Abstract
Over recent decades, widespread declines of kelp forests have been reported along the European coast, prompting the need for effective and scalable restoration strategies. The green gravel technique, in which kelp gametophytes are seeded onto small rocks and cultivated in the lab before being outplanted, has shown promising results. In this study, we tested the effects of four commonly available substrates—granite, limestone, quartz, and schist—on the early development of Laminaria ochroleuca recruits under optimal laboratory conditions. All substrates supported gametophyte adhesion and sporophyte development. By week 6, quartz promoted the greatest recruit length (1.25 ± 0.16 mm), with quartz and limestone (1.54 ± 0.17 and 1.58 ± 0.14 mm, respectively) showing the best overall performance by week 7. Final recruit densities were similar across substrates, indicating multiple materials can support early development. Quartz and limestone showed both biological effectiveness and practical advantages, with limestone emerging as the most cost-effective option. Substrate selection should consider not only biological performance but also economic and logistical factors. These findings contribute to refining green gravel protocols and improving the feasibility of large-scale kelp forest restoration, although field validation is necessary to assess long-term outcomes under natural conditions.
1. Introduction
Kelp forests are suffering a global decline with some regions facing almost complete local disappearances. This decline is not merely due to range shifts but rather the result of a complex set of factors that vary across different environments [1,2,3]. These ecosystems are increasingly threatened by anthropogenic and climate-driven factors, including overfishing, eutrophication, pollution, ocean warming, and extreme events such as storms [4,5]. These stressors can have severe consequences for marine ecosystems, and projections indicate further kelp forest losses in the near future [6,7,8].
Kelp forests provide a broad range of ecosystem goods and services that are ecological, economic, and social. These services include direct benefits, such as kelp harvesting, commercial and recreational fisheries, and ecotourism, as well as indirect benefits like coastal protection, nutrient cycling, carbon storage and sequestration, and mitigation of eutrophication and ocean acidification [9,10,11]. Kelp forests also provide significant non-use values, including the maintenance of biodiversity, opportunities for scientific research, and cultural importance [12]. As habitat-forming species, kelps act as ecosystem engineers, modifying their environment and creating complex three-dimensional structures that support diverse biological communities [13]. These structures provide nursery grounds, feeding areas, and shelter for numerous marine organisms, including invertebrates, fish, and marine mammals [10,14,15].
Beyond their ecological role, kelp forests play an important role in coastal defence. They can attenuate wave intensity and modify hydrodynamic flow, these effects vary with species and individuals’ morphological characteristics, such as height and robustness. By mitigating coastal erosion and stabilizing sediments, kelp forests contribute to maintaining coastal structural integrity [16,17]. Additionally, kelp forests enhance marine biodiversity by modifying local environmental conditions such as temperature, sedimentation, and hydrodynamics. They create complex habitats that support diverse biological communities and facilitate species recruitment [9,10,18]. Given their ecological significance and growing role in coastal protection, safeguarding kelp forests is essential to ensuring the resilience of marine ecosystems [11,19].
As a response to ongoing declines, restoration efforts have been ongoing with various techniques, both resorting to transplantation and non-extractive approaches like green gravel, which builds on well-established propagation techniques from macroalgae aquaculture. Seeding offers several advantages over transplantation. (i) Transplantation requires the removal of individuals from the donor population, which can negatively affect their ability to maintain population stability. (ii) Kelps are adapted to local environmental conditions, often growing slender stipes in areas with low hydrodynamics. When transplanted to a different location, they might not be suited to the new conditions, resulting in elevated mortality. (iii) Seeding, in contrast, allows producing recruits in controlled laboratory settings, enabling reforestation efforts to begin early [20,21,22,23]. These individuals also have the opportunity to adapt to the local conditions from the outset. However, the seeding method is not without disadvantages. As adult kelps provide shelter and protection for juveniles, reforestation efforts based solely on recruits may experience higher mortalities due to the increased exposure to environmental conditions [24]. Yet, summing up the pros and cons, seeding restoration methodologies are overall preferred to extractive approaches due to their almost neglectable effect on the donor populations.
