensitive properties. J. Exp. Biol. 2012, 215, 3881–3894. [Google Scholar] [CrossRef]
- Jonz, M.G.; Nurse, C.A. Peripheral chemoreceptors in air-versus water-breathers. In Arterial Chemoreception: From Molecules to Systems; Springer: Berlin/Heidelberg, Germany, 2012; pp. 19–27. [Google Scholar] [CrossRef]
- Percival, J.M.; Anderson, K.N.; Huang, P.; Adams, M.E.; Froehner, S.C. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J. Clin. Investig. 2010, 120, 816–826. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Aragona, M.; Mhalhel, K.; Pansera, L.; Montalbano, G.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Abbate, F.; Vega, J.A.; Germanà, A. Localization of Piezo 1 and Piezo 2 in Lateral Line System and Inner Ear of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2024, 25, 9204. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Consentius, C.; Mirenska, A.; Jurisch, A.; Reinke, S.; Scharm, M.; Zenclussen, A.C.; Hennig, C.; Volk, H.-D. In situ detection of CD73+ CD90+ CD105+ lineage: Mesenchymal stromal cells in human placenta and bone marrow specimens by chipcytometry. Cytom. Part A 2018, 93, 889–893. [Google Scholar] [CrossRef]
- Fonseca, L.N.; Bolívar-Moná, S.; Agudelo, T.; Beltrán, L.D.; Camargo, D.; Correa, N.; Del Castillo, M.A.; Fernández de Castro, S.; Fula, V.; García, G.; et al. Cell surface markers for mesenchymal stem cells related to the skeletal system: A scoping review. Heliyon 2023, 9, e13464. [Google Scholar] [CrossRef]
- Spooner-Harris, M.; Kerns, K.; Zigo, M.; Sutovsky, P.; Balboula, A.; Patterson, A.L. A re-appraisal of mesenchymal-epithelial transition (MET) in endometrial epithelial remodeling. Cell Tissue Res. 2023, 391, 393–408. [Google Scholar] [CrossRef]
- Abbate, F.; Catania, S.; Germana, A.; González, T.; Diaz-Esnal, B.; Germana, G.; Vega, J. S-100 protein is a selective marker for sensory hair cells of the lateral line system in teleosts. Neurosci. Lett. 2002, 329, 133–136. [Google Scholar] [CrossRef]
- Germana, A.; Abbate, F.; González-Martínez, T.; Del Valle, M.; De Carlos, F.; Germanà, G.; Vega, J. S100 protein is a useful and specific marker for hair cells of the lateral line system in postembryonic zebrafish. Neurosci. Lett. 2004, 365, 186–189. [Google Scholar] [CrossRef]
- Germana, A.; Montalbano, G.; Laura, R.; Ciriaco, E.; Del Valle, M.; Vega, J.A. S100 protein-like immunoreactivity in the crypt olfactory neurons of the adult zebrafish. Neurosci. Lett. 2004, 371, 196–198. [Google Scholar] [CrossRef]
- Germanà, A.; Paruta, S.; Germanà, G.P.; Ochoa-Erena, F.J.; Montalbano, G.; Cobo, J.; Vega, J.A. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio). Brain Res. 2007, 1162, 48–55. [Google Scholar] [CrossRef]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Donato, R.; Geczy, C.L.; Weber, D.J. S100 Proteins. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1863–1874. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Nicol, J.A.C. Observations on Photophores and Luminescence in the Teleost Porichthys. J. Cell Sci. 1957, 3, 179–188. [Google Scholar] [CrossRef]
- Krönström, J. Control of Bioluminescence. Operating the Light Switch in Photophores from Marine Animals. Ph.D. Thesis, University of Gothenburg, Göteborg, Sweden, 2009. [Google Scholar]
- Claes, J.M.; Krönström, J.; Holmgren, S.; Mallefet, J. Nitric oxide in the control of luminescence from lantern shark (Etmopterus spinax) photophores. J. Exp. Biol. 2010, 213, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Holcroft, N.I.; Wiley, E.O.; Sparks, J.S.; Leo Smith, W. Species-specific bioluminescence facilitates speciation in the deep sea. Mar. Biol. 2014, 161, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Irigoien, X.; Klevjer, T.A.; Røstad, A.; Martinez, U.; Boyra, G.; Acuña, J.L.; Bode, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Hernandez-Leon, S.; et al. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 2014, 5, 3271. [Google Scholar] [CrossRef]
- Davison, P.C.; Checkley, D.M.; Koslow, J.A.; Barlow, J. Carbon export mediated by mesopelagic fishes in the northeast Pacific Ocean. Prog. Oceanogr. 2013, 116, 14–30. [Google Scholar] [CrossRef]
- Roda, A.; Pasini, P.; Mirasoli, M.; Michelini, E.; Guardigli, M. Biotechnological applications of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef]

| Scientific Name | Author |
|---|---|
| Ichthyococcus australis | Mukhacheva, 1980 |
| Ichthyococcus elongatus | Imai, 1941 |
| Ichthyococcus intermedius | Mukhacheva, 1980 |
| Ichthyococcus irregularis | Rechnitzer and Böhlke, 1958 |
| Ichthyococcus ovatus | Cocco, 1838 |
| Ichthyococcus parini | Mukhacheva, 1980 |
| Ichthyococcus polli | Blache, 1963 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallaro, M.; Pansera, L.; Mhalhel, K.; Laurà, R.; Levanti, M.; Montalbano, G.; Abbate, F.; Aragona, M.; Guerrera, M.C. Morphological and Immunohistochemical Study of Ventral Photophores of Ichthyococcus ovatus (Cocco, 1838) (Fam: Stomiidae). J. Mar. Sci. Eng. 2025, 13, 1534. https://doi.org/10.3390/jmse13081534
Cavallaro M, Pansera L, Mhalhel K, Laurà R, Levanti M, Montalbano G, Abbate F, Aragona M, Guerrera MC. Morphological and Immunohistochemical Study of Ventral Photophores of Ichthyococcus ovatus (Cocco, 1838) (Fam: Stomiidae). Journal of Marine Science and Engineering. 2025; 13(8):1534. https://doi.org/10.3390/jmse13081534
Chicago/Turabian StyleCavallaro, Mauro, Lidia Pansera, Kamel Mhalhel, Rosaria Laurà, Maria Levanti, Giuseppe Montalbano, Francesco Abbate, Marialuisa Aragona, and Maria Cristina Guerrera. 2025. "Morphological and Immunohistochemical Study of Ventral Photophores of Ichthyococcus ovatus (Cocco, 1838) (Fam: Stomiidae)" Journal of Marine Science and Engineering 13, no. 8: 1534. https://doi.org/10.3390/jmse13081534
APA StyleCavallaro, M., Pansera, L., Mhalhel, K., Laurà, R., Levanti, M., Montalbano, G., Abbate, F., Aragona, M., & Guerrera, M. C. (2025). Morphological and Immunohistochemical Study of Ventral Photophores of Ichthyococcus ovatus (Cocco, 1838) (Fam: Stomiidae). Journal of Marine Science and Engineering, 13(8), 1534. https://doi.org/10.3390/jmse13081534