Because kelps experience their highest mortality during early life stages (particularly at the microscopic phase), restoration projects relying on these stages are inherently high risk [25]. Cultivating kelp in a controlled laboratory setting before outplanting provides a more stable environment, reducing exposure to natural stressors such as grazing, competition, and hydrodynamic forces [20]. This increases the survival rate of juveniles, improving the overall success of restoration efforts. Laboratory conditions enable fine tuning the temperature, light, and nutrient levels, which are critical during early development [26,27]. These advantages help ensure that recruits reach a minimum size and physiological robustness necessary for higher chances of survival upon outplanting. Deploying recruits at an early developmental stage may enhance their ability to adapt to strong currents, decreasing the risk of developing fragile stipes that are more prone to breaking under natural hydrodynamic conditions. Therefore, balancing laboratory growth with timely outplanting is crucial to maximize the resilience and field performance of kelp recruits.
Among the seeding-based restoration techniques, the ‘green gravel’ method has been successfully used to restore kelp forests along the southern coast of Norway [21], on the Portuguese coastline [28], in Danish waters [29], in Australia, and in Canada [30]. This method consists of sowing small rocks with kelp gametophytes and cultivating them in optimal conditions in the laboratory until they reach about 1 cm in length, after which they are outplanted at sea. While several studies have focused of the effectiveness of the method, particularly after outplanting, not many have looked into optimizing the green gravel production, improving its effectiveness and efficiency, reducing costs and creating the basis to allow scaling up the efforts [31,32,33].
The golden kelp L. ochroleuca serves as a model organism in our research. This species can be found along continental Portugal and in the seamounts of Azores, extending northward to the northwest coasts of Spain, Brittany (France), the English and Bristol Channels, and more recently, Ireland. Further south, it has also been reported in the Strait of Messina (Italy) and along the coast of Morocco [34,35]. Ocean warming has been linked to shifts in its distribution range. At its equatorial limit, L. ochroleuca is experiencing declines, while at its northern edge, it is expanding and increasingly outcompeting Laminaria hyperborea [36]. These shifts highlight the species’ ability to persist under warming conditions, particularly at its southern distribution limit, where its forests may be increasingly vulnerable to environmental change. L. ochroleuca usually inhabits deep intertidal pools and the subtidal down to 30 m deep. Along the Portuguese coast, they are distributed between lower intertidal pools and 3–23 m deep with an average depth of about 10 m [37].
The main goal of this study was to optimize the green gravel method by examining how different substrates (granite, limestone, quartz, and schist) can affect kelp recruitment. While most studies testing the green gravel method have used granite, no prior research has explicitly evaluated whether it is the most efficient substrate for this technique. To address this gap, these substrates were selected as they are some of the most common in the intertidal and shallow subtidal areas and are easy to find at an accessible cost in hardware stores. Although this work focuses on the golden kelp Laminaria ochroleuca, the gained insights may be applicable to other kelp species given their comparable ecological functions and physiological traits.
2. Materials and Methods
2.1. Sample Collection and Laboratory Procedures
Fertile tissue from 12 individuals of L. ochroleuca was collected in the spring of 2021, from intertidal pools in Carreço, northern Portugal (41°44′30.3″ N 8°52′38.3″ W). On the day of collection, the tissue was rinsed with filtered seawater to remove epiphytes and protozoa. It was then pat-dried and stored wrapped in paper towel at 5 °C for 30 min to cause a slight osmotic stress. Sporulation was subsequently induced by submerging the tissue in autoclaved seawater for 2 h at 5 °C. The resulting cultures were transferred to t-flasks with 0.2 µM Provasoli Enriched Seawater (PES) [38] and maintained under white light (40 µmol m−2 s−1) to stimulate spore germination. Once spores had developed into gametophytes, after 5 days, the stock cultures were transferred to low-intensity red light (12 µmol m−2 s−1) to suppress gametogenesis while promoting vegetative growth. The culture was kept in these conditions, with weekly medium changes, for one month. This gametophyte solution, with an estimated density of 110 gametophytes mL−1, was sprayed directly onto granite, limestone, quartz, and schist stones (5 cm length), covering the bottom of 50 L tanks (Figure 1). Three tanks were used for each gravel type (n = 3). The tanks were filled one hour after spraying to allow them to better fix to the substrate. The system was kept at 15 °C, the optimum temperature for this species [39,40]. All tanks were connected to a common recirculating seawater system, which was sterilised with Titan UV Steriliser P2 110 W (Tropical Marine Centre, S. Julião do Tojal, Portugal). The tanks were illuminated with OSRAM L 58 W /965 Biolux fluorescent lights (OSRAM Licht AG, Munich, Germany) at 100 µmol m−2 s−1, with a 12:12 h light: dark photoperiod, to match the spring conditions in Portugal, when recruitment usually peaks.

Figure 1.
Schematic representation of the green gravel experimental setup using four substrate types: granite, limestone, quartz, and schist.
Environmental conditions, including dissolved oxygen, pH, salinity, and temperature, were monitored using a Hach HQ40D Portable Multi Meter (Hach Company, Colorado, USA). To ensure an adequate nutrient supply, PES medium was used, and monitoring and water renewal were carried out on a weekly basis. Recruitment and size were monitored every week by randomly selecting 3 stones from each replicate and observing them under a Leica EZ4W Stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany) at a 35× magnification.
2.2. Data Analysis Kelp
Length and density were measured using 15 pictures from randomly selected areas on 3 stones per tank (totaling 45 pictures per tank) and analysed with ImageJ (version 1.53e, US National Institutes of Health, Bethesda, MD, USA). A linear mixed-effects model (LMM) was performed using R software (version 4.5.1), with the lme4 package [41] to fit the model. The LMM was used to assess the effect of different substrates (limestone, granite, quartz, and schist) and time on recruit length. The model was constructed to test the influence of substrates, time, and their interaction, where substrates and time were treated as fixed effects, and tanks were included as a random effect to account for the variation between tanks. The response variable was the recruit’s length. The same model was also employed to evaluate the impact of substrate and time on recruit density. Model assumptions, including normality and homoscedasticity, were checked through residual analysis. Tukey’s HSD post hoc test was applied for pairwise comparisons to identify significant differences (with p-value < 0.05) between treatments, where applicable.
3. Results
3.1. Sporophyte Length
The trial started in mid-April and lasted a total of 8 weeks. The first kelp recruitment was observed in the 3rd week (Figure 2).
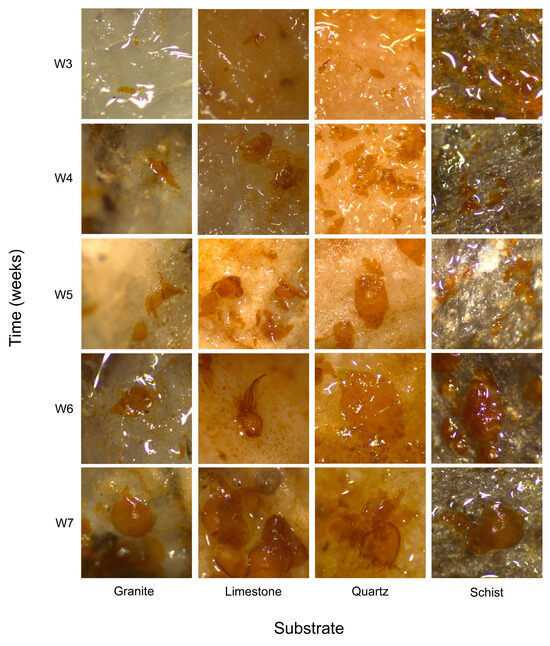
Figure 2.
Microscopic images of L. ochroleuca recruits on different substrates (granite, limestone, quartz, and schist), taken during the sampling period following the first observation of individuals. Images captured using a Leica EZ4W stereomicroscope.
The analysis revealed that time and substrate type are statistically significant factors influencing kelp size (p < 0.001) (Figure 3). From week 3 to week 5, no significant differences were observed among the substrates. In week 6, quartz exhibited significantly higher kelp length (1.25 ± 0.16 mm) compared with the remaining substrates. Limestone exhibited the lowest length also compared with schist (0.67 ± 0.05 mm, p < 0.001), while no significant difference was observed with granite. Finally, in week 7, limestone (1.58 ± 0.14 mm) outperformed schist (1.2 ± 0.1 mm; p = 0.029) and granite (1.14 ± 0.09 mm; p = 0.09) while showing no significant differences compared to quartz (1.54 ± 0.17 mm). Schist and granite exhibited similar length (1.20 ± 0.1 and 1.14 ± 0.09 mm, respectively) during this period.
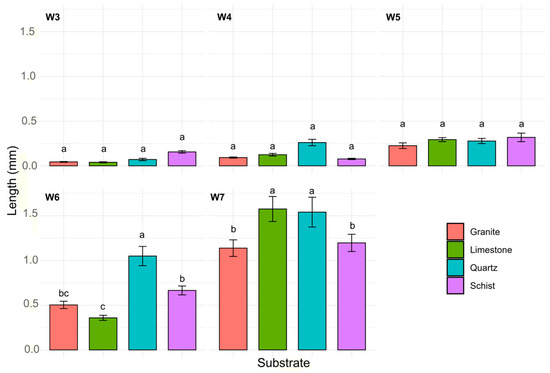
Figure 3.
L. ochroleuca length variability (mm) across different substrates (granite, limestone, quartz, and schist) for each experimental week (W3 to W7). Bars represent the mean L. ochroleuca length with error bars indicating the standard error of the mean (±SE). Different letters represent significant differences between treatments.
3.2. Sporophyte Density
The analysis showed that the interaction between time and substrate type is a statistically significant factor influencing kelp density (p < 0.001; Figure 4). In weeks 3, 6, and 7, no significant difference was observed between the substrates. In week 4, significant differences were observed between quartz and schist (0.90 ± 0.27 individuals mm−2 vs. 0.50 ± 0.05 individuals mm−2, p = 0.0023) and between limestone and quartz (0.51 ± 0.10 individuals mm−2 vs. 0.50 ± 0.05 individuals mm−2, p = 0.0022). However, no significant differences were detected between the remaining substrates. In week 5, significant differences were observed between limestone and granite (0.84 ± 0.08 individuals mm−2 vs. 0.49 ± 0.05 individuals mm−2, p = 0.0025) and between limestone and quartz (0.84 ± 0.08 individuals mm−2 vs. 0.45 ± 0.05 individuals mm−2, p = 0.0027). The results indicate that limestone stood out as a substrate with significantly higher densities compared to granite and quartz during this experiment stage. By week 6 and 7, however, no significant difference were observed among substrates.
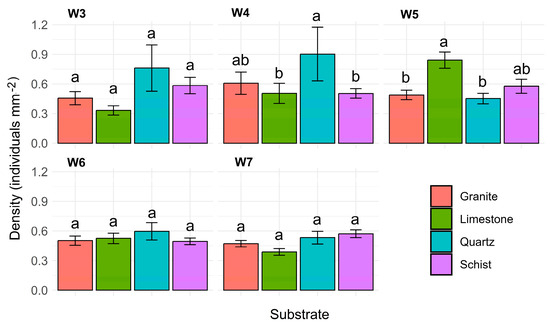
Figure 4.
L. ochroleuca density in different substrates (granite, limestone, quartz, and schist) for each experimental week (W3 to W7). Bars represent the mean L. ochroleuca density with error bars indicating the standard error of the mean (±SE). Different letters denote significant differences between treatments.
4. Discussion
This study aimed to optimize the green gravel method, focusing on the kelp species L. ochroleuca, by investigating how different substrata (granite, limestone, quartz, and schist) affect recruitment success particularly in terms of timing, recruit size, and adherence to the substrate.
In terms of recruit density, the overall assessment indicated that substrate type did not have an outstanding effect, suggesting that all tested materials can support the initial settlement and early development of L. ochroleuca under controlled conditions. However, looking at kelp length, our results demonstrated that the type of substrate plays a significant role in early kelp growth. Among the tested substrates, quartz exhibited the overall highest sporophyte size. By week 6, quartz emerged as the most favourable substrate for recruitment, outperforming the remaining substrates. However, by week 7, limestone demonstrated comparable efficiency, suggesting that multiple substrate types may be suitable for the green gravel technique.
Our findings align with previous research demonstrating that substrate characteristics, such as roughness and colour, can potentially play a crucial role in macroalgal settlement and early growth [42]. Past studies on other kelp species have highlighted the importance of surface roughness in enhancing early-stage settlement and recruitment [43]. Roughened surfaces are believed to strengthen juvenile attachment by increasing the available area for holdfast anchorage and allowing rhizoids to interlock within microscopic crevices, thereby improving stability [44,45]. The differences observed between limestone and granite, in week 7, may be partially explained by the concept of ‘geophycology’, which suggests that macroalgae interact with substrate surfaces through both physical and chemical mechanisms. According to Morrison et al. [45], kelp holdfasts can penetrate bedrock by exploiting the physical characteristics of rock-forming minerals, such as inter- and intra-crystalline boundaries. In their study, holdfasts penetrated up to 1.5 mm in granite and 4 mm in limestone, demonstrating that substrate type can significantly influence anchoring success. Although our study did not measure holdfast penetration, the superior performance of quartz and limestone over granite may reflect such microstructural differences, which could enhance early-stage settlement and recruitment. Additionally, previous research also suggested that colour, through its influence on light absorption and temperature, may affect algal settlement and growth. Darker substrates have been associated with higher settlement rates, potentially due to localized temperature increases that enhance zoospore adhesion [46,47]. However, our results suggest a more complex pattern, as among the darker substrates, schist performed well overall but did not exhibit the best performance. In contrast, the lighter substrate limestone showed inconsistent results, with improved performance only emerging in the final week. While no single substrate had the highest values for both recruit length and density, quartz exhibited the most consistent overall performance. This may be attributed to a combination of factors, such as surface texture, mineral composition, and chemical properties, which together could create more favourable conditions for attachment and early development. These findings emphasize the complex interactions between substrate properties and macroalgal recruitment, highlighting the need for further research to better understand the underlying mechanisms.
From both practical and economic perspectives, substrate selection is crucial for optimizing large-scale kelp restoration. The green gravel method is increasingly recognized as a cost-effective and scalable technique, reducing reliance on labour- and resource-intensive transplantation methods. Since quartz showed promising results by week 6, its use could enable earlier deployment, thereby significantly reducing the duration and cost of laboratory cultivation. This supports the broader goal of making kelp restoration more economically viable and accessible for conservation initiatives.
Results from week 7 indicate that limestone may be an equally viable alternative. Both quartz and limestone are generally more readily available in hardware stores, although quartz tends to be more expensive. Given that quartz shares similar physical characteristics with granite (such as size, weight, and surface roughness), and that granite has been commonly used in previous green gravel efforts, the favourable results observed for quartz, particularly in comparison with granite, suggest it may be a better alternative substrate, in terms of kelp performance, for this technique.
From a practical perspective, the economic cost of the substrates is an important consideration for large-scale application of this technique. Based on average prices from local hardware suppliers, granite and limestone are typically the most affordable options. Quartz is moderately priced (≈0.29 €/kg), while schist is the most expensive among the tested materials (≈0.54 €/kg), costing more than twice as much as granite (≈0.24 €/kg) or limestone (≈0.25 €/kg). Although quartz exhibited the most consistent biological performance in this study, its higher cost compared with limestone and granite may limit its applicability for large-scale restoration efforts. Therefore, practitioners must weigh biological effectiveness against economic constraints, in selecting substrates, especially in large-scale projects where material costs can be significant. Similarly, while PES medium was effective for this short-term controlled experiments, future large-scale applications should consider more cost-effective and less contamination-prone alternatives (e.g., Erdschreiber’s (ESS) culture medium).
Given the nuanced differences in suitability among quartz, limestone, and schist, the final substrate selection will likely depend on multiple factors, including site-specific hydrodynamic conditions, material availability, and overall cost. Despite granite being the most commonly used substrate in reforestation efforts [21], our findings do not support its use. These findings may encourage future studies to explore alternative materials that enhance the efficiency and success of green gravel technique. Ultimately, long-term monitoring following outplanting will be essential for refining substrate selection and improving restoration outcomes.
5. Conclusions
This study contributes to the optimization of the green gravel technique for Laminaria ochroleuca restoration by evaluating the effects of different substrates on early sporophyte development. While all materials supported kelp recruitment, quartz and limestone promoted greater sporophyte length by the final week, suggesting multiple substrates may be viable under controlled conditions. These results also indicate that earlier deployment of seeded gravel could reduce cultivation time and associated costs. Although further validation under field conditions is needed, particularly regarding hydrodynamics and herbivory, these findings provide practical insights for cost-effective and scalable kelp restoration.
Author Contributions
Conceptualization, S.C. and T.R.P.; methodology, T.F.P., S.C. and T.R.P.; validation, S.C., T.R.P. and I.S.-P.; formal analysis, T.F.P.; investigation, S.C. and T.R.P.; resources, I.S.-P.; writing—original draft preparation, T.F.P.; writing—review and editing, S.C. and T.R.P.; supervision, I.S.-P.; project administration, I.S.-P.; funding acquisition, I.S.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Seaforests for blue carbon—natural capital from nature-based solutions” funded by EEA Grants (PT-INNOVATION-0081).
Data Availability Statement
The original contributions presented in this study are included in the complimentary article. Further enquiries may be addressed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Fujita, D. Management of kelp ecosystem in Japan. Cah. Biol. Mar. 2011, 52, 499–505. [Google Scholar]
- Rogers-Bennett, L.; Catton, C. Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Sci. Rep. 2019, 9, 15050. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Marzinelli, E.; Vergés, A.; Coleman, M.; Steinberg, P. Towards Restoration of Missing Underwater Forests. PLoS ONE 2014, 9, e84106. [Google Scholar] [CrossRef]
- Araújo, R.; Assis, J.; Aguillar, R.; Airoldi, L.; Bárbara, I.; Bartsch, I.; Bekkby, T.; Christie, H.; Davoult, D.; Derrien-Courtel, S.; et al. Status, trends and drivers of kelp forests in Europe: An expert assessment. Biodivers. Conserv. 2016, 25, 1319–1348. [Google Scholar] [CrossRef]
- Krumhansl, K.A.; Okamoto, D.; Rassweiler, A.; Novak, M.; Bolton, J.; Cavanaugh, K.; Connell, S.; Johnson, C.R.; Konar, B.; Ling, S.; et al. Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. USA 2016, 113, 13785–13790. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Rise of Turfs: A New Battlefront for Globally Declining Kelp Forests. BioScience 2018, 68, 64–76. [Google Scholar] [CrossRef]
- Martinez, B.; Radford, B.; Thomsen, M.; Connell, S.; Carreño, F.; Bradshaw, C.; Fordham, D.; Russell, B.; Gurgel, C.; Wernberg, T. Distribution models predict large contractions of habitat-forming seaweeds in response to ocean warming. Divers. Distrib. 2018, 24, 1350–1366. [Google Scholar] [CrossRef]
- Christie, H.; Norderhaug, K.; Fredriksen, S. Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 2009, 396, 221–233. [Google Scholar] [CrossRef]
- Teagle, H.; Hawkins, S.J.; Moore, P.; Smale, D. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Biol. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T.; Barreiro, R.; Coleman, M.A.; Bettignies, T.d.; Feehan, C.J.; Franco, J.N.; Hasler, B.; Louro, I.; Norderhaug, K.M.; et al. Leveraging the blue economy to transform marine forest restoration. J. Phycol. 2022, 58, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Wernberg, T.; Connell, S.; Hobday, A.J.; Johnson, C.R.; Poloczanska, E.S. The ‘Great Southern Reef’: Social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshw. Res. 2016, 67, 47–56. [Google Scholar] [CrossRef]
- Jones, C.; Lawton, J.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 130–147. [Google Scholar] [CrossRef]
- Graham, M. Effects of Local Deforestation on the Diversity and Structure of Southern California Giant Kelp Forest Food Webs. Ecosystems 2003, 7, 341–357. [Google Scholar] [CrossRef]
- Bertocci, I.; Araújo, R.; Oliveira, P.J.; Sousa-Pinto, I. Review: Potential effects of kelp species on local fisheries. J. Appl. Ecol. 2015, 52, 1216–1226. [Google Scholar] [CrossRef]
- Løvås, S.M.; Tørum, A. Effect of the kelp Laminaria hyperborea upon sand dune erosion and water particle velocities. Coast. Eng. 2001, 44, 37–63. [Google Scholar] [CrossRef]
- Mork, M. The effect of kelp in wave damping. Sarsia 1996, 80, 323–327. [Google Scholar] [CrossRef]
- Thomsen, M.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; McGlathery, K.; Holmer, M.; Silliman, B. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 2010, 50, 158–175. [Google Scholar] [CrossRef]
- Feehan, C.J.; Filbee-Dexter, K.; Wernberg, T. Embrace kelp forests in the coming decade. Science 2021, 373, 863. [Google Scholar] [CrossRef]
- Eger, A.M.; Layton, C.; McHugh, T.A.; Gleason, M.; Eddy, N. Kelp Restoration Guidebook: Lessons Learned from Kelp Projects Around the World; The Nature Conservancy: Arlington, VA, USA, 2022. [Google Scholar]
- Fredriksen, S.; Filbee-Dexter, K.; Norderhaug, K.M.; Steen, H.; Bodvin, T.; Coleman, M.A.; Moy, F.E.; Wernberg, T. Green gravel: A novel restoration tool to combat kelp forest decline. Sci. Rep. 2020, 10, 3983. [Google Scholar] [CrossRef]
- Eger, A.M.; Marzinelli, E.M.; Christie, H.; Fagerli, C.W.; Fujita, D.; Gonzalez, A.P.; Hong, S.W.; Kim, J.H.; Lee, L.C.; McHugh, T.A.; et al. Global kelp forest restoration: Past lessons, present status, and future directions. Biol. Rev. 2022, 97, 1449–1475. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.L.; Hale, R.; Strain, E.M.A.; Reeves, S.E.; Vergés, A.; Marzinelli, E.M.; Layton, C.; Shelamoff, V.; Graham, T.D.J.; Chevalier, M.; et al. Key Principles for Managing Recovery of Kelp Forests through Restoration. BioScience 2020, 70, 688–698. [Google Scholar] [CrossRef]
- Layton, C.; Shelamoff, V.; Cameron, M.J.; Tatsumi, M.; Wright, J.T.; Johnson, C.R. Resilience and stability of kelp forests: The importance of patch dynamics and environment-engineer feedbacks. PLoS ONE 2019, 14, e0210220. [Google Scholar] [CrossRef] [PubMed]
- Schiel, D.R.; Foster, M.S. The Population Biology of Large Brown Seaweeds: Ecological Consequences of Multiphase Life Histories in Dynamic Coastal. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 343–372. [Google Scholar] [CrossRef]
- Reed, D.C. The Effects of Variable Settlement and Early Competition on Patterns of Kelp Recruitment. Ecology 1990, 71, 776–787. [Google Scholar] [CrossRef]
- Perrow, M.R.; Davy, A.J. Handbook of Ecological Restoration; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Marques, A.; Sanchéz-Gallego, Á.; Correia, R.; Sousa Pinto, I.; Chemello, S.; Louro, I.; Lemos, M.; Franco, J. Assessing Atlantic Kelp Forest Restoration Efforts in Southern Europe. Sustainability 2024, 16, 9176. [Google Scholar] [CrossRef]
- Enevoldsen, K. Green Gravel—A Novel Kelp Forest Restoration Method Tested on an Artificial Boulder Reef in Danish Waters. Master’s Thesis, Aarhus University, Aarhus, Denmark, 2022. [Google Scholar]
- Eger, A.; Eddy, N.; Gleason, M.; Layton, C.; McHugh, T.; Steinberg, P.; Vergés, A. The Kelp Forest Alliance: A Global Community of Practice to Understand, Advise, and Motivate Kelp Forest Conservation and Restoration. Limnol. Oceanogr. Bull. 2022, 31, 130–132. [Google Scholar] [CrossRef]
- Alsuwaiyan, N.; Filbee-Dexter, K.; Vranken, S.; Burkholz, C.; Cambridge, M.; Coleman, M.; Wernberg, T. Green gravel as a vector of dispersal for kelp restoration. Front. Mar. Sci. 2022, 9, 910417. [Google Scholar] [CrossRef]
- Chemello, S.; Pinto, I.S.; Pereira, T.R. Optimising Kelp Cultivation to Scale up Habitat Restoration Efforts: Effect of Light Intensity on “Green Gravel” Production. Hydrobiology 2023, 2, 347–353. [Google Scholar] [CrossRef]
- Chemello, S.; Santos, I.; Sousa Pinto, I.; Pereira, T. Unlocking the Potential of Green Gravel Production for Efficient Kelp Restoration: How Seeding Density Affects the Development of the Golden Kelp Laminaria ochroleuca. Phycology 2024, 4, 443–449. [Google Scholar] [CrossRef]
- Birkett, D.; Maggs, C.; Dring, M.; Boaden, P. An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs. Scott. Assoc. Mar. Sci. SAMS 1998, 5, 1–174. [Google Scholar]
- Schoenrock, K.; O’Callaghan, T.; O’Callaghan, R.; Krueger-Hadfield, S. First record of Laminaria ochroleuca Bachelot de la Pylaie in Ireland in Béal an Mhuirthead, county Mayo. Mar. Biodivers. Rec. 2019, 12, 9. [Google Scholar] [CrossRef]
- Smale, D.; Wernberg, T.; Yunnie, A.L.E.; Vance, T. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Mar. Ecol. 2015, 36, 1033–1044. [Google Scholar] [CrossRef]
- Tuya, F.; Cacabelos, E.; Duarte, P.; Jacinto, D.; Castro, J.J.; Silva, T.; Bertocci, I.; Franco, J.N.; Arenas, F.; Coca, J.; et al. Patterns of landscape and assemblage structure along a latitudinal gradient in ocean climate. Mar. Ecol. Prog. Ser. 2012, 466, 9–19. [Google Scholar] [CrossRef]
- Provasoli, L. Media and prospects for the cultivation of marine algae. Cult. Collect. Algae 1966, 63–75. [Google Scholar]
- Izquierdo, J.; Pérez-ruzafa, I.; Gallardo, T. Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie. Helgol. Mar. Res. 2001, 55, 285–292. [Google Scholar] [CrossRef]
- Biškup, S.; Bertocci, I.; Arenas, F.; Tuya, F. Functional responses of juvenile kelps, Laminaria ochroleuca and Saccorhiza polyschides, to increasing temperatures. Aquat. Bot. 2014, 113, 117–122. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kerrison, P.; Stanley, M.; Kelly, M.; MacLeod, A.; Black, K.; Hughes, A. Optimising the settlement and hatchery culture of Saccharina latissima (Phaeophyta) by manipulation of growth medium and substrate surface condition. J. Appl. Phycol. 2015, 28, 1181–1191. [Google Scholar] [CrossRef]
- Muth, A.F. Effects of Zoospore Aggregation and Substrate Rugosity on Kelp Recruitment Success. J. Phycol. 2012, 48, 1374–1379. [Google Scholar] [CrossRef]
- Milligan, K.; DeWreede, R.E. Variations in holdfast attachment mechanics with developmental stage, substratum-type, season, and wave-exposure for the intertidal kelp species Hedophyllum sessile (C. Agardh) Setchell. J. Exp. Mar. Biol. Ecol. 2000, 254, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.; Feely, M.; Stengel, D.; Blamey, N.; Dockery, P.; Sherlock, A.; Timmins, E. Seaweed attachment to bedrock: Biophysical evidence for a new geophycology paradigm. Geobiology 2009, 7, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Dahlem, C.; Moran, P.J.; Grant, T. Larval settlement of marine sessile invertebrates on surfaces of different colour and position. Ocean. Sci. Eng. 1984, 9, 225–236. [Google Scholar]
- Swain, G.W.; Herpe, S.; Ralston, E.; Tribou, M. Short-term testing of antifouling surfaces: The importance of colour. Biofouling 2006, 22, 425–429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).